Abstract
Importance
One-fifth of patients with peripheral artery disease (PAD) experience depression and stress. Depression and stress may impact patients’ abilities to be physically active, a key recommendation for supporting overall PAD management to improve symptoms and reduce the risk of cardiovascular events.
Objective
To study interrelationships between 1-year longitudinal trajectories of depression, stress, and physical activity following a PAD diagnosis.
Design
Longitudinal observational multi-center registry. Patient interviews were completed at baseline, 3, 6, and 12 months.
Setting
Patients were enrolled in the PORTRAIT study from 10 US vascular specialty clinics.
Participants
Patients with new or worsening lower extremity PAD with claudication symptoms seen from June 2, 2011, to December 3, 2015 were included.
Main Outcome and Measures
Depressive symptoms (measured with the 8-item Patient Health Questionnaire) and perceived stress (measured with the 4-item Perceived Stress Scale). Physical activity, assessed with INTERHEART study questions. All measures were assessed at 3, 6, and 12 months after presentation.
Results
A total of 766 patients were included (mean age of 68.2 (± 9.4) years, 57.7% male). Overall, 17.8% reported significant depressive symptoms, 36.0% experienced increased perceived stress, 44.1% were sedentary upon PAD diagnosis. A decrease in physical activity preceded a rise in subsequent depressive symptoms (β ranges −0.45; −0.81) over the course of one year. A decrease in physical activity was followed by an increase in perceived stress from the initial presentation to 3 months (β=−0.44).
Conclusions and Relevance
In symptomatic PAD, a decrease in physical activity was followed by an increased risk of depressive symptoms and perceived stress within one year following diagnosis. Increasing physical activity and improving depression and chronic stress within the overall PAD management are important collective goals to address.
INTRODUCTION
Peripheral artery disease (PAD) is a chronic condition1 that affects more than 230 million people worldwide.2,3 Its prevalence is increasing due to an aging population and the growth of lifestyle-related comorbidities such as diabetes and obesity.3,4 The management of atherosclerotic lower extremity PAD focuses on managing symptoms and reducing the risk of cardiovascular and limb events through medical therapy, revascularization, and lifestyle changes.5
Increased physical activity, preferably through a walking regimen, is a critical lifestyle change recommended for patients with PAD.6–12 These patients face many obstacles to becoming more active, including pain with walking, overall lower-limb dysfunction, and increased vulnerability to mental health concerns like depression and anxiety.13 About 20% of patients with PAD experience depression,14 and a similar proportion of patients experiences chronic stress.15 Both depression and stress in PAD have been associated with worse disease outcomes, including diminished PAD-specific health status, and increased long-term mortality.15–18
Depression and/or stress interfere with self-regulatory behaviors due to depletion of energy and poor concentration and may undermine the ability to manage a chronic condition, such as PAD.19 In patients with coronary artery disease (CAD), depression and stress have been shown to be inversely associated with maintaining self-regulatory behaviors, including exercise.19–21 Exercise and stress management interventions have been shown to reduce emotional distress and improve markers of cardiovascular risk in CAD.22 Reductions in depressive symptoms in patients with CAD have also been shown to precede improvements in self-regulatory behaviors.23
For PAD, however, neither the association between depression, stress, and self-regulatory behaviors such as maintaining an active lifestyle, nor the directionality of these associations, have been examined. This knowledge would help inform clinicians on how to focus their efforts to manage both physical activity and mental health treatment goals in the context of overall PAD disease management. Therefore, we aimed to examine the longitudinal interrelationships between self-reported depressive symptoms, perceived stress, and physical activity levels in the year following new or worsening PAD in the PORTRAIT (Patient-Centered Outcomes Related to Treatment Practices in Peripheral Arterial Disease: Investigating Trajectories) study using path analysis.
METHODS
Study Design and Patients
The US PORTRAIT study is a longitudinal registry that collected serial health status information from 10 vascular specialty clinics in the US between June 2, 2011, and December 3, 2015.24 All patients were enrolled during their initial clinical work-up for new or worsening PAD symptoms. The inclusion criteria were ≥18 years of age with new or worsening symptoms of PAD, and an abnormal resting ankle-brachial index ≤0.90 or a drop in post-exercise ankle pressure of ≥20mmHg.24 Exclusion criteria were a non-compressible ankle-brachial index ≥1.30, critical limb ischemia, ipsilateral lower-limb revascularization within a year of screening, having a hearing impairment, imprisonment at the time of enrollment, previous enrollment in the PORTRAIT study, an inability to provide informed consent, and inability to speak either English or Spanish. Patients with missing baseline physical activity, depression, and perceived stress data were excluded from our analysis.
Demographic, socioeconomic information, PAD characteristics, and medical history were abstracted from patients’ electronic medical records by trained data collectors. A standardized interview was conducted by study coordinators at the enrolling sites to collect information about psychosocial factors, health status, PAD-specific symptoms, and cardiovascular lifestyle factors at the time of new or worsening PAD diagnosis. Centralized follow-up phone interviews were administered at 3, 6, and 12 months following enrollment to collect serial information. All study participants provided informed consent and IRB approval was obtained from all participating centers. 24
Measures
Depressive Symptoms and Perceived Stress
The 8-item Patient Health Questionnaire (PHQ-8) was used to assess depressive symptoms at the initial visit and at 3-, 6-, and 12-month follow-ups. The PHQ-8 consists of 8 items measured on a 4-point Likert scale evaluating depression symptoms experienced in the past two weeks, following the Diagnostic and Statistical Manual of Mental Disorders25 framework for a major depressive disorder. Scores on the PHQ-8 range from 0 to 24, with higher scores indicating more severe depressive symptoms. Patients with scores equal to or above 10 were identified as having high levels of depressive symptoms. The sensitivity and specificity of a PHQ-8 score ≥ 10 for major depressive disorder has been demonstrated at 100% and 95%, respectively.26
To quantify patients’ perceived stress, the 4-item Perceived Stress Scale (PSS-4) was administered.27 Patients were asked to rate the frequency with which they experienced each item in the last month on a 5-point scale, ranging from 0 (Never) to 4 (Very Often). Scores range from 0 to 16, with higher scores indicating a higher stress burden and worse coping. High stress was defined as a score of 6 or above, consistent with those used in previous studies, and was predictive of prognostic outcomes regarding patients’ health status trajectories and long-term mortality.24,27
Physical Activity
To assess patients’ exercise and physical activity status, self-reported questions from the INTERHEART study were used.28,29 The questions were graded from 1 to 4, with Grade 1 indicating mainly sedentary lifestyle, Grade 2 indicating mild activity, Grade 3 indicating moderate activity, and Grade 4 indicating strenuous exercise. In the path analysis, physical activity was treated as an ordinal variable, with Grades 3 and 4 grouped due to the small number of patients in Grade 4. Previous studies have shown that the INTERHEART questions for physical activity predictive of incident coronary artery disease, all-cause mortality, and cardiovascular mortality.30,31
Statistical Analysis
Demographic characteristics were described for the overall population and by depression and stress status. Continuous variables were presented as means with standard deviations and as medians and interquartile ranges. Categorical variables were described as frequencies and percentages. Standardized differences were calculated to describe differences between groups, with Cohen’s d 0.20, 0.50, or 0.80 being considered small, moderate, or large effect sizes, respectively.32–34
To examine the longitudinal relationships between depression and physical activity, assessments at the initial work-up, 3-month, 6-month, and 12-month visits were used. Given the repeated measures and types of variables (i.e., continuous variables for the depression, and ordinal variables for physical activity), a cross-lagged panel design using generalized structural equation modeling (GSEM) was used to accommodate the different measurement scales and distributions.35 GSEM analyzes the predictive association of two variables while controlling for effects at earlier time points and combines the power and flexibility of both structural equation modeling and generalized linear modeling in a unified framework.35 GSEM uses a combination of linear and logistic regressions and considers both direct and indirect effects of multiple interacting factors (i.e. each variable serving as the independent and dependent variable for all possible pathways between the variables entered) to evaluate potential causal pathways. Effect sizes of individual pathways, β= 0.10, 0.30, and 0.50, were interpreted as small, medium, and large effect sizes respectively. 33
For our main analyses, and for ease of interpretation of the model by showing only the strongest associations, we derived a backward selection model, which eliminates non-significant correlation pathways step-by-step (p-value > 0.05). Complete case analyses were performed for our main analyses.
As a sensitivity analysis, we also completed a complete GSEM model to verify whether additional paths would be identified.36 As a measure of model performance, we calculated the Akaike Information Criterion (AIC)37, with lower values (>−2 points difference) representing a better fit.
Sensitivity analysis was also performed using imputed datasets for PHQ-8, PSS-4, and physical activity scores at follow-up, generated by a series of multiple imputations through a chained equation.38 The missing PHQ-8, PSS-4, and physical activity scores at 3, 6, and 12 months were imputed from the following variables: age, sex, race, insurance, smoking status, hypertension, congestive heart failure, diabetes, chronic kidney disease, stroke, chronic lung disease, CAD, Rutherford stage39, and baseline PHQ-8, PSS-4, and physical activity scores. A total of 165 complete datasets were generated. Coefficients estimated for each pathway were combined using Rubin’s rules.40,41
All analyses were completed in Stata version 17 (StataCorp, College Station TX).
Results
Among 887 eligible patients, 121 were excluded from our analyses because of missing data at the time of initial assessment on either physical activity (n=93), depression (n=21), or perceived stress (n=7), leaving 766 patients for the analytic cohort (Figure 1). Minor differences were observed for baseline characteristics between patients with follow-up at 12 months of at least one measure (i.e., PSS-4, PHQ-8, physical activity questions) and patients with no follow-up at 12 months (Table S1). Patients missing at least one measure at follow-up reported higher rates of sedentary behavior as well as higher depressive symptoms and higher stress levels.
Figure 1.
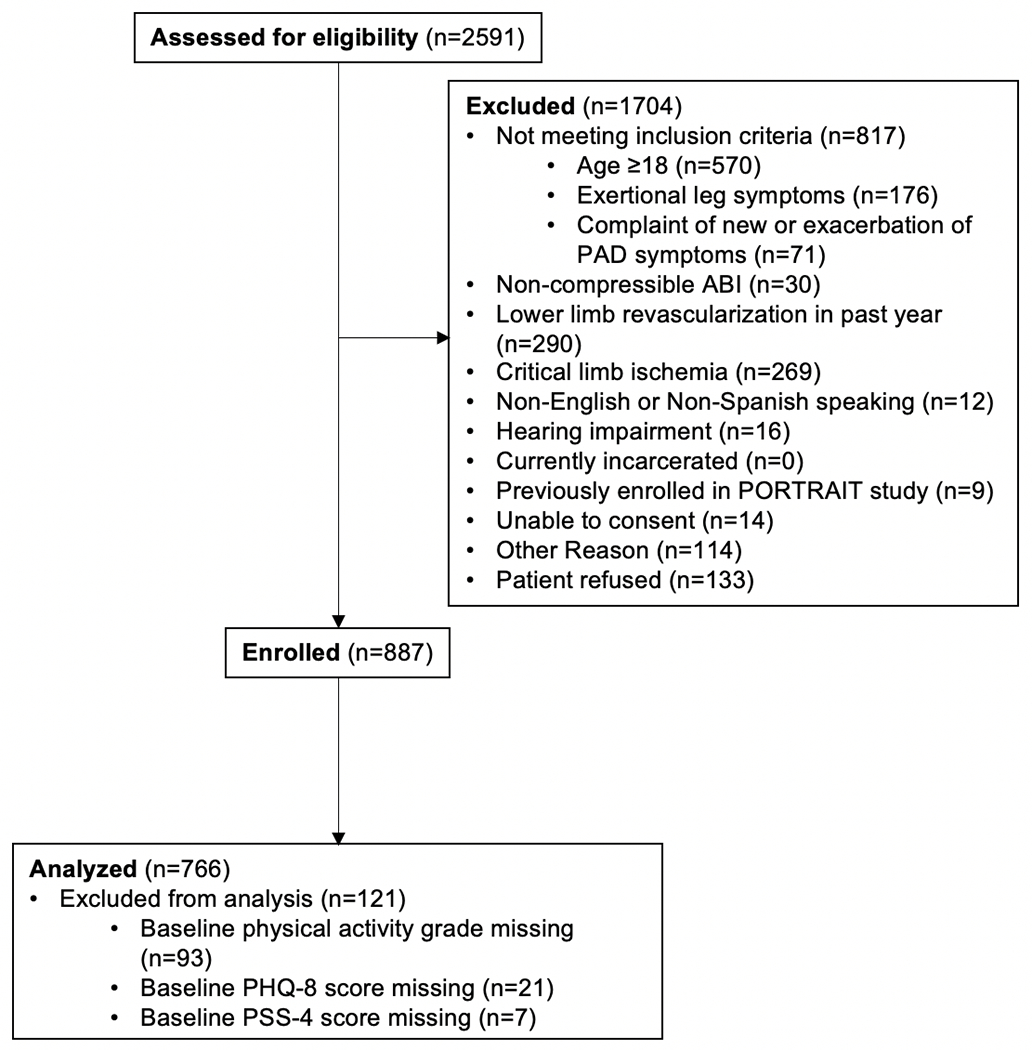
Derivation of the Analytic Cohort.
The mean age of the overall cohort was 68.2 ± 9.4 years. A majority of patients were white (71.8%) and male (57.7%), with high rates of medical comorbidities such as hypertension (80.9%), congestive heart failure (14.4%), CAD (49.0%), diabetes (38.4%), chronic kidney disease (15.0%), cerebrovascular disease (11.7%), and chronic lung disease (15.7%). Patients with a positive depression screen were younger and were more likely to be white and current or past smokers compared to those with high perceived stress scores (d < 0.200) (Table 1).
Table 1.
Patient Characteristics For the Overall Sample, and Characteristics of Those with a Positive Depression Screen, and Those with Increased Perceived Stress Scores.
| Total N=766 |
Depression cohort N=137 |
Stress cohort N=276 |
d | |
|---|---|---|---|---|
| Demographics | ||||
| Age | −0.36 | |||
| Mean (SD) | 68.15 (9.43) | 62.47 (9.30) | 65.89 (9.66) | |
| Median [IQR] | 69.00 [61.00; 75.00] | 61.00 [56.00; 68.00] | 66.00 [58.00; 73.00] | |
| Sex | 0.16 | |||
| Female | 324 (42.3) | 73 (53.3) | 125 (45.3) | |
| Hispanic or Latino Ethnicity | 0.12 | |||
| No | 746 (98.2) | 133 (98.5) | 264 (96.7) | |
| Yes | 14 (1.8) | 2 (1.5) | 9 (3.3) | |
| Race | 0.24 | |||
| White | 550 (71.8) | 95 (69.3) | 184 (66.7) | |
| Black/African American | 181 (23.6) | 27 (19.7) | 76 (27.5) | |
| Other | 35 (4.6) | 15 (10.9) | 16 (5.8) | |
| Primary insurer | ||||
| Medicare | 535 (69.8) | 81 (59.1) | 178 (64.5) | 0.11 |
| Medicaid | 116 (15.1) | 35 (25.5) | 53 (19.2) | 0.15 |
| Medical history and risk factors | ||||
| Smoking status | 0.23 | |||
| Never | 99 (12.9) | 15 (10.9) | 38 (13.8) | |
| Former | 433 (56.5) | 62 (45.3) | 148 (53.6) | |
| Current | 234 (30.5) | 60 (43.8) | 90 (32.6) | |
| Hypertension | 682 (89.0) | 119 (86.9) | 244 (88.4) | 0.05 |
| Congestive heart failure | 110 (14.4) | 20(14.6) | 46 (16.7) | 0.06 |
| Coronary artery disease | 375 (49.0) | 75 (54.7) | 134 (48.6) | 0.12 |
| Diabetes | 294 (38.4) | 59 (43.1) | 116 (42.0) | 0.02 |
| Chronic kidney disease (eGFR<60) | 115 (15.0) | 18 (13.1) | 41 (14.9) | 0.05 |
| Cerebrovascular disease | 90 (11.7) | 19 (13.9) | 35 (12.7) | 0.04 |
| Chronic lung disease | 120 (15.7) | 31 (22.6) | 48 (17.4) | 0.13 |
| PAD history | ||||
| Prior amputation | 12 (1.6) | 4 (2.9) | 7 (2.5) | 0.02 |
| Prior peripheral procedure | 266 (34.7) | 56 (40.9) | 95 (34.4) | 0.13 |
| Bilateral symptoms | 432 (56.4) | 73 (53.3) | 160 (58.0) | 0.14 |
| Rutherford Stage | 0.13 | |||
| Mild Claudication | 154 (20.3) | 31 (22.8) | 53 (19.3) | |
| Moderate Claudication | 387 (50.9) | 58 (42.6) | 136 (49.5) | |
| Severe Claudication | 219 (28.8) | 47 (34.6) | 86 (31.3) | 0.14 |
All values are presented as n (%) unless otherwise specified. Depression cohort includes patients with an 8-item Patient Health Questionnaire (PHQ-8) score of 10 or above at baseline. Stress cohort includes patients with a 4-item Perceived Stress Scale (PSS-4) score of 6 or above at baseline.
Abbreviations: SD, standard deviation; IQR, interquartile range; eGFR, effective glomerular filtration rate; PAD, peripheral artery disease.
Overall, 17.8% of patients reported clinically relevant depressive symptoms (PHQ-8 score of 10 or above), and 36.0% had a high perceived stress burden (PSS-4 score of 6 or above) upon PAD presentation. A total of 44.1% of patients reported that they were sedentary upon presentation (Grade 1). At 12-months, 9.9% of patients had a positive depression screen, 19% had a high perceived stress burden, and 36.1% were sedentary (Table 2).
Table 2.
Measures of Depression, Perceived Stress, and Physical Activity Upon PAD Presentation, 3-Month, 6-Month, and 12-Month Follow Up.
| Upon PAD Presentation | 3 Months | 6 Months | 12 Months | |
|---|---|---|---|---|
| Depression (PHQ-8 score) | ||||
| Mean (SD) | 4.83 (5.26) | 3.65 (4.67) | 3.57 (4.43) | 3.52 (4.42) |
| Median [Q1; Q3] | 3.00 [1.00; 7.00] | 2.00 [0.00; 5.00] | 2.00 [0.00; 5.00] | 2.00 [0.00; 5.00] |
| 0-5 (none) | 471 (61.5) | 464 (71.2) | 440 (73.2) | 433 (71.1) |
| 5-10 (mild) | 158 (20.6) | 109 (16.7) | 94 (15.6) | 116 (19.0) |
| 10-15 (moderate) | 79 (10.3) | 48 (7.4) | 44 (7.3) | 32 (5.3) |
| 15-20 (moderately severe) | 44 (5.7) | 21 (3.2) | 17 (2.8) | 23 (3.8) |
| 20-24 (severe) | 14 (1.8) | 10 (1.5) | 6 (1.0) | 5 (0.8) |
| Stress (PSS-4 score) | ||||
| Mean (SD) | 4.26 (3.53) | 3.22 (3.10) | 3.35 (3.39) | 3.05 (3.05) |
| Median [IQR] | 4.00 [1.00; 7.00] | 2.00 [1.00; 5.00] | 2.00 [1.00; 5.00] | 2.00 [1.00; 4.00] |
| 0-6 (low stress) | 490 (64.0) | 293 (78.1) | 111 (78.7) | 505 (80.9) |
| 6-16 (high stress) | 276 (36.0) | 82 (21.9) | 30 (21.3) | 119 (19.1) |
| Physical activity | ||||
| Grade 1 (sedentary) | 338 (44.1) | 181 (26.9) | 199 (31.4) | 229 (36.1) |
| Grade 2 (mild activity) | 284 (37.1) | 295 (43.8) | 275 (43.4) | 241 (38.0) |
| Grade 3 and 4 (moderate to strenuous activity) | 144 (18.8) | 197 (29.3) | 160 (25.2) | 164 (25.9) |
All values are presented as n (%) unless otherwise specified.
Abbreviations: PHQ-8, 8-item Patient Health Questionnaire; PSS-4, 4-item Perceived Stress Scale; SD, standard deviation; Q1 and Q3, respectively, first and third quartiles.
There was an inverse relationship between physical activity and subsequent depression score observed in the backward selection model (Figure 2). An increase in physical activity preceded a decrease in depressive symptoms across several pathways: baseline to 3 months: β=−0.52, 95% CI, −0.89;−0.14 (large effect size); baseline to 6 months: β=−0.45, 95% CI, −0.80;−0.09 (medium to large effect size); and from 3 months to 12 months: β=−0.81, 95% CI, −1.19;−0.42 (large effect size). In addition, small effect sizes were observed for the following associations: an increase in depression scores was followed by a decrease in physical activity at follow-up (baseline to 3 months: β=−0.08, 95% CI, −0.11;−0.05; 3 months to 6 months: β=−0.08, 95% CI, −0.12;−0.04); 6 months to 12 months: β=−0.09, 95% CI, −0.13;−0.05).
Figure 2. Longitudinal Association Depression and Physical Activity.
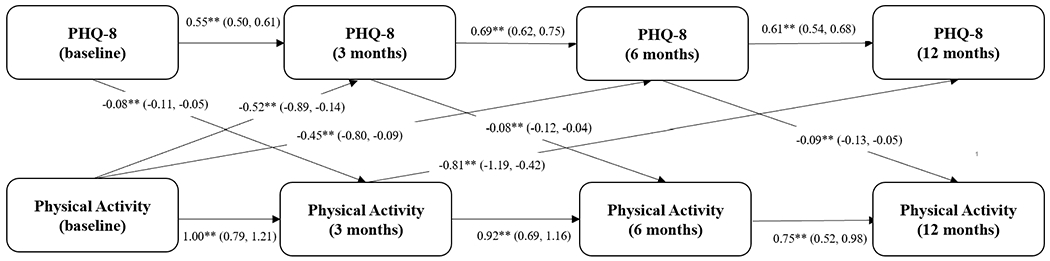
Cross-lagged panel model testing the relationship between depression and physical activity (as ordinal variable) over 12 months of follow-up (backward selection model). Beta coefficients with 95% confidence intervals are presented (straight arrows). ** = p-value <0.05.
Similarly, a decrease in physical activity at the time of PAD presentation was followed by an increase in perceived stress at 3 months (β=−0.44; 95% CI, −0.80; −0.07, medium to large effect size). There was also a small inverse association between perceived stress at PAD presentation at baseline and physical activity at 3 months (β=−0.09; 95% CI, −0.14; −0.05), and between perceived stress at 3 months and physical activity at 6 months (β=−0.12; 95% CI, −0.20; −0.03). These latter effects were trivial and did not reach the threshold for small effect size.
Results from the complete model remained consistent with those of the backward selection model for the association between physical activity and both depression and stress. The fit of the complete model (AIC = 12,446) (Figure S1) was better than that of the backward selection model (AIC = 12,582) for the association between physical activity and depression. The fit of the complete model (AIC = 5,524) (Figure S2) was better than that of the backward selection model (AIC = 6,650) for the association between physical activity and stress. Results remained consistent between the complete model and the backward selection model, with no additional significant paths visualized.
Sensitivity analysis using multiple imputations largely replicated (directionality and strength) the paths visualized in the main analyses, with no additional associations identified for the depression path model. Similarly, for the stress path model, associations were replicated, with additional paths visualized for lowering of physical activity at 3 months and increased stress at 6 months, and 12 months, respectively (Figures S3 and S4).
DISCUSSION
Understanding the complex relationship between physical activity and mental health in patients with PAD can help identify potential strategies to improve both. In our longitudinal study of patients with PAD, we gained important insights into the interrelationship between psychological factors and physical activity levels over the course of PAD patients’ experiences. Our findings demonstrated that after presenting with symptomatic PAD, a decrease in physical activity precedes a rise in depressive symptoms and perceived stress over the course of one year. Particularly for depression, these effects were medium to large at 3 and 6 months after presentation. Similarly, a large effect was observed for the association between decreased physical activity at 3 months and an increase in 12-month depressive symptoms. These associations were also noted between decreased physical activity around the time of initial PAD work-up and increased perceived stress at 3 months, with a medium to large effect size.
Both depression and chronic stress are known to have an inverse association with the ability to engage in physical activity across various populations.20,21,42,43 For the PAD population, the longitudinal associations and directionality between physical activity and psychological factors have never been studied, despite the central role of physical activity in evidence-based management of PAD.5,7,9,11 Insights and programs for patients with coronary artery disease are not directly transferrable to PAD given the complexity and unique risk profile of PAD,44–46 the failure to engage in prescribed physical activity,30 and the absence of integrated behavioral health care within vascular specialty care and rehabilitation programs. Therefore, gaining an understanding of the directionality of these relationships is critical for the future design of effective PAD disease management programs that address depression and chronic stress.
Comorbid depression and chronic stress are each associated with adverse PAD outcomes, including health status outcomes, limb events, and mortality.15,17,18,47–49 Our findings suggest that one possible mechanism that may contribute to adverse PAD outcomes is decreased physical activity, which in return, increases the risk of depression, particularly in patients who have symptomatic PAD. Specifically, the pain experienced due to PAD may lead patients to avoid physical activity, consequently predisposing them to increased risks of developing depression and stress. This suggests that adopting a proactive and integrated approach to the management of PAD, targeting increased physical activity and better motivation, mental health, and pain management, may improve clinical and psychological outcomes.
Relieving PAD symptoms through invasive treatments may not result in a decrease in sedentary behavior, as our prior work has shown.30,31 Psychoeducation and behavioral activation strategies (e.g. cognitive behavioral therapy focusing on problem-solving strategies, motivational interviewing) are needed to promote physical activity. Furthermore, screening patients at risk of developing depression or chronic stress, integrating behavioral health care into PAD care, and monitoring symptoms of depression and stress are areas of need, as both depression and perceived stress are associated with PAD progression, complications, and mortality.15,17,18,47–49 Although evidence-based approaches to treating mental health (i.e., depression, chronic/acute stress) across medical populations50 have not been translated to the care setting of PAD, applying such approaches may further minimize the risk of adverse cardiovascular events and improve quality of life outcomes.18,47 PAD-related treatment and management costs around $6.31 billion without considering indirect costs (e.g. productivity), with costs related to inpatient care and interventions absorbing most of the resources.51 Optimizing strategies for successful PAD outcomes and preventing exacerbations, are key priorities in the pursuit of more value-based PAD care. Centers for Medicare and Medicaid Services (CMS) currently reimburses exercise therapy for patients for PAD,52 thereby making it worthwhile to consider mental health screening at the start of such services in order to determine patient’s readiness and care needs to engage in such PAD exercise regimen, and optimize chances of success. Integrating mental health screening, intervention and addressing obstacles to behavioral changes allows for a more patient-centered approach to PAD disease management with the potential for more efficacious use of health care resources, and value-based care delivery.53,54
Our findings should be interpreted in the context of several potential limitations. Physical activity was self-reported and may not be as accurate as objective measures. However, self-reported physical activity measures have been previously validated in the INTERHEART study and were found to be prognostically reliable for cardiovascular outcomes such as all-cause and cardiovascular mortality.28,30,31 Another limitation is that we used the PSS-4, which has less variability in scores than longer versions of the instrument. Previous research has found, however, that the PSS-4 is a reliable scale 55 and has been a strong predictor of relevant PAD outcomes, including health status and long-term mortality.15,17 Third, the excluded patients who did not record scores for physical activity, depression, and perceived stress may be those with the most extreme scores, which may have contributed to them not adhering to the study protocol. However, these patients made up a small portion of the sample and the path analyses were still valid after multiple imputations. Finally, we were unable to integrate treatment crossovers (i.e. invasive treatments in the course of follow-up) in the derived path estimates, which may explain the variations in the strength of the associations at various follow-up assessments between measures of physical activity and depression/stress.
In conclusion, we reconstructed pathways between physical activity levels, depression, and perceived stress in the year following patients’ PAD diagnosis. Patients with a decline in physical activity, potentially influenced by the experience of symptomatic PAD, were more likely to experience subsequent depressive symptoms and perceived stress. While these conditions already warrant recognition and management in and of themselves, an additional reason to encourage increased physical activity may be to minimize future mental health deterioration in patients with PAD. Integrated treatment interventions that include behavioral healthcare should be developed to improve PAD disease management.
Supplementary Material
Figure 3. Longitudinal Association Perceived Stress and Physical Activity.
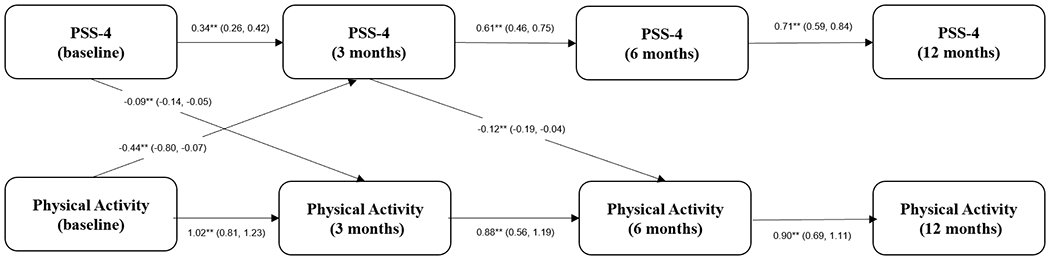
Cross-lagged panel model testing the relationship between depression and physical activity (as an ordinal variable) over 12 months of follow-up (backward selection model). ** = p-value <0.05.
KEY POINTS.
Question
What is the longitudinal relationship between depression, stress, and physical activity in patients with peripheral artery disease (PAD)?
Findings
In a longitudinal observational registry, upon PAD diagnosis, a decrease in physical activity preceded a subsequent rise in depressive symptoms. Similarly, a decrease in physical activity was followed by an increase in perceived stress from the initial presentation to 3 months.
Meaning
For patients with PAD, increasing physical activity alongside with integrated behavioral health approaches to manage depression and stress are critical collective treatment goals.
Funding
Research reported in this article was partially funded through two Patient-Centered Outcomes Research Institute (PCORI) Awards (IP2 PI000753-01; CE-1304–6677), The Netherlands Organization for Scientific Research (VENI Grant No. 916.11.179), and an unrestricted grant from W. L. Gore & Associates, Inc (Flagstaff, AZ).
Disclosures
Dr. Smolderen reports unrestricted research grants from Philips, Merck, Shockwave, and Johnson & Johnson; she is a consultant for Optum Labs, Cook, Tegus, Twill Inc. and Abbott Vascular. Dr. Spertus reports research grants from Janssen, Bristol Meyers Squibb, and Abbott Vascular; consulting for Janssen, Bristol Meyers Squibb, Bayer, United Healthcare, Terumo, Merck, Imbria Pharmaceuticals, Sanofi Aventis; serving on the board of directors for Blue Cross Blue Shield of Kansas City; and owning the copyright to the KCCQ, SAQ, and PAQ; Dr. Mena-Hurtado reports unrestricted research grants from Abbott Vascular, Philips, and Shockwave and is a consultant for Abbott Vascular, Cook, Optum Labs. The other authors report no conflicts of interest.
REFERENCES
- 1.Mustapha JA, Katzen BT, Neville RF, et al. Determinants of Long-Term Outcomes and Costs in the Management of Critical Limb Ischemia: A Population-Based Cohort Study. J Am Heart Assoc. Aug 21 2018;7(16):e009724. doi: 10.1161/JAHA.118.009724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Virani SS, Alonso A, Aparicio HJ, et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. Feb 23 2021;143(8):e254–e743. doi: 10.1161/cir.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 3.Song P, Rudan D, Zhu Y, et al. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health. Aug 2019;7(8):e1020–e1030. doi: 10.1016/S2214-109X(19)30255-4 [DOI] [PubMed] [Google Scholar]
- 4.Matsushita K, Sang Y, Ning H, et al. Lifetime Risk of Lower-Extremity Peripheral Artery Disease Defined by Ankle-Brachial Index in the United States. J Am Heart Assoc. Sep 17 2019;8(18):e012177. doi: 10.1161/JAHA.119.012177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. Mar 21 2017;69(11):1465–1508. doi: 10.1016/j.jacc.2016.11.008 [DOI] [PubMed] [Google Scholar]
- 6.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease. Journal of the American College of Cardiology. 2017;69(11):e71–e126. doi:doi: 10.1016/j.jacc.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 7.Gardner AW, Katzel LI, Sorkin JD, et al. Exercise rehabilitation improves functional outcomes and peripheral circulation in patients with intermittent claudication: a randomized controlled trial. J Am Geriatr Soc. Jun 2001;49(6):755–62. doi: 10.1046/j.1532-5415.2001.49152.x [DOI] [PubMed] [Google Scholar]
- 8.Gardner AW, Montgomery PS, Afaq A. Exercise performance in patients with peripheral arterial disease who have different types of exertional leg pain. J Vasc Surg. Jul 2007;46(1):79–86. doi: 10.1016/j.jvs.2007.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardner AW, Montgomery PS, Parker DE. Physical activity is a predictor of all-cause mortality in patients with intermittent claudication. J Vasc Surg. Jan 2008;47(1):117–22. doi: 10.1016/j.jvs.2007.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardner AW, Montgomery PS, Scott KJ, Afaq A, Blevins SM. Patterns of ambulatory activity in subjects with and without intermittent claudication. J Vasc Surg. Dec 2007;46(6):1208–14. doi: 10.1016/j.jvs.2007.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardner AW, Montgomery PS, Wang M, Shen B. Association between meeting daily step count goals with ambulatory function and quality of life in patients with claudication. J Vasc Surg. Jun 2021;73(6):2105–2113. doi: 10.1016/j.jvs.2020.10.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy TP, Cutlip DE, Regensteiner JG, et al. Supervised exercise, stent revascularization, or medical therapy for claudication due to aortoiliac peripheral artery disease: the CLEVER study. J Am Coll Cardiol. Mar 17 2015;65(10):999–1009. doi: 10.1016/j.jacc.2014.12.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris KM, Mena-Hurtado C, Arham A, et al. Increasing Prevalence of Critical Limb Ischemia Hospitalizations With Distinct Mental Health Burden Among Younger Adults. J Am Coll Cardiol. Nov 23 2021;78(21):2126–2128. doi: 10.1016/j.jacc.2021.09.025 [DOI] [PubMed] [Google Scholar]
- 14.McDermott MM, Guralnik JM, Tian L, et al. Incidence and Prognostic Significance of Depressive Symptoms in Peripheral Artery Disease. J Am Heart Assoc. Mar 18 2016;5(3):e002959. doi: 10.1161/JAHA.115.002959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malik AO, Peri-Okonny P, Gosch K, et al. Association of Perceived Stress Levels With Long-term Mortality in Patients With Peripheral Artery Disease. JAMA Netw Open. Jun 1 2020;3(6):e208741. doi: 10.1001/jamanetworkopen.2020.8741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scierka LE, Mena-Hurtado C, Ahmed ZV, et al. The association of depression with mortality and major adverse limb event outcomes in patients with peripheral artery disease: A systematic review and meta-analysis. J Affect Disord. Sep 28 2022;320:169–177. doi: 10.1016/j.jad.2022.09.098 [DOI] [PubMed] [Google Scholar]
- 17.Malik AO, Poghni P-O, Gosch K, et al. Association of perceived stress with health status outcomes in patients with peripheral artery disease. J Psychosom Res. Jan 2021;140:110313. doi: 10.1016/j.jpsychores.2020.110313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smolderen KG, Plomondon ME, Armstrong EJ, et al. Depression and long-term prognostic outcomes following peripheral endovascular interventions in the VA Healthcare System. Vasc Med. Oct 2018;23(5):454–460. doi: 10.1177/1358863x18770275 [DOI] [PubMed] [Google Scholar]
- 19.Detweiler-Bedell JB, Friedman MA, Leventhal H, Miller IW, Leventhal EA. Integrating co-morbid depression and chronic physical disease management: identifying and resolving failures in self-regulation. Clin Psychol Rev. Dec 2008;28(8):1426–46. doi: 10.1016/j.cpr.2008.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steptoe A, Wardle J, Pollard TM, Canaan L, Davies GJ. Stress, social support and health-related behavior: a study of smoking, alcohol consumption and physical exercise. J Psychosom Res. Aug 1996;41(2):171–80. doi: 10.1016/0022-3999(96)00095-5 [DOI] [PubMed] [Google Scholar]
- 21.Hamer M Psychosocial stress and cardiovascular disease risk: the role of physical activity. Psychosom Med. Nov-Dec 2012;74(9):896–903. doi: 10.1097/PSY.0b013e31827457f4 [DOI] [PubMed] [Google Scholar]
- 22.Blumenthal JA, Sherwood A, Babyak MA, et al. Effects of exercise and stress management training on markers of cardiovascular risk in patients with ischemic heart disease: a randomized controlled trial. Jama. Apr 6 2005;293(13):1626–34. doi: 10.1001/jama.293.13.1626 [DOI] [PubMed] [Google Scholar]
- 23.Rieckmann N, Gerin W, Kronish IM, et al. Course of depressive symptoms and medication adherence after acute coronary syndromes: an electronic medication monitoring study. J Am Coll Cardiol. Dec 5 2006;48(11):2218–22. doi: 10.1016/j.jacc.2006.07.063 [DOI] [PubMed] [Google Scholar]
- 24.Smolderen KG, Gosch K, Patel M, et al. PORTRAIT (Patient-Centered Outcomes Related to Treatment Practices in Peripheral Arterial Disease: Investigating Trajectories): Overview of Design and Rationale of an International Prospective Peripheral Arterial Disease Study. Circ Cardiovasc Qual Outcomes. Feb 2018;11(2):e003860. doi: 10.1161/CIRCOUTCOMES.117.003860 [DOI] [PubMed] [Google Scholar]
- 25.Diagnostic and statistical manual of mental disorders : DSM-5™. 5th edition. ed. DSM-5. American Psychiatric Publishing, a division of American Psychiatric Association; 2013. [Google Scholar]
- 26.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. Apr 2009;114(1-3):163–73. doi: 10.1016/j.jad.2008.06.026 [DOI] [PubMed] [Google Scholar]
- 27.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. Dec 1983;24(4):385–96. [PubMed] [Google Scholar]
- 28.McGorrian C, Yusuf S, Islam S, et al. Estimating modifiable coronary heart disease risk in multiple regions of the world: the INTERHEART Modifiable Risk Score. Eur Heart J. Mar 2011;32(5):581–9. doi: 10.1093/eurheartj/ehq448 [DOI] [PubMed] [Google Scholar]
- 29.Rosengren A, Wilhelmsen L. Physical activity protects against coronary death and deaths from all causes in middle-aged men. Evidence from a 20-year follow-up of the primary prevention study in Goteborg. Ann Epidemiol. Jan 1997;7(1):69–75. doi: 10.1016/s1047-2797(96)00106-8 [DOI] [PubMed] [Google Scholar]
- 30.Peri-Okonny PA, Gosch K, Patel S, et al. Physical Activity in Patients with Symptomatic Peripheral Artery Disease: Insights from the PORTRAIT Registry. Eur J Vasc Endovasc Surg. Dec 2020;60(6):889–895. doi: 10.1016/j.ejvs.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peri-Okonny PA, Patel S, Spertus JA, et al. Physical Activity After Treatment for Symptomatic Peripheral Artery Disease. Am J Cardiol. Jan 1 2021;138:107–113. doi: 10.1016/j.amjcard.2020.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Austin PC. Using the Standardized Difference to Compare the Prevalence of a Binary Variable Between Two Groups in Observational Research. Communications in Statistics - Simulation and Computation. 2009/05/14 2009;38(6):1228–1234. doi: 10.1080/03610910902859574 [DOI] [Google Scholar]
- 33.Cohen DJ. Statistical power analysis for the behavioral sciences. Erlbaum; 1988. [Google Scholar]
- 34.Yang DS DJ. A Unified Approach to Measuring the Effect Size Between Two Groups Using SAS. SAS Global Forum. 2012;Paper 335 [Google Scholar]
- 35.Lombardi S, Santini G, Marchetti GM, Focardi S. Generalized structural equations improve sexual-selection analyses. PLoS One. 2017;12(8):e0181305. doi: 10.1371/journal.pone.0181305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Durlak JA. How to Select, Calculate, and Interpret Effect Sizes. Journal of Pediatric Psychology. 2009;34(9):917–928. doi: 10.1093/jpepsy/jsp004 [DOI] [PubMed] [Google Scholar]
- 37.Vrieze SI. Model selection and psychological theory: a discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Psychol Methods. 2012;17(2):228–243. doi: 10.1037/a0027127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. Mar 2011;20(1):40–9. doi: 10.1002/mpr.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rutherford RB, Flanigan DP, Gupta SK, et al. Suggested standards for reports dealing with lower extremity ischemia. Journal of Vascular Surgery. 1986/07/01/ 1986;4(1):80–94. doi: 10.1016/0741-5214(86)90326-5 [DOI] [PubMed] [Google Scholar]
- 40.Rubin DB. Inference and Missing Data. Biometrika. 1976;63(3):581–592. doi: 10.2307/2335739 [DOI] [Google Scholar]
- 41.Rubin DB. Multiple Imputation for Nonresponse in Surveys. John Wiley & Sons.; 1987. [Google Scholar]
- 42.Griffin KW, Friend R, Eitel P, Lobel M. Effects of environmental demands, stress, and mood on health practices. J Behav Med. Dec 1993;16(6):643–61. doi: 10.1007/bf00844724 [DOI] [PubMed] [Google Scholar]
- 43.Glazer KM, Emery CF, Frid DJ, Banyasz RE. Psychological predictors of adherence and outcomes among patients in cardiac rehabilitation. J Cardiopulm Rehabil. Jan-Feb 2002;22(1):40–6. doi: 10.1097/00008483-200201000-00006 [DOI] [PubMed] [Google Scholar]
- 44.Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation. Aug 15 2006;114(7):688–99. doi: 10.1161/circulationaha.105.593442 [DOI] [PubMed] [Google Scholar]
- 45.Saxon JT, Safley DM, Mena‐Hurtado C, et al. Adherence to Guideline‐Recommended Therapy—Including Supervised Exercise Therapy Referral—Across Peripheral Artery Disease Specialty Clinics: Insights From the International PORTRAIT Registry. Journal of the American Heart Association. 2020;9(3):e012541. doi:doi: 10.1161/JAHA119012541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grenon SM, Vittinghoff E, Owens CD, Conte MS, Whooley M, Cohen BE. Peripheral artery disease and risk of cardiovascular events in patients with coronary artery disease: insights from the Heart and Soul Study. Vasc Med. Aug 2013;18(4):176–84. doi: 10.1177/1358863x13493825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jelani QU, Mena-Hurtado C, Burg M, et al. Relationship Between Depressive Symptoms and Health Status in Peripheral Artery Disease: Role of Sex Differences. J Am Heart Assoc. Aug 18 2020;9(16):e014583. doi: 10.1161/JAHA.119.014583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cherr GS, Wang J, Zimmerman PM, Dosluoglu HH. Depression is associated with worse patency and recurrent leg symptoms after lower extremity revascularization. J Vasc Surg. Apr 2007;45(4):744–50. doi: 10.1016/j.jvs.2006.11.057 [DOI] [PubMed] [Google Scholar]
- 49.Cherr GS, Zimmerman PM, Wang J, Dosluoglu HH. Patients with depression are at increased risk for secondary cardiovascular events after lower extremity revascularization. J Gen Intern Med. May 2008;23(5):629–34. doi: 10.1007/s11606-008-0560-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. Dec 30 2010;363(27):2611–20. doi: 10.1056/NEJMoa1003955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kohn CG, Alberts MJ, Peacock WF, Bunz TJ, Coleman CI. Cost and inpatient burden of peripheral artery disease: Findings from the National Inpatient Sample. Atherosclerosis. Jul 2019;286:142–146. doi: 10.1016/j.atherosclerosis.2019.05.026 [DOI] [PubMed] [Google Scholar]
- 52.Ehrman JK, Gardner AW, Salisbury D, Lui K, Treat-Jacobson D. Supervised Exercise Therapy for Symptomatic Peripheral Artery Disease: A REVIEW OF CURRENT EXPERIENCE AND PRACTICE-BASED RECOMMENDATIONS. J Cardiopulm Rehabil Prev. Sep 16 2022;doi: 10.1097/hcr.0000000000000723 [DOI] [PubMed] [Google Scholar]
- 53.Burton NW, Ademi Z, Best S, et al. Efficacy of brief behavioral counselling by allied health professionals to promote physical activity in people with peripheral arterial disease (BIPP): study protocol for a multi-center randomized controlled trial. BMC Public Health. Nov 9 2016;16(1):1148. doi: 10.1186/s12889-016-3801-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McDermott MM, Liu K, Guralnik JM, et al. Home-based walking exercise intervention in peripheral artery disease: a randomized clinical trial. Jama. Jul 3 2013;310(1):57–65. doi: 10.1001/jama.2013.7231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Warttig SL, Forshaw MJ, South J, White AK. New, normative, English-sample data for the Short Form Perceived Stress Scale (PSS-4). J Health Psychol. Dec 2013;18(12):1617–28. doi: 10.1177/1359105313508346 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


