Abstract
The activity of moxifloxacin (BAY 12-8039) against a Streptococcus pneumoniae type 3 strain (MIC and minimum bactericidal concentration [MBC] of moxifloxacin, 0.06 and 0.25 μg/ml, respectively; MIC and MBC of ceftriaxone, 0.03 and 0.06 μg/ml, respectively) was determined in vitro and in a rabbit model of meningitis. Despite comparable bactericidal activity, 10 μg of moxifloxacin per ml released lipoteichoic and teichoic acids less rapidly than 10 μg of ceftriaxone per ml in vitro. Against experimental meningitis, 10 mg of moxifloxacin per kg of body weight per ml reduced the bacterial titers in cerebrospinal fluid (CSF) almost as rapidly as ceftriaxone did (mean ± standard deviation, −0.32 ± 0.14 versus −0.39 ± 0.11 Δlog CFU/ml/h). The activity of moxifloxacin could be described by a sigmoid dose-response curve with a maximum effect of −0.33 ΔlogCFU/ml/h and with a dosage of 1.4 mg/kg/h producing a half-maximal effect. Maximum tumor necrosis factor activity in CSF was observed later with moxifloxacin than with ceftriaxone (5 versus 2 h after the initiation of treatment). At 10 mg/kg/h, the concentrations of moxifloxacin in CSF were 3.8 ± 1.2 μg/ml. Adjunctive treatment with dexamethasone at 1 mg/kg prior to the initiation of antibiotic treatment only marginally reduced the concentrations of moxifloxacin in CSF (3.3 ± 0.6 μg/ml). In conclusion, moxifloxacin may qualify for use in the treatment of S. pneumoniae meningitis.
Pneumococci moderately or highly resistant to penicillin G and other β-lactam antibiotics are a challenge worldwide (2, 5, 10, 15, 22, 24). A reduced sensitivity to penicillin is parallelled by increases in the MICs of all β-lactam and carbapenem antibiotics, and clinical failures of cefotaxime and ceftriaxone in the treatment of meningitis caused by penicillin-resistant Streptococcus pneumoniae have also been observed (2, 5, 10). Although at present resistance to cephalosporins and carbapenems is very rare, treatment options with antibacterial agents not belonging to the β-lactam and carbapenem groups appear highly desirable.
Treatment of pneumococcal meningitis with the β-lactam antibiotic ceftriaxone leads to a rapid increase in tumor necrosis factor alpha (TNF-α) and interleukin-1β levels in cerebrospinal fluid (CSF) (31). This increase is probably caused by the rapid lysis of bacteria and the release of proinflammatory cell wall components and is thought to contribute to neuronal damage in bacterial meningitis. It can be inhibited by the administration of dexamethasone 15 min prior to antibiotic therapy (31). However, the coadministration of dexamethasone reduces the entry of ceftriaxone into the CSF (22) and may aggravate neuronal damage in the dentate gyrus of the hippocampal formation (33). Quinolones are bactericidal for susceptible bacteria. They are less hydrophilic than β-lactam and carbapenem antibiotics and rapidly enter the subarachnoid space (17, 18). Older members of this class were unsuitable for empiric treatment of bacterial meningitis due to their poor activity against S. pneumoniae (19). A new group of quinolones with improved activity against gram-positive bacteria including S. pneumoniae appears very promising for the treatment of bacterial meningitis. Unlike β-lactam antibiotics, quinolones do not kill bacteria by direct lysis of the cell wall. After the initiation of therapy they release proinflammatory cell wall products less rapidly than β-lactam antibiotics (21, 27).
The present study addresses whether (i) the new quinolone moxifloxacin (BAY 12-8039) possesses adequate in vitro and in vivo activity for the treatment of S. pneumoniae meningitis, (ii) it modulates the inflammatory host response known to occur after the initiation of therapy, and (iii) its penetration into CSF is affected by the coadministration of dexamethasone.
(These data were presented, in part, at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada, 28 September to 1 October 1997).
MATERIALS AND METHODS
In vitro activity.
The MICs and minimum bactericidal concentrations (MBCs) of moxifloxacin and ceftriaxone for the S. pneumoniae type 3 strain used in this and previous studies (19, 21, 29, 33) were determined by the macrodilution method in tryptic soy broth. Furthermore, the bactericidal activity of moxifloxacin was studied at different antibacterial concentrations in tryptic soy broth (27). After overnight growth, the bacteria were suspended in fresh medium at a concentration of approximately 5 × 108 CFU/ml prior to the initiation of treatment. The relatively high inoculum was used to mimic the bacterial titers in CSF prior to the initiation of antibacterial therapy. Thereafter, bacteria were exposed to moxifloxacin at concentrations of 0.06 (i.e., the MIC), 0.25 (i.e., the MBC), 1, 5, and 10 μg/ml. Furthermore, at 10 μg/ml, the release of lipoteichoic acid (LTA) and teichoic acid from S. pneumoniae type 3 by moxifloxacin and ceftriaxone was studied over 12 h (for each group, n = 5).
Sandwich ELISA for the detection of pneumococcal LTA and teichoic acid.
LTA was prepared from S. pneumoniae R6 (1). Polyclonal antibodies were raised in two New Zealand White rabbits immunized subcutaneously with 500 mg of LTA mixed with an equal volume of incomplete Freund’s adjuvant. The enzyme-linked immunosorbent assay (ELISA) used the mouse monoclonal antibody TEPC-15 (Sigma, Deisenhofen, Germany) directed against phosphorylcholine as the capture antibody and the polyclonal rabbit antiserum raised against LTA as the detector antibody. Standard curves were constructed within each assay. Intra-assay and interday coefficients of variation determined by repeatedly measuring spiked quality control samples were 8.4 and 10.9%, respectively, with 300 ng/ml and 7.1 and 7.1%, respectively, with 1,800 ng/ml. The assay was used to determine the in vitro release of LTA and teichoic acid from the S. pneumoniae type 3 strain used for the in vivo experiments after exposure to 10 μg of ceftriaxone or moxifloxacin per ml.
Rabbit model.
After intramuscular induction of anesthesia with ketamine (25 mg/kg of body weight) and xylazine (5 mg/kg), New Zealand White rabbits (weight, approximately 2.5 kg) were anesthetized with intravenous (i.v.) urethane for the whole duration of the experiment (24 h) and were placed in a stereotaxic frame by means of dental acrylic helmet fixed at the scull with four screws as originally described by Dacey and Sande (6). A spinal needle (22 by 3.5 in.; Spinocan; Braun, Melsungen, Germany) was placed in the cisterna magna.
Meningitis was induced by intracisternal injection of 106 CFU of S. pneumoniae type 3 (gift of M. G. Täuber, Division of Infectious Diseases, University of California, San Francisco). Blood (3 ml) and CSF (300 μl) were drawn before and at 12, 14, 17, 20, and 24 h after infection. At 12 h after infection, therapy was initiated: 10 rabbits received a maintenance dose of 10 mg of moxifloxacin per kg/h i.v. for the following 12 h. Six rabbits received the same dosage and in addition an adjunctive treatment of 1.0 mg of dexamethasone (Fortecortin; kindly provided by Merck, Darmstadt, Germany) per kg 15 min prior to the initiation of antibiotic therapy. As maintenance therapy six animals received 2.5 mg/kg/h and four rabbits received 20 mg/kg/h i.v. In order to rapidly reach steady state, therapy was started with an i.v. bolus dose of twice the maintenance dose per hour. Animals treated with an i.v. bolus of 20 mg of ceftriaxone (Rocephin; kindly provided by Hoffmann-La Roche, Grenzach-Wyhlen, Germany) per kg followed by 10 mg/kg/h as maintenance therapy served as controls (n = 10).
Sample processing.
The numbers of leukocytes in CSF were counted in a Fuchs-Rosenthal hemocytometer. After coagulation, blood was centrifuged at 3,000 × g for 5 min, and the supernatant was immediately frozen at −80°C. Pneumococcal titers in CSF were counted by plating 10 μl of serial 10-fold dilutions on blood agar plates, which were then incubated overnight at 37°C with 5% CO2. The bacterial titers at 12, 14, 17, 20, and 24 h were used for log-linear regression analysis. The maximum effect and the dose producing the half-maximum effect were estimated by linear regression analysis of a double-reciprocal plot (1/Δlog CFU per milliliter per hour versus 1/dose).
The remaining CSF was centrifuged at 3,000 × g for 5 min, and the supernatants were stored at −80°C.
Moxifloxacin levels in serum and CSF were measured by high-pressure liquid chromatography (HPLC). For the measurement of the levels in serum, after the addition of 10 μl of an internal standard to 0.2 ml of serum, the samples were deproteinized with 0.2 ml of acetonitrile. After centrifugation, 100 μl of the resulting supernatants was mixed with 300 μl of 0.067 mM disodium hydrogen phosphate buffer (pH 7.5). For the measurement of the levels in CSF, after the addition of 10 μl of an internal standard, 10 μl of CSF was diluted with 0.067 mM disodium hydrogen phosphate buffer (pH 7.5) and acetonitrile (75/25; vol/vol) prior to HPLC analysis. Calibration was done by spiking seven different levels of blank rabbit serum or CSF with concentrations of between 0.005 and 0.7 μg/ml. HPLC separation (Hewlett-Packard model HP 1090A with HP ChemStation software) was performed at room temperature on a reversed-phase Nucleosil C18 column (250 by 4.6 mm; particle size, 5 μm). For serum, the mobile phase consisted of acetonitrile and tetrabutylammonium hydrogen sulfate solution in Mill-Q water (10 g/liter). A gradient from 20% (1 min) to 41% acetronitrile (within 9 min) with a flow rate of 1.0 ml/min was used. Moxifloxacin and the internal standard eluted at approximately 7.3 and 8.1 min, respectively. For CSF, an isocratic elution with acetonitrile–tetrabutylammonium hydrogen sulfate solution (18:82; vol/vol) and a Nucleosil C18 column (125 by 4.6 mm; particle size, 5 μm) with a flow rate of 1.5 ml/min was used. Peaks were detected by fluorescence recording (Jasco Model FP 920-S) at 504 nm with an excitation wavelength of 296 nm. The peak heights were used for the construction of calibration curves by 1/y2 weighted linear regression (Concalc 1.21 software; Bayer AG, Wuppertal, Germany). The limit of quantification was 0.05 μg/ml for serum and 0.01 μg/ml for CSF. The interassay coefficient of variation ranged from 2 to 11% for serum and from 6 to 9% for CSF. The accuracy ranged from +5% to +8% for serum and from ±0 to −12% for CSF. The concentrations of ceftriaxone in serum and CSF were determined by the agar-well diffusion technique in Antibiotic Medium No. 2 (Oxoid) plus 0.4% agar with Escherichia coli 108 (collection of H. Hof, Department of Medical Microbiology, University of Heidelberg-Mannheim, Mannheim, Germany). For serum and CSF samples, different standard curves were constructed with undiluted rabbit serum and rabbit serum diluted 1:20. The quantification limit of the assay was 1 μg/ml. To avoid interassay variation, the levels in serum and CSF samples each were measured in one assay (21). The mean concentrations in serum and CSF were calculated as the arithmetic mean of the moxifloxacin and ceftriaxone concentrations at 14 and 24 h after infection.
TNF-α activity in CSF was measured by a cytolytic assay with the L929 fibroblast cell line (33). Neuron-specific enolase (NSE) concentrations were determined by an immunoluminometric method (LIA-mat NSE Prolifigen; Byk-Sangtec, Dietzenbach, Germany). Lactate was measured enzymatically (Biosen, Dreieich, Germany), and the CSF protein concentration was measured photometrically (BCA-protein-Test; Pierce, Rockford, Ill.).
Statistics.
Data were described as means ± standard deviations (SDs) if the data were normally distributed. Data for the ceftriaxone (10 mg/kg/h) and moxifloxacin (10 mg/kg/h) groups were compared by the two-tailed t test for independent samples. In the absence of a normal distribution, the median and the 25th and 75th percentiles were used, and the data for the ceftriaxone and moxifloxacin (10 mg/kg/h) groups were compared by the U-test of Mann and Whitney.
RESULTS
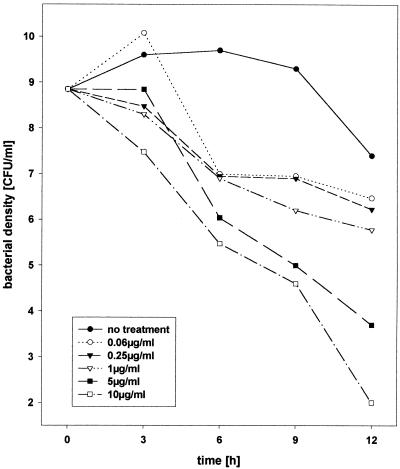
For the S. pneumoniae type 3 strain used in this study, the moxifloxacin MIC and MBC were 0.06 and 0.25 μg/ml, respectively, and the ceftriaxone MIC and MBC were 0.03 and 0.06 μg/ml, respectively. In vitro, with high bacterial concentrations, moxifloxacin showed dose-dependent bactericidal activity (Fig. 1). At 0.06 and 0.25 μg/ml, only a slow reduction of bacterial titers was observed, whereas higher concentrations were active more rapidly. At a concentration of 10 μg/ml, moxifloxacin and ceftriaxone were rapidly bactericidal (−0.60 ± 0.09 and −0.41 ± 0.03 Δlog CFU/ml/h, respectively). Moxifloxacin delayed the release of LTA and teichoic acid from S. pneumoniae type 3 compared to the rate for ceftriaxone (281 ± 117 versus 1,734 ± 820 ng/ml at 1 h [P < 0.01]; 685 ± 236 versus 3,248 ± 1,509 ng/ml at 3 h [P < 0.01]; 2,512 ± 1,195 versus 3,827 ± 1,908 ng/ml at 12 h [difference not significant]).
FIG. 1.
Dose-dependent bactericidal activity of 10, 5, 1, 0.25 (MBC), and 0.06 (MIC) μg of moxifloxacin per liter over 12 h in vitro.
In vivo, the bacterial titer in CSF 12 h after infection, i.e., prior to the initiation of therapy, did not differ significantly in the treatment groups (moxifloxacin at 10 mg/kg/h, 7.82 ± 0.58 log CFU/ml; ceftriaxone at 10 mg/kg/h, 8.00 ± 0.85 log CFU/ml). For rabbits treated with 10 mg of moxifloxacin per kg/h, mean ± SD concentrations were 10.4 ± 3.4 μg/ml in serum and 3.8 ± 1.2 μg/ml in CSF. Dexamethasone (1 mg/kg) given 15 min prior to the initiation of therapy only marginally reduced the mean moxifloxacin concentrations in CSF (3.3 ± 0.6 μg/ml; mean ± SD). The ratio of the concentration in CSF to the concentration in serum for moxifloxacin at 24 h was 0.44 ± 0.08 without the coadministration of dexamethasone and 0.34 ± 0.15 with the coadministration of dexamethasone (Table 1). The mean ± SD concentrations of ceftriaxone were 7.1 ± 3.4 μg/ml in CSF and 159.6 ± 48.4 μg/ml in serum, and the ratio of the concentration in CSF to the concentration in serum was 0.06 ± 0.03 (mean ± SD) 24 h after infection.
TABLE 1.
Pharmacokinetics of moxifloxacin and ceftriaxone in serum and CSF and leukocyte density and protein content in CSF during experimental meningitis caused by S. pneumoniae
| Antibiotic | Dosage (mg/kg/h) | No. of rabbits | Mean ± SD concn (μg/ml)
|
Mean ± SD concn ratio at 24 ha | Median (25th/75th percentile) leukocyte counts in CSF
|
Mean ± SD protein content (mg/liter) in CSF
|
|||
|---|---|---|---|---|---|---|---|---|---|
| Serum | CSF | 12h | 24h | 12h | 24h | ||||
| Ceftriaxone | 10 | 10 | 159.6 ± 48.4 | 7.1 ± 3.4 | 0.06 ± 0.03 | 1,123 (341/2,125) | 14,037 (7,547/21,078) | 862 ± 1,102 | 4,159 ± 1,881 |
| Moxifloxacin | 2.5 | 6 | 3.3 ± 0.3 | 1.9 ± 0.2 | 0.36 ± 0.04 | 3,499 (1,371/6,592) | 11,258 (8,748/17,110) | 957 ± 403 | 3,904 ± 1,849 |
| Moxifloxacin | 10 | 10 | 10.4 ± 3.4 | 3.8 ± 1.2 | 0.44 ± 0.08 | 3,147 (771/6,358) | 7,210 (2,799/13,555) | 1,533 ± 1,170 | 4,902 ± 3,971 |
| Moxifloxacin | 20 | 4 | 19.8 ± 5.1 | 8.5 ± 3.2 | 0.47 ± 0.08 | 6,262 (4,675/9,366) | 4,560 (3,523/6,640) | 1,063 ± 632 | 11,875 ± 11,803 |
| Moxifloxacin + dexamethasone | 10 | 6 | 12.3 ± 4.2 | 3.3 ± 0.6 | 0.34 ± 0.15 | 1,975 (604/7,999) | 4,886 (2,528/5,823) | 603 ± 279 | 2,119 ± 891 |
Ratio of concentration in CSF to concentration in serum.
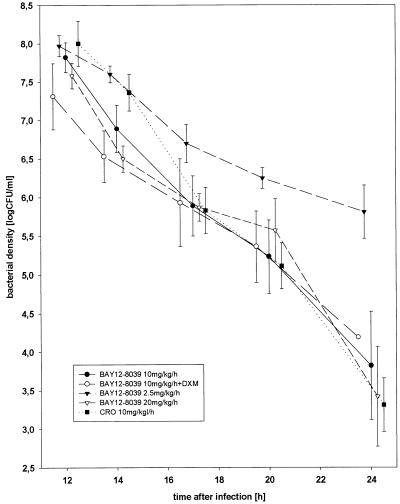
The bactericidal activity of 10 mg of moxifloxacin per kg/h without and with adjunctive treatment with dexamethasone was almost as high as the bactericidal activity of ceftriaxone (−0.32 ± 0.14 and −0.25 ± 0.06 Δlog CFU/ml/h versus −0.39 ± 0.11 Δlog CFU/ml/h [mean ± SD]). Doubling of the moxifloxacin dosage (20 mg/kg/h) did not result in an increase in the bactericidal activity (−0.31 ± 0.10 Δlog CFU/ml/h) (Fig. 2). Moxifloxacin at 2.5 mg/kg/h (−0.19 ± 0.06 Δlog CFU/ml/h) was less effective than moxifloxacin at 10 mg/kg/h. The dose-dependent bactericidal activity of moxifloxacin against experimental S. pneumoniae meningitis could be described by a sigmoid function: the maximum effect was −0.33 Δlog CFU/ml/h, and the dose producing a half-maximal effect was estimated to be 1.4 mg/kg/h.
FIG. 2.
Bactericidal activities of different concentrations of moxifloxacin against experimental S. pneumoniae meningitis (error bars represent standard errors of means). BAY 12-8039, moxifloxacin; CRO, ceftriaxone; DXM, dexamethasone, given at 1 mg/kg 15 min prior to antibiotic treatment.
In rabbits treated with moxifloxacin at 10 mg/kg/h, maximum TNF-α concentrations in CSF were observed later (17 h after infection) than the times for rabbits treated with ceftriaxone (14 h after infection), but the magnitudes of the TNF-α peaks were almost identical (median [25th/75th percentiles], 54 [29/123] U/ml for moxifloxacin versus 66 [14/108] U/ml for ceftriaxone). Other parameters of inflammation (leukocyte counts in CSF, lactate concentrations in CSF, protein content in CSF) were not different in moxifloxacin- and ceftriaxone-treated animals.
The NSE concentration in CSF as a parameter of neuronal damage was not significantly different (median [25th/75th percentiles]: for moxifloxacin at 10 mg/kg/h, 54.4 [20.5/160.7] ng/ml; for ceftriaxone at 10 mg/kg/h, 21.8 [16.2/123.5] ng/ml).
DISCUSSION
In the rabbit model of experimental meningitis, moxifloxacin was as active as ceftriaxone against a penicillin-sensitive strain of S. pneumoniae. Since the activities of quinolones in general (24, 25) and moxifloxacin in particular (3, 13) are not affected by the development of resistance to penicillin or cephalosporins, these results are relevant for the treatment of meningitis caused by penicillin-sensitive and -resistant S. pneumoniae strains. We administered moxifloxacin by continuous infusion to facilitate the comparison with other compounds which have been studied with a continuous i.v. infusion in this model (19, 21, 29). In particular, several quinolones have been investigated at a dosage of 10 mg/kg/h (19, 21). Furthermore, the use of continuous instead of bolus infusions at the same daily dose will reduce the maximum concentrations in serum and may be a strategy for decreasing the incidence of toxic side effects.
Moxifloxacin is a compound with a low level of toxicity. Single doses of up to 800 mg producing maximum concentrations in serum of 4.73 ± 1.16 mg/liter have been tolerated without serious side effects in humans. The levels that we observed in serum during the infusion of 2.5 mg of moxifloxacin per kg/h, which was bactericidal in vivo, were lower than those maximum concentrations. Higher concentrations of moxifloxacin may be tolerated in critically ill humans with acceptable side effects.
The rates of bactericidal activity observed in this study were lower than those seen before with other quinolones and ceftriaxone (19, 29). This is probably due to the approximately 2-log higher bacterial density in CSF at the start of antibiotic treatment in the present study. Since high bacterial titers are frequently encountered in humans, we aimed at high bacterial titers and kept the animals under anesthesia for 24 h, thereby preventing an increase in body temperature above 40°C. The rates of bactericidal activity in the present study are in accordance with those in a comparison of ceftriaxone and trovafloxacin in animals anesthetized for 24 h (21).
In addition to the high bacterial densities, other factors may affect the bactericidal actions of antibacterial agents in CSF compared with those in broth: bacteria multiply less rapidly in CSF than in broth and therefore are less susceptible to the inhibition of penicillin-binding proteins and DNA gyrases. The acidic pH of CSF in the presence of meningeal inflammation decreases the activities of some antibacterial agents, in particular, of aminoglycosides. The in vitro activity of trovafloxacin, however, was not affected by a lowering of the pH from 7.4 to 6.8 (12). A high level of protein binding may decrease the concentration of the active fraction of the antibacterial agent. Although no data are available on the protein binding of moxifloxacin in rabbits, in humans the level of protein binding in serum has been reported to be approximately 30%. The addition of human serum had little or no effect upon the antimicrobial activity of moxifloxacin in vitro (32). For this reason, it appears unlikely that protein binding may diminish the action of moxifloxacin in the CSF compartment.
For quinolones, half-maximal killing of the S. pneumoniae organisms causing meningitis was observed with concentrations in the CSF of approximately five times the MBC (19). In the present study, the moxifloxacin dosage producing a half-maximal effect was estimated to be 1.4 mg/kg/h. Moxifloxacin at 2.5 mg/kg/h produced concentrations in CSF of 1.9 ± 0.2 μg/ml (i.e., (seven to eight times the MBC) and a rate of bactericidal activity distinctly above the half-maximal effect.
The entry of moxifloxacin into the CSF compared well with the penetration of other quinolones into the CSF in the rabbit model of meningitis (19). In contrast to the hydrophilic β-lactam antibiotics, the less hydrophilic quinolones enter the CSF more readily in bacterial meningitis, and the concentrations in CSF are less influenced by the state of the blood-CSF barrier. (i) In the present study, the ratio of the concentration of moxifloxacin is CSF to that in serum was not substantially reduced by the coadministration of dexamethasone. In contrast, the same dose of dexamethasone reduced the level of entry of ceftriaxone into the CSF by 30 to 50% (20). (ii) The ratio of the area under the concentration-time curve for CSF to that for serum for ceftriaxone (18) and ofloxacin (17) in humans with minor impairment of the blood-CSF barrier was almost identical to the ratios of the concentrations in CSF to those in serum for these antibacterial agents at steady state in the rabbit model of pneumococcal meningitis (19). This implies that quinolones are suitable for the treatment of central nervous system infections when the disturbance of the blood-CSF barrier is less pronounced or resolves rapidly, such as during adjunctive treatment with dexamethasone.
At present, only indirect evidence that a delay of the release of proinflammatory bacterial compounds may have clinical significance is available. In adults with bacterial meningitis, evidence of brain herniation at autopsy was present in 8 of 27 patients who died within 7 days of presentation (8). More than 50% of the brain herniations occurred later than 2 h after lumbar puncture (7). At this time, antibiotic therapy presumably had been initiated. Since endotoxin itself is able to increase the water content in the brain (28), it has been suspected that the antibiotic-induced release of proinflammatory cell wall products contributed to brain herniation in some of these patients (30). Quinolones are rapidly bactericidal but do not directly affect cell wall synthesis. For this reason, they are promising candidates for reducing the level of release of proinflammatory cell wall products during antibiotic killing. For Escherichia coli exposed to ciprofloxacin for 60 min, a 3-log-order decrease in the numbers of CFU was found, but no equivalent decrease in bacterial numbers was found, as determined by light microscopy and flow cytometry (16). Although the absolute amount of endotoxin released from E. coli as a result of ciprofloxacin exposure was similar to that liberated as a result of ceftazidime exposure (9, 23), in timed experiments ciprofloxacin, despite its rapid bactericidal activity, released only 12.7% of the lipopolysaccharide within the first hour of exposure, whereas ceftazidime released 61.9% (9).
LTA and teichoic acid are considered the most potent proinflammatory products of S. pneumoniae (31). Peptidoglycans (11) contribute to the inflammatory potency of the S. pneumoniae cell wall (4). Recently, the DNA of gram-positive bacteria has been shown to cause septic shock in mice (26). We were able to quantify the concentration of free LTA and teichoic acid only by a newly developed enzyme immunoassay (27). The measurement of peptidoglycan and free bacterial DNA levels would provide valuable additional information. In the present study, during in vitro exposure, 10 μg of moxifloxacin per ml released LTA and teichoic acid less rapidly than ceftriaxone did, even though the compounds had equal bactericidal activities. This compares well the delayed release of these compounds by trovafloxacin at an equal concentration (27). At lower concentrations, trovafloxacin releases larger quantities of LTA and teichoic acid than it releases at 10 μg/ml (27).
In experimental S. pneumoniae meningitis, maximum TNF-α concentrations in CSF were observed 2 h after the initiation of ceftriaxone treatment and 5 h after the start of moxifloxacin treatment. The delayed increase in the TNF-α concentration after the initiation of therapy, however, did not modulate other parameters of inflammation in CSF. The leukocyte count in CSF, the protein content of CSF, and the lactate concentration in CSF were almost identical during treatment with moxifloxacin and ceftriaxone, and the NSE level in CSF (NSE is a measure of neuronal damage in meningitis [14]) was not reduced.
In conclusion, at high concentrations moxifloxacin was as effective as ceftriaxone in the rabbit model of meningitis with a penicillin-sensitive S. pneumoniae strain. Considering the close relation between the in vitro and in vivo activities of antibacterial agents against experimental meningitis (19, 22, 29; the present study), moxifloxacin will probably be active against penicillin-resistant strains causing experimental meningitis, provided that the strain is sufficiently sensitive in vitro. The level of penetration of moxifloxacin into CSF was only slightly reduced by the coadministration of dexamethasone. In vitro, moxifloxacin released proinflammatory teichoic acid and LTA from S. pneumoniae less rapidly than ceftriaxone did. In vivo, however, we were unable to demonstrate a substantial attenuation of the inflammatory response associated with the initiation of antibiotic therapy or a reduction of NSE in CSF as a parameter of neuronal damage.
ACKNOWLEDGMENTS
This work was supported by Bayer AG, Wuppertal, Germany, and by the Deutsche Forschungsgemeinschaft (grant Na165/2-2).
REFERENCES
- 1.Behr T, Fischer W, Peter-Katalinic J, Egge H. The structure of pneumococcal lipoteichoic acid. Eur J Biochem. 1992;207:1063–1075. doi: 10.1111/j.1432-1033.1992.tb17143.x. [DOI] [PubMed] [Google Scholar]
- 2.Bradley J S, Connor J D. Ceftriaxone failure in meningitis caused by Streptococcus pneumoniae with reduced susceptibility to beta-lactam antibiotics. Pediatr Infect Dis. 1991;10:871–873. [PubMed] [Google Scholar]
- 3.Brueggemann A B, Kugler K C, Doern G V. In vitro activity of Bay 12-8039, a novel 8-methoxyquinolone, compared to activities of six fluoroquinolones against Streptococcus pneumoniae, Haemophilus influenzae, and Moxarella catarrhalis. Antimicrob Agents Chemother. 1997;41:1594–1597. doi: 10.1128/aac.41.7.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burroughs M, Rozdzinski E, Geelen S, Tuomanen E. A structure-activity relationship for induction of meningeal inflammation by muramyl peptides. J Clin Invest. 1993;92:297–302. doi: 10.1172/JCI116565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canton E. Cefotaxime breakpoint for Streptococcus pneumoniae. Antimicrob Agents Chemother. 1993;37:616–617. doi: 10.1128/aac.37.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dacey R G, Sande M A. Effect of probenecid on cerebrospinal fluid concentrations of penicillin and cephalosporin derivatives. Antimicrob Agents Chemother. 1974;6:437–441. doi: 10.1128/aac.6.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dodge P R, Swartz M N. Bacterial meningitis—a review of selected aspects. II. Special neurologic problems, postmeningitic complications and clinicopathological correlations. N Engl J Med. 1965;272:954–960. doi: 10.1056/NEJM196505062721806. , 1003–1010. [DOI] [PubMed] [Google Scholar]
- 8.Durand M L, Calderwood S B, Weber D J, Miller S I, Southwick F S, Caviness V S, Swartz M N. Acute bacterial meningitis in adults. A review of 493 episodes. N Engl J Med. 1993;328:21–28. doi: 10.1056/NEJM199301073280104. [DOI] [PubMed] [Google Scholar]
- 9.Evans M E, Pollack M. Effect of antibiotic class and concentration on the release of lipopolysaccharide from Escherichia coli. J Infect Dis. 1993;167:1336–1343. doi: 10.1093/infdis/167.6.1336. [DOI] [PubMed] [Google Scholar]
- 10.Figueiredo A M S, Connor J D, Severin A, Vaz Pat M V, Tomasz A. A pneumococcal clinical isolate with high-level resistance to cefotaxime and ceftriaxone. Antimicrob Agents Chemother. 1992;36:886–889. doi: 10.1128/aac.36.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Bustos J F, Chait B T, Tomasz A. Structure of the peptide network of pneumococcal peptidoglycan. J Biol Chem. 1987;262:15400–15405. [PubMed] [Google Scholar]
- 12.Giovannopoulos A, Eiffert H, Reinert R R, Hof H, Nau R. 15. Arbeitstagung der Arbeitsgemeinschaft für Neurologische Intensivmedizin; 1998. In azider Umgebung sinkt die Empfindlichkeit von Erregern von ZNS-Infektionen gegenüber Aminoglykosiden und Erythromycin, poster. [Google Scholar]
- 13.Goldstein E J C, Citron D M, Hudspeth M, Hunt Gerardo S, Merriam C V. In vitro activity of Bay 12-8039, a new 8-methoxyquinolone, compared to the activities of 11 other oral antimicrobial agents against 390 aerobic and anaerobic bacteria isolated from human and animal bite wound skin and soft tissue infections in humans. Antimicrob Agents Chemother. 1997;41:1552–1557. doi: 10.1128/aac.41.7.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue S, Takahashi H, Kaneko K I. The fluctuations of neuron-specific enolase (NSE) levels of cerebrospinal fluid during bacterial meningitis: the relationship between the fluctuations of NSE levels and neurological complications or outcome. Acta Paediatr Japon. 1994;36:485–488. doi: 10.1111/j.1442-200x.1994.tb03230.x. [DOI] [PubMed] [Google Scholar]
- 15.Marton A, Gulyas M, Munoz R, Tomasz A. Extremely high incidence of antibiotic resistance in clinical isolates of Streptococcus pneumoniae in Hungary. Clin Infect Dis. 1991;163:542–548. doi: 10.1093/infdis/163.3.542. [DOI] [PubMed] [Google Scholar]
- 16.Mason D J, Power E G M, Talsania H, Phillips I, Gant V A. Antibacterial action of ciprofloxacin. Antimicrob Agents Chemother. 1995;39:2257–2258. doi: 10.1128/aac.39.12.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nau R, Kinzig M, Dreyhaupt T, Kolenda H, Sörgel F, Prange H W. Cerebrospinal fluid kinetics of ofloxacin and its metabolites after a single intravenous infusion of 400 milligrams of ofloxacin. Antimicrob Agents Chemother. 1994;38:1849–1853. doi: 10.1128/aac.38.8.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nau R, Prange H W, Martell J, Sharifi S, Kolenda H, Bircher J. Penetration of ciprofloxacin into the cerebrospinal fluid of patients with uninflamed meninges. J Antimicrob Chemother. 1990;25:965–973. doi: 10.1093/jac/25.6.965. [DOI] [PubMed] [Google Scholar]
- 19.Nau R, Schmidt T, Kaye K, Froula J L, Täuber M G. Quinolone antibiotics in therapy of experimental pneumococcal meningitis in rabbits. Antimicrob Agents Chemother. 1995;39:593–597. doi: 10.1128/AAC.39.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nau R, Zysk G, Börner T, Prange H. Deutscher Kongreβ für Infektions-und Tropenmedizin. 1995. Einfluβ einer Immunsuppression auf die Liquorpenetration von Ceftriaxon bei der experimentellen Pneumokokken-Meningitis. Oral presentation 3. [Google Scholar]
- 21.Nau R, Zysk G, Schmidt H, Fischer F R, Stringaris A, Stuertz K, Brück W. Trovafloxacin delays the antibiotic-induced inflammatory response in experimental pneumococcal meningitis. J Antimicrob Chemother. 1997;39:781–788. doi: 10.1093/jac/39.6.781. [DOI] [PubMed] [Google Scholar]
- 22.Paris M M, Hickey S M, Uscher M I, Shelton S, Olsen K D, McCracken G H J. Effect of dexamethasone on therapy of experimental penicillin- and cephalosporin-resistant pneumococcal meningitis. Antimicrob Agents Chemother. 1994;38:1320–1324. doi: 10.1128/aac.38.6.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prins J M, Kuijper E J, Mevissen M L, Speelman P, van Deventer S J. Release of tumor necrosis factor alpha and interleukin 6 during antibiotic killing of Escherichia coli in whole blood: influence of antibiotic class, antibiotic concentration, and presence of septic serum. Infect Immun. 1995;63:2236–2242. doi: 10.1128/iai.63.6.2236-2242.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spangler S K, Jacobs M R, Appelbaum P C. Susceptibilities of penicillin-susceptible and -resistant strains of Streptococcus pneumoniae to RP 59500, vancomycin, erythromycin, PD 131628, sparfloxacin, temafloxacin, Win 57273, ofloxacin, and ciprofloxacin. Antimicrob Agents Chemother. 1992;36:856–859. doi: 10.1128/aac.36.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spangler S K, Jacobs M R, Appelbaum P C. Comparative activity of the new fluoroquinolone Bay Y3118 against 177 penicillin susceptible and resistant pneumococci. Eur J Clin Microbiol Infect Dis. 1993;12:965–967. doi: 10.1007/BF01992176. [DOI] [PubMed] [Google Scholar]
- 26.Sparwasser T, Miethke T, Lipford G B, Borschert K, Häcker H, Heeg K, Wagner H. Bacterial DNA cause septic shock. Nature. 1997;386:336–337. doi: 10.1038/386336a0. [DOI] [PubMed] [Google Scholar]
- 27.Stuertz K, Schmidt H, Eiffert H, Schwartz P, Mäder M, Nau R. Differential release of lipoteichoic and teichoic acids from Streptococcus pneumoniae as a result of exposure to β-lactam antibiotics, rifamycins, trovafloxacin, and quinupristin-dalfopristin. Antimicrob Agents Chemother. 1998;42:277–281. doi: 10.1128/aac.42.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Syrogiannopoulos G A, Hansen E J, Erwin A L, Munford R S, Rutledge J, Reisch J S, McCracken G H. Haemophilus influenzae type b lipooligosaccharide induces meningeal inflammation. J Infect Dis. 1988;157:237–244. doi: 10.1093/infdis/157.2.237. [DOI] [PubMed] [Google Scholar]
- 29.Täuber M G, Doroshow C A, Hackbarth C J, Rusnak M G, Drake T A, Sande M A. Antibacterial activity of beta-lactam antibiotics in experimental meningitis due to Streptococcus pneumoniae. J Infect Dis. 1984;149:568–574. doi: 10.1093/infdis/149.4.568. [DOI] [PubMed] [Google Scholar]
- 30.Täuber M G, Shibl A M, Hackbarth C J, Larrick J W, Sande M A. Antibiotic therapy, endotoxin concentration in cerebrospinal fluid, and brain edema in experimental Escherichia coli meningitis in rabbits. J Infect Dis. 1987;156:456–462. doi: 10.1093/infdis/156.3.456. [DOI] [PubMed] [Google Scholar]
- 31.Tuomanen E, Liu H, Hengstler B, Zak O, Tomasz A. The induction of meningeal inflammation by components of the pneumococcal cell wall. J Infect Dis. 1985;151:859–868. doi: 10.1093/infdis/151.5.859. [DOI] [PubMed] [Google Scholar]
- 32.Woodcock J M, Andrews J M, Boswell F J, Brenwald N P, Wise R. In vitro activity of BAY 12-8039, a new fluoroquinolone. Antimicrob Agents Chemother. 1997;41:101–106. doi: 10.1128/aac.41.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zysk G, Brück W, Gerber J, Brück Y, Prange H W, Nau R. Anti-inflammatory treatment influences neuronal apoptotic cell death in the dentate gyrus in experimental pneumococcal meningitis. J Neuropathol Exp Neurol. 1996;55:722–728. doi: 10.1097/00005072-199606000-00006. [DOI] [PubMed] [Google Scholar]