Abstract
Background
Overactive bladder (OAB) is a common chronic and bothersome condition. Bladder training is widely prescribed as a first‐line treatment for OAB, but the efficacy has been systematically evaluated for urinary incontinence rather than OAB alone.
Objectives
To evaluate the benefits and harms of bladder training for treating adults with OAB compared to no treatment, anticholinergics, β3‐adrenoceptor agonists, or pelvic floor muscle training (PFMT) alone or in combination.
Search methods
We used standard, extensive Cochrane search methods. The latest search date was 6 November 2022.
Selection criteria
We included randomized controlled trials involving adults aged 18 years or older with non‐neurogenic OAB. We excluded studies of participants whose symptoms were caused by factors outside the urinary tract (e.g. neurologic disorders, cognitive impairment, gynecologic diseases).
Data collection and analysis
We used standard Cochrane methods. Our primary outcomes were 1. participant‐reported cure or improvement, 2. symptom‐ and condition‐related quality of life (QoL), and 3. adverse events. Secondary outcomes included 4. participant‐reported satisfaction, 5. number of incontinence episodes, 6. number of urgency episodes, and 7. number of micturition episodes. For the purpose of this review, we considered two time points: immediately after the treatment (early phase) and at least two months after the treatment (late phase). We used GRADE to assess certainty of evidence for each outcome.
Main results
We included 15 trials with 2007 participants; participants in these trials were predominantly women (89.3%). We assessed the risk of bias of results for primary and secondary outcomes, which across all studies was similar and predominantly of high risk of bias, and none were at low risk of bias. The certainty of evidence was low to very low, with some moderate, across measured outcomes.
Bladder training versus no treatment: three studies involving 92 participants compared bladder training to no treatment. The evidence is very uncertain about the effects of bladder training on cure or improvement at the early phase (risk ratio (RR) 17.00, 95% confidence interval (CI) 1.13 to 256.56; 1 study, 18 participants; very low‐certainty evidence). Bladder training may reduce the number of incontinence episodes (mean difference (MD) −1.86, 95% CI −3.47 to −0.25; 1 study, 14 participants; low‐certainty evidence). No studies measured symptom‐ and condition‐related QoL, number of adverse events, participant‐reported satisfaction, number of urgency episodes, or number of micturition episodes in the early phase.
Bladder training versus anticholinergics: seven studies (602 participants) investigated the effects of bladder training versus anticholinergic therapy. Bladder training may be more effective than anticholinergics on cure or improvement at the early phase (RR 1.37, 95% CI 1.10 to 1.70; 4 studies, 258 participants; low‐certainty evidence). The evidence is very uncertain about the effects of bladder training on symptom‐ and condition‐related QoL (standardized mean difference (SMD) −0.06, 95% CI −0.89 to 0.77; 2 studies, 117 participants; very low‐certainty evidence). Although the evidence is very uncertain, there were fewer adverse events in the bladder training group than in the anticholinergics group (RR 0.03, 95% CI 0.01 to 0.17; 3 studies, 187 participants; very low‐certainty evidence). The evidence is very uncertain about the effects of the number of incontinence episodes per 24 hours (MD 0.36, 95% CI −0.27 to 1.00; 2 studies, 117 participants; very low‐certainty evidence), the number of urgency episodes per 24 hours (MD 0.70, 95% CI −0.62 to 2.02; 2 studies, 92 participants; very low‐certainty evidence), and the number of micturition episodes per 24 hours (MD −0.35, 95% CI −1.90 to 1.20; 3 studies, 175 participants; very low‐certainty evidence). No studies measured participant‐reported satisfaction in the early phase.
Bladder training versus PFMT: three studies involving 203 participants compared bladder training to PFMT. The evidence is very uncertain about the different effects between bladder training and PFMT on symptom‐ and condition‐related QoL at the early phase (SMD 0.10, 95% CI −0.19 to 0.40; 2 studies, 178 participants; very low‐certainty evidence). There were no adverse events in either group at the early phase (1 study, 97 participants; moderate‐certainty evidence). The evidence is uncertain about the effects of the number of incontinence episodes per 24 hours (MD 0.02, 95% CI −0.35 to 0.39, 1 study, 81 participants; low‐certainty evidence) and very uncertain about the number of micturition episodes per 24 hours (MD 0.10, 95% CI −1.44 to 1.64; 1 study, 81 participants; very low‐certainty evidence). No studies measured cure or improvement, participant‐reported satisfaction, or number of urgency episodes in the early phase.
Although we were interested in studies examining bladder training versus β3‐adrenoceptor agonists, in combination with β3‐adrenoceptor agonists versus β3‐adrenoceptor agonists alone, and in combination with PFMT versus PFMT alone, we did not identify any eligible studies for these comparisons.
Authors' conclusions
This review focused on the effect of bladder training to treat OAB. However, most of the evidence was low or very‐low certainty. Based on the low‐ or very low‐certainty evidence, bladder training may cure or improve OAB compared to no treatment. Bladder training may be more effective to cure or improve OAB than anticholinergics, and there may be fewer adverse events. There may be no difference in efficacy or safety between bladder training and PFMT. More well‐designed trials are needed to reach a firm conclusion.
Keywords: Adult; Female; Humans; Male; Cholinergic Antagonists; Cholinergic Antagonists/therapeutic use; Electric Stimulation Therapy; Electric Stimulation Therapy/methods; Pelvic Floor; Quality of Life; Receptors, Adrenergic; Urinary Bladder; Urinary Bladder, Overactive; Urinary Bladder, Overactive/therapy; Urinary Incontinence; Urinary Incontinence/therapy
Plain language summary
Bladder training for treating overactive bladder in adults
What did we want to find out?
We wanted to compare the effectiveness of bladder training to other treatments for adults with overactive bladder (OAB).
Background
OAB is a common chronic condition involving daytime frequent urination, urination during sleep, and sudden urge to urinate with or without urinary incontinence (unintentional passing of urine). The disorder reduces quality of life and results in a significant economic burden on society. Bladder training is a behavioral therapy that establishes treatment goals and uses techniques to modify inappropriate responses to urinary urgency. The aim is to improve OAB symptoms by minimizing the frequent urge to urinate. Although clinical guidelines recommend bladder training to treat OAB, there is no review to evaluate the efficacy systematically.
What did we do?
We searched for studies that investigated bladder training in the following seven interventions: 1. compared to no treatment, 2. compared to medicines called anticholinergics, 3. compared to medicines called β3‐adrenoceptor agonists, 4. compared to pelvic floor muscle training (PFMT; strengthening of the muscles around the bladder, anus, and vagina or penis), 5. in combination with anticholinergics versus anticholinergics alone, 6. in combination with β3‐adrenoceptor agonists versus β3‐adrenoceptor agonists alone, and 7. in combination with PFMT versus PFMT alone.
What did we find?
We found 15 eligible studies involving 2007 participants. Most participants were women. The studies compared bladder training to three comparisons: no treatment, anticholinergics, and PFMT in adults with OAB. No studies investigated the other four comparisons. Seven studies were publicly funded. Two studies received grants from drug companies. Six studies did not declare their funding sources.
Key results
Bladder training versus no treatment: bladder training may cure or improve OAB symptoms, but we are very uncertain about the results. Bladder training may reduce the number of incontinence episodes. We found no studies to help us answer our question on the other outcomes.
Bladder training versus anticholinergics: bladder training may cure or improve OAB symptoms more than anticholinergics. We do not know whether bladder training has an effect on the other outcomes, and we found no studies to help us answer our question on patient‐reported satisfaction.
Bladder training versus PFMT: bladder training may make little to no difference to quality of life or the number of incontinence episodes per 24 hours. The only study that looked at side effects reported zero events. It is unclear if bladder training has an effect on urination episodes. We found no studies that measured the other outcomes.
What are the limitations of the evidence?
Most of the included studies were limited due to small numbers of participants and poor reporting of study details, which lead to uncertainty in the evidence. The evidence to date is insufficient to show the effectiveness of bladder training to treat OAB and more well‐designed studies are needed to reach a firm conclusion.
How up to date is this review?
The evidence is up to date to 6 November 2022.
Summary of findings
Summary of findings 1. Summary of findings table ‐ Bladder training compared to no treatment for overactive bladder.
| Bladder training compared to no treatment for overactive bladder | ||||||
| Patient or population: overactive bladder Setting: outpatient Intervention: bladder training Comparison: no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no treatment | Risk with bladder training | |||||
| Participant‐reported cure or improvement: immediately after treatment | 0 per 1000 | 0 per 1000 (0 to 0) | RR 17.00 (1.13 to 256.56) | 18 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | Although bladder training may result in cure or improvement of an overactive ladder, the evidence is very uncertain as it was based on only 1 study that is leading to very wide CIs. |
| Symptom‐ and condition‐related quality of life: immediately after treatment | ‐ | (0 studies) | ‐ | |||
| Number of any adverse events: immediately after treatment | ‐ | (0 studies) | ‐ | |||
| Participant‐reported satisfaction: immediately after treatment | ‐ | (0 studies) | ‐ | |||
| Number of incontinence episodes per 24 hours: immediately after treatment | The mean number of incontinence episodes per 24 hours: immediately after treatment was 2.57 | MD 1.86 lower (3.47 lower to 0.25 lower) | ‐ | 14 (1 RCT) | ⊕⊕⊝⊝ Lowb | Although bladder training may reduce the number of incontinence episodes, it was based on only 1 study with very wide CIs. |
| Number of urgency episodes per 24 hours: immediately after treatment | ‐ | (0 studies) | ‐ | |||
| Number of micturition episodes per 24 hours: immediately after treatment | ‐ | (0 studies) | ‐ | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_423485434552773248. | ||||||
a Downgraded one level due to risk of bias: high risk of bias in only one included study. b Downgraded two levels due to imprecision: small sample size (fewer than 400 participants) with low number of events that is leading to very wide CIs.
Summary of findings 2. Summary of findings table ‐ Bladder training compared to anticholinergics for overactive bladder.
| Bladder training compared to anticholinergics for overactive bladder | ||||||
| Patient or population: overactive bladder Setting: outpatient Intervention: bladder training Comparison: anticholinergics | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with anticholinergics | Risk with bladder training | |||||
| Participant‐reported cure or improvement: immediately after treatment | 602 per 1000 | 824 per 1000 (662 to 1000) | RR 1.37 (1.10 to 1.70) | 258 (4 RCTs) | ⊕⊕⊝⊝ Lowa,b | Bladder training may result in cure or improvement of an overactive bladder compared with anticholinergics. |
| Symptom‐ and condition‐related quality of life: immediately after treatment | ‐ | SMD 0.06 SD lower (0.89 lower to 0.77 higher) | ‐ | 117 (2 RCTs) | ⊕⊝⊝⊝ Very lowc,d,e | The evidence is uncertain about the effect of bladder training on symptom‐related quality of life compared with anticholinergics. |
| Number of any adverse events: immediately after treatment | 434 per 1000 | 13 per 1000 (4 to 74) | RR 0.03 (0.01 to 0.17) | 187 (3 RCTs) | ⊕⊝⊝⊝ Very lowb,c | The evidence is uncertain about the effect of bladder training on adverse events compared with anticholinergics. |
| Participant‐reported satisfaction: immediately after treatment | ‐ | (0 studies) | ‐ | |||
| Number of incontinence episodes per 24 hours: immediately after treatment | The mean number of incontinence episodes per 24 hours: immediately after treatment was 0.10 to 0.51 | MD 0.36 higher (0.27 lower to 1 higher) | ‐ | 117 (2 RCTs) | ⊕⊝⊝⊝ Very lowb,c,f | The evidence is uncertain about the effect of bladder training on the number of incontinence episodes compared with anticholinergics. |
| Number of urgency episodes per 24 hours: immediately after treatment | The mean number of urgency episodes per 24 hours: immediately after treatment was 1.1 to 1.5 | MD 0.7 higher (0.62 lower to 2.02 higher) | ‐ | 92 (2 RCTs) | ⊕⊝⊝⊝ Very lowb,c | The evidence is uncertain about the effect of bladder training on the number of urgency episodes compared with anticholinergics. |
| Number of micturition episodes per 24 hours: immediately after treatment | The mean number of micturition episodes per 24 hours: immediately after treatment was 6.3 to 11.3 | MD 0.35 lower (1.9 lower to 1.2 higher) | ‐ | 175 (3 RCTs) | ⊕⊝⊝⊝ Very lowb,c | The evidence is uncertain about the effect of bladder training on the number of micturition episodes compared with anticholinergics. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_423485506556390153. | ||||||
a Downgraded one level due to risk of bias: overall high risk of bias in at least one study but less than half of the included studies. b Downgraded one level due to imprecision: small sample size (fewer than 400 participants). c Downgraded two levels due to risk of bias: overall high risk of bias in all included studies. d Downgraded one level due to inconsistency: serious heterogeneity was shown visually. e Downgraded two levels due to imprecision: small sample size (fewer than 400 participants) and the CIs were consistent with both benefit and harm. f Downgraded one level due to inconsistency: there was statistical heterogeneity (I2 = 80%).
Summary of findings 3. Summary of findings table ‐ Bladder training compared to pelvic floor muscle training (PFMT) for overactive bladder.
| Bladder training compared to pelvic floor muscle training (PFMT) for overactive bladder | ||||||
| Patient or population: overactive bladder Setting: outpatients Intervention: bladder training Comparison: pelvic floor muscle training (PFMT) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with pelvic floor muscle training (PFMT) | Risk with bladder training | |||||
| Participant‐reported cure or improvement: immediately after treatment | ‐ | (0 studies) | ‐ | |||
| Symptom‐related quality of life: immediately after treatment | ‐ | SMD 0.1 SD higher (0.19 lower to 0.4 higher) | ‐ | 178 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | There may be no difference in symptom‐related quality of life between bladder training and PFMT. |
| Number of adverse events: immediately after treatment | Not pooled | Not pooled | Not pooled | 97 (1 RCT) | ⊕⊕⊕⊝ Moderateb | Although there were 0 adverse events in either group, it was based on only 1 study. |
| Participant‐reported satisfaction: immediately after treatment | ‐ | (0 studies) | ‐ | |||
| Number of incontinence episodes per 24 hours: immediately after treatment | The mean number of incontinence episodes per 24 hours: immediately after treatment was 0.54 | MD 0.02 higher (0.35 lower to 0.39 higher) | ‐ | 81 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | There may be no difference in the number of incontinence episodes between bladder training and PFMT. |
| Number of urgency episodes per 24 hours: immediately after treatment | ‐ | (0 studies) | ‐ | |||
| Number of micturition episodes per 24 hours: immediately after treatment | The mean number of micturition episodes per 24 hours: immediately after treatment was 9.6 | MD 0.1 higher (1.44 lower to 1.64 higher) | ‐ | 81 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c | The evidence is uncertain about the effect of bladder training on micturition episodes compared with PFMT. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_423864967873274305. | ||||||
a Downgraded one level due to risk of bias: overall high risk of bias in at least one study but less than half of the included studies. b Downgraded one level due to imprecision: small numbers of events (fewer than 400 participants). c Downgraded two levels due to imprecision: small sample size (fewer than 400 participants) and the CIs were consistent with both benefit and harm.
Background
For a glossary of terms used, see Appendix 1.
Description of the condition
Overactive bladder (OAB) is a common chronic condition associated with voiding dysfunction. The disorder reduces health‐related quality of life (HRQoL) and results in a significant economic burden on society (Irwin 2011; Vaughan 2011).
According to the International Continence Society (ICS), OAB is defined as "urinary urgency, usually accompanied by increased daytime frequency and/or nocturia, with urinary incontinence (UI) (OAB‐wet) or without (OAB‐dry), in the absence of urinary tract infection or other detectable diseases" (Haylen 2010). OAB can be classified into two subtypes: OAB with UI (OAB‐wet) and OAB without incontinence (OAB‐dry). The pathophysiologic mechanisms contributing to OAB are categorized as neuropathic and non‐neuropathic mechanisms, although there is a lack of conclusive evidence in this regard. Neurogenic mechanisms operate through the following pathways: supraspinal (e.g. Parkinson's disease), spinal (e.g. spinal cord injury), and peripheral nerve (e.g. diabetes mellitus) (Kennelly 2008). OAB that occurs in the absence of such neurogenic mechanisms is attributed to non‐neurogenic pathology and the contributory pathophysiologic mechanisms remain unclear.
Depending on the definition of OAB, the estimated prevalence of OAB varies from 11.8% to 35.6% across studies (Coyne 2011; Irwin 2006; Milsom 2001; Stewart 2003). However, most observe that the prevalence of OAB increases with age. One study reported that the prevalence of OAB among people from Asia was lower than that in other races in both men and women (Coyne 2012), while another reported an increase in the global prevalence of OAB, particularly in low‐ and middle‐income countries within Africa, South America, and Asia (Irwin 2011).
The symptoms of OAB have a significant negative impact on a patient's physical, social, and emotional well‐being and thus OAB is considered a major public health concern, with one study reporting that OAB might significantly diminish HRQoL (Vaughan 2011). In addition, OAB results in a notable economic burden on society. The EPIC study reported that the estimated total expenditure on OAB in six Western countries included in the study was EUR 9.7 billion in 2005 (Irwin 2009). As stated above, the prevalence of OAB is increasing in an aging society and the economic burden is expected to become a more serious issue.
Description of the intervention
Treatment options for OAB include lifestyle interventions, behavioral therapy, pharmacotherapy, onabotulinum toxin A administration, peripheral tibial nerve stimulation, sacral neuromodulation, and surgery (Corcos 2017; Lightner 2019). The American Urology Association (AUA) guidelines recommend behavioral therapy as the first‐line treatment for OAB and pharmacotherapy as the second‐line treatment (Lightner 2019). Compared with antimuscarinics, behavioral therapy is associated with a lower risk of adverse events (Rai 2012). This is a significant advantage because OAB is a benign condition. Occasionally, behavioral intervention and pharmacotherapy are used in combination to provide an additive effect.
Bladder training (sometimes called 'bladder drill', 'bladder retraining', or 'bladder re‐education') is one component of behavioral therapy for OAB that can help by minimizing the frequent urge to urinate. Although bladder training has no standardized definition or standardized administration, this review defined bladder training to include the following components (Fantl 1996).
Patient education: explaining the mechanism of bladder action and voiding function to enable patients to gain a better understanding of their excretory function.
Scheduled voiding: training to void at fixed voiding intervals while awake, which progressively lengthens as successful control is achieved.
Positive reinforcement: psychological support to patients to encourage them to continue the practice.
Despite the similarity in the basic framework, treatment protocols often differ, particularly with respect to where the intervention is delivered (such as outpatient, inpatient, and home environments). Bladder training is usually provided directly by healthcare providers, although pamphlets, educational materials, or information and communication technology (ICT) are occasionally used. It can be performed as either individual or group therapy. The duration of the therapy can vary, but is usually recommended for eight to 12 weeks (Lightner 2019).
How the intervention might work
Bladder training in people with OAB helps to control urgency by diverting their attention (e.g. performing mental arithmetic or pelvic floor muscle contractions) and helping them to relax (e.g. with deep breathing activities), and gradually prolonging the voiding interval by 15 minutes (Nygaard 2010). Eventually, the patient may be able to void every three to four hours without the frequent urge to urinate.
Although the mechanism of action remains unclear, the specific goals of bladder training are to adjust habit patterns of frequent urination, improve control over bladder urgency, prolong voiding intervals, increase bladder capacity, reduce incontinent episodes, and restore patient confidence in controlling bladder function (Bo 2017).
Bladder training is occasionally combined with other therapies, such as pelvic floor muscle training (PFMT) and pharmacotherapy, for an additive effect. In clinical practice, bladder training and PFMT are prescribed in combination and European Association of Urology (EAU) guidelines introduced both therapies as "behavioural and physical therapies" (Nambiar 2018). Pharmacotherapy, especially anticholinergics, is also combined with bladder training in clinical practice; AUA guidelines recommend the combination, but the evidence is of low quality (Lightner 2019).
Why it is important to do this review
Although several systematic reviews have discussed bladder training for UI and limited evidence has suggested its effectiveness (Roe 2007; Shamliyan 2008; Wallace 2004), few have assessed this intervention for OAB. Despite the clinical overlap, OAB does not necessarily accompany UI and the symptoms associated with both conditions can range in severity from mild (OAB) to moderate‐to‐severe (UI). Therefore, it is appropriate to investigate the effectiveness of bladder training in people presenting with OAB.
Objectives
To evaluate the benefits and harms of bladder training for treating adults with OAB compared to no treatment, anticholinergics, β3‐adrenoceptor agonists, or pelvic floor muscle training (PFMT) alone or in combination.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) assessing bladder training in adults with non‐neurogenic OAB. We also included cross‐over RCTs and cluster‐RCTs. For randomized cross‐over trials, we used data from the first period of treatment only. We included studies that use the terms 'bladder drill', 'bladder retraining', or 'bladder re‐education'.
We excluded quasi‐RCTs as their method of randomization leaves these studies open to a high risk of selection bias.
Types of participants
We included studies of adults (aged over 18 years, or according to the study authors' definition of 'adult') with non‐neurogenic OAB. We also included studies of urge urinary incontinence (UUI) and detrusor instability (DI) as OAB because the three are not clearly distinguishable disease concepts and overlap with each other.
We excluded studies of participants whose symptoms were caused by factors outside the urinary tract (e.g. neurologic disorders, cognitive impairment, gynecologic diseases). We also excluded studies that recruited specific populations, such as people with nocturnal enuresis, people who had undergone urinary tract surgery or vaginal surgery, and prenatal or postnatal women.
Types of interventions
We included studies with at least one study arm involving bladder training for treating OAB, as well as studies that investigated the additive effect with another treatment compared with monotherapy.
We also included studies where the interventions were termed 'patient education', 'scheduled voiding', and 'positive reinforcement'.
As recommended in the AUA guidelines (Lightner 2019), behavioral therapy and pharmacologic treatment are often prescribed in combination in clinical practice. However, the optimal treatment combination remains uncertain (Chancellor 2008). Therefore, we included the following comparisons.
Bladder training versus no treatment
Bladder training versus anticholinergics
Bladder training versus β3‐adrenoceptor agonists
Bladder training versus PFMT
Bladder training combined with anticholinergics versus anticholinergics alone
Bladder training combined with β3‐adrenoceptor agonists versus β3‐adrenoceptor agonists alone
Bladder training combined with PFMT versus PFMT alone
We believe that the comparisons of particular interest to patients and clinicians are 'bladder training versus no treatment', 'bladder training versus anticholinergics', 'bladder training versus β3‐adrenoceptor agonists', and 'bladder training versus PFMT'.
Types of outcome measures
Primary outcomes
Participant‐reported cure or improvement (assessed by validated self‐reported questionnaires such as the Patient Global Impression of Improvement (PGI‐I) Index (Busner 2007). In studies that did not use validated scales, we included author‐defined data regarding the number of participants who perceived cure or improvement. For studies in which participants reported more than a single level of improvement (e.g. much better and somewhat better), we entered data for the greater degree of improvement reported).
Symptom‐ and condition‐related quality of life (QoL) (assessed using validated questionnaires, such as Overactive Bladder Questionnaire (Coyne 2002) and King's Health Questionnaire (Kelleher 1997)).
Any adverse events (e.g. dry mouth, constipation, nausea, headache, dizziness, deceased visual acuity, and urinary tract infection).
Secondary outcomes
Participant‐reported satisfaction
Number of incontinence episodes per 24 hours
Number of urgency episodes per 24 hours
Number of micturition episodes per 24 hours
Timing of outcome measurement
We considered two time points for all primary and secondary outcomes: immediately after treatment and at least two months after treatment, to assess longer‐term effects. For adverse events, we also sought data during treatment and at follow‐up.
Main outcomes for summary of findings tables
We assessed all primary and secondary outcomes for the summary of findings tables immediately after treatment with the exception of adverse events, which we assessed during treatment.
Search methods for identification of studies
We did not impose any restrictions, for example language or publication status, on the searches described below.
Electronic searches
We identified relevant trials from the Cochrane Incontinence Specialised Register, which contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL) (on CRS Web), MEDLINE (on Ovid), MEDLINE In‐Process, In‐Data‐Review & Other Non‐Indexed Citations (on Ovid), MEDLINE Epub Ahead of Print (on Ovid), MEDLINE Daily (on Ovid), ClinicalTrials.gov (clinicaltrials.gov), World Health Organization International Clinical Trials Registry Platform (trialsearch.who.int), and handsearching of journals and conference proceedings. Many of the trials in the Cochrane Incontinence Specialised Register are also contained in CENTRAL. The date of the most recent search of the Register was 6 November 2022. The terms we used to search the Cochrane Incontinence Specialised Register are in Appendix 2.
Searching other resources
We searched the reference lists of relevant articles for potentially eligible studies.
Data collection and analysis
As reported in our protocol (Funada 2020), we conducted data collection and analysis in accordance with methods specified in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022).
Selection of studies
Two review authors (SF and TY) independently screened the list of titles and abstracts identified by our literature search and assessed the eligibility of full‐text articles for inclusion in the review. Where necessary, we contacted study investigators for further information. We resolved disagreements through discussion with a third review author (YL). We recorded the reasons for the exclusion of excluded studies at the full‐text screening in the Characteristics of excluded studies table.
Data extraction and management
Two review authors (SF and YL) independently extracted data onto a prepiloted form, which was cross‐checked by a third review author (TY). For trials with multiple publications, we used only the most up‐to‐date data or complete data for each outcome. Where the necessary data were not reported or were not reported in a form that could be directly used for meta‐analysis, we contacted the trial authors for further information.
Assessment of risk of bias in included studies
At least two review authors (SF and YL) independently assessed the risk of bias of included studies using Cochrane's RoB 2 tool (Higgins 2022). We used the Excel tool to implement RoB 2 and store our data (available at www.riskofbias.info/welcome/rob-2-0-tool). The types of bias include the following: bias arising from the randomization process; bias due to deviations from the intended intervention; bias due to outcome data; bias in measurement of the outcome; bias in selection of the reported results; and overall bias.
We assessed the outcomes and time points included in the summary of findings tables, and focused on the assessment of the effect of assignment to the interventions at baseline.
We categorized each potential domain of bias as follows.
Low risk of bias: the study is considered to show a low risk of bias.
Some concerns: a few concerns are expected to be associated with the study in at least one domain, but it does not warrant categorization as a study with a high risk of bias with regard to any domain.
High risk of bias: the study is considered at high risk of bias in at least one domain; or a few concerns with regard to multiple domains are observed in the study such that these concerns significantly lower confidence in the study results.
We summarized our findings in the risk of bias tables. We expressed the percentage of agreement about the judgment of risk of bias and resolved any disagreements by consulting a third review author (TY).
Measures of treatment effect
For categorical data, we used the ratio of the number of people who presented with the outcome to the number of people at risk in each group to calculate a risk ratio (RR) with 95% confidence intervals (CI) (Higgins 2022).
For continuous data, we used means and standard deviations (SDs) to calculate a mean difference (MD) with 95% CI. When studies used different scales, we reported standardized mean differences (SMD) (Higgins 2022).
If data to calculate RRs or MDs were not reported, we used the most appropriate numerical data available to calculate the actual numbers or means and SDs (e.g. test statistics and P values) (Higgins 2022).
Unit of analysis issues
We analyzed trials that included multiple treatment groups by treating each pair of trial arms as a separate comparison. In such cases, we divided the number of comparison groups dependent on the multiple intervention groups to avoid double counting. For randomized cross‐over trials, we only used data from the first period of treatment. For cluster‐randomized trials, we made corrections using an intracluster correlation coefficient (ICC). If this was not possible, we calculated the ICC based on similar studies included in the review, or we extracted primary data and calculated RRs with 95% CIs. We selected 'after treatment' as a single time point and analyzed data obtained only at this time.
Dealing with missing data
We attempted to obtain missing data from the trial authors. Where this was not possible, we analyzed the trial data based on the intention‐to‐treat (ITT) approach. We included summary statistics when studies used approaches including mixed‐effects models for repeated measurements or multiple imputation methods. If studies reported sufficient details to calculate MDs or SMDs but not the associated SD, we assumed the outcome to show an SD equal to the highest SD from other trials within the same analysis. For studies with missing SDs, we pursued simple imputation by using the SDs from studies in another published meta‐analysis as per the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022), and pursued sensitivity analysis to explore the impact of imputed SDs (Sensitivity analysis).
To obtain daily means for our outcomes of interest, we divided weekly means and the SD by seven.
Assessment of heterogeneity
We assessed heterogeneity between trials by visual inspection of plots of the data, the Chi² test for heterogeneity, and the I² statistic. We interpreted the I² statistic using the thresholds in the Cochrane Handbook for Systematic Reviews of Interventions, with substantial heterogeneity defined as I² values between 50% and 90%, and considerable heterogeneity as I² values more than 75% (Higgins 2022). We aimed to determine and discuss possible explanations for heterogeneity.
Assessment of reporting biases
Had sufficient data been available, we planned to assess potential publication bias using funnel plots and by performing an Egger's test when the meta‐analysis included 10 studies or more (Egger 1997).
Data synthesis
The main analysis included all studies that provided data regardless of the overall risk of bias as assessed by the RoB 2 tool.
We used Review Manager 2014 for data analysis. We performed a meta‐analysis if participants, interventions, comparisons, and outcomes were sufficiently similar. We pooled RRs using the Mantel‐Haenszel method for dichotomous outcomes and presented MDs or SMDs using inverse variance for continuous outcomes. We used a random‐effects model to perform a meta‐analysis (Higgins 2022).
Subgroup analysis and investigation of heterogeneity
If data allowed, we planned to perform the following subgroup analyses.
Heterogeneity among participants: sex and age (less than 65 years, 65 years or greater).
Heterogeneity in treatments: intervention (face‐to‐face, pamphlets, ICT); types of sessions (individual versus group sessions); and duration of therapy (less than 12 weeks, 12 weeks or greater).
Sensitivity analysis
Where sufficient data are available in future updates, we plan to test the robustness of our results using the following sensitivity analyses.
Exclusion of cross‐over RCTs and cluster‐RCTs.
Exclusion of studies in which there was no imputation of missing data.
For outcomes included in the summary of findings tables, we will include data from studies judged at low risk of bias or with some concerns for that outcome. Data from studies judged at high risk of bias for that outcome will be excluded from the analysis.
We performed a post doc sensitivity analysis to assess the robustness of results due to missing SDs in an included study (Dealing with missing data).
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to assess the certainty of evidence related to the primary and secondary outcomes as listed in the Types of outcome measures (Schünemann 2019). We used the five GRADE considerations (study limitations, inconsistency of effect, indirectness, imprecision, and publication bias) to assess the certainty of the body of evidence for the prespecified outcomes as outlined in Appendix 3 (Guyatt 2011a).
We justified all decisions to downgrade the certainty of the evidence using footnotes. Where there was sufficient evidence, we prepared summary of findings tables for the following main comparisons as stated in the Types of interventions using GRADEpro GDT software (GRADEpro GDT).
Bladder training versus no treatment
Bladder training versus anticholinergics
Bladder training versus β3‐adrenoceptor agonists
Bladder training versus PFMT
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; and Characteristics of studies awaiting classification tables.
Results of the search
We identified 856 references through our electronic and manual searches. After deduplication and title and abstract screening, we retrieved 243 references. After screening the full text, we included 15 RCTs from 38 references and excluded 144 studies from 200 references (see Figure 1).
1.
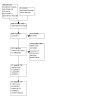
PRISMA study flow diagram.
Included studies
Design
All 15 studies were parallel RCTs. Two studies had four arms (Kafri 2013; Zhang 2012), three studies had three arms (Lauti 2008; Rizvi 2018; Song 2006), and the remaining 10 were two‐armed studies (Colombo 1995; Fantl 1991; Jarvis 1980; Jarvis 1981; Lagro‐Janssen 1992; Lentz 1994; Mattiasson 2003; Mattiasson 2010; McCreanor 1998; Milani 1987).
Sample sizes
The studies included 2007 participants. Mattiasson 2010 had the largest study population with 643 participants randomized. Lagro‐Janssen 1992 had the smallest study population as only 18/110 participants were diagnosed with DI and thus randomized to bladder training versus no treatment.
Setting
Four studies were conducted in the UK (Jarvis 1980; Jarvis 1981; Lentz 1994; McCreanor 1998), two in other European countries (Mattiasson 2003; Mattiasson 2010), two in Italy (Colombo 1995; Milani 1987), one in the USA (Fantl 1991), one in Israel (Kafri 2013), one in the Netherlands (Lagro‐Janssen 1992), one in Korea (Song 2006), one in New Zealand (Lauti 2008), one in China (Zhang 2012), and one in Pakistan (Rizvi 2018).
Five studies were conducted in multiple centers (Kafri 2013; Lagro‐Janssen 1992; Mattiasson 2003; Mattiasson 2010; Milani 1987), four were single‐center studies (Colombo 1995; Lauti 2008; McCreanor 1998; Rizvi 2018), and six were unclear (Fantl 1991; Jarvis 1980; Jarvis 1981; Lentz 1994; Song 2006; Zhang 2012).
Participants
All participants were women except in two trials; there were 378 women and 123 men in one study (Mattiasson 2003), and 551 women and 92 men in the other study (Mattiasson 2010).
The mean or median age of participants ranged from 40 to 49 years in eight studies (Colombo 1995; Jarvis 1980; Jarvis 1981; Lagro‐Janssen 1992; Lentz 1994; Milani 1987; Rizvi 2018; Song 2006), 50 to 59 years in three studies (Kafri 2013; Lauti 2008; Mattiasson 2010), 60 to 69 years in two studies (Fantl 1991; Mattiasson 2003), and unclear in two studies (McCreanor 1998; Zhang 2012).
The diagnosis was OAB by symptoms in seven studies (Lentz 1994; Mattiasson 2003; Mattiasson 2010; Milani 1987; Rizvi 2018; Song 2006; Zhang 2012), UUI by urodynamics in two studies (Colombo 1995; Lagro‐Janssen 1992), UUI by symptoms in three studies (Kafri 2013; Lauti 2008; McCreanor 1998), and DI by urodynamics in three studies (Fantl 1991; Jarvis 1980; Jarvis 1981). In two of these studies, other types of incontinence (stress or mixed incontinence) were included and the results of UUI or DI were extracted from all participants (Fantl 1991; Lagro‐Janssen 1992).
Interventions
Descriptions of bladder training
Twelve studies prescribed bladder training face‐to‐face (Colombo 1995; Fantl 1991; Jarvis 1980; Jarvis 1981; Kafri 2013; Lagro‐Janssen 1992; Lentz 1994; McCreanor 1998; Milani 1987; Rizvi 2018; Song 2006; Zhang 2012), two studies by leaflet (Mattiasson 2003; Mattiasson 2010), and one study by face‐to‐face and leaflet (Lauti 2008).
In terms of provider, three studies used a nurse to provide bladder training (McCreanor 1998; Song 2006; Zhang 2012), one study used a general practitioner (Lagro‐Janssen 1992), two studies used a physical therapist (Kafri 2013; Lauti 2008), one study used a nurse and physician (Rizvi 2018), two studies used a leaflet but personnel unknown (Mattiasson 2003; Mattiasson 2010), and six studies were unclear (Colombo 1995; Fantl 1991; Jarvis 1980; Jarvis 1981; Lentz 1994; Milani 1987). No study performed group sessions.
Three studies had a duration of therapy of less than 12 weeks (Colombo 1995; Fantl 1991; McCreanor 1998), nine studies of more than 12 weeks (Kafri 2013; Lagro‐Janssen 1992; Lauti 2008; Mattiasson 2003; Mattiasson 2010; Milani 1987; Rizvi 2018; Song 2006; Zhang 2012), and three studies were unclear (Jarvis 1980; Jarvis 1981; Lentz 1994).
The details of bladder training were as follows.
Nine studies prescribed participant education (Colombo 1995; Fantl 1991; Jarvis 1980; Kafri 2013; Lagro‐Janssen 1992; Lauti 2008; Mattiasson 2003; Mattiasson 2010; Song 2006).
Eleven studies prescribed scheduled voiding (Colombo 1995; Fantl 1991; Jarvis 1980; Kafri 2013; Lagro‐Janssen 1992; Lauti 2008; Mattiasson 2003; Mattiasson 2010; Milani 1987; Rizvi 2018; Song 2006).
Six studies prescribed positive reinforcement (Colombo 1995; Fantl 1991; Jarvis 1980; Kafri 2013; Lauti 2008; Mattiasson 2010).
Nine studies prescribed self‐monitoring (Fantl 1991; Jarvis 1980; Lagro‐Janssen 1992; Kafri 2013; Mattiasson 2003; Mattiasson 2010; Milani 1987; Rizvi 2018; Song 2006).
Three studies performed pelvic floor muscle squeeze to palliate urgency (Lauti 2008; Mattiasson 2010; Song 2006).
Description of comparators
Bladder training versus no treatment (Fantl 1991; Jarvis 1980; Lagro‐Janssen 1992): participants in the control groups received no treatment during the intervention phase.
Bladder training versus anticholinergics (Colombo 1995; Jarvis 1981; Kafri 2013; Lauti 2008; McCreanor 1998; Milani 1987; Song 2006): four studies prescribed oxybutynin (Colombo 1995; Lauti 2008; McCreanor 1998; Milani 1987), two studies prescribed tolterodine (Kafri 2013; Song 2006), and one study prescribed flavoxate hydrochloride plus imipramine (Jarvis 1981).
Bladder training versus β3‐adrenoceptor agonists: no studies identified.
Bladder training versus PFMT (Kafri 2013; Lentz 1994; Rizvi 2018): one study performed PFMT via vaginal cone (Lentz 1994).
Bladder training combined with anticholinergics versus anticholinergics alone (Lauti 2008; Mattiasson 2003; Mattiasson 2010; Song 2006; Zhang 2012): four studies prescribed tolterodine (Mattiasson 2003; Song 2006; Zhang 2012), two studies prescribed oxybutynin (Lauti 2008), one study prescribed solifenacin (Mattiasson 2010).
Bladder training combined with β3‐adrenoceptor agonists versus β3‐adrenoceptor agonists alone: no studies identified.
Bladder training combined with PFMT versus PFMT: no studies identified.
Outcomes
For primary outcomes, eight studies reported participant‐reported cure or improvement immediately after treatment (Colombo 1995; Fantl 1991; Jarvis 1981; Lagro‐Janssen 1992; Lentz 1994; Mattiasson 2003; Milani 1987; Song 2006), and five studies more than two months after treatment (Colombo 1995; Jarvis 1980; Lagro‐Janssen 1992; Lentz 1994; Milani 1987). Six studies reported symptom‐related QoL immediately after treatment (Fantl 1991; Kafri 2013; Lauti 2008; Mattiasson 2010; Rizvi 2018; Zhang 2012), and three studies more than two months after treatment (Kafri 2013; Lauti 2008; Mattiasson 2010). Seven studies reported adverse events immediately after treatment (Colombo 1995; Jarvis 1981; Lauti 2008; Mattiasson 2003; Milani 1987; Rizvi 2018; Song 2006), and two studies more than two months after treatment (Lauti 2008; Mattiasson 2010).
For secondary outcomes, one study reported participant‐reported satisfaction immediately after treatment and more than two months after treatment (Mattiasson 2010). Six studies reported the number of incontinence episodes immediately after treatment (Fantl 1991; Kafri 2013; Lagro‐Janssen 1992; Lauti 2008; Mattiasson 2003; Mattiasson 2010), and three studies more than two months after treatment (Kafri 2013; Lauti 2008; Mattiasson 2010). Three studies reported the number of urgency episodes immediately after treatment (Lauti 2008; Mattiasson 2003; Mattiasson 2010). One study reported an urgency score, not the number of urgency episodes, that was defined as follows; 0 being no symptoms, 1 rarely, 2 occasionally, 3 often, and 4 always (Song 2006). Two studies reported the number of urgency episodes more than two months after treatment (Lauti 2008; Mattiasson 2010). Five studies reported the number of micturition episodes immediately after treatment (Kafri 2013; Lauti 2008; Mattiasson 2003; Mattiasson 2010; Song 2006), and three studies more than two months after treatment (Kafri 2013; Lauti 2008; Mattiasson 2010).
Although we contacted 18 study authors to seek unpublished/missing information and received responses from three authors, the available data were insufficient. We obtained the missing SDs in Song 2006 from those reported in a published Cochrane Review (Rai 2012). Although we contacted the authors of the Cochrane Review (Rai 2012) to ask how they obtained the missing SDs from Song 2006, we did not receive a response.
Funding sources
Nine studies reported their funding sources (Fantl 1991; Kafri 2013; Lagro‐Janssen 1992; Lauti 2008; Mattiasson 2003; Mattiasson 2010; McCreanor 1998; Rizvi 2018; Zhang 2012).
Excluded studies
We excluded 144 studies (200 full‐text articles), and the details were shown in the Characteristics of excluded studies table. The main reasons were non‐RCTs, participants not having OAB, and irrelevant types of intervention.
Studies awaiting classification
Five studies are awaiting classification (Characteristics of studies awaiting classification table).
Ongoing studies
We identified no ongoing studies.
Risk of bias in included studies
Risk of bias assessments for each outcome, including all domain judgments and support for judgment, is located in the risk of bias section, and visually represented as traffic lights in forest plots. To access detailed risk of bias assessment data see: 10.6084/m9.figshare.21623364.
Risk of bias of outcomes across all studies was similar and predominantly of high risk of bias and none were at low risk of bias. Many studies did not report the details of randomization and allocation concealment. Due to the nature of our interventions and comparators of interest, blinding was difficult and that may cause more deviation from intervention and missing outcomes. Moreover, most studies did not perform adequate imputation for missing data. As all were participant‐reported outcomes, it was difficult to ensure blinding of outcome assessors. None of the included studies reported a prespecified analysis plan with sufficient details.
Effects of interventions
See: Table 1; Table 2; Table 3
Bladder training versus no treatment
Three studies compared bladder training versus no treatment (Fantl 1991; Jarvis 1980; Lagro‐Janssen 1992). See Table 1.
Primary outcomes
Participant‐reported cure or improvement
Bladder training may be more effective than no treatment in increasing cure/improvement rates immediately after treatment (RR 17.00, 95% CI 1.13 to 256.56; 1 study, 18 participants; Analysis 1.1; very low‐certainty evidence) and at more than two months after the treatment (RR 3.86, 95% CI 1.99 to 7.46; 1 study, 60 participants; Analysis 1.2; very low‐certainty evidence), but the evidence is very uncertain. Both results were based on one study. As the ranges of the 95% CIs were wide, the results were imprecise. We judged the certainty of the evidence to be very low immediately after treatment and more than two months after the treatment due to serious concerns regarding risk of bias and imprecision.
1.1. Analysis.

Comparison 1: Bladder training versus no treatment, Outcome 1: Participant‐reported cure or improvement: immediately after treatment
1.2. Analysis.

Comparison 1: Bladder training versus no treatment, Outcome 2: Participant‐reported cure or improvement: long‐term effect (> 2 months after treatment)
Symptom‐ and condition‐related quality of life
No studies reported symptom‐ and condition‐related QoL.
Adverse events
No studies reported adverse events.
Secondary outcomes
Participant‐reported satisfaction
No studies reported participant‐reported satisfaction.
Number of incontinence episodes per 24 hours
Bladder training may reduce the number of incontinence episodes per 24 hours when compared to no treatment immediately after treatment (MD −1.86, 95% CI −3.47 to −0.25; 1 study, 14 participants; Analysis 1.3; low‐certainty evidence). The result was based on one study, the range of the CIs was wide, and the result was imprecise. There were no eligible trials assessing this outcome at more than two months after the treatment. Using GRADE, we judged the certainty of the evidence to be low immediately after treatment due to serious concerns regarding imprecision.
1.3. Analysis.

Comparison 1: Bladder training versus no treatment, Outcome 3: Number of incontinence episodes per 24 hours: immediately after treatment
Number of urgency episodes per 24 hours
No studies reported number of urgency episodes per 24 hours.
Number of micturition episodes per 24 hours
No studies reported number of micturition episodes per 24 hours.
Bladder training versus anticholinergics
Seven studies compared bladder training versus anticholinergics (Colombo 1995; Jarvis 1981; Kafri 2013; Lauti 2008; McCreanor 1998; Milani 1987; Song 2006). See Table 2.
Primary outcomes
Participant‐reported cure or improvement
Bladder training may be slightly more effective than anticholinergic therapy on cure/improvement immediately after treatment (RR 1.37, 95% CI 1.10 to 1.70; 4 studies, 258 participants; Analysis 2.1; low‐certainty evidence). Bladder training may be more effective than anticholinergic therapy at more than two months after the treatment (RR 1.61, 95% CI 1.18 to 2.18; 2 studies, 150 participants; Analysis 2.2; low‐certainty evidence). There was considerable heterogeneity in the early phase (I2 = 52%), but there was no heterogeneity in the long‐term effect (I2 = 0%).
2.1. Analysis.

Comparison 2: Bladder training versus anticholinergics, Outcome 1: Participant‐reported cure or improvement: immediately after treatment
2.2. Analysis.

Comparison 2: Bladder training versus anticholinergics, Outcome 2: Participant‐reported cure or improvement: long‐term effect (> 2 months after treatment)
McCreanor 1998 reported "symptom score" and "VAS" (Visual Analog Scale) at eight weeks and 16 weeks; however, the outcomes data could not be extracted. The mean symptom score was higher in bladder training than in oxybutynin at week eight and lower at week 16. The mean VAS scale was lower in bladder training than in oxybutynin at week eight and equal at week 16.
Symptom‐ and condition‐related quality of life
There may be little or no difference between bladder training and anticholinergic therapy on symptom‐ and condition‐related QoL immediately after treatment (SMD −0.06, 95% CI −0.89 to 0.77; 2 studies, 117 participants; Analysis 2.3; very low‐certainty evidence) and more than two months after the treatment (SMD 0.15, 95% CI −0.22 to 0.52; 2 studies, 112 participants; Analysis 2.4; very low‐certainty evidence), but the evidence is very uncertain. There was considerable heterogeneity in the early phase (I2 = 76%), but no evidence of heterogeneity in the long‐term effect (I2 = 0%).
2.3. Analysis.

Comparison 2: Bladder training versus anticholinergics, Outcome 3: Symptom‐related quality of life (QoL): immediately after treatment
2.4. Analysis.

Comparison 2: Bladder training versus anticholinergics, Outcome 4: Symptom‐related QoL: long‐term effect (> 2 months after treatment)
Adverse events
The evidence is very uncertain about the effect of bladder training on adverse events when compared to anticholinergic therapy on adverse events immediately after treatment (RR 0.03, 95% CI 0.01 to 0.17; 3 studies, 187 participants; Analysis 2.5; very low‐certainty evidence) and at more than two months after the treatment (RR 0.04, 95% CI 0.00 to 0.57; 1 study, 75 participants; Analysis 2.6; very low‐certainty evidence). There was no heterogeneity in the early phase (I2 = 0%). The range of the CIs was narrow enough that the result was precise in the early phase.
2.5. Analysis.

Comparison 2: Bladder training versus anticholinergics, Outcome 5: Adverse events: immediately after treatment
2.6. Analysis.

Comparison 2: Bladder training versus anticholinergics, Outcome 6: Adverse events: long‐term effect (> 2 months after treatment)
Lauti 2008 reported only all adverse events and not the number of participants with adverse events; therefore, this study was not included in our meta‐analyses. The most frequently reported adverse events by Lauti 2008 were dry mouth and constipation.
Secondary outcomes
Participant‐reported satisfaction
No studies reported participant‐reported satisfaction.
Number of incontinence episodes per 24 hours
The evidence is very uncertain about the effect of bladder training as compared to anticholinergic therapy on incontinence episodes per 24 hours immediately after treatment (MD 0.36, 95% CI −0.27 to 1.00; 2 studies, 117 participants; Analysis 2.7; very low‐certainty evidence) and at more than two months after the treatment (MD −0.22, 95% CI −0.64 to 0.20; 2 studies, 112 participants; Analysis 2.8; very low‐certainty evidence). There was substantial heterogeneity in the early phase (I2 = 84%), but there was no evidence of heterogeneity in the long‐term effect (I2 = 0%).
2.7. Analysis.

Comparison 2: Bladder training versus anticholinergics, Outcome 7: Number of incontinence episodes per 24 hours: immediately after treatment
2.8. Analysis.

Comparison 2: Bladder training versus anticholinergics, Outcome 8: Number of incontinence episodes per 24 hours: long‐term effect (> 2 months after treatment)
Number of urgency episodes per 24 hours
The evidence is very uncertain about the effect of bladder training when compared to anticholinergics on urgency episodes per 24 hours immediately after treatment (MD 0.70, 95% CI −0.62 to 2.02; 2 studies, 92 participants; Analysis 2.9; very low‐certainty evidence) and at more than two months after the treatment (MD 0.40, 95% CI −1.27 to 2.07; 1 study, 29 participants; Analysis 2.10; very low‐certainty evidence).
2.9. Analysis.

Comparison 2: Bladder training versus anticholinergics, Outcome 9: Number of urgency episodes per 24 hours: immediately after treatment
2.10. Analysis.

Comparison 2: Bladder training versus anticholinergics, Outcome 10: Number of urgency episodes per 24 hours: long‐term effect (> 2 months after treatment)
Song 2006 reported the urgency scores immediately after treatment (at 12 weeks), which were 1.4 in the bladder training group and 1.1 in the anticholinergic (tolterodine) group, without SDs.
Number of micturition episodes per 24 hours
The evidence is very uncertain about the effect of bladder training versus anticholinergic therapy on micturition immediately after treatment (MD −0.35, 95% CI −1.90 to 1.20; 3 studies, 175 participants; Analysis 2.11; very low‐certainty evidence) and at more than two months after the treatment (MD 0.26, 95% CI −0.60 to 1.12; 2 studies, 112 participants; Analysis 2.12; very low‐certainty evidence). There was moderate heterogeneity in the early phase (I2 = 51%), but there was no evidence of heterogeneity in the long‐term effect (I2 = 0%). As we did not extract the SDs from the original study report of Song 2006 but instead imputed/borrowed data from a published Cochrane Review (Rai 2012), we performed a post hoc sensitivity analysis by excluding Song 2006 from Analysis 2.11 and confirmed that the results remained consistent (MD −0.51, 95% CI −2.46 to 1.44; 2 studies, 117 participants).
2.11. Analysis.

Comparison 2: Bladder training versus anticholinergics, Outcome 11: Number of micturition episodes per 24 hours: immediately after treatment
2.12. Analysis.

Comparison 2: Bladder training versus anticholinergics, Outcome 12: Number of micturition episodes per 24 hours: long‐term effect (> 2 months after treatment)
Bladder training versus pelvic floor muscle training
Three trials compared bladder training versus PFMT (Kafri 2013; Lentz 1994; Rizvi 2018). See Table 3.
Primary outcomes
Participant‐reported cure or improvement
Lentz 1994 reported cure or improvement rates of 80% at one month and 100% at three months in the bladder training group and 78% at one month and 60% at three months among participants in the PFMT group. There were no details about the events/total number.
Symptom‐ and condition‐related quality of life
There may be little or no difference between bladder training and PFMT on symptom‐ and condition‐related QoL immediately after treatment (SMD 0.10, 95% CI −0.19 to 0.40; 2 studies, 178 participants; Analysis 3.1; low‐certainty evidence) and at more than two months after the treatment (SMD −0.09, 95% CI −0.52 to 0.35; 1 study, 81 participants; Analysis 3.2; very low‐certainty evidence). There was no evidence of heterogeneity in the early phase (I2 = 0%).
3.1. Analysis.

Comparison 3: Bladder training versus pelvic floor muscle training (PFMT), Outcome 1: Symptom‐related quality of life (QoL): immediately after treatment
3.2. Analysis.

Comparison 3: Bladder training versus pelvic floor muscle training (PFMT), Outcome 2: Symptom‐related QoL: long‐term effect (> 2 months after treatment)
Adverse events
There were no adverse events in the bladder training and PFMT groups immediately after treatment (1 study, 97 participants; Analysis 3.3); we judged the evidence to be of moderate certainty due to imprecision.
3.3. Analysis.

Comparison 3: Bladder training versus pelvic floor muscle training (PFMT), Outcome 3: Adverse events: immediately after treatment
Secondary outcomes
Participant‐reported satisfaction
No studies reported participant‐reported satisfaction.
Number of incontinence episodes per 24 hours
There may be little or no difference between bladder training and PFMT on incontinence episodes immediately after treatment (MD 0.02, 95% CI −0.35 to 0.39; 1 study, 81 participants; Analysis 3.4; low‐certainty evidence) and more than two months after the treatment (MD −0.20, 95% CI −2.46 to 2.06; 1 study, 81 participants; Analysis 3.5; very low‐certainty evidence). Both results were based on one study. As the ranges of the CIs were wide, the results were imprecise.
3.4. Analysis.

Comparison 3: Bladder training versus pelvic floor muscle training (PFMT), Outcome 4: Number of incontinence episodes per 24 hours: immediately after treatment
3.5. Analysis.

Comparison 3: Bladder training versus pelvic floor muscle training (PFMT), Outcome 5: Number of incontinence episodes per 24 hours: long‐term effect (> 2 months after treatment)
Number of urgency episodes per 24 hours
No studies reported number of urgency episodes per 24 hours.
Number of micturition episodes per 24 hours
The evidence is very uncertain about the effect of bladder training as compared to PFMT on micturition episodes immediately after treatment (MD 0.10, 95% CI −1.44 to 1.64; 1 study, 81 participants; Analysis 3.6; very low‐certainty evidence) and at more than two months after the treatment (MD 0.50, 95% CI −1.39 to 2.39; 1 study, 81 participants; Analysis 3.7; very low‐certainty evidence). Both results were based on one study. As the ranges of the CIs were wide, the results were imprecise.
3.6. Analysis.

Comparison 3: Bladder training versus pelvic floor muscle training (PFMT), Outcome 6: Number of micturition episodes per 24 hours: immediately after treatment
3.7. Analysis.

Comparison 3: Bladder training versus pelvic floor muscle training (PFMT), Outcome 7: Number of micturition episodes per 24 hours: long‐term effect (> 2 months after treatment)
Bladder training combined with anticholinergics versus anticholinergics alone
Five trials compared bladder training combined with anticholinergics versus anticholinergics alone (Lauti 2008; Mattiasson 2003; Mattiasson 2010; Song 2006; Zhang 2012).
Primary outcomes
Participant‐reported cure or improvement
There may be little or no difference between bladder training combined with anticholinergics and anticholinergics alone on cure/improvement immediately after treatment (RR 1.08, 95% CI 0.97 to 1.19; 2 studies, 564 participants; Analysis 4.1; low‐certainty evidence). There was no evidence of heterogeneity (I2 = 0%).
4.1. Analysis.

Comparison 4: Bladder training plus anticholinergics versus anticholinergics alone, Outcome 1: Participant‐reported cure or improvement: immediately after treatment
Symptom‐ and condition‐related quality of life
There may be little or no difference between bladder training combined with anticholinergics and anticholinergics alone on symptom‐ and condition‐related QoL immediately after treatment (SMD 0.07, 95% CI −0.09 to 0.22; 2 studies, 630 participants; Analysis 4.2; moderate‐certainty evidence) and more than two months after the treatment (SMD 0.45, 95% CI −0.34 to 1.25; 2 studies, 627 participants; Analysis 4.3; low‐certainty evidence). There was no evidence of heterogeneity in the early phase (I2 = 0%).
4.2. Analysis.

Comparison 4: Bladder training plus anticholinergics versus anticholinergics alone, Outcome 2: Symptom‐related quality of life (QoL): immediately after treatment
4.3. Analysis.

Comparison 4: Bladder training plus anticholinergics versus anticholinergics alone, Outcome 3: Symptom‐related QOL: long‐term effect (> 2 months after treatment)
Mattiasson 2010 reported QoL using the Incontinence Quality of Life (I‐QoL) questionnaire more than two months after the treatment (16 weeks) (scores: 25.3 in the bladder training plus anticholinergic (solifenacin) group versus 24.5 in the anticholinergic alone group; SDs not reported).
Zhang 2012 reported the rate of increases in the participant perception of bladder condition (PPBC) was 66% in the bladder training plus anticholinergic (tolterodine) group and 53% in the anticholinergic alone group at 12 weeks.
Adverse events
There was probably little or no difference between bladder training combined with anticholinergics and anticholinergics alone on adverse events immediately after treatment (RR 0.94, 95% CI 0.83 to 1.06; 2 studies, 564 participants; Analysis 4.4; moderate‐certainty evidence) and at more than two months after the treatment (RR 1.00, 95% CI 0.85 to 1.18; 1 study, 643 participants; Analysis 4.5; moderate‐certainty evidence). There was no heterogeneity in the early phase (I2 = 0%). The most frequently reported adverse events were dry mouth and constipation.
4.4. Analysis.

Comparison 4: Bladder training plus anticholinergics versus anticholinergics alone, Outcome 4: Adverse events: immediately after treatment
4.5. Analysis.

Comparison 4: Bladder training plus anticholinergics versus anticholinergics alone, Outcome 5: Adverse events: long‐term effect (> 2 months after treatment)
Secondary outcomes
Participant‐reported satisfaction
Mattiasson 2010 reported satisfaction using a VAS immediately after treatment (eight weeks) (scores: 3.5 in the bladder training plus anticholinergic (solifenacin) group versus 3.3 in the anticholinergic alone group; SDs not reported; Analysis 4.6; moderate‐certainty evidence), and at more than two months after the treatment (16 weeks) (scores: 4.18 in the bladder training plus anticholinergic group versus 3.72 in the anticholinergic alone group; SDs not reported; Analysis 4.7; moderate‐certainty evidence).
4.6. Analysis.

Comparison 4: Bladder training plus anticholinergics versus anticholinergics alone, Outcome 6: Participant‐reported satisfaction: immediately after treatment
4.7. Analysis.

Comparison 4: Bladder training plus anticholinergics versus anticholinergics alone, Outcome 7: Participant‐reported satisfaction: long‐term effect (> 2 months after treatment)
Number of incontinence episodes per 24 hours
There may be little or no difference between bladder training combined with anticholinergics and anticholinergics alone on incontinence episodes immediately after treatment (MD 0.50, 95% CI 0.02 to 0.98; 3 study, 931 participants; Analysis 4.8; low‐certainty evidence) and more than two months after the treatment (MD −0.10, 95% CI −0.77 to 0.57; 2 studies, 627 participants; Analysis 4.9; low‐certainty evidence).
4.8. Analysis.

Comparison 4: Bladder training plus anticholinergics versus anticholinergics alone, Outcome 8: Number of incontinence episodes per 24 hours: immediately after treatment
4.9. Analysis.

Comparison 4: Bladder training plus anticholinergics versus anticholinergics alone, Outcome 9: Number of incontinence episodes per 24 hours: long‐term effect (> 2 months after treatment)
Mattiasson 2003 reported median incontinence episodes per 24 hours immediately after treatment (24 weeks) (scores: 0.3 (range 0 to 14.7) in the bladder training plus anticholinergic (tolterodine) group versus 0.3 (range 0 to 14.7) in the anticholinergic alone group; SDs not reported).
Mattiasson 2010 reported a change of incontinence episodes per 24 hours immediately after treatment (8 weeks) (scores: −1.3 in the bladder training plus anticholinergic (solifenacin) group versus −1.2 in the anticholinergic alone group; SDs not reported), and more than two months after the treatment (16 weeks) (scores: −1.5 in the bladder training plus anticholinergic group versus −1.5 in the anticholinergic alone group; SDs not reported).
Zhang 2012 reported the reduction of pad use was 71% in the bladder training plus anticholinergic (tolterodine) group versus 56% in the anticholinergic group at 12 weeks.
Number of urgency episodes per 24 hours
There may be little or no difference between bladder training combined with anticholinergics and anticholinergics alone on urgency episodes immediately after treatment (MD 0.20, 95% CI −1.25 to 1.65; 4 studies, 1177 participants; Analysis 4.10; low‐certainty evidence) and more than two months after the treatment (MD 0.10, 95% CI −1.20 to 1.40; 2 studies, 627 participants; Analysis 4.11; low‐certainty evidence). There was no evidence of heterogeneity in the early phase or long‐term effect (I2 = 0%).
4.10. Analysis.

Comparison 4: Bladder training plus anticholinergics versus anticholinergics alone, Outcome 10: Number of urgency episodes per 24 hours: immediately after treatment
4.11. Analysis.

Comparison 4: Bladder training plus anticholinergics versus anticholinergics alone, Outcome 11: Number of urgency episodes per 24 hours: long‐term effect (> 2 months after treatment)
Song 2006 reported urgency episodes per 24 hours immediately after treatment (12 weeks) (scores: 1.2 in the bladder training plus anticholinergic (tolterodine) group versus 1.1 in the anticholinergic alone group; SDs not reported).
Mattiasson 2003 reported median urgency episodes per 24 hours immediately after treatment (24 weeks) (scores: 4.0 (range 0 to 15.7) in the bladder training plus anticholinergic (tolterodine) group versus 4.0 (range 0 to 18.7) in the anticholinergic alone group; SDs not reported).
Mattiasson 2010 reported a change of urgency episodes per 24 hours immediately after treatment (eight weeks) (scores: −2.0 in the bladder training plus anticholinergic (solifenacin) group versus −2.0 in the anticholinergic alone group; SDs not reported), and more than two months after the treatment (16 weeks) (scores: −2.5 in the bladder training plus anticholinergic group versus −2.2 in the anticholinergic alone group; SDs not reported).
Zhang 2012 reported the reduction of urgency episodes was 71% in the bladder training plus anticholinergic (tolterodine) group versus 58% in the anticholinergic alone group at 12 weeks.
Number of micturition episodes per 24 hours
There may be little or no difference between bladder training combined with anticholinergics and anticholinergics alone immediately after treatment (MD 0.40, 95% CI −1.07 to 1.87; 4 studies, 1182 participants; Analysis 4.12; low‐certainty evidence) and more than two months after the treatment (MD 0.80, 95% CI −0.34 to 1.94; 2 studies, 627 participants; Analysis 4.13; low‐certainty evidence).
4.12. Analysis.

Comparison 4: Bladder training plus anticholinergics versus anticholinergics alone, Outcome 12: Number of micturition episodes per 24 hours: immediately after treatment
4.13. Analysis.

Comparison 4: Bladder training plus anticholinergics versus anticholinergics alone, Outcome 13: Number of micturition episodes per 24 hours: long‐term effect (> 2 months after treatment)
Song 2006 reported micturition episodes per 24 hours immediately after treatment (12 weeks) (scores: 7.9 in the bladder training plus anticholinergic (tolterodine) group versus 8.1 in the anticholinergic alone group; SDs not reported).
Mattiasson 2003 reported median micturition episodes per 24 hours immediately after treatment (24 weeks) (scores: 7.0 (range 3 to 15.3) in the bladder training plus anticholinergic (tolterodine) group versus 8.0 (range 3 to 25.0) in the anticholinergic group; SDs not reported).
Mattiasson 2010 reported a change of micturition episodes per 24 hours immediately after treatment (eight weeks) (scores: −2.9 in the bladder training plus anticholinergic (solifenacin) group versus −2.2 in the anticholinergic alone group; SDs not reported), and more than two months after the treatment (16 weeks) (score: −2.5 in the bladder training plus anticholinergic group versus −2.2 in the anticholinergic alone group; SDs not reported).
Subgroup analysis
There were insufficient data to perform prespecified subgroup analyses.
Sensitivity analysis
There were insufficient data to perform prespecified sensitivity analyses.
Discussion
Summary of main results
We included 15 studies with 2007 participants in this review. Participants in these trials were predominantly women (89.3%). We assessed the risk of bias of results for primary and secondary outcomes, and across all studies these were similar and predominantly of high risk of bias and none were at low risk of bias. Of the results assessed as 'some concern', most studies did not treat missing data appropriately and did not perform trial registry or prespecify an analysis plan. Many studies were at high risk of bias due to deviation from intervention and measurement of outcomes due to an open‐label design. Most results were judged with serious imprecision because of the small sample size or number of events. Using the GRADE method, we assessed the certainty of evidence as low to very low, with some as moderate, across measured outcomes.
Based on low‐ or very low‐certainty evidence, bladder training may cure or improve OAB compared to no treatment. Moreover, bladder training may be more effective to cure or improve OAB than anticholinergics and there may be fewer adverse events. There may be no difference in efficacy or safety between bladder training and PFMT. When compared to anticholinergics alone, combination therapy with bladder training and anticholinergics had little or no effect on cure or improvement, symptom‐related QoL, or adverse events. Three trials recruited a large sample size (Mattiasson 2003; Mattiasson 2010; Song 2006); however, they did not report SDs of the reported outcomes, and we did not receive any responses to our attempts to obtain additional information from the study authors.
Overall completeness and applicability of evidence
Although we were interested in trials examining bladder training versus β3‐adrenoceptor agonists, in combination with β3‐adrenoceptor agonists versus β3‐adrenoceptor agonists alone, and in combination with PFMT versus PFMT alone, we did not identify any eligible studies for these comparisons.
Even in the comparisons with eligible studies, we were unable to perform sufficient quantitative synthesis. Although two studies compared bladder training versus no treatment, they assessed only two outcomes: participant‐reported cure or improvement, and number of incontinence episodes (Jarvis 1980; Lagro‐Janssen 1992). In the comparison 'bladder training versus anticholinergics', seven studies reported the primary and secondary outcomes, except for participant‐reported satisfaction (Colombo 1995; Jarvis 1981; Kafri 2013; Lauti 2008; McCreanor 1998; Milani 1987; Song 2006). In the comparison of bladder training versus PFMT, three trials assessed participant‐reported cure/improvement, symptom‐related QoL, adverse events, number of incontinence episodes, and micturition episodes (Kafri 2013; Lentz 1994; Rizvi 2018). In the comparison 'bladder training combined with anticholinergics versus anticholinergics', five trials reported all our predefined primary and secondary outcomes (Lauti 2008; Mattiasson 2003; Mattiasson 2010; Song 2006; Zhang 2012).
Quality of the evidence
We assessed the certainty of evidence using GRADE, which was rated as low and very low, with some moderate, for most outcomes and comparisons. Our judgment of the downgrade of the evidence was based on the following reasons.
Study limitation (risk of bias): mainly high risk of bias on deviation from the intervention, missing outcome, outcome measurement. The overall bias judgments were either 'some concern' or 'high risk'.
Inconsistency: clinically relevant heterogeneity based on high I2 values (greater than 75%).
Imprecision: small sample size and CIs that were wide and crossed assumed thresholds of clinically important differences.
Potential biases in the review process
We assessed the potential biases in this review process based on ROBIS (Whiting 2016). We have some concerns about the restriction of study eligibility criteria. We set relatively broad eligibility criteria, which may lead to some bias from our research question. For example, we included participants with slightly different diagnoses (OAB, UUI, DI), the contents of bladder training, and the comparisons were different among included studies.
With respect to our methods of study identification and selection, we performed a comprehensive literature search including trial registries and gray literature for unpublished studies. Two review authors independently selected and assessed trials for inclusion using prespecified criteria, extracted data, and assessed the quality of the studies to minimize potential biases in the review process. We attempted to contact study authors to obtain additional information about several studies (6 October 2020 to 13 March 2021), but we did not receive answers from them. This could lead to a potential bias with selective reporting.
Agreements and disagreements with other studies or reviews
We identified two Cochrane Reviews assessing the efficacy and safety of bladder training for OAB (Rai 2012; Wallace 2004). Compared to these, this review updated the literature search and used the RoB 2 tool for assessing risk of bias.
Wallace 2004 excluded people with OAB from the review, but UUI was overlapped with this review. The previous review suggested that bladder training may be effective to treat UI, but the result was based on variable quality and small size. Furthermore, evidence on UUI was limited.
Rai 2012 compared anticholinergic drugs versus non‐drug active therapies for non‐neurogenic OAB, and the review compared bladder training versus anticholinergics. The review suggested the opposite, that participants were more likely to improve when treated with anticholinergics rather than bladder training, but there were more adverse events with anticholinergics. Our review updated the literature search and added three new studies to the analysis (Kafri 2013; Rizvi 2018; Zhang 2012). The results were not in line with the previous reviews, but still with low‐certainty evidence.
We identified two pairwise meta‐analyses (Roe 2007; Shamliyan 2008), and one network meta‐analysis (Balk 2019), exploring the effects of bladder training for UI. As Roe 2007 and Shamliyan 2008 did not classify UI into UUI, or behavioral therapy into bladder training, we cannot compare these with our results.
Limitations
First, as there is no standardized definition of bladder training, our definition may cause misclassification of bladder training and induce bias. Second, we selected the outcomes for the summary of findings tables at immediately after treatment instead of long‐term follow‐up. Although long‐term outcomes would be more clinically relevant to treat OAB, it is difficult to evaluate the long‐term effects of all the comparisons. For example, the effects of pharmacologic treatment disappear once the treatment is completed, and it is near impossible to evaluate long‐term outcomes associated with interventions for OAB. Finally, we imputed the missing SDs of an included study (Song 2006) by imputing them from a published Cochrane Review (Rai 2012). We were unable to clarify data origin and methods of imputation from review authors of Rai 2012 and thus acknowledge the potential bias associated with incomplete outcome data. As recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022), we addressed this by performing a sensitivity analysis and noted no substantial changes in the results. It is worth highlighting that all imputation techniques of SDs involve making assumptions about unknown statistics, and it is best to avoid imputation wherever possible.
Authors' conclusions
Implications for practice.
The findings of this review were based on low‐ or very low‐certainty evidence that bladder training may be beneficial in treating overactive bladder (OAB) compared to no treatment. When compared to anticholinergics, bladder training may be more effective in curing or improving OAB symptoms, and safer. There were no differences in the effects of bladder training compared to pelvic floor muscle training (PFMT) and the additive effect of bladder training on anticholinergics was unclear.
Implications for research.
This review showed that the best available evidence to date is insufficient to fully explore the benefits and harms of bladder training to treat OAB, and well‐designed trials are needed to reach a firm conclusion. Specifically, the target population should be clearly defined, the details of bladder training should be established, the required outcomes should be measured in the short and long time points, and the sample size should be estimated to be sufficient to evaluate the effectiveness. Furthermore, multiple trials with the same design should be conducted to synthesize the results. For bladder training, it is unclear who or what should provide it and for how long, and it is also necessary to determine the optimal bladder training delivery by comparing the effects under different conditions.
History
Protocol first published: Issue 4, 2020
Risk of bias
Risk of bias for analysis 1.1 Participant‐reported cure or improvement: immediately after treatment.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Lagro‐Janssen 1992 | Some concerns | There was no detail about the random sequence generation and the allocation concealment. The baseline characteristics were similar between both groups. | Low risk of bias | Although no detail about masking, it is difficult for participants and carers to be masked in this setting. Only one participant deviated from the intervention because of pregnancy. The outcome was analyzed on an ITT basis. | Low risk of bias | All participants (n=110) that were randomised were included in the analysis. | High risk of bias | The outcome was patient reported outcome (PRO) and the assessors (= participants) were aware of their interventions. Control group were not taken for an active treatment during 3 months and the awareness likely affected the outcome. | Some concerns | As there were no protocols, statistical analysis plan, it is difficult to make a judgement. | High risk of bias | Overall high due to the nature of self reported outcome data and the participants are aware of the intervention. |
Risk of bias for analysis 1.2 Participant‐reported cure or improvement: long‐term effect (> 2 months after treatment).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Jarvis 1980 | Some concerns | There was no detail about the random sequence generation and the allocation concealment, however, the baseline characteristics were similar. | Low risk of bias | Although no detail about masking, it is difficult for participants and carers to be masked in this setting. There was no deviation from intervention. The outcome was analyzed on an ITT basis. | Low risk of bias | The outcome data were available for all participants. | High risk of bias | The outcome was the patient‐reported outcome (PRO) and the assessors were aware of their interventions. The control group was not received active treatment and the outcome was likely affected by the non‐blinding. | Some concerns | As there were no protocols, statistical analysis plan or trial registry, it is difficult to make a judgment. | High risk of bias | Overall high due to the nature of self reported outcome data and the participants are aware of the intervention. |
Risk of bias for analysis 1.3 Number of incontinence episodes per 24 hours: immediately after treatment.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Fantl 1991 | Some concerns | There was no detail about the random sequence generation and the allocation concealment. The baseline characteristics were similar. | Low risk of bias | Although no detail about masking, it is difficult for participants and carers to be masked in this setting. The deviations were five in the treatment group and three in the control group. This trial would be acceptable for modified ITT. | Low risk of bias | The outcome data were available for all DI participants. | Low risk of bias | The outcome was the patient‐reported outcome (PRO) and the assessors were aware of their interventions. FVC is a relatively hard outcome and would not be less susceptible to awareness. | Some concerns | As there were no protocols, statistical analysis plan or trial registry, it is difficult to make a judgment. | Some concerns | Due to no detail of the randomization process and potential bias in the selection of the reported result. |
Risk of bias for analysis 2.1 Participant‐reported cure or improvement: immediately after treatment.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Colombo 1995 | Some concerns | The random sequence was generated by computer. There was no detail about the allocation concealment and the baseline characteristics between the groups. | Low risk of bias | Although no detail about masking, it is difficult for participants and carers to be masked in this setting. The treatment was discontinued in 6 cases: 4 in the oxybutynin arm because of severe anticholinergic adverse effects and 2 in the bladder training group because the patients claimed that the treatment was time‐consuming. These were not trial contexts. This trial would be acceptable for modified ITT. | Low risk of bias | The follow‐up rates were 90% (38/42) in GroupI, 95% (37/39) in GroupII. The observed number of events is much greater than the number of participants with missing outcome data. | Some concerns | The outcome was the patient‐reported outcome (PRO) and the assessors were aware of their interventions. Both groups were taken for active treatment and it was difficult to decide that the awareness affected the outcome. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | Some concerns | Due to no detail of the randomization process, non‐blinding of outcome assessors and potential bias in the selection of the reported result. |
| Jarvis 1981 | Some concerns | There was no detail about the random sequence generation and the allocation concealment. The baseline characteristics were similar, but only age and duration of disease were reported. There was too little information to make a judgment. | Low risk of bias | Although no detail about masking, it is difficult for participants and carers to be masked in this setting. The reason for all five deviations from intervention was the AEs of a drug, not the trial context. This trial would be acceptable for modified ITT. | Low risk of bias | The outcome data were available for all participants. | Some concerns | The outcome was the patient‐reported outcome (PRO) and the assessors were aware of their interventions. Both groups were taken for active treatment and it was difficult to decide that the awareness affected the outcome. | Some concerns | As there were no protocols, statistical analysis plan or trial registry, it is difficult to make a judgment. | Some concerns | Due to no detail of randomization process, non‐blinding of outcome assessors and potential bias in the selection of the reported result. |
| Milani 1987 | Some concerns | There was no detail about the random sequence generation and the allocation concealment. The baseline characteristics were similar, but only age and duration of disease were reported. There was too little information to make a judgment. | Low risk of bias | Although no detail about masking, it is difficult for participants and carers to be masked in this setting. The two deviations from BT was for long lasting therapy and four from oxybutynin for adverse events. These were not trial contexts. | Low risk of bias | The outcome was dichotomous and the number of events was much greater than missing numbers, two in BT and four in oxybutynin. | High risk of bias | Although the end of therapy of BT was 12 weeks, that of oxybutynin was 4 weeks. The time point could be different between the two groups. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | High risk of bias | Overall high due to the different time point of outcome assessment between two groups. |
| Song 2006 | Some concerns | There was no detail about the random sequence generation and the allocation concealment. The baseline characteristics were not so different between groups. | High risk of bias | There was no detail about masking. Many participants dropped out from intervention (43.5% in BT, 31.9% in tolterodine, 32.6% in combination) and there was no detail about the deviation. Only the patients who completed the protocol were analyzed in this trial, and it was not ITT analysis. | High risk of bias | Many participants dropped out from outcome assessment (43.5% in BT, 31.9% in tolterodine, 32.6% in combination) and it was likely that missingness affected its true value. | Some concerns | The outcome was the patient‐reported outcome (PRO) and the assessors were aware of their interventions. Both groups were taken for active treatment and it was difficult to decide that the awareness affected the outcome. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | High risk of bias | Overall high due to the many deviations from the intervention and the much missing outcome data. |
Risk of bias for analysis 2.2 Participant‐reported cure or improvement: long‐term effect (> 2 months after treatment).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Colombo 1995 | Some concerns | The random sequence was generated by computer. There was no detail about the allocation concealment and the baseline characteristics between the groups. | Low risk of bias | Although no detail about masking, it is difficult for participants and carers to be masked in this setting. The treatment was discontinued in 6 cases: 4 in the oxybutynin arm because of severe anticholinergic adverse effects and 2 in the bladder training group because the patients claimed that the treatment was time‐consuming. These were not trial contexts. This trial would be acceptable for modified ITT. | Low risk of bias | The follow‐up rates were 90% (38/42) in GroupI, 95% (37/39) in GroupII. The observed number of events is much greater than the number of participants with missing outcome data. | Some concerns | The outcome was the patient‐reported outcome (PRO) and the assessors were aware of their interventions. Both groups were taken for active treatment and it was difficult to decide that the awareness affected the outcome. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | Some concerns | Due to no detail of the randomization process, non‐blinding of outcome assessors and potential bias in the selection of the reported result. |
| Milani 1987 | Some concerns | There was no detail about the random sequence generation and the allocation concealment. The baseline characteristics were similar, but only age and duration of disease were reported. There was too little information to make a judgment. | Low risk of bias | Although no detail about masking, it is difficult for participants and carers to be masked in this setting. The two deviations from BT was for long lasting therapy and four from oxybutynin for adverse events. These were not trial contexts. This trial would be acceptable for modified ITT. | Low risk of bias | The outcome was dichotomous and the number of events was much greater than missing numbers, two in BT and four in oxybutynin. | High risk of bias | Although the end of therapy of BT was 12 weeks, that of oxybutynin was 4 weeks. The time point could be different between the two groups. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | High risk of bias | Overall high due to the different time point of outcome assessment between two groups. |
Risk of bias for analysis 2.3 Symptom‐related quality of life (QoL): immediately after treatment.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kafri 2013 | Low risk of bias | The participants were by randomly permuted blocks of four, with random assignment concealed in tamper‐proof envelopes. The baseline characteristics were similar between groups. | High risk of bias | Although no detail about masking, it is difficult for participants and carers to be masked in this setting. The deviations from the intervention were higher in the drug therapy group than in other groups because of dissatisfaction with the allocation. All data were analyzed on an ITT basis. | High risk of bias | The rate of available data was 95% (39/41) in bladder training and 64% (27/42) in anticholinergics. Missing outcome data were imputed by last observation carried forward (LOCF) and the missing numbers were different between groups, especially many missing in the drug therapy group. This missingness was likely dependent on their true value. | Some concerns | The outcome was the patient‐reported outcome (PRO) and the assessors (= participants) were aware of their interventions. Both groups were taken for active treatment and it was difficult to decide that the awareness affected the outcome. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | High risk of bias | Overall high due to the deviation from the intervention and the missing outcome data. |
| Lauti 2008 | Low risk of bias | Participants were randomly allocated, using a password protected web page that remotely accessed a computer‐generated randomisation list. However, the baseline characteristics (e.g. randomised number, age) were different between groups. | Low risk of bias | This was an open‐label trial. All women received their allocated treatment, except for one who opted for tolterodine. This trial would be acceptable for modified ITT. | High risk of bias | The follow‐up rates were 86% (18/21) in GroupI, 100% (16/16) in GroupII and 63% (12/19) in GroupIII. There was no analysis for missing data and the missing likely affected the true value. | Some concerns | The outcome was the patient‐reported outcome (PRO) and the assessors were aware of their interventions. Both groups were taken for active treatment and it was difficult to decide that the awareness affected the outcome. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | High risk of bias | Overall high due to the missing outcome data. |
Risk of bias for analysis 2.4 Symptom‐related QoL: long‐term effect (> 2 months after treatment).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kafri 2013 | Low risk of bias | The participants were by randomly permuted blocks of four, with random assignment concealed in tamper‐proof envelopes. The baseline characteristics were similar between groups. | High risk of bias | Although no detail about masking, it is difficult for participants and carers to be masked in this setting. The deviations from the intervention were higher in the drug therapy group than in other groups because of dissatisfaction with the allocation. All data were analyzed on an ITT basis. | High risk of bias | The rate of available data was 95% (39/41) in bladder training and 64% (27/42) in anticholinergics. Missing outcome data were imputed by last observation carried forward (LOCF) and the missing numbers were different between groups, especially many missing in the drug therapy group. This missingness was likely dependent on their true value. | Some concerns | The outcome was the patient‐reported outcome (PRO) and the assessors (= participants) were aware of their interventions. Both groups were taken for active treatment and it was difficult to decide that the awareness affected the outcome. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | High risk of bias | Overall high due to the deviation from the intervention and the missing outcome data. |
| Lauti 2008 | Low risk of bias | Participants were randomly allocated, using a password protected web page that remotely accessed a computer‐generated randomisation list. However, the baseline characteristics (e.g. randomised number, age) were different between groups. | Low risk of bias | This was an open‐label trial. All women received their allocated treatment, except for one who opted for tolterodine. This trial would be acceptable for modified ITT. | High risk of bias | The follow‐up rates were 86% (18/21) in GroupI, 100% (16/16) in GroupII and 63% (12/19) in GroupIII. There was no analysis for missing data and the missing likely affected the true value. | Some concerns | The outcome was patient reported outcome (PRO) and the assessors were aware of their interventions. Both groups were taken for active treatment and it was difficult to decide that the awareness affected the outcome. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | High risk of bias | Overall high due to the missing outcome data. |
Risk of bias for analysis 2.5 Adverse events: immediately after treatment.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Colombo 1995 | Some concerns | The random sequence was generated by computer. There was no detail about the allocation concealment and the baseline characteristics between the groups. | Low risk of bias | Although no detail about masking, it is difficult for participants and carers to be masked in this setting. The treatment was discontinued in 6 cases: 4 in the oxybutynin arm because of severe anticholinergic adverse effects and 2 in the bladder training group because the patients claimed that the treatment was time‐consuming. These were not trial contexts. This trial would be acceptable for modified ITT. | Low risk of bias | The follow‐up rates were 90% (38/42) in GroupI, 95% (37/39) in GroupII. The observed number of events is much greater than the number of participants with missing outcome data. | High risk of bias | The outcome was patient reported outcome (PRO) and the assessors were aware of their interventions. As adverse events were specific to anticholinergics, the awareness would affect the outcome. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | Some concerns | Overall high due to the nature of self reported outcome data and the participants are aware of the intervention. |
| Jarvis 1981 | Some concerns | There was no detail about the random sequence generation and the allocation concealment. The baseline characteristics were similar, but only age and duration of disease were reported. There was too little information to make a judgment. | Low risk of bias | Although no detail about masking, it is difficult for participants and carers to be masked in this setting. The reason for all five deviations from intervention was the AEs of a drug, not the trial context. This trial would be acceptable for modified ITT. | Low risk of bias | The outcome data were available for all participants. | High risk of bias | The outcome was patient reported outcome (PRO) and the assessors were aware of their interventions. As adverse events were specific to anticholinergics, the awareness would affect the outcome. | Some concerns | As there were no protocols, statistical analysis plan or trial registry, it is difficult to make a judgment. | High risk of bias | Overall high due to the nature of self reported outcome data and the participants are aware of the intervention. |
| Song 2006 | Some concerns | There was no detail about the random sequence generation and the allocation concealment. The baseline characteristics were not so different between groups. | High risk of bias | Although no detail about masking, it is difficult for participants and carers to be masked in this setting. Many participants dropped out from intervention (43.5% in BT, 31.9% in tolterodine, 32.6% in combination) and there was no detail about the deviation. Only the patients who completed the protocol were analyzed in this trial, and it was not ITT analysis. | High risk of bias | Many participants dropped out from outcome assessment (43.5% in BT, 31.9% in tolterodine, 32.6% in combination) and it was likely that missingness affected its true value. | High risk of bias | The outcome was patient reported outcome (PRO) and the assessors were aware of their interventions. As adverse events were specific to anticholinergics, the awareness would affect the outcome. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | High risk of bias | Overall high due to the many deviation from the intervention, the much missing outcome data and non‐blinding of outcome assessors. |
Risk of bias for analysis 2.6 Adverse events: long‐term effect (> 2 months after treatment).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Milani 1987 | Some concerns | There was no detail about the random sequence generation and the allocation concealment. The baseline characteristics were similar, but only age and duration of disease were reported. There was too little information to make a judgment. | Low risk of bias | Although no detail about masking, it is difficult for participants and carers to be masked in this setting. The two deviations from BT was for long lasting therapy and four from oxybutynin for adverse events. These were not trial contexts. This trial would be acceptable for modified ITT. | Low risk of bias | All adverse events were probably reported. | High risk of bias | The outcome was patient reported outcome (PRO) and the assessors (= participants) were aware of their interventions. Adverse events were specific to oxybutynin and the awareness likely affected the outcome. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | High risk of bias | Overall high due to the nature of self reported outcome data and the participants are aware of the intervention. |
Risk of bias for analysis 2.7 Number of incontinence episodes per 24 hours: immediately after treatment.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kafri 2013 | Low risk of bias | The participants were by randomly permuted blocks of four, with random assignment concealed in tamper‐proof envelopes. The baseline characteristics were similar between groups. | High risk of bias | Although no detail about masking, it is difficult for participants and carers to be masked in this setting. The deviations from the intervention were higher in the drug therapy group than in other groups because of dissatisfaction with the allocation. All data were analyzed on an ITT basis. | High risk of bias | The rate of available data was 95% (39/41) in bladder training and 64% (27/42) in anticholinergics. Missing outcome data were imputed by last observation carried forward (LOCF) and the missing numbers were different between groups, especially many missing in the drug therapy group. This missingness was likely dependent on their true value. | Low risk of bias | The outcome was the patient‐reported outcome (PRO) and the assessors (= participants) were aware of their interventions. However, FVC is a relatively hard outcome and is not likely affected by awareness. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | High risk of bias | Overall high due to the deviation from the intervention and the missing outcome data. |
| Lauti 2008 | Low risk of bias | Participants were randomly allocated, using a password protected web page that remotely accessed a computer‐generated randomisation list. However, the baseline characteristics (e.g. randomised number, age) were different between groups. | Low risk of bias | This was an open‐label trial. All women received their allocated treatment, except for one who opted for tolterodine. This trial would be acceptable for modified ITT. | High risk of bias | The follow‐up rates were 86% (18/21) in GroupI, 100% (16/16) in GroupII and 63% (12/19) in GroupIII. There was no analysis for missing data and the missing likely affected the true value. | Low risk of bias | The outcome was the patient reported outcome (PRO) and the assessors were aware of their interventions. FVC is a relatively hard outcome and the awareness would not affect the outcome. | Some concerns | As there were no protocols, statistical analysis plan, it is difficult to make a judgment. | High risk of bias | Overall high due to the missing outcome data. |
Risk of bias for analysis 2.8 Number of incontinence episodes per 24 hours: long‐term effect (> 2 months after treatment).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kafri 2013 | Low risk of bias | The participants were by randomly permuted blocks of four, with random assignment concealed in tamper‐proof envelopes. The baseline characteristics were similar between groups. | High risk of bias | Although no detail about masking, it is difficult for participants and carers to be masked in this setting. The deviations from the intervention were higher in the drug therapy group than in other groups because of dissatisfaction with the allocation. All data were analyzed on an ITT basis. | High risk of bias | The rate of available data was 95% (39/41) in bladder training and 64% (27/42) in anticholinergics. Missing outcome data were imputed by last observation carried forward (LOCF) and the missing numbers were different between groups, especially many missing in the drug therapy group. This missingness was likely dependent on their true value. | Low risk of bias | The outcome was the patient‐reported outcome (PRO) and the assessors (= participants) were aware of their interventions. However, FVC is a relatively hard outcome and is not likely affected by awareness. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | High risk of bias | Overall high due to the deviation from the intervention and the missing outcome data. |
| Lauti 2008 | Low risk of bias | Participants were randomly allocated, using a password protected web page that remotely accessed a computer‐generated randomisation list. However, the baseline characteristics (e.g. randomised number, age) were different between groups. | Low risk of bias | This was an open‐label trial. All women received their allocated treatment, except for one who opted for tolterodine. This trial would be acceptable for modified ITT. | High risk of bias | The follow‐up rates were 86% (18/21) in GroupI, 100% (16/16) in GroupII and 63% (12/19) in GroupIII. There was no analysis for missing data and the missing likely affected the true value. | Low risk of bias | The outcome was the patient reported outcome (PRO) and the assessors were aware of their interventions. FVC is a relatively hard outcome and the awareness would not affect the outcome. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | High risk of bias | Overall high due to the missing outcome data. |
Risk of bias for analysis 2.9 Number of urgency episodes per 24 hours: immediately after treatment.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Lauti 2008 | Low risk of bias | Participants were randomly allocated, using a password protected web page that remotely accessed a computer‐generated randomisation list. However, the baseline characteristics (e.g. randomised number, age) were different between groups. | Low risk of bias | This was an open‐label trial. All women received their allocated treatment, except for one who opted for tolterodine. This trial would be acceptable for modified ITT. | High risk of bias | The follow‐up rates were 86% (18/21) in GroupI, 100% (16/16) in GroupII and 63% (12/19) in GroupIII. There was no analysis for missing data and the missing likely affected the true value. | Low risk of bias | The outcome was the patient reported outcome (PRO) and the assessors were aware of their interventions. FVC is a relatively hard outcome and the awareness would not affect the outcome. | Some concerns | As there were no protocols, statistical analysis plan, it is difficult to make a judgment. | High risk of bias | Overall high due to the missing outcome data. |
| Song 2006 | Some concerns | There was no detail about the random sequence generation and the allocation concealment. The baseline characteristics were not so different between groups. | High risk of bias | Although no detail about masking, it is difficult for participants and carers to be masked in this setting. Many participants dropped out from intervention (43.5% in BT, 31.9% in tolterodine, 32.6% in combination) and there was no detail about the deviation. Only the patients who completed the protocol were analyzed in this trial, and it was not ITT analysis. | High risk of bias | Many participants dropped out from outcome assessment (43.5% in BT, 31.9% in tolterodine, 32.6% in combination) and it was likely that missingness affected its true value. | Low risk of bias | The outcome was the patient reported outcome (PRO) and the assessors were aware of their interventions. FVC is a relatively hard outcome and the awareness would not affect the outcome. | Some concerns | As there were no protocols, statistical analysis plan, it is difficult to make a judgment. | High risk of bias | Overall high due to the many deviation from the intervention and the much missing outcome data. |
Risk of bias for analysis 2.10 Number of urgency episodes per 24 hours: long‐term effect (> 2 months after treatment).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Lauti 2008 | Low risk of bias | Participants were randomly allocated, using a password protected web page that remotely accessed a computer‐generated randomisation list. However, the baseline characteristics (e.g. randomised number, age) were different between groups. | Low risk of bias | This was an open‐label trial. All women received their allocated treatment, except for one who opted for tolterodine. This trial would be acceptable for modified ITT. | High risk of bias | The follow‐up rates were 86% (18/21) in GroupI, 100% (16/16) in GroupII and 63% (12/19) in GroupIII. There was no analysis for missing data and the missing likely affected the true value. | Low risk of bias | The outcome was the patient reported outcome (PRO) and the assessors were aware of their interventions. FVC is a relatively hard outcome and the awareness would not affect the outcome. | Some concerns | As there were no protocols, statistical analysis plan, it is difficult to make a judgment. | High risk of bias | Overall high due to the missing outcome data. |
Risk of bias for analysis 2.11 Number of micturition episodes per 24 hours: immediately after treatment.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kafri 2013 | Low risk of bias | The participants were by randomly permuted blocks of four, with random assignment concealed in tamper‐proof envelopes. The baseline characteristics were similar between groups. | High risk of bias | Although no detail about masking, it is difficult for participants and carers to be masked in this setting. The deviations from the intervention were higher in the drug therapy group than in other groups because of dissatisfaction with the allocation. All data were analyzed on an ITT basis. | High risk of bias | The rate of available data was 95% (39/41) in bladder training and 64% (27/42) in anticholinergics. Missing outcome data were imputed by last observation carried forward (LOCF) and the missing numbers were different between groups, especially many missing in the drug therapy group. This missingness was likely dependent on their true value. | Low risk of bias | The outcome was the patient‐reported outcome (PRO) and the assessors (= participants) were aware of their interventions. However, FVC is a relatively hard outcome and is not likely affected by awareness. | Some concerns | As there were no protocols, statistical analysis plan, it is difficult to make a judgment. | High risk of bias | Overall high due to the deviation from the intervention and the missing outcome data. |
| Lauti 2008 | Low risk of bias | Participants were randomly allocated, using a password protected web page that remotely accessed a computer‐generated randomisation list. However, the baseline characteristics (e.g. randomised number, age) were different between groups. | Low risk of bias | This was an open‐label trial. All women received their allocated treatment, except for one who opted for tolterodine. This trial would be acceptable for modified ITT. | High risk of bias | The follow‐up rates were 86% (18/21) in GroupI, 100% (16/16) in GroupII and 63% (12/19) in GroupIII. There was no analysis for missing data and the missing likely affected the true value. | Low risk of bias | The outcome was the patient‐reported outcome (PRO) and the assessors were aware of their interventions. FVC is a relatively hard outcome and would not be less susceptible to awareness. | Some concerns | As there were no protocols, statistical analysis plan, it is difficult to make a judgment. | High risk of bias | Overall high due to the missing outcome data. |
| Song 2006 | Some concerns | There was no detail about the random sequence generation and the allocation concealment. The baseline characteristics were not so different between groups. | High risk of bias | Although no detail about masking, it is difficult for participants and carers to be masked in this setting. Many participants dropped out from intervention (43.5% in BT, 31.9% in tolterodine, 32.6% in combination) and there was no detail about the deviation. Only the patients who completed the protocol were analyzed in this trial, and it was not ITT analysis. | High risk of bias | Many participants dropped out from outcome assessment (43.5% in BT, 31.9% in tolterodine, 32.6% in combination) and it was likely that missingness affected its true value. | Low risk of bias | The outcome was the patient reported outcome (PRO) and the assessors were aware of their interventions. FVC is a relatively hard outcome and the awareness would not affect the outcome. | Some concerns | As there were no protocols, statistical analysis plan, it is difficult to make a judgment. | High risk of bias | Overall high due to the many deviation from the intervention and the much missing outcome data. |
Risk of bias for analysis 2.12 Number of micturition episodes per 24 hours: long‐term effect (> 2 months after treatment).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kafri 2013 | Low risk of bias | The participants were by randomly permuted blocks of four, with random assignment concealed in tamper‐proof envelopes. The baseline characteristics were similar between groups. | High risk of bias | Although no detail about masking, it is difficult for participants and carers to be masked in this setting. The deviations from the intervention were higher in the drug therapy group than in other groups because of dissatisfaction with the allocation. All data were analyzed on an ITT basis. | High risk of bias | The rate of available data was 95% (39/41) in bladder training and 64% (27/42) in anticholinergics. Missing outcome data were imputed by last observation carried forward (LOCF) and the missing numbers were different between groups, especially many missing in the drug therapy group. This missingness was likely dependent on their true value. | Low risk of bias | The outcome was the patient‐reported outcome (PRO) and the assessors (= participants) were aware of their interventions. However, FVC is a relatively hard outcome and is not likely affected by awareness. | Some concerns | As there were no protocols, statistical analysis plan, it is difficult to make a judgment. | High risk of bias | Overall high due to the deviation from the intervention and the missing outcome data. |
| Lauti 2008 | Low risk of bias | Participants were randomly allocated, using a password protected web page that remotely accessed a computer‐generated randomisation list. However, the baseline characteristics (e.g. randomised number, age) were different between groups. | Low risk of bias | This was an open‐label trial. All women received their allocated treatment, except for one who opted for tolterodine. This trial would be acceptable for modified ITT. | High risk of bias | The follow‐up rates were 86% (18/21) in GroupI, 100% (16/16) in GroupII and 63% (12/19) in GroupIII. There was no analysis for missing data and the missing likely affected the true value. | Low risk of bias | The outcome was the patient‐reported outcome (PRO) and the assessors were aware of their interventions. FVC is a relatively hard outcome and would not be less susceptible to awareness. | Some concerns | As there were no protocols, statistical analysis plan, it is difficult to make a judgment. | High risk of bias | Overall high due to the missing outcome data. |
Risk of bias for analysis 3.1 Symptom‐related quality of life (QoL): immediately after treatment.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kafri 2013 | Low risk of bias | The participants were by randomly permuted blocks of four, with random assignment concealed in tamper‐proof envelopes. The baseline characteristics were similar between groups. | Low risk of bias | Although no detail about masking, it is difficult for participants and carers to be masked in this setting. The reason for deviations from both interventions was a medical condition or no response to treatment, and these could not be because of trial context. All data were analyzed on an ITT basis. | High risk of bias | The rate of available data was 95% (39/41) in bladder training and 85% (34/40) in PFMT. Missing outcome data were imputed by last observation carried forward (LOCF) and the missing numbers were different between groups, especially many missing in the drug therapy group. This missingness was likely dependent on their true value. | Some concerns | The outcome was the patient‐reported outcome (PRO) and the assessors (= participants) were aware of their interventions. Both groups were taken for an active treatment and it was difficult to decide that the awareness affected the outcome. | Some concerns | As there were no protocols, statistical analysis plan, it is difficult to make a judgment. | High risk of bias | Overall high due to the missing outcome data. |
| Rizvi 2018 | Low risk of bias | Randomization numbers were computer generated and the allocation was undertaken by sequentially opening a numbered sealed envelope. The baseline characteristics were similar between groups. | Low risk of bias | There was no detail about "single blinded". Three women in the BT group were lost to follow‐up due to no effects of treatment and these were not by trial context. This trial would be acceptable for modified ITT. | Low risk of bias | There were only three (47/50) missing outcome data. | Some concerns | The outcome was the patient‐reported outcome (PRO) and the assessors were aware of their interventions. Both groups were taken for active treatment and it was difficult to decide that the awareness affected the outcome. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | Some concerns | Due to potential bias in the selection of the reported result. |
Risk of bias for analysis 3.2 Symptom‐related QoL: long‐term effect (> 2 months after treatment).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kafri 2013 | Low risk of bias | The participants were by randomly permuted blocks of four, with random assignment concealed in tamper‐proof envelopes. The baseline characteristics were similar between groups. | Low risk of bias | Although no detail about masking, it is difficult for participants and carers to be masked in this setting. The reason for deviations from both interventions was a medical condition or no response to treatment, and these could not be because of trial context. All data were analyzed on an ITT basis. | High risk of bias | The rate of available data was 95% (39/41) in bladder training and 85% (34/40) in PFMT. Missing outcome data were imputed by last observation carried forward (LOCF) and the missing numbers were different between groups, especially many missing in the drug therapy group. This missingness was likely dependent on their true value. | Some concerns | The outcome was the patient‐reported outcome (PRO) and the assessors (= participants) were aware of their interventions. Both groups were taken for active treatment and it was difficult to decide that the awareness affected the outcome. | Some concerns | As there were no protocols, statistical analysis plan, it is difficult to make a judgment. | High risk of bias | Overall high due to the missing outcome data. |
Risk of bias for analysis 3.3 Adverse events: immediately after treatment.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Rizvi 2018 | Low risk of bias | Randomization numbers were computer generated and the allocation was undertaken by sequentially opening a numbered sealed envelope. The baseline characteristics were similar between groups. | Low risk of bias | There was no detail about "single blinded". Three women in the BT group were lost to follow‐up due to no effects of treatment and these were not by trial context. This trial would be acceptable for modified ITT. | Low risk of bias | There were only three (47/50) missing outcome data. | Some concerns | The outcome was the patient‐reported outcome (PRO) and the assessors were aware of their interventions. Both groups were taken for active treatment and it was difficult to decide that the awareness affected the outcome. | Some concerns | As there were no protocols, statistical analysis plan, it is difficult to make a judgment. | Some concerns | Due to potential bias in the selection of the reported result. |
Risk of bias for analysis 3.4 Number of incontinence episodes per 24 hours: immediately after treatment.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kafri 2013 | Low risk of bias | The participants were by randomly permuted blocks of four, with random assignment concealed in tamper‐proof envelopes. The baseline characteristics were similar between groups. | Low risk of bias | Although no detail about masking, it is difficult for participants and carers to be masked in this setting. The reason for deviations from both interventions was a medical condition or no response to treatment, and these could not be because of trial context. All data were analyzed on an ITT basis. | High risk of bias | The rate of available data was 95% (39/41) in bladder training and 85% (34/40) in PFMT. Missing outcome data were imputed by last observation carried forward (LOCF) and the missing numbers were different between groups, especially many missing in the drug therapy group. This missingness was likely dependent on their true value. | Low risk of bias | The outcome was the patient‐reported outcome (PRO) and the assessors (= participants) were aware of their interventions. However, FVC is a relatively hard outcome and is not likely affected by awareness. | Some concerns | As there were no protocols, statistical analysis plan, it is difficult to make a judgment. | High risk of bias | Overall high due to the missing outcome data. |
Risk of bias for analysis 3.5 Number of incontinence episodes per 24 hours: long‐term effect (> 2 months after treatment).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kafri 2013 | Low risk of bias | The participants were by randomly permuted blocks of four, with random assignment concealed in tamper‐proof envelopes. The baseline characteristics were similar between groups. | Low risk of bias | Although no detail about masking, it is difficult for participants and carers to be masked in this setting. The reason for deviations from both interventions was a medical condition or no response to treatment, and these could not be because of trial context. All data were analyzed on an ITT basis. | High risk of bias | The rate of available data was 95% (39/41) in bladder training and 85% (34/40) in PFMT. Missing outcome data were imputed by last observation carried forward (LOCF) and the missing numbers were different between groups, especially many missing in the drug therapy group. This missingness was likely dependent on their true value. | Low risk of bias | The outcome was the patient‐reported outcome (PRO) and the assessors (= participants) were aware of their interventions. However, FVC is a relatively hard outcome and is not likely affected by awareness. | Some concerns | As there were no protocols, statistical analysis plan, it is difficult to make a judgment. | High risk of bias | Overall high due to the missing outcome data. |
Risk of bias for analysis 3.6 Number of micturition episodes per 24 hours: immediately after treatment.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kafri 2013 | Low risk of bias | The participants were by randomly permuted blocks of four, with random assignment concealed in tamper‐proof envelopes. The baseline characteristics were similar between groups. | Low risk of bias | Although no detail about masking, it is difficult for participants and carers to be masked in this setting. The reason for deviations from both interventions was a medical condition or no response to treatment, and these could not be because of trial context. All data were analyzed on an ITT basis. | High risk of bias | The rate of available data was 95% (39/41) in bladder training and 85% (34/40) in PFMT. Missing outcome data were imputed by last observation carried forward (LOCF) and the missing numbers were different between groups, especially many missing in the drug therapy group. This missingness was likely dependent on their true value. | Low risk of bias | The outcome was the patient‐reported outcome (PRO) and the assessors (= participants) were aware of their interventions. However, FVC is a relatively hard outcome and is not likely affected by awareness. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | High risk of bias | Overall high due to the missing outcome data. |
Risk of bias for analysis 3.7 Number of micturition episodes per 24 hours: long‐term effect (> 2 months after treatment).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kafri 2013 | Low risk of bias | The participants were by randomly permuted blocks of four, with random assignment concealed in tamper‐proof envelopes. The baseline characteristics were similar between groups. | Low risk of bias | Although no detail about masking, it is difficult for participants and carers to be masked in this setting. The reason for deviations from both interventions was a medical condition or no response to treatment, and these could not be because of trial context. All data were analyzed on an ITT basis. | High risk of bias | The rate of available data was 95% (39/41) in bladder training and 85% (34/40) in PFMT. Missing outcome data were imputed by last observation carried forward (LOCF) and the missing numbers were different between groups, especially many missing in the drug therapy group. This missingness was likely dependent on their true value. | Low risk of bias | The outcome was the patient‐reported outcome (PRO) and the assessors (= participants) were aware of their interventions. However, FVC is a relatively hard outcome and is not likely affected by awareness. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | High risk of bias | Overall high due to the missing outcome data. |
Risk of bias for analysis 4.1 Participant‐reported cure or improvement: immediately after treatment.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Mattiasson 2003 | Low risk of bias | The participants were randomized in balanced blocks of four, according to a computer‐generated randomization list. The baseline characteristics were similar between groups. | Low risk of bias | There was no detail about "single blinded". The completion rates were comparable for the two groups (tolterodine + BT, 77%; tolterodine alone). The main reasons for withdrawal were: adverse events (15%), lack of efficacy (3%), withdrawal of consent (2%), protocol violations (1%), and not the trial context. This trial would be acceptable for modified ITT. | High risk of bias | There was no detail about the missing number and reasons. Missing outcome data were imputed by last observation carried forward (LOCF). | Some concerns | The outcome was the patient‐reported outcome (PRO) and the assessors (= participants) were aware of their interventions. Both groups were taken for an active treatment and it was difficult to decide that the awareness affected the outcome. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | High risk of bias | Overall high due to the method of missing imputation. |
| Song 2006 | Some concerns | There was no detail about the random sequence generation and the allocation concealment. The baseline characteristics were not so different between groups. | High risk of bias | Although no detail about masking, it is difficult for participants and carers to be masked in this setting. Many participants dropped out from intervention (43.5% in BT, 31.9% in tolterodine, 32.6% in combination) and there was no detail about the deviation. Only the patients who completed the protocol were analyzed in this trial, and it was not ITT analysis. | High risk of bias | Many participants dropped out from outcome assessment (43.5% in BT, 31.9% in tolterodine, 32.6% in combination) and it was likely that missingness affected its true value. | Some concerns | The outcome was the patient‐reported outcome (PRO) and the assessors were aware of their interventions. Both groups were taken for active treatment and it was difficult to decide that the awareness affected the outcome. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | High risk of bias | Overall high due to the many deviation from the intervention and the much missing outcome data. |
Risk of bias for analysis 4.2 Symptom‐related quality of life (QoL): immediately after treatment.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Lauti 2008 | Low risk of bias | Participants were randomly allocated, using a password protected web page that remotely accessed a computer‐generated randomisation list. However, the baseline characteristics (e.g. randomised number, age) were different between groups. | Low risk of bias | This was an open‐label trial. All women received their allocated treatment, except for one who opted for tolterodine. This trial would be acceptable for modified ITT. | High risk of bias | The follow‐up rates were 86% (18/21) in GroupI, 100% (16/16) in GroupII and 63% (12/19) in GroupIII. There was no analysis for missing data and the missing likely affected the true value. | Some concerns | The outcome was patient reported outcome (PRO) and the assessors were aware of their interventions. Both groups were taken for active treatment and it was difficult to decide that the awareness affected the outcome. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | High risk of bias | Overall high due to the missing outcome data. |
| Mattiasson 2010 | Some concerns | There was no detail about the random sequence generation and the allocation concealment. The baseline characteristics were similar. | Low risk of bias | There was no detail about "single blinded". The follow‐up rate was 77% in GroupI and 79% in GroupII. The reasons of drop out were AE (15%), no‐efficacy (3%), no‐consent (2%), protocol violation (1%), not the trial context. This trial would be acceptable for modified ITT. | Some concerns | The rate of available data was 93% (297/320) in GroupI and 94% (305/323). There was no analysis for the missing outcome. The missing data could affect true value, however, the numbers were small and similar between groups. | Some concerns | The outcome was the patient‐reported outcome (PRO) and the assessors were aware of their interventions. Both groups were taken for active treatment and it was difficult to decide that the awareness affected the outcome. | Some concerns | As there were no protocols or statistical analysis, it is difficult to make a judgment. | Some concerns | Due to no detail of the randomization process, non‐blinding of outcome assessors and potential bias in the selection of the reported result. |
Risk of bias for analysis 4.3 Symptom‐related QOL: long‐term effect (> 2 months after treatment).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Lauti 2008 | Low risk of bias | Participants were randomly allocated, using a password protected web page that remotely accessed a computer‐generated randomisation list. However, the baseline characteristics (e.g. randomised number, age) were different between groups. | Low risk of bias | This was an open‐label trial. All women received their allocated treatment, except for one who opted for tolterodine. This trial would be acceptable for modified ITT. | High risk of bias | This was an open‐label trial. All women received their allocated treatment, except for one who opted for tolterodine. This trial would be acceptable for modified ITT. | Some concerns | The outcome was patient reported outcome (PRO) and the assessors were aware of their interventions. Both groups were taken for active treatment and it was difficult to decide that the awareness affected the outcome. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | High risk of bias | Overall high due to the missing outcome data. |
| Mattiasson 2010 | Some concerns | There was no detail about the random sequence generation and the allocation concealment. The baseline characteristics were similar. | Low risk of bias | There was no detail about "single blinded". The follow‐up rate was 77% in GroupI and 79% in GroupII. The reasons of drop out were AE (15%), no‐efficacy (3%), no‐consent (2%), protocol violation (1%), not the trial context. This trial would be acceptable for modified ITT. | Some concerns | The rate of available data was 93% (297/320) in GroupI and 94% (305/323). There was no analysis for the missing outcome. The missing data could affect true value, however, the numbers were small and similar between groups. | Some concerns | The outcome was the patient‐reported outcome (PRO) and the assessors were aware of their interventions. Both groups were taken for active treatment and it was difficult to decide that the awareness affected the outcome. | Some concerns | As there were no protocols or statistical analysis, it is difficult to make a judgment. | Some concerns | Due to no detail of the randomization process, non‐blinding of outcome assessors and potential bias in the selection of the reported result. |
Risk of bias for analysis 4.4 Adverse events: immediately after treatment.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Mattiasson 2003 | Low risk of bias | The participants were randomized in balanced blocks of four, according to a computer‐generated randomization list. The baseline characteristics were similar between groups. | Low risk of bias | There was no detail about "single blinded". The completion rates were comparable for the two groups (tolterodine + BT, 77%; tolterodine alone). The main reasons for withdrawal were: adverse events (15%), lack of efficacy (3%), withdrawal of consent (2%), protocol violations (1%), and not the trial context. This trial would be acceptable for modified ITT. | Low risk of bias | All participants (n=501) that were randomised were monitored for adverse events (Table 1). | Some concerns | The outcome was the patient‐reported outcome (PRO) and the assessors (= participants) were aware of their interventions. Both groups were taken for an active treatment and it was difficult to decide that the awareness affected the outcome. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | Some concerns | Due to non‐blinding of outcome assessors and potential bias in the selection of the reported result. |
| Song 2006 | Some concerns | There was no detail about the random sequence generation and the allocation concealment. The baseline characteristics were not so different between groups. | High risk of bias | Although no detail about masking, it is difficult for participants and carers to be masked in this setting. Many participants dropped out from intervention (43.5% in BT, 31.9% in tolterodine, 32.6% in combination) and there was no detail about the deviation. Only the patients who completed the protocol were analyzed in this trial, and it was not ITT analysis. | High risk of bias | Many participants dropped out from outcome assessment (43.5% in BT, 31.9% in tolterodine, 32.6% in combination) and it was likely that missingness affected its true value. | Some concerns | The outcome was the patient‐reported outcome (PRO) and the assessors were aware of their interventions. Both groups were taken for active treatment and it was difficult to decide that the awareness affected the outcome. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | High risk of bias | Overall high due to the many deviations from the intervention and the much missing outcome data. |
Risk of bias for analysis 4.5 Adverse events: long‐term effect (> 2 months after treatment).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Mattiasson 2010 | Some concerns | There was no detail about the random sequence generation and the allocation concealment. The baseline characteristics were similar. | Low risk of bias | There was no detail about "single blinded". The follow‐up rate was 77% in GroupI and 79% in GroupII. The reasons of drop out were AE (15%), no‐efficacy (3%), no‐consent (2%), protocol violation (1%), not the trial context. This trial would be acceptable for modified ITT. | Low risk of bias | All participants (n=643) that were randomised were monitored for adverse events. | Some concerns | The outcome was the patient‐reported outcome (PRO) and the assessors were aware of their interventions. Both groups were taken for active treatment and it was difficult to decide that the awareness affected the outcome. | Some concerns | As there were no protocols or statistical analysis, it is difficult to make a judgment. | Some concerns | Due to no detail of the randomization process, non‐blinding of outcome assessors and potential bias in the selection of the reported result. |
Risk of bias for analysis 4.6 Participant‐reported satisfaction: immediately after treatment.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Mattiasson 2010 | Some concerns | There was no detail about the random sequence generation and the allocation concealment. The baseline characteristics were similar. | Low risk of bias | There was no detail about "single blinded". The follow‐up rate was 77% in GroupI and 79% in GroupII. The reasons for drop out were AE (15%), no‐efficacy (3%), no‐consent (2%), protocol violation (1%), not the trial context. This trial would be acceptable for modified ITT. | Some concerns | The rate of available data was 93% (297/320) in GroupI and 94% (305/323). There was no analysis for the missing outcome. The missing data could affect true value, however, the numbers were small and similar between groups. | Some concerns | The outcome was the patient‐reported outcome (PRO) and the assessors were aware of their interventions. Both groups were taken for active treatment and it was difficult to decide that the awareness affected the outcome. | Some concerns | As there were no protocols or statistical analysis, it is difficult to make a judgment. | Some concerns | Due to no detail of the randomization process, non‐blinding of outcome assessors and potential bias in the selection of the reported result. |
Risk of bias for analysis 4.7 Participant‐reported satisfaction: long‐term effect (> 2 months after treatment).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Mattiasson 2010 | Some concerns | There was no detail about the random sequence generation and the allocation concealment. The baseline characteristics were similar. | Low risk of bias | There was no detail about "single blinded". The follow‐up rate was 77% in GroupI and 79% in GroupII. The reasons for drop out were AE (15%), no‐efficacy (3%), no‐consent (2%), protocol violation (1%), not the trial context. This trial would be acceptable for modified ITT. | Some concerns | The rate of available data was 93% (297/320) in GroupI and 94% (305/323). There was no analysis for the missing outcome. The missing data could affect true value, however, the numbers were small and similar between groups. | Some concerns | The outcome was the patient‐reported outcome (PRO) and the assessors were aware of their interventions. Both groups were taken for active treatment and it was difficult to decide that the awareness affected the outcome. | Some concerns | As there were no protocols or statistical analysis, it is difficult to make a judgment. | Some concerns | Due to no detail of the randomization process, non‐blinding of outcome assessors and potential bias in the selection of the reported result. |
Risk of bias for analysis 4.8 Number of incontinence episodes per 24 hours: immediately after treatment.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Lauti 2008 | Low risk of bias | Participants were randomly allocated, using a password protected web page that remotely accessed a computer‐generated randomisation list. However, the baseline characteristics (e.g. randomised number, age) were different between groups. | Low risk of bias | This was an open‐label trial. All women received their allocated treatment, except for one who opted for tolterodine. This trial would be acceptable for modified ITT. | High risk of bias | The follow‐up rates were 86% (18/21) in GroupI, 100% (16/16) in GroupII and 63% (12/19) in GroupIII. There was no analysis for missing data and the missing likely affected the true value. | Low risk of bias | The outcome was the patient‐reported outcome (PRO) and the assessors were aware of their interventions. FVC is a relatively hard outcome and would not be less susceptible to awareness. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | High risk of bias | Overall high due to the missing outcome data. |
| Mattiasson 2003 | Low risk of bias | The participants were randomized in balanced blocks of four, according to a computer‐generated randomization list. The baseline characteristics were similar between groups. | Low risk of bias | There was no detail about "single blinded". The completion rates were comparable for the two groups (tolterodine + BT, 77%; tolterodine alone). The main reasons for withdrawal were: adverse events (15%), lack of efficacy (3%), withdrawal of consent (2%), protocol violations (1%), and not the trial context. This trial would be acceptable for modified ITT. | High risk of bias | There was no detail about the missing number and reasons. Missing outcome data were imputed by last observation carried forward (LOCF). | Low risk of bias | The outcome was the patient‐reported outcome (PRO) and the assessors (= participants) were aware of their interventions. However, FVC is a relatively hard outcome and is not likely affected by awareness. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | High risk of bias | Overall high due to the method of missing imputation. |
| Mattiasson 2010 | Some concerns | There was no detail about the random sequence generation and the allocation concealment. The baseline characteristics were similar. | Low risk of bias | There was no detail about "single blinded". The follow‐up rate was 77% in GroupI and 79% in GroupII. The reasons for drop out were AE (15%), no‐efficacy (3%), no‐consent (2%), protocol violation (1%), not the trial context. This trial would be acceptable for modified ITT. | Some concerns | The rate of available data was 93% (297/320) in GroupI and 94% (305/323). There was no analysis for the missing outcome. The missing data could affect true value, however, the numbers were small and similar between groups. | Low risk of bias | The outcome was the patient‐reported outcome (PRO) and the assessors were aware of their interventions. FVC is a relatively hard outcome and would not be less susceptible to awareness. | Some concerns | As there were no protocols or statistical analysis, it is difficult to make a judgment. | Some concerns | Due to no detail of the randomization process, non‐blinding of outcome assessors and potential bias in the selection of the reported result. |
Risk of bias for analysis 4.9 Number of incontinence episodes per 24 hours: long‐term effect (> 2 months after treatment).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Lauti 2008 | Low risk of bias | Participants were randomly allocated, using a password protected web page that remotely accessed a computer‐generated randomisation list. However, the baseline characteristics (e.g. randomised number, age) were different between groups. | Low risk of bias | This was an open‐label trial. All women received their allocated treatment, except for one who opted for tolterodine. This trial would be acceptable for modified ITT. | High risk of bias | The follow‐up rates were 86% (18/21) in GroupI, 100% (16/16) in GroupII and 63% (12/19) in GroupIII. There was no analysis for missing data and the missing likely affected the true value. | Low risk of bias | The outcome was patient reported outcome (PRO) and the assessors were aware of their interventions. FVC is a relatively hard outcome and the awareness would not affect the outcome. | Some concerns | As there were no protocols, statistical analysis plan, it is difficult to make a judgment. | High risk of bias | Overall high due to the missing outcome data. |
| Mattiasson 2010 | Some concerns | There was no detail about the random sequence generation and the allocation concealment. The baseline characteristics were similar. | Low risk of bias | There was no detail about "single blinded". The follow‐up rate was 77% in GroupI and 79% in GroupII. The reasons for drop out were AE (15%), no‐efficacy (3%), no‐consent (2%), protocol violation (1%), not the trial context. This trial would be acceptable for modified ITT. | Some concerns | The rate of available data was 93% (297/320) in GroupI and 94% (305/323). There was no analysis for the missing outcome. The missing data could affect true value, however, the numbers were small and similar between groups. | Low risk of bias | The outcome was the patient‐reported outcome (PRO) and the assessors were aware of their interventions. FVC is a relatively hard outcome and would not be less susceptible to awareness. | Some concerns | As there were no protocols, statistical analysis plans it is difficult to make a judgment. | Some concerns | Due to no detail of the randomization process, non‐blinding of outcome assessors and potential bias in the selection of the reported result. |
Risk of bias for analysis 4.10 Number of urgency episodes per 24 hours: immediately after treatment.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Lauti 2008 | Low risk of bias | Participants were randomly allocated, using a password protected web page that remotely accessed a computer‐generated randomisation list. However, the baseline characteristics (e.g. randomised number, age) were different between groups. | Low risk of bias | This was an open‐label trial. All women received their allocated treatment, except for one who opted for tolterodine. This trial would be acceptable for modified ITT. | High risk of bias | The follow‐up rates were 86% (18/21) in GroupI, 100% (16/16) in GroupII and 63% (12/19) in GroupIII. There was no analysis for missing data and the missing likely affected the true value. | Low risk of bias | The outcome was the patient‐reported outcome (PRO) and the assessors were aware of their interventions. FVC is a relatively hard outcome and would not be less susceptible to awareness. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | High risk of bias | Overall high due to the missing outcome data. |
| Mattiasson 2003 | Low risk of bias | The participants were randomized in balanced blocks of four, according to a computer‐generated randomization list. The baseline characteristics were similar between groups. | Low risk of bias | There was no detail about "single blinded". The completion rates were comparable for the two groups (tolterodine + BT, 77%; tolterodine alone). The main reasons for withdrawal were: adverse events (15%), lack of efficacy (3%), withdrawal of consent (2%), protocol violations (1%), and not the trial context. This trial would be acceptable for modified ITT. | High risk of bias | There was no detail about the missing number and reasons. Missing outcome data were imputed by last observation carried forward (LOCF). | Low risk of bias | The outcome was the patient‐reported outcome (PRO) and the assessors (= participants) were aware of their interventions. However, FVC is a relatively hard outcome and is not likely affected by awareness. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | High risk of bias | Overall high due to the method of missing imputation. |
| Mattiasson 2010 | Some concerns | There was no detail about the random sequence generation and the allocation concealment. The baseline characteristics were similar. | Low risk of bias | There was no detail about "single blinded". The follow‐up rate was 77% in GroupI and 79% in GroupII. The reasons for drop out were AE (15%), no‐efficacy (3%), no‐consent (2%), protocol violation (1%), not the trial context. This trial would be acceptable for modified ITT. | Some concerns | The rate of available data was 93% (297/320) in GroupI and 94% (305/323). There was no analysis for the missing outcome. The missing data could affect true value, however, the numbers were small and similar between groups. | Low risk of bias | The outcome was the patient‐reported outcome (PRO) and the assessors were aware of their interventions. FVC is a relatively hard outcome and would not be less susceptible to awareness. | Some concerns | As there were no protocols or statistical analysis, it is difficult to make a judgment. | Some concerns | Due to no detail of the randomization process, non‐blinding of outcome assessors and potential bias in the selection of the reported result. |
| Song 2006 | Some concerns | There was no detail about the random sequence generation and the allocation concealment. The baseline characteristics were not so different between groups. | High risk of bias | There was no detail about masking. Many participants dropped out from intervention (43.5% in BT, 31.9% in tolterodine, 32.6% in combination) and there was no detail about the deviation. Only the patients who completed the protocol were analyzed in this trial, and it was not ITT analysis. | High risk of bias | Many participants dropped out from outcome assessment (43.5% in BT, 31.9% in tolterodine, 32.6% in combination) and it was likely that missingness affected its true value. | Low risk of bias | The outcome was the patient reported outcome (PRO) and the assessors were aware of their interventions. FVC is a relatively hard outcome and the awareness would not affect the outcome. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | High risk of bias | Overall high due to the many deviation from the intervention and the much missing outcome data. |
Risk of bias for analysis 4.11 Number of urgency episodes per 24 hours: long‐term effect (> 2 months after treatment).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Lauti 2008 | Low risk of bias | Participants were randomly allocated, using a password protected web page that remotely accessed a computer‐generated randomisation list. However, the baseline characteristics (e.g. randomised number, age) were different between groups. | Low risk of bias | This was an open‐label trial. All women received their allocated treatment, except for one who opted for tolterodine. This trial would be acceptable for modified ITT. | High risk of bias | The follow‐up rates were 86% (18/21) in GroupI, 100% (16/16) in GroupII and 63% (12/19) in GroupIII. There was no analysis for missing data and the missing likely affected the true value. | Low risk of bias | The outcome was the patient‐reported outcome (PRO) and the assessors were aware of their interventions. FVC is a relatively hard outcome and would not be less susceptible to awareness. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | High risk of bias | Overall high due to the missing outcome data. |
| Mattiasson 2010 | Some concerns | There was no detail about the random sequence generation and the allocation concealment. The baseline characteristics were similar. | Low risk of bias | There was no detail about "single blinded". The follow‐up rate was 77% in GroupI and 79% in GroupII. The reasons for drop out were AE (15%), no‐efficacy (3%), no‐consent (2%), protocol violation (1%), not the trial context. This trial would be acceptable for modified ITT. | Some concerns | The rate of available data was 93% (297/320) in GroupI and 94% (305/323). There was no analysis for the missing outcome. The missing data could affect true value, however, the numbers were small and similar between groups. | Low risk of bias | The outcome was the patient‐reported outcome (PRO) and the assessors were aware of their interventions. FVC is a relatively hard outcome and would not be less susceptible to awareness. | Some concerns | As there were no protocols or statistical analysis, it is difficult to make a judgment. | Some concerns | Due to no detail of the randomization process, non‐blinding of outcome assessors and potential bias in the selection of the reported result. |
Risk of bias for analysis 4.12 Number of micturition episodes per 24 hours: immediately after treatment.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Lauti 2008 | Low risk of bias | Participants were randomly allocated, using a password protected web page that remotely accessed a computer‐generated randomisation list. However, the baseline characteristics (e.g. randomised number, age) were different between groups. | Low risk of bias | This was an open‐label trial. All women received their allocated treatment, except for one who opted for tolterodine. This trial would be acceptable for modified ITT. | High risk of bias | The follow‐up rates were 86% (18/21) in GroupI, 100% (16/16) in GroupII and 63% (12/19) in GroupIII. There was no analysis for missing data and the missing likely affected the true value. | Low risk of bias | The outcome was the patient‐reported outcome (PRO) and the assessors were aware of their interventions. FVC is a relatively hard outcome and would not be less susceptible to awareness. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | High risk of bias | Overall high due to the missing outcome data. |
| Mattiasson 2003 | Low risk of bias | The participants were randomized in balanced blocks of four, according to a computer‐generated randomization list. The baseline characteristics were similar between groups. | Low risk of bias | There was no detail about "single blinded". The completion rates were comparable for the two groups (tolterodine + BT, 77%; tolterodine alone). The main reasons for withdrawal were: adverse events (15%), lack of efficacy (3%), withdrawal of consent (2%), protocol violations (1%), and not the trial context. This trial would be acceptable for modified ITT. | High risk of bias | There was no detail about the missing number and reasons. Missing outcome data were imputed by last observation carried forward (LOCF). | Low risk of bias | The outcome was the patient‐reported outcome (PRO) and the assessors (= participants) were aware of their interventions. However, FVC is a relatively hard outcome and is not likely affected by awareness. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | High risk of bias | Overall high due to the method of missing imputation. |
| Mattiasson 2010 | Some concerns | There was no detail about the random sequence generation and the allocation concealment. The baseline characteristics were similar. | Low risk of bias | There was no detail about "single blinded". The follow‐up rate was 77% in GroupI and 79% in GroupII. The reasons for drop out were AE (15%), no‐efficacy (3%), no‐consent (2%), protocol violation (1%), not the trial context. This trial would be acceptable for modified ITT. | Some concerns | The rate of available data was 93% (297/320) in GroupI and 94% (305/323). There was no analysis for the missing outcome. The missing data could affect true value, however, the numbers were small and similar between groups. | Low risk of bias | The outcome was the patient‐reported outcome (PRO) and the assessors were aware of their interventions. FVC is a relatively hard outcome and would not be less susceptible to awareness. | Some concerns | As there were no protocols or statistical analysis, it is difficult to make a judgment. | Some concerns | Due to no detail of the randomization process, non‐blinding of outcome assessors and potential bias in the selection of the reported result. |
| Song 2006 | Some concerns | There was no detail about the random sequence generation and the allocation concealment. The baseline characteristics were not so different between groups. | High risk of bias | Although no detail about masking, it is difficult for participants and carers to be masked in this setting. Many participants dropped out from intervention (43.5% in BT, 31.9% in tolterodine, 32.6% in combination) and there was no detail about the deviation. Only the patients who completed the protocol were analyzed in this trial, and it was not ITT analysis. | High risk of bias | Many participants dropped out from outcome assessment (43.5% in BT, 31.9% in tolterodine, 32.6% in combination) and it was likely that missingness affected its true value. | Low risk of bias | The outcome was the patient reported outcome (PRO) and the assessors were aware of their interventions. FVC is a relatively hard outcome and the awareness would not affect the outcome. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | High risk of bias | Overall high due to the many deviation from the intervention and the much missing outcome data. |
Risk of bias for analysis 4.13 Number of micturition episodes per 24 hours: long‐term effect (> 2 months after treatment).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Lauti 2008 | Low risk of bias | Participants were randomly allocated, using a password protected web page that remotely accessed a computer‐generated randomisation list. However, the baseline characteristics (e.g. randomised number, age) were different between groups. | Low risk of bias | This was an open‐label trial. All women received their allocated treatment, except for one who opted for tolterodine. This trial would be acceptable for modified ITT. | High risk of bias | The follow‐up rates were 86% (18/21) in GroupI, 100% (16/16) in GroupII and 63% (12/19) in GroupIII. There was no analysis for missing data and the missing likely affected the true value. | Low risk of bias | The outcome was the patient‐reported outcome (PRO) and the assessors were aware of their interventions. FVC is a relatively hard outcome and would not be less susceptible to awareness. | Some concerns | As there were no protocols, statistical analysis plans, it is difficult to make a judgment. | High risk of bias | Overall high due to the missing outcome data. |
| Mattiasson 2010 | Some concerns | There was no detail about the random sequence generation and the allocation concealment. The baseline characteristics were similar. | Low risk of bias | There was no detail about "single blinded". The follow‐up rate was 77% in GroupI and 79% in GroupII. The reasons for drop out were AE (15%), no‐efficacy (3%), no‐consent (2%), protocol violation (1%), not the trial context. This trial would be acceptable for modified ITT. | Some concerns | The rate of available data was 93% (297/320) in GroupI and 94% (305/323). There was no analysis for the missing outcome. The missing data could affect true value, however, the numbers were small and similar between groups. | Low risk of bias | The outcome was the patient‐reported outcome (PRO) and the assessors were aware of their interventions. FVC is a relatively hard outcome and would not be less susceptible to awareness. | Some concerns | As there were no protocols or statistical analysis, it is difficult to make a judgment. | Some concerns | Due to no detail of the randomization process, non‐blinding of outcome assessors and potential bias in the selection of the reported result. |
Acknowledgements
Cochrane Incontinence supported the authors in the development of this review. The following people conducted the editorial process for the review.
Sign‐off Editor (final editorial decision): Luke Vale, Faculty of Medical Sciences, Newcastle University; Co‐ordinating Editor of Cochrane Incontinence (closed March 2023)
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial comments/guidance to authors, edited the article): Joey Kwong, Cochrane Central Editorial Service
Editorial Assistant (conducted editorial policy checks, collated peer‐reviewer comments, supported editorial team): Leticia Rodrigues, Cochrane Central Editorial Service
Copy Editor (copy‐editing and production): Anne Lawson, Cochrane Central Production Service
Peer‐reviewers (provided comments and recommended an editorial decision): Andika Afriansyah, Persahabatan General Hospital Jakarta – Universitas Indonesia, Indonesia (clinical/content review), Priya Kannan, Department of Rehabilitation Sciences, The Hong Kong Polytechnic University (clinical/content review), Becca Reisch, Pacific University Oregon (clinical/content review), Giulia I Lane, Department of Urology, University of Michigan (clinical/content review), Brian Stafford (consumer review), Theresa HM Moore NIHR ARC West Bristol; Population Health Sciences Institute, University of Bristol (methods review), Anne Littlewood, Cochrane Oral Health (closed March 2023) (search review)
Appendices
Appendix 1. Glossary of terms
Neurogenic detrusor overactivity: detrusor muscle contractions occur during filling cystometry in the setting of a clinically relevant neurologic disorder.
Nocturia: the act of waking up in the night to pass urine. Having woken to pass urine for the first time, each urination must be followed by sleep or the intention to sleep. This should be quantified using a bladder diary.
Overactive bladder (OAB): urinary urgency, usually accompanied by increased daytime frequency or nocturia, or both, with urinary incontinence (OAB‐wet) or without (OAB‐dry), in the absence of urinary tract infection or other detectable disease.
Appendix 2. Cochrane Incontinence Specialised Register search strategy
We used the following search terms to search the Cochrane Incontinence Specialised Register of clinical effects:
((design.cct*) OR (design.rct*)) AND ((topic.urine.incon*) OR (topic.urine.overactive*)) AND ((intvent.psych.bladdrill*) OR (intvent.psych.behav*)) All searches were of the keywords field of EndNote (EndNote 2018).
The date of the most recent search of the Specialised Register for this review was on 6 November 2022. At the time of this updated search the Cochrane Incontinence Specialised Register had been updated to 1 November 2022.
The Cochrane Incontinence Specialised Register search does not include a search of Embase as the Cochrane Centralised Search Service includes Embase in its search for records to be included in CENTRAL. During informal testing for a number of our Cochrane reviews we have found that additional searches of Embase do not locate additional relevant records for our Cochrane reviews.
Appendix 3. GRADE assessment
Study limitations (risk of bias)
Not serious: overall risk of bias is 'low' in more than half of included studies and no 'high' in the included studies.
Serious: overall risk of bias is 'high' in at least one study but less than half of the included studies.
Very serious: overall risk of bias is 'high' in half or more than half of included studies.
Inconsistency
Not serious: no visual and no statistical (I2 greater than 75%) heterogeneity are present.
Serious: visual or statistical (I2 greater than 75%) heterogeneity is present.
Very serious: visual and statistical (I2 greater than 75%) heterogeneity are present.
Indirectness
Not serious: no difference in PICO (patient/population, intervention, comparison, and outcomes)/setting from those of interest.
Serious: one or a slight difference in PICO/setting from those of interest.
Very serious: more than two or serious differences in PICO/setting from those of interest.
Imprecision
Not serious: enough sample size (more than 400 events in dichotomous data or more than 400 participants in continuous data) and clinical importance of 95% CI (e.g. 'the upper 95% CI of RR is smaller than 0.75', 'the lower 95% CI is larger than 1.25', or 'the lower 95% CI is larger than 0.75 and the upper is smaller than 1.25').
Serious: small sample size (less than 400 events in dichotomous data or less than 400 participants in continuous data) or not clinical important 95% CI (the lower 95% CI is below 0.75 while the upper is larger than 1.25.).
Very serious: small sample size (less than 400 events in dichotomous data or less than 400 participants in continuous data) and not clinical important CI ('the upper CI is smaller than 0.75', 'the lower CI is larger than 1.25', or the lower CI is larger than 0.75 and the upper CI is smaller than 1.25').
Publication bias
Not serious: none of the following examples is met.
Serious: one of the following examples is met.
Very serious: more than two of the following examples is met.
There are study protocols in a trial registry, but no subsequent publications.
Meta‐analysis includes small studies consistently showing different effects than the larger studies.
Small studies were funded by people who could gain from the results.
Adapted from Guyatt 2011a; Guyatt 2011b; Schünemann 2013.
Data and analyses
Comparison 1. Bladder training versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Participant‐reported cure or improvement: immediately after treatment | 1 | 18 | Risk Ratio (M‐H, Random, 95% CI) | 17.00 [1.13, 256.56] |
| 1.2 Participant‐reported cure or improvement: long‐term effect (> 2 months after treatment) | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 3.86 [1.99, 7.46] |
| 1.3 Number of incontinence episodes per 24 hours: immediately after treatment | 1 | 14 | Mean Difference (IV, Random, 95% CI) | ‐1.86 [‐3.47, ‐0.25] |
Comparison 2. Bladder training versus anticholinergics.
Comparison 3. Bladder training versus pelvic floor muscle training (PFMT).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Symptom‐related quality of life (QoL): immediately after treatment | 2 | 178 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.19, 0.40] |
| 3.2 Symptom‐related QoL: long‐term effect (> 2 months after treatment) | 1 | 81 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.52, 0.35] |
| 3.3 Adverse events: immediately after treatment | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 3.4 Number of incontinence episodes per 24 hours: immediately after treatment | 1 | 81 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.35, 0.39] |
| 3.5 Number of incontinence episodes per 24 hours: long‐term effect (> 2 months after treatment) | 1 | 81 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐2.46, 2.06] |
| 3.6 Number of micturition episodes per 24 hours: immediately after treatment | 1 | 81 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐1.44, 1.64] |
| 3.7 Number of micturition episodes per 24 hours: long‐term effect (> 2 months after treatment) | 1 | 81 | Mean Difference (IV, Random, 95% CI) | 0.50 [‐1.39, 2.39] |
Comparison 4. Bladder training plus anticholinergics versus anticholinergics alone.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Colombo 1995.
| Study characteristics | |
| Methods |
Study design: 2‐arm, parallel design RCT Dates study conducted: May 1990 to March 1993 |
| Participants |
Number of participants: 81 women with UUI Setting: outpatient, single‐center, national Country: Italy Method of diagnosis: DI diagnosed by pressure flow study Age (mean (range)): Group I 49 (24–65) years; Group II 48 years (31–65) years Sex: female Duration from onset (mean (SD)): not reported Prevalence of target participants (OAB, DI, or UUI): Group I 100% (39/39); Group II 100% (42/42) Prevalence of incontinence: Group I 100% (39/39); Group II 100% (42/42) Any relevant details of health status of participants: not reported Inclusion criteria: symptoms of severe urge incontinence 'socially embarrassing' and urodynamic diagnosis of 'detrusor instability, low‐compliance bladder or sensory bladder' Exclusion criteria: age > 65 years; stable bladder at cystometry; neurologic disease; co‐existing GSI; genital prolapse; previous urogynecologic surgery; prior drug use for urge incontinence; postvoid residual volume > 50 mL; urethral diverticula, fistulas, urinary tract neoplasia; bladder stones; bacterial or interstitial cystitis; previous pelvic radiation therapy. |
| Interventions |
Group I (n = 39): bladder training. Inpatient bladder drill. No details of the method reported or how long the participants stayed in the hospital.
Group II (n = 42): oxybutynin 5 mg (3 times a day) for 6 weeks. If there were 'substantial side effects', dose reduced to 2.5 mg 3 times per day; 2‐weekly follow‐up during treatment. |
| Outcomes |
Primary endpoint: immediately after intervention (6 weeks) Primary outcome: not reported Participant‐reported cure or improvement: yes, measured by a question, "cured", "improved", or "failed", immediately after intervention (6 weeks) and at 6 months Symptom‐related QoL: not reported Adverse events: yes, probably during intervention (6 weeks) Satisfaction: not reported Number of incontinence episodes: not reported Number of urgency episodes: not reported Number of micturition episodes: not reported |
| Notes |
Funding: not reported COI description: unclear |
Fantl 1991.
| Study characteristics | |
| Methods |
Study design: 2‐arm, parallel design RCT Dates study conducted: not reported |
| Participants |
Number of participants: 131 females with incontinence Setting: outpatient, number of centers/facilities not reported, national Country: USA Method of diagnosis: DI was diagnosed by pressure flow study Age (mean): Group I 66 (SD 8) years; Group II 68 (SD 9) years Sex: female Duration from onset (mean): Group I 13 (SD 11) years; Group II 8 (SD 10) years Prevalence of target participants (OAB, DI, or UUI): Group I 12% (7/60); Group II 11% (7/63) Prevalence of incontinence: Group I 100% (60/60); Group II 100% (63/63) Any relevant details of health status of participants: not reported Inclusion criteria: independent community‐dwelling women; ≥ 55 years; ≥ 1 episode of involuntary urine loss a week; mentally intact (Mini‐Mental State Examination score > 23); able to perform toileting independently Exclusion criteria: metabolic decompensation (e.g. uncontrolled diabetes mellitus); lower UTI; urinary obstruction; diverticulum; fistula; reversible cause of UI (e.g. fecal impaction); permanent indwelling catheter; not fulfilling pre‐established objective urodynamic criteria for either DI or GSI (or both) |
| Interventions |
Group I (n = 60): bladder training
Group II (n = 63): no further contact and asked to return in 6 weeks. |
| Outcomes |
Primary endpoint: immediately after intervention (6 weeks) Primary outcome: number of weekly incontinent episodes Participant‐reported cure or improvement: yes, measured by "50% or greater reduction in incontinent episodes" immediately after intervention (6 weeks). Outcomes of DI could not be extracted. Symptom‐related QoL: yes, IIQ‐R immediately after intervention (6 weeks). Outcomes of DI could not be extracted. Adverse events: not reported Satisfaction: not reported Number of incontinence episodes: yes, FVC immediately after intervention (6 weeks) Number of urgency episodes: not reported Number of micturition episodes: not reported |
| Notes |
Funding: National Institute on Aging and National Center on Nursing Research, USA COI description: unclear |
Jarvis 1980.
| Study characteristics | |
| Methods |
Study design: 2‐arm, parallel design RCT Dates study conducted: not reported |
| Participants |
Number of participants: 60 incontinent women diagnosed as DI Setting: inpatient in Group I and at home in Group II, number of centers/facilities not reported, national Country: UK Method of diagnosis: DI was diagnosed by pressure flow study Age (mean (range)): Group I 49.7 (35–74) years; Group II 46.7 (27–79) years Sex: female Duration from onset (mean (range)): Group I 2.3 (0.4–10.0) years; Group II 2.7 (0.5–10.0) years Prevalence of target participants (OAB, DI, or UUI): Group I 100% (30/30); Group II 100% (30/30) Prevalence of incontinence: Group I 100% (30/30); Group II 100% (30/30) Any relevant details of health status of participants: not reported Inclusion criteria: UI due to idiopathic DI diagnosed by pressure‐flow study Exclusion criteria: UTIs; any woman taking a drug suspected of affecting lower urinary tract function; any woman with co‐existing GSI |
| Interventions |
Group I (n = 30): bladder drill
Group II (n = 30): no treatment The women were advised that they should be able to hold urine for 4 hours and be continent, then sent home. |
| Outcomes |
Primary endpoint: post‐treatment (6 months) Primary outcome: not reported Participant‐reported cure or improvement: yes, measured by "continence" or "symptom free" immediately after intervention (6 months) Symptom‐related QoL: not reported Adverse events: not reported Satisfaction: not reported Number of incontinence episodes: not reported Number of urgency episodes: not reported Number of micturition episodes: not reported |
| Notes |
Funding: not reported COI description: unclear |
Jarvis 1981.
| Study characteristics | |
| Methods |
Study design: 2‐arm, parallel design RCT Dates study conducted: not reported |
| Participants |
Number of participants: 50 incontinent women diagnosed as DI Setting: inpatient in Group I and at home in Group II, number of centers/facilities not reported, national Country: UK Method of diagnosis: DI diagnosed by pressure flow study Age (mean): Group I 47 (SD 15.4) years; Group II 46 (SD 12.8) years Sex: female Duration from onset (mean): Group I 4.3 (SD 2.7) years; Group II 5.4 (SD 3.2) years Prevalence of target participants (OAB, DI, or UUI): Group I 100% (25/25); Group II 100% (25/25) Prevalence of incontinence: Group I 100% (25/25); Group II 100% (25/25) Any relevant details of health status of participants: not reported Inclusion criteria: UI with DI diagnosed by UDS Exclusion criteria: co‐existing GSI; neurologic abnormalities; diabetes mellitus; UTIs; woman taking a drug suspected of affecting lower urinary tract function |
| Interventions |
Group I (n = 25): bladder training. Inpatient bladder drill. No details of the method given or how long the women stayed in the hospital.
Group II (n = 25): flavoxate hydrochloride plus imipramine Flavoxate hydrochloride 200 mg 3 times a day plus imipramine 25 mg 3 times a day for 4 weeks |
| Outcomes |
Primary endpoint: immediately after intervention (4 weeks) Primary outcome: not reported Participant‐reported cure or improvement: yes, measured by "continence" or "symptom free" immediately after intervention (4 weeks) Symptom‐related QoL: not reported Adverse events: yes, probably during intervention (4 weeks) Group I (n = 0/25) Group II (n = 14/25): dizziness (8), dry mouth (6), headache (6), nausea (4), drowsiness (2), vomiting (1) Satisfaction: not reported Number of incontinence episodes: not reported Number of urgency episodes: not reported Number of micturition episodes: not reported |
| Notes |
Funding: not reported COI description: unclear |
Kafri 2013.
| Study characteristics | |
| Methods |
Study design: 4‐arm, parallel design RCT Dates study conducted: June 2007 to January 2012 |
| Participants |
Participants: 164 women with UUI symptoms Setting: outpatient, multicenter, national Country: Israel Method of diagnosis: UUI diagnosed by healthcare professionals based on questionnaires Age (mean): Group I 57.2 (SD 8.2) years; Group II 57.1 (SD 9.0) years; Group III 56.4 (SD 7.1) years; Group IV 56.2 (SD 7.8) years Sex: female Duration from onset (mean (SD)): not reported Prevalence of target participants (OAB, DI, or UUI): Group I 100% (41/41); Group II 100% (42/42); Group III 100% (40/40); Group IV 100% (41/41) Prevalence of incontinence: Group I 100% (41/41); Group II 100% (42/42); Group III 100% (40/40); Group IV 100% (41/41) Any relevant details of health status of participants: not reported Inclusion criteria: women aged 45–75 years who experienced ≥ 3 episodes of UUI that were not completely explained by SUI symptoms over the previous 4 weeks; PFM contraction, Oxford Strength Scale ≥ 2; no vaginal prolapse; RU volume by ultrasound < 100 mL Exclusion criteria: not being independent; contraindications to drug therapy; current UTI; neurologic disease; diagnosed with psychiatric or depressive disorder; previous pelvic floor surgery; previous pelvic floor physical therapy |
| Interventions |
Group I (n = 41): bladder training
Group II (n = 42): tolterodine SR 4 mg once a day for 3 months Group III (n = 40): PFMT. The PFMT protocol was based on the National Institute for Health and Clinical Excellence recommendations. At each appointment, the women practiced 3 sets of 8–12 slow maximal contractions sustained for 6–8 seconds in different functional body positions, progressing from lying to standing. The maximum prescribed PFMT duration progressed to 10 seconds of contractions followed by 10 seconds of relaxation. Participants then continued a daily PFMT home‐based program and recorded their home exercise sessions using an exercise log. Participants were also taught to contract these muscles repeatedly to diminish urgency and prevent UI as suggested by Burgio 2008.
Group IV (n = 41): CPFR The CPFR protocol included BT, PFMT, and behavioral advice, including bowel education to avoid constipation, advising modification of fluid intake, daily activity, and ergonomic consultation. Bladder diaries were completed between appointments to record time and volumes of voids per 24 hours
|
| Outcomes |
Primary endpoint: after treatment (12 months) Primary outcome: number of micturitions in a 24‐hour bladder diary; number of UUI episodes by participants' reports per week Participant‐reported cure or improvement: not reported Symptom‐related QoL: yes, I‐QOL (3 months and 12 months) Adverse events: not reported Satisfaction: not reported Number of incontinence episodes: yes, FVC (3 months and 12 months) Number of urgency episodes: yes, FVC (3 months and 12 months) Number of micturition episodes: yes, FVC (3 months and 12 months) |
| Notes |
Funding: Maccabbi Healthcare Services, Israel COI description: none |
Lagro‐Janssen 1992.
| Study characteristics | |
| Methods |
Study design: 2‐arm, parallel design RCT (the control group received treatment after the 3 months' evaluation) Dates study conducted: not reported |
| Participants | 110 incontinent women (Group I 54; Group II 56) Setting: outpatient, multicenter, national Country: Netherlands Method of diagnosis: DI diagnosed by pressure flow study. Of all participants, 18 were diagnosed as DI (GSI 66, DI 18, MUI 20, other 6) Age (mean): Group I 44.6 (SD 10.4) years; Group II 42.3 (SD 10.0) years Sex: female Duration from onset (mean):
Prevalence of target participants (OAB, DI, or UUI): Group I 17% (9/54); Group II 16% (9/56) Prevalence of incontinence: Group I 100% (54/54); Group II 100% (56/56) Any relevant details of health status of participants: not reported Inclusion criteria: incontinence was defined as the involuntary loss of urine twice or more per month Exclusion criteria: surgery for incontinence; neurologic diseases which may cause incontinence; UTIs |
| Interventions |
Group I (n = 9): bladder training
Group II (n = 9): no treatment The control group received bladder training after the 3‐month evaluation. |
| Outcomes |
Primary endpoint: immediately after intervention Primary outcome: not reported Participant‐reported cure or improvement: yes, at 3 months Symptom‐related QoL: not reported Adverse events: not reported Satisfaction: not reported Number of incontinence episodes: yes, at 3 months Number of urgency episodes: not reported Number of micturition episodes: not reported |
| Notes |
Funding: Dutch Prevention Fund. COI description: unclear The outcome of UUI cannot be excluded from the original paper. However, a previous Cochrane Review searched for additional published data about UUI. Based on the review, 8 in treatment group (n = 9) and none in control group (n = 9) were cured or improved at 3 months. |
Lauti 2008.
| Study characteristics | |
| Methods |
Study design: 3‐arm, parallel design RCT Dates study conducted: February 2003 to July 2003 |
| Participants |
Number of participants: 57 UUI females Setting: outpatient, single‐center, national Country: New Zealand Method of diagnosis: no detail (eligibility was confirmed by a urogynecologist) Age (mean): Group I 53.8 (SD 14.8) years; Group II 63.9 years (SD 17.2) years; Group III 47.6 (SD 16.3) years Sex: female Duration from onset, mean: Group I 8.1 (SD 7.1) years; Group II 3.2 (SD 5.5) years; Group III 3.8 (SD 2.9) years Prevalence of target participants (OAB, DI, or UUI): Group I 100% (21/21); Group II 100% (17/17); Group III 100% (19/19) Prevalence of incontinence: Group I 100% (21/21); Group II 100% (17/17); Group III 100% (19/19) Any relevant details of health status of participants: not reported Inclusion criteria: predominant UUI experiencing at least monthly leakage and aged > 18 years Exclusion criteria: predominant SUI; contraindications to anticholinergic drugs; current UTI; neurologic disease; psychiatric disorder; untreated co‐existing pelvic organ prolapse below the hymenal ring; obstructed voiding; functional–reversible cause of incontinence; inability to toilet independently; limited fluency in written or spoken (or both) English or current or recent use of either of the trial interventions |
| Interventions |
Group I (n = 21): bladder training
Group II (n = 16): oxybutynin 2.5 mg once a day to 5 mg 3 times a day for 3 months Group III (n = 19): bladder training plus oxybutynin. Both were administered in the same way as the other 2 arms. |
| Outcomes |
Primary endpoint: 3 months Primary outcome: OAB‐q Participant‐reported cure or improvement: not reported Symptom‐related QoL: yes, OAB‐q at 3 months and 12 months Adverse events: yes, at 3 months and 12 months Group I vs Group II vs Group III Dry mouth: 19% (3/16) vs 93% (14/15) vs 83% (10/12) at 3 months, 21% (3/14) vs 46% (5/11) vs 42% (5/12) at 12 months Headaches: 33% (6/18) vs 31% (4/13) vs 33% (4/12) at 3 months, 43% (6/14) vs 11% (1/9) vs 58% (7/12) at 12 months Dizziness–vertigo: 13% (2/16) vs 17% (2/12) vs 18% (2/11) at 3 months, 29% (4/14) vs 20% (2/10) vs 25% (3/12) at 12 months Constipation: 18% (3/17) vs 20% (3/12) vs 42% (5/12) at 3 months, 21% (3/14) vs 27% (3/11) vs 27% (3/11) at 12 months Fatigue: 28% (5/18) vs 62% (8/13) vs 25% (3/12) at 3 months, 64% (9/14) vs 46% (5/11) vs 64% (7/11) at 12 months Satisfaction: not reported Number of incontinence episodes: yes, FVC at 3 months and 12 months Number of urgency episodes: yes, FVC at 3 months and 12 months Number of micturition episodes: yes, FVC at 3 months and 12 months |
| Notes |
Funding: University of Otago, Otago Research Grant COI description: unclear |
Lentz 1994.
| Study characteristics | |
| Methods |
Study design: 2‐arm, parallel design RCT Dates study conducted: not reported |
| Participants |
Number of participants: 22 women with OAB Setting: outpatient, not reported, national Country: UK Method of diagnosis: not reported Age (mean (range)): total: 42 (19–64) years Sex: female Duration from onset (mean (range)): total: 6.5 (1–10) years Prevalence of target participants (OAB, DI, or UUI): Group I 100% (11/11); Group II 100% (11/11) Prevalence of incontinence: not reported Any relevant details of health status of participants: not reported Inclusion criteria: frequency, urgency, urge (or a combination) incontinence were present; 1‐week urinary diary confirmed > 7 voids/day Exclusion criteria: subtracted cystometry was abnormal; urine culture, cystoscopy and cytology were abnormal; vaginal infection |
| Interventions |
Group I (n = 11): bladder training Inpatient bladder drill. No detail of the method given or how long the participants stayed in the hospital.
Group II (n = 11): PFMT provided with vaginal cones. There were no other details. |
| Outcomes |
Primary endpoint: not reported Primary outcome: not reported Participant‐reported cure or improvement: yes, measured by "cure" or "improvement" at 1 month and 3 months Group I: 80% at 1 month and 100% at 3 months Group II: 78% at 1 month and 60% at 3 months Symptom‐related QoL: not reported Adverse events: not reported Satisfaction: not reported Number of incontinence episodes: not reported Number of urgency episodes: not reported Number of micturition episodes: not reported, only daytime micturition at 3 months |
| Notes |
Funding: not reported COI description: unclear |
Mattiasson 2003.
| Study characteristics | |
| Methods |
Study design: 2‐arm, parallel design RCT Dates study conducted: October 1999 to December 2000 |
| Participants |
Number of participants: 501 adults with OAB Setting: outpatient, multicenter, international Countries: Sweden, Norway, Denmark Method of diagnosis: questionnaires Age, median (range): Group I 62 (19–86) years; Group II 63 (22–86) years Sex:
Duration from onset (> 5 years): Group I 120 (49%); Group II 124 (48%) Prevalence of target participants (OAB, DI, or UUI): Group I 100% (244/244); Group II 100% (257/257) Prevalence of incontinence: Group I 59% (143/244); Group II 64% (165/257) Any relevant details of health status of participants: not reported Inclusion criteria: aged ≥ 18 years; urinary frequency (≥ 8 micturitions/24 hours on average); urgency (a strong and sudden desire to urinate); with or without urge incontinence Exclusion criteria: stress or mixed incontinence; contraindication to antimuscarinic therapy; use of electrostimulation therapy or BT within the previous 3 months; patients with an indwelling catheter or on intermittent catheterization; pregnancy and lactation; and use of anticholinergic agents or concomitant treatment for an OAB (other than estrogen replacement therapy started ≥ 2 months before study commencement) |
| Interventions |
Group I (n = 244): bladder training + tolterodine Inpatient bladder drill. No detail of the method given or how long the participants stayed in the hospital.
Group II (n = 257): tolterodine 2 mg 2 times a day for 24 weeks |
| Outcomes |
Primary endpoint: immediately after intervention (24 weeks) Primary outcome: number of micturition per day Participant‐reported cure or improvement: yes, improvement was defined as a decrease in the 6‐point score of ≥ 1 point at immediately after intervention (24 weeks) Symptom‐related QoL: not reported Adverse events: yes, probably during intervention (24 weeks) Group I (n = 158/244): dry mouth (76), headache (15), constipation (7) Group II (n = 177/257): dry mouth (90), headache (8), constipation (5) Satisfaction: not reported Number of incontinence episodes: yes, FVC (24 weeks) Number of urgency episodes: yes, FVC (24 weeks) Number of micturition episodes: yes, FVC (24 weeks) |
| Notes |
Funding: supported by Pharmacia Corporation COI description: unclear |
Mattiasson 2010.
| Study characteristics | |
| Methods |
Study design: 2‐arm, parallel design RCT Dates study conducted: May 2006 to May 2007 |
| Participants |
Number of participants: 643 people with OAB Setting: outpatient, multicenter, international Countries: 16 countries in Europe and Australia Method of diagnosis: questionnaires Age (mean (range)): Group I 58.6 (18–85) years; Group II 58.2 (20–87) years Sex:
Duration from onset, mean: Group I 49.0 months; Group II 48.7 months Prevalence of target participants (OAB, DI, or UUI): Group I 100% (323/323); Group II 100% (320/320) Prevalence of incontinence: Group I 50.2%; Group II 50.2% Any relevant details of health status of participants: not reported Inclusion criteria: aged ≥ 18 years; ≥ 3 episodes of urgency or urgency incontinence; mean ≥ 8 micturitions/24 hours during the 3‐day voiding diary period Exclusion criteria: BOO; postvoid residual volume > 200 mL; significant stress incontinence or mixed stress/urgency incontinence where stress was the predominant factor; evidence of UTI; bladder stones; chronic interstitial cystitis; neurologic causes of abnormal detrusor activity; previous irradiation; malignant disease (previous or current); child‐bearing potential; pregnancy; lactation; conditions contraindicating use of anticholinergic medication; myasthenia gravis or diabetic neuropathy; non‐drug OAB treatments (including electrostimulation therapy and pelvic floor exercise in the 4 weeks before study start); cognitive bladder training in the last 6 months or who intended to start bladder training (other than the study regimen) during the study; use of drugs intended to treat UI; concomitant use of a strong CYP3A4 inhibitor |
| Interventions |
Group I (n = 320): bladder training + solifenacin
Group II (n = 323): solifenacin 5 mg once a day for 8 weeks, solifenacin 5 mg or 10 mg once a day from 8th week to 16th week |
| Outcomes |
Primary endpoint: 8 weeks Primary outcome: change from baseline in the mean number of micturitions per 24 hours Participant‐reported cure or improvement: yes, I‐QOL at 8 weeks and 16 weeks Symptom‐related QoL: not reported Adverse events: yes, 16 weeks Satisfaction: yes, VAS at 8 weeks and 16 weeks Number of incontinence episodes: yes, FVC at 8 weeks and 16 weeks Number of urgency episodes: yes, FVC at 8 weeks and 16 weeks Number of micturition episodes: yes, FVC at 8 weeks and 16 weeks |
| Notes |
Funding: research grant from Astellas Pharma Europe Ltd COI description: authors declared COIs for research, consultancy, advisory work, or a combination of these. |
McCreanor 1998.
| Study characteristics | |
| Methods |
Study design: 2‐arm, parallel design RCT Dates study conducted: unclear |
| Participants |
Number of participants: 31 women with UUI Setting: outpatient, single‐center Country: UK Method of diagnosis: questionnaires Exclusion criteria: not reported Age (mean (range)): not reported Sex: female Duration from onset (mean): not reported Prevalence of target participants (OAB, DI, or UUI): Group I 100% (17/17); Group II 100% (17/17) Prevalence of incontinence: not reported Any relevant details of health status of participants: not reported Inclusion criteria: women aged 30–70 years, urge with or without incontinence, normal cystoscopy, sterile MSU, static UDS, RU < 100 mL Exclusion criteria: not reported |
| Interventions |
Group I (n = 17): bladder training
Group II (n = 14): oxybutynin 2.5–5 mg 3 times a day for 16 weeks |
| Outcomes |
Primary endpoint: 8 weeks and 16 weeks Primary outcome: unclear Participant‐reported cure or improvement: not reported Symptom‐related QoL: not reported Adverse events: not reported Satisfaction: not reported Number of incontinence episodes: using an original score Number of urgency episodes: not reported Number of micturition episodes: not reported |
| Notes |
Funding: Scottish Home and Health Department Disability and Continuing Health Care Research Grant COI description: unclear |
Milani 1987.
| Study characteristics | |
| Methods |
Study design: 2‐arm, parallel design RCT Dates study conducted: May 1983 to December 1985 |
| Participants |
Number of participants: 81 women with idiopathic urge syndrome Setting: outpatient, multicenter, national Country: Italy Method of diagnosis: not reported Age (mean): Group I (SD 15.4) years; Group II 46 (SD 12.8) years Sex: female Duration from onset (mean): Group I 4.3 (SD 2.7) years; Group II 5.4 (SD 3.2) years Prevalence of target participants (OAB, DI, or UUI): Group I 100% (39/39); Group II 100% (42/42) Prevalence of incontinence: not reported Any relevant details of health status of participants: not reported Inclusion criteria: women with idiopathic urge syndrome Exclusion criteria: aged > 65 years; previous pelvic radiotherapy; pelvic masses or malignancy; urinary tract or kidney pathology; nervous system diseases; 2nd or 3rd degree genital prolapse |
| Interventions |
Group I (n = 39): bladder retraining
Group II (n = 42): oxybutynin 15 mg 3 times a day for 4 weeks |
| Outcomes |
Primary endpoint: not reported Primary outcome: not reported Participant‐reported cure or improvement: yes, at the end of therapy and 3 months' follow‐up Symptom‐related QoL: not reported Adverse events: yes, probably during intervention (4 weeks) Group I (n = 0/39) Group II (n = 14/42): mainly dry mouth Satisfaction: not reported Number of incontinence episodes: not reported Number of urgency episodes: not reported Number of micturition episodes: not reported Note: the time point of collecting outcome might be different between 2 groups (the end of bladder retraining was 12 weeks and that of oxybutynin was 4 weeks). |
| Notes |
Funding: not reported COI description: unclear |
Rizvi 2018.
| Study characteristics | |
| Methods |
Study design: 2‐arm, parallel design RCT Dates study conducted: not reported |
| Participants |
Number of participants: 150 women with OAB Setting: outpatient, single‐center, national Country: Pakistan Method of diagnosis: questionnaires (frequency, urgency, and nocturia with or without UUI for ≥ 6 months) Age (mean): Group I 55.7 (SD 14.7); Group II 49.1 (SD 14.9) years; Group III 49.3 (SD 14.7) years Sex: female Duration from onset (mean (SD)): not reported Prevalence of target participants (OAB, DI, or UUI): Group I 100% (50/50); Group II 100% (50/50); Group III 100% (50/50) Prevalence of incontinence: Group I 74.0% (37/50); Group II 36.0% (18/50); Group III 72.0% (36/50) Any relevant details of health status of participants: not reported Inclusion criteria: aged 25–65 years with symptoms of OAB Exclusion criteria: anticholinergic or tricyclic antidepressants use; treated with pelvic floor exercises or BT; pregnancy; UTI; under current urologic care; urinary obstruction with persistent indwelling catheter; uncontrolled diabetes mellitus; neurologic disorders; history of pelvic surgery; prolapse greater than POP‐Q stage 2. |
| Interventions |
Group I (n = 50): bladder training
Group II (n = 50): PFMT They were instructed to hold submaximal to maximal PFM contractions for 6 seconds, 5 times and to perform 10 fast contractions per session. All participants were instructed to practice this regimen at home ≥ 3 times daily in the lying, standing, or sitting position and were assessed for any improvement during subsequent visits. Group III (n = 50): PFMT + biofeedback Each participant was instructed to contract or relax her PFMs following the audiovisual signals. The PERFECT scheme was used to assess muscle strength both before and after sessions. |
| Outcomes |
Primary endpoint: immediately after intervention (12 weeks) Primary outcome: QoL using scores of UDI‐SF6/IIQ‐SF7 and urgency scores for these treatment modalities Participant‐reported cure or improvement: not reported Symptom‐related QoL: yes, UDI‐SF6/IIQ‐SF7 (12 weeks) Adverse events: yes (12 weeks) Group I (n = 0/47) Group II (n = 0/50) Group III (n = 1/50): unspecified pelvic pain (1) Satisfaction: not reported Number of incontinence episodes: yes, but only figure, no available data (12 weeks) Number of urgency episodes: yes, but only figure, no available data (12 weeks) Number of micturition episodes: yes, but only figure, no available data (12 weeks) |
| Notes |
Funding: the first author received financial support from Women and Health Alliance USA (WAHA), International. This extramural grant was approved by clinical trial unit of AKU (P‐15928). COI: none |
Song 2006.
| Study characteristics | |
| Methods |
Study design: 3‐arm, parallel design RCT Dates study conducted: May 2001 to April 2002 |
| Participants |
Number of participants: 139 OAB females Setting: outpatient, number of centers/facilities not reported, national Country: Korea Method of diagnosis: questionnaires Age (mean): Group I 45.7 (SD 12.7) years; Group II 48.4 (SD 9.4) years; Group III 45.4 (SD 9.5) years Sex: female Duration from onset (mean): Group I 6.4 (SD 6.8) years; Group II 4.5 (SD 5.2) years; Group III 4.1 (SD 4.0) years Prevalence of target participants (OAB, DI, or UUI): Group I 100% (46/46); Group II 100% (47/47); Group III 100% (47/47) Prevalence of incontinence: not reported Any relevant details of health status of participants: not reported Inclusion criteria: aged > 18 years; > 8 urination frequency per day; urge symptoms with or without incontinence; symptom duration > 3 months; no prior history of treatment for OAB Exclusion criteria: UTI; SUI; BOO, interstitial cystitis, glaucoma, or megacolon; maximal urine flow rate < 10 mL/second; postvoid RU amount that was more than 30% of the total amount voided on uroflowmetry |
| Interventions |
Group I (n = 46): bladder training
Group II (n = 47): tolterodine 2 mg (twice a day) for 12 weeks Group III (n = 46): bladder training + tolterodine for 12 weeks. Both administered in the same way as the other 2 arms |
| Outcomes |
Primary endpoint: immediately after intervention (12 weeks) Primary outcome: not reported Participant‐reported cure or improvement: yes, measured by "satisfaction score". A change of ≥ 2 points was considered symptom improvement at 12 weeks Symptom‐related QOL: not reported Adverse events: yes Group I (n = 0/26) Group II (n = 13/32): dry mouth (7), constipation (2), headache (1), hesitancy (3) Group III (n = 12/31): dry mouth (9), constipation (2), hesitancy (2) Satisfaction: not reported Number of incontinence episodes: not reported Number of urgency episodes: yes, FVC (12 weeks) Number of micturition episodes: yes, FVC (12 weeks) |
| Notes |
Funding: not reported COI description: unclear |
Zhang 2012.
| Study characteristics | |
| Methods |
Study design: 4‐arm, parallel design RCT Dates study conducted: March 2011 to February 2012 |
| Participants |
Number of participants: 165 women with OAB Setting: outpatient, number of centers/facilities not reported, national Country: China Method of diagnosis: questionnaires Age (mean (SD)): not reported Sex: female Duration from onset, mean (SD): not reported Prevalence of target participants (OAB, DI, or UUI): Group I 100% (47/47); Group II 100% (41/41); Group III 100% (41/41); Group IV 100% (34/34) Prevalence of incontinence: not reported Any relevant details of health status of participants: postmenopausal Inclusion criteria: presenting with symptoms of urgency and frequency, with or without urge incontinence Exclusion criteria: not reported |
| Interventions |
Group I (n = 47): bladder training + anticholinergics. Tolterodine 4 mg once a day for 12 weeks
Group II (n = 41): anticholinergics. Tolterodine 4 mg once a day for 12 weeks Group III (n = 43): tolterodine 4 mg for 12 weeks + low‐dose vaginal estrogen treatment (vaginal conjugated equine estrogen 0.625 mg locally applied twice a week) Group IV (n = 34): Tolterodine 4 mg for 12 weeks + low‐dose vaginal estrogen treatment (vaginal conjugated equine estrogen 0.625 mg locally applied twice a week) + bladder training (same as Group I) |
| Outcomes |
Primary endpoint: not reported Primary outcome: 12 weeks Participant‐reported cure or improvement: yes, measured by the median percentage increases of PPBC immediately after intervention (12 weeks) 66% (Group I) vs 53% (Group II) vs 50% (Group III) vs 82% (Group IV) Symptom‐related QoL: yes, PPBC at 12 weeks Adverse events: not reported Satisfaction: not reported Number of incontinence episodes: yes, median percentage reductions of pads usage 71% (Group I) vs 56% (Group II) vs 60% (Group III) vs 78% (Group IV) Number of urgency episodes: yes, median percentage reductions of urgency episodes 71% (Group I) vs 58% (Group II) vs 60% (Group III) vs 87% (Group IV) Number of micturition episodes: not reported |
| Notes |
Funding: The Pujiang Talent Plan of the Shanghai Municipal Government (PJ14027) COI description: unclear |
BOO: bladder outlet obstruction; COI: conflict of interest; CPFR: combined pelvic floor rehabilitation; DI: detrusor instability; FVC: frequency volume chart; GSI: genuine stress incontinence; IIQ‐SF7: Incontinence Impact Questionnaire, Short Form; IIQ‐R: Incontinence Impact Questionnaire – Revised; MSU: midstream urine; MUI: mixed urinary incontinence; n: number of participants; I‐QOL: Urinary Incontinence Quality of Life Scale; QoL: quality of life; PERFECT: Power (or Pressure), Endurance, Repetition, Fast contraction, and Every Contraction Timed; PFM: pelvic floor muscle; PFMT: pelvic floor muscle training; PPBC: participant perception of bladder condition; POP‐Q: Pelvic Organ Prolapse Quantification; OAB: overactive bladder; OAB‐q: overactive bladder questionnaire; RCT: randomized controlled trial; RU: residual urine; SD: standard deviation; SR: slow release; SUI: stress urinary incontinence; UDI: Urogenital Distress Inventory; UDI‐SF6: Urinary Distress Inventory, Short Form; UDS: urodynamic study; UI: urinary incontinence; UTI: urinary tract infection; UUI: urge urinary incontinence; VAS: Visual Analog Scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12606000511538 | Poststroke/neurogenic OAB |
| Andrade 2015 | Intervention not relevant |
| Aslan 2008 | Intervention not relevant |
| Assassa 2010 | Intervention not relevant |
| Azizi 2020 | Intervention not relevant |
| Barber 2002 | Not an RCT |
| Barber 2009 | Not OAB or UUI |
| Bell‐Kotwall 2003 | Intervention not relevant |
| Berghmans 2002 | Intervention not relevant |
| Borrie 2002 | Intervention not relevant |
| Breyer 2018 | Intervention not relevant |
| Brown 2009 | Not OAB or UUI |
| Brubaker 2009 | Intervention not relevant |
| Burgio 2000 | Intervention not relevant |
| Burgio 2003 | Review paper |
| Burgio 2006 | Review paper |
| Burgio 2008 | Intervention not relevant |
| Burgio 2010 | Intervention not relevant |
| Burgio 2011 | Intervention not relevant |
| Burgio 2020 | Intervention not relevant |
| Burgio 2002 | Intervention not relevant |
| Castleden 1986 | Intervention not relevant |
| Castleden 1987 | Intervention not relevant |
| Chanfreau‐Rona 1984 | Not an RCT |
| Chanfreau‐Rona 1986 | Not an RCT |
| Chesworth 2015 | Poststroke/neurogenic OAB |
| Cho 2016 | Intervention not relevant |
| Chu 2019 | Intervention not relevant |
| Colling 1992 | Quasi‐RCT |
| Colling 2003 | Quasi‐RCT |
| Davila 1998 | Intervention not relevant |
| Diokno 2004 | Intervention not relevant. Not OAB or UUI |
| Diokno 2010 | Intervention not relevant |
| Diokno 2018 | Intervention not relevant |
| Dougherty 2002 | Intervention not relevant |
| Dowd 2000 | Intervention not relevant |
| Dyer 2011 | Intervention not relevant |
| Elser 1995 | Not OAB or UUI |
| Engberg 2002 | Intervention not relevant |
| Fonda 1994 | Intervention not relevant |
| Frost 2019 | Not OAB or UUI |
| Gezginci 2018 | Intervention not relevant |
| Glazener 2010 | Postsurgery |
| Golmakani 2014 | Not OAB or UUI |
| Gonzalez 2015 | Intervention not relevant |
| Goode 2002 | Intervention not relevant |
| Goode 2003 | Intervention not relevant |
| Goode 2004 | Not an RCT |
| Griebling 2018 | Intervention not relevant |
| Ha 2008 | Intervention not relevant |
| Haywood 2008 | Not an RCT |
| Henalla 1991 | Intervention not relevant |
| Herschorn 2004 | Intervention not relevant |
| Hill 2007 | Intervention not relevant |
| Hines 2007 | Intervention not relevant |
| Holtedahl 2000 | Intervention not relevant |
| Hu 1989 | Not OAB or UUI |
| Huang 2012 | Intervention not relevant |
| Hui 2006 | Intervention not relevant |
| ISRCTN62679410 | Intervention not relevant |
| ISRCTN62722772 | Intervention not relevant |
| Janssen 2001 | Intervention not relevant |
| Jirovec 2001 | Not OAB or UUI |
| Johnson 2005 | Intervention not relevant |
| Kafri 2008 | Intervention not relevant |
| Kangchai 2002 | Quasi‐RCT |
| Kaya 2011 | Intervention not relevant |
| Kaya 2015 | Intervention not relevant |
| Kilinc 2019 | Intervention not relevant |
| Kim 2001 | Intervention not relevant |
| Kim 2008 | Intervention not relevant |
| Kincade 2007 | Intervention not relevant |
| Klarskov 1984 | Intervention not relevant |
| Kobayashi 2009 | Intervention not relevant |
| Komesu 2011 | Intervention not relevant |
| Komesu 2017 | Intervention not relevant |
| Komesu 2020 | Intervention not relevant |
| Kraus 2007 | Intervention not relevant |
| Kumari 2008 | Intervention not relevant |
| Lai 2017 | Intervention not relevant |
| Lee 2005 | Intervention not relevant |
| Lee 2018 | Intervention not relevant |
| Leong 2014 | Intervention not relevant |
| Linn 1995 | Not OAB or UUI |
| Locher 2002 | Intervention not relevant |
| Loohuis 2019 | Intervention not relevant |
| Macaulay 1988 | No separate data available for DI |
| Madersbacher 2004 | Intervention not relevant |
| Margolis 2009 | Intervention not relevant |
| McAdam 2013 | Poststroke/neurogenic OAB |
| McDowell 1996 | Not an RCT |
| McFall 2000 | Intervention not relevant |
| Messer 2006 | Aim was to prevent UI |
| NCT00821184 | Intervention not relevant |
| NCT01032265 | Intervention not relevant |
| NCT01187082 | Intervention not relevant |
| NCT02107820 | Intervention not relevant |
| NCT02202031 | Intervention not relevant |
| NCT02206958 | Intervention not relevant |
| NCT02505607 | Intervention not relevant |
| NCT02511314 | Intervention not relevant |
| NCT03176901 | Intervention not relevant |
| NCT03797365 | Intervention not relevant |
| NCT04068025 | Intervention not relevant |
| NCT04237753 | Intervention not relevant |
| Nikoletti 2004 | Intervention not relevant |
| NL2075 | Intervention not relevant |
| O'Brien 1991 | Intervention not relevant |
| O'Sullivan 2000 | Intervention not relevant |
| Oh‐Oka 2007 | Intervention not relevant |
| Ouslander 1988 | Intervention not relevant |
| Ouslander 1995 | Intervention not relevant |
| Pahwa 2016 | Intervention not relevant |
| Ramsay 1996 | Intervention not relevant |
| RBR‐64wczh | Intervention not relevant |
| Sackley 2008 | Intervention not relevant |
| Sale 1994 | Not an RCT |
| Saltmarche 1991 | Intervention not relevant |
| Sampselle 2017 | Intervention not relevant |
| Schnelle 1983 | Not OAB or UUI |
| Schnelle 1990 | Not OAB or UUI |
| Schnelle 1995 | Not OAB or UUI |
| Schnelle 2003 | Intervention not relevant |
| Seers 2018 | Not OAB or UUI |
| Sereika 2003 | Not OAB or UUI |
| Sherburn 2011 | Not OAB or UUI |
| Shirreff 2020 | Intervention not relevant |
| Sran 2016 | Intervention not relevant |
| Subak 2002 | Intervention not relevant |
| Sung 2015 | Intervention not relevant |
| Surdy 1992 | Intervention not relevant |
| Suzuki 2019 | Intervention not relevant |
| Szonyi 1995 | Intervention not relevant |
| Tak 2012 | Intervention not relevant. Not OAB or UUI |
| Tobin 1986 | Intervention not relevant |
| Tomlinson 1999 | Not an RCT |
| Voorham 2017 | Intervention not relevant |
| Wadensten 2019 | Intervention not relevant |
| Wagg 2007 | Intervention not relevant |
| Williams 2005 | Intervention not relevant |
| Wiseman 1991 | Intervention not relevant |
| Wyman 1998 | No separate data available for DI |
| Yoon 2003 | Not OAB or UUI |
| Yu 1991 | Intervention not relevant |
DI: detrusor instability; OAB: overactive bladder; RCT: randomized controlled trial; UI: urinary incontinence; UUI: urge urinary incontinence.
Characteristics of studies awaiting classification [ordered by study ID]
ChiCTR‐TRC‐14004921.
| Methods |
Study design: 2‐arm, parallel design RCT Dates study conducted: 2 June 2014 to 31 December 2015 |
| Participants |
Participants: 94 participants with OAB Setting: not reported Country: China Method of diagnosis: not reported Age (mean (SD)): not reported Sex: male or female Duration from onset (mean (SD)): not reported Prevalence of target participants (OAB, DI, or UUI): not reported Prevalence of incontinence: not reported Any relevant details of health status of participants: not reported Inclusion criteria: adults aged > 18 years; symptoms of OAB (including urgency, urinary frequency, nocturnal enuresis, with or without urge incontinence); within the last week, OABSS > 3 points and the Urgency Score > 2 points; capable of understanding research questions; written informed consent has been obtained. Exclusion criteria: current urinary infection; associated with neurologic disease such as Parkinson disease and spinal cord injury; pelvic organ prolapse or pelvic surgery; prostate cancer and bladder cancer; end‐stage renal disease; pregnancy; had received similar behavioral intervention in the past year. |
| Interventions | Cognitive behavioral intervention. No other details reported |
| Outcomes |
Primary endpoint: not reported Primary outcome: OABSS, IUSS Participant‐reported cure or improvement: not stated Symptom‐related QoL: yes, PPBC Adverse events: not stated Satisfaction: not stated Number of incontinence episodes: yes, FVC Number of urgency episodes: yes, FVC Number of micturition episodes: yes, FVC |
| Notes |
Funding: not reported COI description: unclear There is only registration. |
Lee 1995.
| Methods |
Study design: 2‐arm, parallel design RCT Dates study conducted: not reported |
| Participants |
Participants: 101 women with UI Setting: not reported Country: Canada Method of diagnosis: not reported Age (mean): 67.4 (SD 15.0) years Sex: female Duration from onset (mean (SD)): not reported Prevalence of target participants (OAB, DI, or UUI): 37.6% Prevalence of incontinence: 100% Any relevant details of health status of participants: not reported Inclusion criteria: ≥ 2 episodes of urinary incontinence per week; no other acute conditions requiring medical treatment (e.g. urinary tract infection, urinary obstruction, residual urine > 150 mL, medication adverse effects); neither language nor cognitive barriers to the treatment intervention Exclusion criteria: not reported |
| Interventions |
Group I: behavioral techniques to self‐manage one's incontinence with monthly follow‐up Group II: no treatment |
| Outcomes |
Primary endpoint: after treatment (3 months) Primary outcome: evaluation of subjective cure Participant‐reported cure or improvement: yes Symptom‐related QoL: yes, IIQ Adverse events: not stated Satisfaction: not stated Number of incontinence episodes: yes, FVC Number of urgency episodes: not stated Number of micturition episodes: not stated |
| Notes |
Funding: not reported COI description: unclear No detail about intervention (behavioral techniques) |
NCT03331081.
| Methods |
Study design: 3‐arm, parallel design RCT Dates study conducted: November 2017 to December 2018 |
| Participants |
Participants: 45 women with OAB Setting: not reported Country: Brazil Method of diagnosis: not reported Age (mean (SD)): not reported Sex: female Duration from onset (mean (SD)): not reported Prevalence of target participants (OAB, DI, or UUI): not reported Prevalence of incontinence: not reported Any relevant details of health status of participants: not reported Inclusion criteria: women aged 18–80 years with IUU or IUM (or both) with a predominance of urinary urgency, capable of contracting MAPs adequately, and who agree to participate in the study, signing the informed consent form. Exclusion criteria: women with a diagnosis of glaucoma, myasthenia gravis, urinary tract obstruction, neurologic and chronic‐degenerative diseases, people with decompensated diabetes and people with complete denervation of the pelvic floor, pregnancy, abnormal genital bleeding, impairment of cognition, inability to fill in the diary voiding, genital dystopias beyond the vaginal introitus and urethral sphincter defect. Used or had used anticholinergics, tricyclic antidepressants, or local hormone therapy within the 6 months prior to the study. |
| Interventions |
Group I: bladder training Group II: pelvic floor muscle training Group III: bladder training + pelvic floor muscle training |
| Outcomes |
Primary endpoint: after treatment (3 months) Primary outcome: evaluation of subjective cure Participant‐reported cure or improvement: yes Symptom‐related QoL: yes, I‐QOL Adverse events: not stated Satisfaction: not stated Number of incontinence episodes: yes, FVC Number of urgency episodes: yes, FVC Number of micturition episodes: yes, FVC |
| Notes |
Funding: not reported COI description: unclear There is only a registration. |
Rajeev 2017.
| Methods |
Study design: not reported Dates study conducted: from May 2017 |
| Participants |
Participants: 196 women with UI Setting: not reported Country: India Method of diagnosis: not reported Age (mean (SD)): not reported Sex: female Duration from onset (mean (SD)): not reported Prevalence of target participants (OAB, DI, or UUI): not reported Prevalence of incontinence: not reported Any relevant details of health status of participants: not reported Inclusion criteria: women aged 40–60 years diagnosed with urinary incontinence; who are likely to attend follow‐up; able to practice the intervention as per the guideline Exclusion criteria: undergone surgical intervention for urinary incontinence; aged > 60 years; not attending follow‐up clinic; with severe medical illness and on treatment |
| Interventions | Pelvic floor exercise, bladder retraining program, combined method. No details about intervention groups. |
| Outcomes |
Primary endpoint: 2 years Primary outcome: reduction in symptoms of urinary incontinence Participant‐reported cure or improvement: not stated Symptom‐related QOL: not stated Adverse events: not stated Satisfaction: not stated Number of incontinence: not stated Number of urgency: not stated Number of micturition: not stated |
| Notes |
Funding: not reported COI description: unclear |
Teo 2008.
| Methods |
Study design: 3‐arm, parallel design RCT Dates study conducted: November 2017 to December 2018 |
| Participants |
Number of participants: 92 people with OAB Setting: not stated Country: Singapore Method of diagnosis: not stated Age (mean (range)): not stated Sex: not stated Duration from onset (mean (SD)): not stated Prevalence of target participants (OAB, DI, or UUI): 100% (92/92) Prevalence of incontinence: not reported Any relevant details of health status of participants: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions |
Group I: multicomponent behavioral training Group II: Detrusitol Group III: combination of bladder training and Detrusitol |
| Outcomes |
Primary endpoint: 12 week Primary outcome: not stated Participant‐reported cure or improvement: not stated Symptom‐related QoL: yes, BFLUTS, KHQ Adverse events: not stated Satisfaction: not stated Number of incontinence episodes: yes, FVC Number of urgency episodes: yes, FVC Number of micturition episodes: yes, FVC |
| Notes |
Funding: no funding COI description: unclear No details about intervention. |
BFLUTS: Bristol Female Lower Urinary Tract Symptoms; COI: conflict of interest; DI: detrusor instability; FVC: frequency volume chart; I‐QOL: Urinary Incontinence Quality of Life Scale; IUM: incontinencia urinaria mixta; IUSS: Indevus Urgency Severity Scale; KHQ: King's Health Questionnaire; MAPS: men after prostate surgery; OAB: overactive bladder; OABSS: Overactive Bladder Symptom Score; PPBC: participant perception of bladder condition; QoL: quality of life; SD: standard deviation; UUI: urge urinary incontinence.
Differences between protocol and review
Criteria for considering studies for this review: we included participants with urge urinary incontinence (UUI) and detrusor instability (DI) as well as OAB because the three are not clearly distinguishable disease concepts and overlap with each other.
Data collection and analysis: to calculate the daily mean of frequency‐volume chart frequencies, we divided the weekly mean and standard deviation (SD) by seven. We also imputed missing SDs in Song 2006 from a published Cochrane Review (Rai 2012); we conducted a post hoc sensitivity analysis to explore the impact of imputed SDs.
GRADE: although we did not prespecify in the protocol, we set the standard of evaluating each domain of the GRADE system as outlined in Appendix 3. The GRADE system is the most widely adopted tool for grading the certainty of evidence and for making recommendations (Guyatt 2011a).
Brief economics commentary: due to time constraints, we omitted our plans to conduct a brief economics commentary in this area. Should capacity allow, we will include this in future versions of this review.
Contributions of authors
SF completed title and abstract screening, full‐text review, risk of bias assessments, data extraction and GRADE, analyzed the data and wrote the manuscript.
TY completed title and abstract screening, full‐text review, risk of bias assessments, and data extraction.
YL completed title and abstract screening, full‐text review, risk of bias assessments, and data extraction.
AS reviewed and provided feedback on the draft version of the manuscript.
SA gave specialist clinical insight into the literature and population with OAB.
NW reviewed and provided feedback on the draft version of the manuscript.
Sources of support
Internal sources
-
No, Other
This project was not internally supported.
External sources
-
National Institute for Health Research (NIHR), UK
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Incontinence. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, National Health Service, or the Department of Health and Social Care.
Declarations of interest
SF: KDDI Foundation (grant/contract); Ministry of Education, Culture, Sports, Science and Technology (grant/contract).
TY: Japan Society for the Promotion of Science (grant/contract); National Cancer Center (grant/contract); The Japanese Society of Urolithiasis Research (grant/contract); works as a medical doctor (urologist) in Abiko Toho Hospital, Japan.
YL: Japan Society for the Promotion of Science (grant/contract).
AS: none.
SA: Astellas Pharma (honoraria, grant/contract); AstraZeneca (honoraria, grant/contract); Janssen Pharmaceuticals (honoraria); Tosoh (grant/contract); Urologic Surgeon at Kyoto University Hospital.
NW: no relevant interests; works as a health professional at Soseikai General Hospital.
New
References
References to studies included in this review
Colombo 1995 {published data only}
- Colombo M, Zanetta G, Scalambrino S, Milani R. Oxybutynin and bladder training in the management of female urinary urge incontinence: a randomized study. International Urogynecology Journal 1995;6(2):63-7. [sr-incont6056] [Google Scholar]
Fantl 1991 {published data only}
- Fantl JA, Wyman JF, Harkins SW, Taylor JR, et al [sic]. Bladder training in women with urinary incontinence (Abstract). Neurourology and Urodynamics 1988;7(3):276-7. [sr-incont3821] [Google Scholar]
- Fantl JA, Wyman JF, McClish DK, Harkins SW, Elswick RK, Taylor JR, et al. Efficacy of bladder training in older women with urinary incontinence. Journal of the American Medical Association 1991;265(5):609-13. [sr-incont281] [PubMed] [Google Scholar]
- McClish DK, Fantl JA, Wyman JF, Pisani G, Bump RC. Bladder training in older women with urinary incontinence: relationship between outcome and changes in urodynamic observations. Obstetrics and Gynecology 1991;77(2):281-6. [sr-incont279] [DOI] [PubMed] [Google Scholar]
- Wyman JF, Fantl JA, McClish DK, Harkins SW, Uebersax JS, Ory MG. Quality of life following bladder training in older women with urinary incontinence. International Urogynecology Journal and Pelvic Floor Dysfunction 1997;8(4):223-9. [sr-incont5459] [DOI] [PubMed] [Google Scholar]
- Wyman JF, McClish DK, Ory MG, Fantl JA. Changes in quality of life following bladder training in older women with urinary incontinence (Abstract). Neurourology and Urodynamics 1992;11(4):426-7. [sr-incont4637] [Google Scholar]
Jarvis 1980 {published data only}
- Jarvis GJ, Millar DR. Controlled trial of bladder drill for detrusor instability. British Medical Journal 1980;281(6251):1322-3. [sr-incont778] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis GJ, Millar DR. The treatment of incontinence due to detrusor instability by bladder drill. Progress in Clinical and Biological Research 1981;78:341-3. [sr-incont731] [PubMed] [Google Scholar]
Jarvis 1981 {published data only}
- Jarvis GJ. A controlled trial of bladder drill and drug therapy in the management of detrusor instability. British Journal of Urology 1981;53(6):565-6. [sr-incont741] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Jarvis GJ. The unstable bladder – a psychosomatic disease? (Abstract). In: International Continence Society (ICS), 11th Annual Meeting; 1981 Sept 3-5; Lund, Sweden. 1981:45-6. [sr-incont10972]
Kafri 2013 {published data only}
- Kafri R, Deutscher D, Shames J, Golombp J, Melzer I. Randomized trial of a comparison of rehabilitation or drug therapy for urgency urinary incontinence: 1-year follow-up [Hebrew] [reprint of Int Urogynaecol J 2013;24(7):1181-9]. Journal of the Israeli Physical Therapy Society (JIPTS) 2013;15(2):30-40. [NCT00498888] [sr-incont61844] [DOI] [PubMed] [Google Scholar]
- Kafri R, Deutscher D, Shames J, Golombp J, Melzer I. Randomized trial of a comparison of rehabilitation or drug therapy for urgency urinary incontinence: 1-year follow-up. International Urogynecology Journal 2013;24(7):1181-9. [NCT00498888] [sr-incont48023] [DOI] [PubMed] [Google Scholar]
- Kafri R, Deutscher D, Shames J, Greenberg D, Kodesh A, Golomb J, et al. A randomized trial comparing rehabilitation and drug therapy for urgency urinary incontinence: 1 year follow up (Abstract number 404). Neurourology and Urodynamics 2014;33(6):869-70. [NCT00498888] [sr-incont64411] [Google Scholar]
- Kafri R, Greenberg D, Shames J, Novack L, Melzer I. Cost and cost-effectiveness of treating urgency stress incontinence-results from a randomized controlled trial (Abstract number PUK16). Value in Health 2013;16(7):A632-3. [NCT00498888] [sr-incont61878] [Google Scholar]
- Kafri R, Kodesh A, Shames J, Golomb J, Melzer I. Depressive symptoms and treatment of women with urgency urinary incontinence. International Urogynecology Journal 2013;24(11):1953-9. [NCT00498888] [sr-incont49451] [DOI] [PubMed] [Google Scholar]
- NCT00498888. The long term outcomes of rehabilitation and drug treatment for in urgency urinary incontinence (UUI) [Is there a difference between rehabilitation treatment, pelvic floor muscle training, bladder training and anticholinergic drug treatment in UUI in the long term: a study of impairment, quality of life, and cost effectiveness]. clinicaltrials.gov/show/NCT00498888 (first received 11 July 2007). [NCT00498888] [sr-incont47848]
Lagro‐Janssen 1992 {published data only}
- Lagro-Janssen AL, Debruyne FM, Smits AJ, Weel C. The effects of treatment of urinary incontinence in general practice. Family Practice 1992;9(3):284-9. [sr-incont36] [DOI] [PubMed] [Google Scholar]
- Lagro-Janssen T, Weel C. Long-term effect of treatment of female incontinence in general practice. British Journal of General Practice 1998;48(436):1735-8. [sr-incont9902] [PMC free article] [PubMed] [Google Scholar]
- Lagro-Janssen TL, Debruyne FM, Smits AJ, Weel C. Controlled trial of pelvic floor exercises in the treatment of urinary stress incontinence in general practice. British Journal of General Practice 1991;41(352):445-9. [sr-incont232] [PMC free article] [PubMed] [Google Scholar]
Lauti 2008 {published data only}ISRCTN66713401
- Herbison GP, Lauti M, Hay-Smith J, Wilson D. Three month results from the URGENT pilot study: a randomised controlled trial comparing drug therapy, bladder retraining and their combination in patients with urge urinary incontinence (Abstract number 174). In: Joint Meeting of the International Continence Society (ICS), 34th Annual Meeting, and the International UroGynecological Association (IUGA); 2004 Aug 23-27; Paris, France. 2004. [ISRCTN66713401] [sr-incont19033]
- ISRCTN66713401. Pilot study for the comparison of drug treatment with conservative treatment for people with overactive bladders. isrctn.com/ISRCTN66713401 (first received 29 November 2002). [DOI: ] [ISRCTN66713401] [sr-incont64653]
- Lauti M, Herbison P, Hay-Smith J, Ellis G, Wilson D. Anticholinergic drugs, bladder retraining and their combination for urge urinary incontinence: a pilot randomised trial. International Urogynecology Journal 2008;19(11):1533-43. [ISRCTN66713401] [sr-incont27730] [DOI] [PubMed] [Google Scholar]
Lentz 1994 {published data only}
- Lentz G, Plevnik S, Stanton SL. Vaginal cones versus bladder drill for sensory urgency treatment. In: International Continence Society (ICS), 24th Annual Meeting; 1994 Aug 30-Sep 2; Prague, Czech Republic. 1994:35-6. [sr-incont10937]
Mattiasson 2003 {published data only}
- Mattiasson A, Blaakaer J, Høye K, Wein AJ, Tolterodine Scandinavian Study Group. Simplified bladder training augments the effectiveness of tolterodine in patients with an overactive bladder. BJU International 2003;91(1):54-60. [sr-incont15701] [DOI] [PubMed] [Google Scholar]
- Mattiasson A. Effect of simplified bladder training and tolterodine treatment in overactive bladder patients (Abstract number 59). In: 2nd International Consultation on Incontinence (ICS); 2001 July 1-3; Paris, France (poster presentations). 2001. [sr-incont15389]
- Mattiasson A. Effect of tolterodine with or without simplified bladder training in overactive bladder patients (Abstract). International Urogynecology Journal 2001;12(Suppl 3):S42. [sr-incont16365] [Google Scholar]
- Mattiasson A. Simplified bladder training augments tolterodine treatment in overactive bladder patients (Abstract number 22). Neurourology and Urodynamics 2001;20(4):403-4. [sr-incont14380] [Google Scholar]
Mattiasson 2010 {published data only}
- EUCTR2005-005546-39-BE. Solifenacin succinate in a flexible dose regimen with simplified bladder training versus solifenacin succinate in a flexible dose regimen alone in a prospective, randomized, parallel group, overactive bladder symptom study – SOLAR. clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2005-005546-39 (first received 31 May 2006). [905-EC-003] [EUCTR2005-005546-39-BE] [NCT00337558] [TrialID.SOLAR] [sr-incont64617]
- Mattiasson A, Masala A, Morton R, Bolodeoku J, SOLAR Study Group. Efficacy of simplified bladder training in patients with overactive bladder receiving a solifenacin flexible-dose regimen: results from a randomized study. BJU International 2010;105(8):1126-35. [EUCTR2005-005546-39-BE] [NCT00337558] [sr-incont41586] [DOI] [PubMed] [Google Scholar]
- Mattiasson A, Morton R, Bolodeoku J. Solifenacin alone and with simplified bladder re-training in overactive bladder syndrome: the prospective, randomised SOLAR study (Abstract number 179). In: International Continence Society (ICS); 38th Annual Meeting; 2008 Oct 20-24; Cairo, Egypt. 2008. [EUCTR2005-005546-39-BE] [NCT00337558] [TrialID.SOLAR] [sr-incont29141]
- NCT00337558. A study of solifenacin with bladder training versus solifenacin alone in patients with overactive bladder (SOLAR) (SOLAR) [Solifenacin succinate in a flexible dose regimen with simplified bladder training versus solifenacin succinate in a flexible dose regimen alone in a prospective, randomized, parallel group, overactive bladder symptom study]. clinicaltrials.gov/show/NCT00337558 (first received 16 June 2006). [EUCTR2005-005546-39-BE] [NCT00337558] [TrialID.SOLAR] [sr-incont61766]
McCreanor 1998 {published data only}
- McCreanor J, Aitchison M, Woods M. Comparing therapies for incontinence. Professional Nurse 1998;13(4):215, 217-9. [sr-incont24106] [Google Scholar]
Milani 1987 {published data only}
- Milani R, Scalambrino S, Carrera S, Quadri G, Riva D, Casolati E. A randomised trial of bladder retraining versus oxybutynin in the treatment of idiophatic urge syndrome: early results. In: International Continence Society (ICS), 16th Annual Meeting; 1986 Sept 17-19; Boston, Massachusetts. 1986:488-90. [sr-incont12030]
- Milani R, Scalambrino S, Quadri G, Carrera S, Riva D, Casolati E. Randomized drug therapy and bladder retraining in urge syndrome: late results. In: International Continence Society (ICS), 17th Annual Meeting; 1987 Sept 2-5; Bristol, UK. 1987:133-4. [sr-incont9033]
Rizvi 2018 {published data only}
- Rizvi RM, Chughtai NG, Kapadia N. Effects of bladder training and pelvic floor muscle training in female patients with overactive bladder syndrome: a randomized controlled trial. Urologia Internationalis 2018;100(4):420-7. [sr-incont77994] [DOI] [PubMed] [Google Scholar]
- Rizvi RM, Chughtai NG, Kapadia NN. Effects of bladder training and behavioural intervention in female patients with overactive bladder syndrome: a randomized controlled trial at AKU (Abstract number 183). International Urogynecology Journal and Pelvic Floor Dysfunction 2017;28(1 Suppl 1):S122-S3. [sr-incont77995] [Google Scholar]
Song 2006 {published data only}
- Song C, Park JT, Heo KO, Lee KS, Choo MS. Effects of bladder training and/or tolterodine in female patients with overactive bladder syndrome: a prospective, randomized study. Journal of Korean Medical Science 2006;21(6):1060-3. [sr-incont22561] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2012 {published data only}
- Zhang Z, Zheng J, Pan Z, Sun Z. The efficacy of tolterodine ER was enhanced by combining low-dose vaginal estrogen treatment and bladder training for postmenopausal women with overactive bladder (OAB) (Abstract number 355). In: International Continence (ICS), 42nd Annual Meeting; 2012 Oct 15-19; Beijing, China. 2012. [sr-incont47797]
References to studies excluded from this review
ACTRN12606000511538 {published data only}
- ACTRN12606000511538. Stroke incontinence study [A randomised controlled trial to evaluate the effects of a continence promotion program including real time ultrasound as biofeedback for pelvic floor muscle rehabilitation, delivered to stroke survivors to reduce urinary incontinence and lower urinary tract symptoms.]. anzctr.org.au/ACTRN12606000511538.aspx (first received 7 December 2006).
Andrade 2015 {published data only}
- Andrade AD, Anam R, Karanam C, Downey P, Ruiz JG. An overactive bladder online self-management program with embedded avatars: a randomized controlled trial of efficacy. Urology 2015;85(3):561-7. [DOI] [PubMed] [Google Scholar]
Aslan 2008 {published data only}
- Aslan E, Komurcu N, Beji NK, Yalcin O. Bladder training and Kegel exercises for women with urinary complaints living in a rest home. Gerontology 2008;54(4):224-31. [DOI] [PubMed] [Google Scholar]
Assassa 2010 {published data only}ISRCTN58226681
- Assassa P, Williams K, Lambert P, Abrams K, Turner D, Shaw C, et al. A double blind randomised placebo controlled trial of the effectiveness of bladder training with oxybutynin or imipramine in the management of detrusor overactivity (DO) (Abstract number 330). In: Joint Meeting of the International Continence Society (ICS) and the International Urogynecological Association; 2010 Aug 23-27; Toronto, Canada. 2010.
- ISRCTN58226681. Evaluation of the efficacy of oxybutynin and imipramine in the management of detrusor instability. isrctn.com/ISRCTN58226681 (first received 25 October 2000).
Azizi 2020 {published data only}
- Azizi M, Azadi A, Otaghi M. The effect of a self-care programme on urinary incontinence and self-esteem in elderly men dwelling in nursing homes in Iran. Aging Male 2020;23(5):687-93. [DOI] [PubMed] [Google Scholar]
Barber 2002 {published data only}
- Barber MD, Visco AG, Wyman JF, Fantl JA, Bump RC, Continence Program for Women Research Group. Sexual function in women with urinary incontinence and pelvic organ prolapse. Obstetrics and Gynecology 2002;99(2):281-9. [DOI] [PubMed] [Google Scholar]
Barber 2009 {published data only}
- Barber MD, Spino C, Janz NK, Brubaker L, Nygaard I, Nager CW, et al. The minimum important differences for the urinary scales of the pelvic floor distress inventory and pelvic floor impact questionnaire. American Journal of Obstetrics and Gynecology 2009;200(5):580.e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bell‐Kotwall 2003 {published data only}
- Bell-Kotwall LM. Factors That Predict Change in Urinary Incontinence in Older Rural Women [PhD thesis]. Chapel Hill, North Carolina: The University of North Carolina at Chapel Hill, 2003. [sr-incont26961] [Google Scholar]
Berghmans 2002 {published data only}
- Berghmans B, Waalwijk van Doorn E, Nieman F, Bie R, den Brandt P, Van Kerrebroeck P. Efficacy of physical therapeutic modalities in women with proven bladder overactivity. European Urology 2002;41(6):581-7. [DOI] [PubMed] [Google Scholar]
Borrie 2002 {published data only}
- Bawden ME, Kartha AS, Borrie MJ, Kerr PS, Durko NA, Haslam IF, et al. Treating women with stress incontinence in a multidisciplinary clinic: a randomized study (Abstract number 276). In: International Continence Society (ICS), 22nd Annual Meeting; 1992 Sept 1-4; Halifax, UK. 1992.
- Borrie MJ, Bawden M, Speechley M, Kloseck M. Interventions led by nurse continence advisers in the management of urinary incontinence: a randomized controlled trial. CMAJ: Canadian Medical Association Journal 2002;166(10):1267-73. [PMC free article] [PubMed] [Google Scholar]
- Borrie MJ, Bawden ME, Kartha AS, Kerr PS. A nurse/physician continence clinic triage approach for urinary incontinence: a 25 week randomized trial. Neurourology and Urodynamics 1992;11(4):364-5. [Google Scholar]
Breyer 2018 {published data only}
- Breyer BN, Creasman JM, Richter HE, Myers D, Burgio KL, Wing RR, et al, PRIDE. A behavioral weight loss program and nonurinary incontinence lower urinary tract symptoms in overweight and obese women with urinary incontinence: a secondary data analysis of PRIDE. Journal of Urology 2018;199(1):215-22. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2009 {published data only}
- Brown CT, Emberton M. Self-management for men with lower urinary tract symptoms. Current Urology Reports 2009;10(4):261-6. [DOI] [PubMed] [Google Scholar]
- Brown CT, Yap T, Cromwell DA, Rixon L, Steed L, Mulligan K, et al. Self management for men with lower urinary tract symptoms: randomised controlled trial. BMJ (Clinical Research Ed.) 2007;334(7583):25. [NCT00270309] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00270309. Self-management for men with uncomplicated lower urinary tract symptoms [Self-management for men with uncomplicated lower urinary tract symptoms. A randomised controlled trial against standard therapy]. clinicaltrials.gov/show/NCT00270309 (first received 26 December 2005).
Brubaker 2009 {published data only}
- Brubaker L, Moalli P, Richter HE, Albo M, Sirls L, Chai T, et al. Challenges in designing a pragmatic clinical trial: the mixed incontinence – medical or surgical approach (MIMOSA) trial experience. Clinical Trials 2009;6(4):355-64. [DOI: 10.1177/1740774509339239] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burgio 2000 {published data only}
- Burgio KL, Locher JL, Goode PS. Combined behavioral and drug therapy for urge incontinence in older women. Journal of the American Geriatrics Society 2000;48(4):370-4. [DOI] [PubMed] [Google Scholar]
Burgio 2002 {published data only}
- Burgio KL, Goode PS, Locher JL, Umlauf MG, Roth DL, Richter HE, et al. Behavioral training with and without biofeedback in the treatment of urge incontinence in older women: a randomized controlled trial. JAMA 2002;288(18):2293-9. [DOI] [PubMed] [Google Scholar]
Burgio 2003 {published data only}
- Burgio KL, Goode PS, Locher JL, Richter HE, Roth DL, Wright KC, et al. Predictors of outcome in the behavioral treatment of urinary incontinence in women. Obstetrics and Gynecology 2003;102(5 Pt 1):940-7. [DOI] [PubMed] [Google Scholar]
Burgio 2006 {published data only}
- Burgio KL, Goode PS, Richter HE, Locher JL, Roth DL. Global ratings of patient satisfaction and perceptions of improvement with treatment for urinary incontinence: validation of three global patient ratings. Neurourology and Urodynamics 2006;25(5):411-7. [DOI] [PubMed] [Google Scholar]
Burgio 2008 {published data only}
- Bailey F, Brubaker L. The BE-DRI study (behaviour enhances drug reduction of incontinence). A clinical trial of the NIH/NIDDK urinary incontinence treatment Network (UITN) (Abstract number 39). Progrès en Urologie 2004;14(3 Suppl 3):15. [Google Scholar]
- Borello-France D, Burgio KL, Goode PS, Markland AD, Kenton K, Balasubramanyam A, et al, Urinary Incontinence Treatment Network. Adherence to behavioral interventions for urge incontinence when combined with drug therapy: adherence rates, barriers, and predictors. Physical Therapy 2010;90(10):1493-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker L, Lukacz ES, Burgio K, Zimmern P, Norton P, Leng W, et al. Mixed incontinence: comparing definitions in non-surgical patients. Neurourology and Urodynamics 2011;30(1):47-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgio K, Richter H, Brubaker L, Kraus S, Chai T, Nyberg L, et al. Predictors of outcomes of drug therapy, combined drug and behavioural therapy and drug discontinuation in the treatment of urge urinary incontinence in women (Abstract number: poster# 63). Neurourology and Urodynamics 2009;28(2):144. [Google Scholar]
- Burgio K, Urinary Incontinence Treatment Network. Combining behavior and drug therapy to improve drug withdrawal in the treatment of urge incontinence: a randomized trial (Abstract number 21). Neurourology and Urodynamics 2007;26(5):616-7. [Google Scholar]
- Burgio KL, Kraus SR, Borello-France D, Chai TC, Kenton K, Goode PS, et al, Urinary Incontinence Treatment Network. The effects of drug and behavior therapy on urgency and voiding frequency. International Urogynecology Journal 2010;21(6):711-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgio KL, Kraus SR, Menefee S, Borello-France D, Corton M, Johnson HW, et al, Urinary Incontinence Treatment Network. Behavioral therapy to enable women with urge incontinence to discontinue drug treatment: a randomized trial. Annals of Internal Medicine 2008;149(3):161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBeau C, FitzGerald MP, Johnson H, Kraus S, Lemack G, Mallett V, et al. Expectations of urge incontinence treatment and their relationship to outcomes (Abstract number 59). Neurourology and Urodynamics 2009;28(7):642-3. [Google Scholar]
- Dyer KY, Xu Y, Brubaker L, Nygaard I, Markland A, Rahn D, et al, Urinary Incontinence Treatment Network (UITN). Minimum important difference for validated instruments in women with urge incontinence. Neurourology and Urodynamics 2011;30(7):1319-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode PS, Burgio KL, Kraus SR, Kenton K, Litman HJ, Richter HE, Urinary Incontinence Treatment Network. Correlates and predictors of patient satisfaction with drug therapy and combined drug therapy and behavioral training for urgency urinary incontinence in women. International Urogynecology Journal 2011;22(3):327-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller ER, Litman H, Zimmern PE, Norton P, Goode P. Impact of fluid management on fluid intake and urge incontinence in the BE-DRI trial for OAB in women (Abstract number 1530). Journal of Urology 2009;181(4 Suppl):547-8. [Google Scholar]
- NCT00090584. Behavior enhances drug reduction of incontinence (BE-DRI) (BE-DRI). clinicaltrials.gov/study/NCT00090584 (first received 31 August 2004).
- Richter H, Burgio K, Brubaker L, Chai T, Kraus S, Nyberg L, et al. Predictors of outcomes of drug therapy, combined drug and behavioral therapy and drug discontinuation in the treatment of urge urinary incontinence in women (Abstract number 39). Journal of Pelvic Medicine and Surgery 2009;15(2):73-4. [Google Scholar]
- Richter HE, Burgio KL, Chai TC, Kraus SR, Xu Y, Nyberg L, et al. Predictors of outcomes in the treatment of urge urinary incontinence in women. International Urogynecology Journal 2009;20(5):489-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urinary Incontinence Treatment Network (UITN). Design of the Behavior Enhances Drug Reduction of Incontinence (BE-DRI) study. Contemporary Clinical Trials 2007;28(1):48-58. [DOI] [PubMed] [Google Scholar]
- Zimmern P, Litman H, Mueller E, Norton P, Goode P. Impact of fluid management on fluid intake and urge incontinence in the BE-DRI trial for OAB in women (Abstract number: podium #30). Neurourology and Urodynamics 2009;28(2):163. [Google Scholar]
- Zimmern P, Litman HJ, Mueller E, Norton P, Goode P, Urinary Incontinence Treatment Network. Effect of fluid management on fluid intake and urge incontinence in a trial for overactive bladder in women. BJU International 2010;105(12):1680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burgio 2010 {published data only}
- Burgio KL, Goode PS, Richter HE, Markland AD, Johnson TM, Redden DT. Combined behavioral and individualized drug therapy versus individualized drug therapy alone for urge urinary incontinence in women. Journal of Urology 2010;184(2):598-603. [DOI] [PubMed] [Google Scholar]
Burgio 2011 {published data only}
- Burgio KL, Goode PS, Johnson TM, Hammontree L, Ouslander JG, Markland AD, et al. Behavioral versus drug treatment for overactive bladder in men: the male overactive bladder treatment in veterans (MOTIVE) trial. Journal of the American Geriatrics Society 2011;59(12):2209-16. [DOI] [PubMed] [Google Scholar]
Burgio 2020 {published data only}
- Burgio KL, Kraus SR, Johnson TM, Markland AD, Vaughan CP, Li P, et al. Effectiveness of combined behavioral and drug therapy for overactive bladder symptoms in men: a randomized clinical trial. JAMA Internal Medicine 2020;180(3):411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castleden 1986 {published data only}
- Castleden CM, Duffin HM, Gulati RS. Double-blind study of imipramine and placebo for incontinence due to bladder instability. Age Ageing 1986;15(5):299-303. [DOI] [PubMed] [Google Scholar]
Castleden 1987 {published data only}
- Castleden CM, Duffin HM, Millar AW. A controlled clinical pilot study of dicyclomine in detrusor instability. In: 16th Annual Meeting of the International Continence Society; 1986 Sept 17-19; Boston, Massachusetts. 1986:373-5.
- Castleden CM, Duffin HM, Millar AW. Dicyclomine hydrochloride in detrusor instability – a controlled clinical pilot study. Journal of Clinical Experimental Gerontology 1987;9(4):265-70. [Google Scholar]
Chanfreau‐Rona 1984 {published data only}
- Chanfreau-Rona D, Bellwood S, Wylie B. Assessment of a behavioural programme to treat incontinent patients in psychogeriatric wards. British Journal of Clinical Psychology 1984;23(4):273-9. [DOI] [PubMed] [Google Scholar]
Chanfreau‐Rona 1986 {published data only}
- Chanfreau-Rona D, Wylie B, Bellwood S. Behaviour treatment of daytime incontinence in elderly male and female patients. Behavioural Psychotherapy 1986;14(1):13-20. [Google Scholar]
Chesworth 2015 {published data only}
- Chesworth BM, Leathley MJ, Thomas LH, Sutton CJ, Forshaw D, Watkins CL, et al. Assessing fidelity to treatment delivery in the ICONS (Identifying Continence OptioNs after Stroke) cluster randomised feasibility trial. BMC Medical Research Methodology 2015;15:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cho 2016 {published data only}
- Cho ST, Kwon O, Choi DK, Lee YG, Kim KK, Kim KH, et al. Is pelvic floor muscle exercise effective for urinary incontinence in elderly women with cognitive impairment (Abstract number 433). Neurourology and Urodynamics 2016;35(S4):S382-3. [Google Scholar]
Chu 2019 {published data only}
- Chu C, Schmitz K, Khanijow K, Stambakio H, Newman D, Andy U, et al. Feasibility and outcomes in a pilot randomized controlled trial of a home-based integrated physical exercise and bladder training program versus usual care for community-dwelling older women with urinary incontinence (Abstract number PD06-12). Journal of Urology 2019;201(4):e141. [DOI] [PubMed] [Google Scholar]
- Chu CM, Schmitz KH, Khanijow K, Stambakio H, Newman DK, Arya LA, et al. Feasibility and outcomes: pilot randomized controlled trial of a home-based integrated physical exercise and bladder-training program vs usual care for community-dwelling older women with urinary incontinence. Neurourology and Urodynamics 2019;38(5):1399-408. [DOI] [PubMed] [Google Scholar]
- NCT03869918. Physical exercise and bladder training program for urinary incontinence [Comparative effectiveness of integrated exercise and urge suppression verses usual care for reducing the risk of falls in women with urgency urinary incontinence]. clinicaltrials.gov/show/NCT03869918 (first received 11 March 2019).
Colling 1992 {published data only}
- Colling J, Ouslander J, Hadley BJ, Eisch J, Campbell E. The effects of patterned urge-response toileting (PURT) on urinary incontinence among nursing home residents. Journal of the American Geriatrics Society 1992;40(2):135-41. [DOI] [PubMed] [Google Scholar]
Colling 2003 {published data only}
- Colling J, Owen TR, McCreedy M, Newman D. The effects of a continence program on frail community-dwelling elderly persons. Urologic Nursing 2003;23(2):117-22, 27-31. [PubMed] [Google Scholar]
Davila 1998 {published data only}
- Davila GW, Primozich J. Prospective randomized trial of bladder retraining using an electronic voiding device versus self-administered bladder drills in women with detrusor instability (Abstract). Neurourology and Urodynamics 1998;17(4):324-5. [Google Scholar]
Diokno 2004 {published data only}
- Diokno AC, Sampselle CM, Herzog AR, Raghunathan TE, Hines S, Messer K, et al. Prevention of urinary incontinence by behavioral modification program: a randomized, controlled trial among older women in the community. Journal of Urology 2004;171(3):1165-71. [NCT00075114] [DOI] [PubMed] [Google Scholar]
- Diokno AC, Sampselle CM, Hines S, Herzog AR, Raghunathan T, Messer K, et al. Prevention of urinary incontinence by group behavioral modification program: a prospective randomized controlled trial among older women in the community (Abstract number 479). Journal of Urology 2003;169(4 Suppl):124. [NCT00075114] [DOI] [PubMed] [Google Scholar]
- NCT00075114. Prevent inability to control urination [Promoting self-care to prevent urinary incontinence]. clinicaltrials.gov/show/NCT00075114 (first received 5 January 2004). [NCT00075114]
- NCT00506766. Promoting self care to prevent urinary incontinence (UI): a four-year follow-up. clinicaltrials.gov/show/NCT00506766 (first received 25 July 2007). [NCT00075114] [NCT00506766]
- Sampselle CM, Messer KL, Herzog R, Hines SJ, Karl C, Diokno AA. Group teaching of pelvic floor and bladder training: function and knowledge outcomes (Abstract). Neurourology and Urodynamics 2003;22(5):545-6. [Google Scholar]
- Sampselle CM, Messer KL, Seng JS, Raghunathan TE, Hines SH, Diokno AC. Learning outcomes of a group behavioral modification program to prevent urinary incontinence. International Urogynecology Journal and Pelvic Floor Dysfunction 2005;16(6):441-6. [DOI] [PubMed] [Google Scholar]
Diokno 2010 {published data only}
- Diokno AC, Ocampo MS, Ibrahim IA, Karl CR, Lajiness MJ, Hall SA. Group session teaching of behavioral modification program (BMP) for urinary incontinence: a randomized controlled trial among incontinent women. International Urology and Nephrology 2010;42(2):375-81. [DOI] [PubMed] [Google Scholar]
Diokno 2018 {published data only}
- Diokno AC, Newman DK, Low LK, Griebling TL, Maddens ME, Goode PS, et al. Effect of group-administered behavioral treatment on urinary incontinence in older women: a randomized clinical trial. JAMA Internal Medicine 2018;178(10):1333-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dougherty 2002 {published data only}
- Dougherty MC, Dwyer JW, Pendergast JF, Boyington AR, Tomlinson BU, Coward RT, et al. A randomized trial of behavioral management for continence with older rural women. Research in Nursing and Health 2002;25(1):3-13. [DOI] [PubMed] [Google Scholar]
Dowd 2000 {published data only}
- Dowd T, Kolcaba K, Steiner R. Using cognitive strategies to enhance bladder control and comfort. Holistic Nursing Practice 2000;14(2):91-103. [DOI] [PubMed] [Google Scholar]
Dyer 2011 {published data only}
- Dyer KY, Xu Y, Brubaker L, Nygaard I, Markland A, Rahn D, et al, Urinary Incontinence Treatment Network (UITN). Minimum important difference for validated instruments in women with urge incontinence: minimum important differences for Urge Incontinence Questionnaires. Neurourology and Urodynamics 2011;30(7):1319-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elser 1995 {published data only}
- Elser DM, Fantl JA, McClish DK. Comparison of "subjective" and "objective" measures of severity of urinary incontinence in women. Neurourology and Urodynamics 1995;14(4):311-6. [DOI] [PubMed] [Google Scholar]
Engberg 2002 {published data only}
- Engberg S, Sereika SM, McDowell BJ, Weber E, Brodak I. Effectiveness of prompted voiding in treating urinary incontinence in cognitively impaired homebound older adults. Journal of Wound, Ostomy and Continence Nursing 2002;29(5):252-65. [DOI] [PubMed] [Google Scholar]
Fonda 1994 {published data only}
- Fonda D, Woodward M, D'Astoli M, Chin W F. The continued success of conservative management for established urinary incontinence in older people. Australian Journal on Ageing 1994;13(1):12-6. [Google Scholar]
Frost 2019 {published data only}
- Frost J, Athene Lane J, Cotterill N, Fader M, Hackshaw-Mcgeagh L, Hashim H, et al. TReatIng Urinary symptoms in Men in Primary Healthcare using non-pharmacological and non-surgical interventions (TRIUMPH) compared with usual care: study protocol for a cluster randomised controlled trial. Trials 2019;20(1):546. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gezginci 2018 {published data only}
- Gezginci E, Iyigun E, Yilmaz S, Aydur E. Comparative effectiveness of three different teaching methods in behavioural therapy program for female overactive bladder: a randomized controlled trial (Abstract number 263). European Urology Supplements 2015;14(2):e263-a. [Google Scholar]
- Gezginci E, Iyigun E, Yilmaz S, Aydur E. Comparative effectiveness of three different teaching methods in behavioural therapy program for female overactive bladder: a randomized controlled trial (Abstract number PD27-03). Journal of Urology 2015;193(4):e572. [Google Scholar]
- Gezginci E, Iyigun E, Yilmaz S. Comparison of 3 different teaching methods for a behavioral therapy program for female overactive bladder: a randomized controlled trial. Journal of Wound, Ostomy and Continence Nursing 2018;45(1):68-74. [NCT02701010] [DOI] [PubMed] [Google Scholar]
- NCT02701010. Behavioural therapy program for female overactive bladder [Comparative effectiveness of three different teaching methods in behavioural therapy program for female overactive bladder: a randomized controlled trial]. clinicaltrials.gov/show/NCT02701010 (first received 7 March 2016).
Glazener 2010 {published data only}
- Glazener C, Boachie C, Buckley B, Cochran C, Dorey G, Grant A, et al. A randomized controlled trial of conservative treatment (pelvic floor muscle training and bladder training) for urinary incontinence in men after prostate surgery (MAPS) (Abstract number 200). Neurourology and Urodynamics 2010;29(6):1093-4. [Google Scholar]
Golmakani 2014 {published data only}
- Golmakani N, Khadem N, Arabipoor A, Kerigh BF, Esmaily H. Behavioral intervention program versus vaginal cones on stress urinary incontinence and related quality of life: a randomized clinical trial. Oman Medical Journal 2014;29(1):32-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gonzalez 2015 {published data only}
- Gonzalez S, Rondini C, Urzúa M J, Alva J, Braun H, Kaplan F, et al. Effect of behavioral therapy versus transcutaneous posterior tibial nerve stimulation in the management of overactive bladder: a prospective randomized cross-over study (Abstract PP 07). International Urogynecology Journal and Pelvic Floor Dysfunction 2015;26(1 Suppl 1):S31-2. [Google Scholar]
Goode 2002 {published data only}
- Goode PS, Burgio KL, Locher JL, Umlauf MG, Lloyd LK, Roth DL. Urodynamic changes associated with behavioral and drug treatment of urge incontinence in older women. Journal of the American Geriatrics Society 2002;50(5):808-16. [DOI] [PubMed] [Google Scholar]
Goode 2003 {published data only}
- Goode PS, Burgio KL, Locher JL, Roth DL, Umlauf MG, Richter HE, et al. Effect of behavioral training with or without pelvic floor electrical stimulation on stress incontinence in women: a randomized controlled trial. JAMA 2003;290(3):345-2. [DOI] [PubMed] [Google Scholar]
Goode 2004 {published data only}
- Goode PS. Behavioral and drug therapy for urinary incontinence. Urology 2004;63(3 Suppl 1):58-64. [DOI] [PubMed] [Google Scholar]
Griebling 2018 {published data only}
- Griebling TL, Diokno A, Newman D, Burgio K, Low L, Maddens M, et al. Training fidelity and quality control in clinical behavioral research for urinary incontinence: the GLADIOULUS trial (Abstract number poster #NM54). Neurourology and Urodynamics 2018;37:S633-4. [NCT02001714] [sr-incont78249] [Google Scholar]
Ha 2008 {published data only}
- Ha Y, Yun SJ, Kim Y, Lee S, Jung W, Kim W, et al. The development and effect of Ubiquitous program for female patients with overactive bladder: prospective, randomized 8-weeks follow up with questionnaire and voiding diary based assessment (Abstract number 611). In: 38th Annual Meeting of the International Continence Society (ICS); 2008 Oct 20-24; Cairo, Egypt. 2008. [sr-incont29138]
Haywood 2008 {published data only}
- Haywood KL, Garratt AM, Lall R, Smith JF, Lamb SE. EuroQol EQ-5D and condition-specific measures of health outcome in women with urinary incontinence: reliability, validity and responsiveness. Quality of Life Research 2008;17(3):475-83. [DOI] [PubMed] [Google Scholar]
Henalla 1991 {published data only}
- Henalla SM, Millar DR, Moon PV. Medical or surgical augmentation of bladder drill for detrusor instability. Journal of Obstetrics and Gynaecology 1991;11(2):128-30. [Google Scholar]
Herschorn 2004 {published data only}
- Herschorn S, Becker D, Miller B, Thompson M, Forte L. The impact of a simple health education intervention in overactive bladder patients (Abstract). In: International Continence Society (ICS), 33rd Annual Meeting; 2003 Oct 5-9; Florence, Italy. 2003:352-3. [sr-incont16988]
- Herschorn S, Becker D, Miller E, Thompson M, Forte L. Impact of a health education intervention in overactive bladder patients. Canadian Journal of Urology 2004;11(6):2430-7. [sr-incont20194] [PubMed] [Google Scholar]
Hill 2007 {published data only}
- Hill LA, Fereday-Smith J, Credgington C, Woodward AF, Knight JC, Williams AJ, et al. Bladders behaving badly: a randomized controlled trial of group versus individual interventions in the management of female urinary incontinence. Journal of the Association of Chartered Physiotherapists in Women's Health 2007;101:30-6. [Google Scholar]
- Hill LA. Bladders behaving badly: a randomized controlled trial of group versus individual interventions in the management of female urinary incontinence (Abstract). Journal of the Association of Chartered Physiotherapists in Women's Health 2007;100:37. [Google Scholar]
- Pepper J, Lamb SE, Doughty G, Fereday Smith J. Group treatment: an acceptable and effective method of physiotherapy for bladder problems? Journal of the Association of Chartered Physiotherapists in Women's Health 2003;93:15-8. [Google Scholar]
Hines 2007 {published data only}
- Hines SH, Seng JS, Messer KL, Raghunathan TE, Diokno AC, Sampselle CM. Adherence to a behavioral program to prevent incontinence. Western Journal of Nursing Research 2007;29(1):36-56. [DOI] [PubMed] [Google Scholar]
Holtedahl 2000 {published data only}
- Holtedahl K, Verelst M, Schiefloe A, Hunskaar S. Usefulness of urodynamic examination in female urinary incontinence – lessons from a population-based, randomized, controlled study of conservative treatment. Scandinavian Journal of Urology and Nephrology 2000;34(3):169-74. [DOI] [PubMed] [Google Scholar]
Hu 1989 {published data only}
- Hu TW, Igou JF, Kaltreider DL, Yu LC, Rohner TJ, Dennis PJ, et al. A clinical trial of a behavioral therapy to reduce urinary incontinence in nursing homes. Outcome and implications. JAMA 1989;261(18):2656-62. [PubMed] [Google Scholar]
Huang 2012 {published data only}
- Huang X, Xu T, Lv B, Xie L. Effects of medical interfered-pelvic floor muscle training in behavioral therapy and/or tolterodine in female patients with primary overactive bladder syndrome: a single-blind randomized study (Abstract number 351). In: 42nd Annual Meeting of the International Continence (ICS); 2012 Oct 15-19; Beijing, China. 2012.
Hui 2006 {published data only}
- Hui E, Lee PS, Woo J. Management of urinary incontinence in older women using videoconferencing versus conventional management: a randomized controlled trial. Journal of Telemedicine and Telecare 2006;12(7):343-7. [DOI] [PubMed] [Google Scholar]
ISRCTN62679410 {published data only}ISRCTN62679410
- ISRCTN62679410. Effectiveness of an urinary incontinence program for Chinese elderly women in a community setting [Effectiveness of an urinary continence physiotherapy program (UCPP) for Chinese elderly women in a community setting: a randomised controlled trial]. isrctn.com/ISRCTN62679410 (first received 30 June 2011). [ISRCTN62679410]
ISRCTN62722772 {published data only}ISRCTN62722772
- ISRCTN62722772. The effects of involving a nurse practitioner in primary care for adult patients with urinary incontinence. isrctn.com/ISRCTN62722772 (first received 20 December 2005).
- NL232. The effects of involving a nurse practitioner in primary care for adult patients with urinary incontinence. trialregister.nl/trial/232 (first received 1 December 2004). [NTR269]
Janssen 2001 {published data only}
- Janssen CC, Lagro-Janssen AL, Felling AJ. The effects of physiotherapy for female urinary incontinence: individual compared with group treatment. BJU International 2001;87(3):201-6. [DOI] [PubMed] [Google Scholar]
Jirovec 2001 {published data only}
- Jirovec MM, Templin T. Predicting success using individualized scheduled toileting for memory-impaired elders at home. Research in Nursing and Health 2001;24(1):1-8. [DOI] [PubMed] [Google Scholar]
Johnson 2005 {published data only}
- Johnson TM, Burgio KL, Redden DT, Wright KC, Goode PS. Effects of behavioral and drug therapy on nocturia in older incontinent women. Journal of the American Geriatrics Society 2005;53(5):846-50. [DOI] [PubMed] [Google Scholar]
Kafri 2008 {published data only}
- Kafri R, Shames J, Raz M, Katz-Leurer M. Rehabilitation versus drug therapy for urge urinary incontinence: long-term outcomes. International Urogynecology Journal 2008;19(1):47-52. [DOI] [PubMed] [Google Scholar]
Kangchai 2002 {published data only}
- Kangchai W, Srisuphun W, Kompayak J, Charoenyooth C, Jitapunkul S. Efficacy of self-management promotion program for elderly women with urinary incontinence. Thai Journal of Nursing Research 2002;6(3):101-14. [Google Scholar]
Kaya 2011 {published data only}
- Kaya S, Akbayrak T, Beksaç S. Comparison of different treatment protocols in the treatment of idiopathic detrusor overactivity: a randomized controlled trial. Clinical Rehabilitation 2011;25(4):327-38. [DOI] [PubMed] [Google Scholar]
Kaya 2015 {published data only}
- Kaya S, Akbayrak T, Gursen C, Beksac S. Short-term effect of adding pelvic floor muscle training to bladder training for female urinary incontinence: a randomized controlled trial. International Urogynecology Journal 2015;26(2):285-93. [DOI] [PubMed] [Google Scholar]
Kilinc 2019 {published data only}
- Kilinc MF, Doluoglu OG, Yildiz Y, Yuceturk CN, Hascicek AM. Using a checklist to increase the effectiveness of behavioral therapy for overactive bladder: a prospective randomized controlled trial. Neurourology and Urodynamics 2019;38(4):1152-9. [DOI] [PubMed] [Google Scholar]
Kim 2001 {published data only}
- Kim JI. Continence efficacy intervention program for community residing women with stress urinary incontinence in Japan. Public Health Nursing 2001;18(1):64-72. [DOI] [PubMed] [Google Scholar]
Kim 2008 {published data only}
- Kim SW, Song SH, Ku JH. Bladder training versus combination of propiverine with bladder training for female urinary frequency. Gynecologic and Obstetric Investigation 2008;65(2):123-7. [DOI] [PubMed] [Google Scholar]
Kincade 2007 {published data only}
- Kincade JE, Dougherty MC, Carlson JR, Hunter GS, Busby-Whitehead J. Randomized clinical trial of efficacy of self-monitoring techniques to treat urinary incontinence in women. Neurourology and Urodynamics 2007;26(4):507-11. [DOI] [PubMed] [Google Scholar]
Klarskov 1984 {published data only}
- Klarskov P, Gerstenberg T, Hald T. Bladder training and terodiline on urge incontinence in females with stable detrusor function. In: International Continence Society (ICS), 14th Annual Meeting; 1984 Sept 13-15; Innsbruck, Austria. 1984:404-5.
Kobayashi 2009 {published data only}
- Kobayashi H, Sawada N, Zakohji H, Yoshiyama M, Araki I, Takeda M. Researches on the improvement of QOL in both patients with overactive bladder syndrome and their caregivers. Comparison between pharmacotherapy alone and combination of pharmacotherapy, physio-therapy, and education of both patients and caregiver (Abstract number 516). In: 39th Annual Meeting of the International Continence Society (ICS); 2009 Sept 29-Oct 3; San Francisco, California. 2009.
Komesu 2011 {published data only}
- Komesu YM, Sapien RE, Rogers RG, Ketai LH. Hypnotherapy for treatment of overactive bladder: a randomized controlled trial pilot study. Female Pelvic Medicine and Reconstructive Surgery 2011;17(6):308-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Komesu 2017 {published data only}
- Komesu YM, Richter HE, Dinwiddie DL, Siddiqui NY, Sung VW, Lukacz ES, et al. Methodology for a vaginal and urinary microbiome study in women with mixed urinary incontinence. International Urogynecology Journal 2017;28(5):711-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Komesu 2020 {published data only}
- Komesu YM, Schrader RM, Rogers RG, Sapien RE, Mayer AR, Ketai LH. Hypnotherapy or medications: a randomized noninferiority trial in urgency urinary incontinent women. American Journal of Obstetrics and Gynecology 2020;222(2):159.e1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kraus 2007 {published data only}
- Kraus SR, Urinary Incontinence Treatment Network (UITN). Combining behavior and drug therapy to improve drug withdrawal in the treatment of urge incontinence: a randomized controlled trial (Abstract number 8 Oral). Journal of Pelvic Medicine and Surgery 2007;13(5):233-4. [Google Scholar]
Kumari 2008 {published data only}
- Kumari S, Jain V, Mandal AK, Singh A. Behavioral therapy for urinary incontinence in India. International Journal of Gynecology and Obstetrics 2008;103(2):125-30. [DOI] [PubMed] [Google Scholar]
Lai 2017 {published data only}
- Lai CK, Wan X. Using prompted voiding to manage urinary incontinence in nursing homes: can it be sustained? Journal of the American Medical Directors Association 2017;18(6):509-14. [DOI] [PubMed] [Google Scholar]
Lee 2005 {published data only}
- Lee C, Johnson C, Chiarelli P. Women's waterworks: evaluating an early intervention for incontinence among adult women. Australian and New Zealand Continence Journal 2005;11(1):11-6. [Google Scholar]
Lee 2018 {published data only}
- Lee HY, Yun YJ, Choi JY, Hong JW, Lee I, Park SH, et al. Effectiveness and safety of moxibustion for alleviating symptoms of overactive bladder: a prospective, randomized controlled, crossover-design, pilot study. Medicine 2018;97(34):e12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leong 2014 {published data only}
- Leong BS, Mok NW. Effectiveness of a new standardised Urinary Continence Physiotherapy Programme for community-dwelling older women in Hong Kong. Hong Kong Medical Journal 2014;21(1):30-7. [EMBASE: 602268989] [DOI] [PubMed] [Google Scholar]
Linn 1995 {published data only}
- Linn JG, Best HL, Holzapfel KM. Geriatrics. Behavioral treatment of urinary incontinence (Abstract). Rehabilitation: R&D Progress Reports 1994;Dec 30-31:106-7. [Google Scholar]
- Linn JG, Best HL, Holzapfel KM. Geriatrics: behavioral treatment of urinary incontinence. Rehabilitation R&D Progress Reports 1996;33:100-1. [Google Scholar]
- Linn JG. Prompted voiding in the treatment of urinary incontinence. Rehabilitation: R&D Progress Reports 1995;32:323. [Google Scholar]
Locher 2002 {published data only}
- Locher JL, Burgio KL, Goode PS, Roth DL, Rodriguez E. Effects of age and causal attribution to aging on health-related behaviors associated with urinary incontinence in older women. Gerontologist 2002;42(4):515-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loohuis 2019 {published data only}
- Loohuis A, Wessels N, Dekker J, Merode N, Slieker-Ten Hove M, Kollen B, et al. App-based treatment for urinary incontinence in women: a pragmatic, randomized, controlled, non-inferiority trial in primary care setting (Abstract number 488). Neurourology and Urodynamics 2019;38:S363-5. [Google Scholar]
- Loohuis AM, Wessels NJ, Jellema P, Vermeulen KM, Slieker-Ten Hove MC, Gemert-Pijnen JE, et al. The impact of a mobile application-based treatment for urinary incontinence in adult women: design of a mixed-methods randomized controlled trial in a primary care setting. Neurourology and Urodynamics 2018;37(7):2167-76. [DOI] [PubMed] [Google Scholar]
- NL4948. Randomized controlled trial in adult women with urinary incontinence comparing treatment delivered through a mobile application versus standard care (URinControl) [RCT comparing treatment based on a mobile application (App: URinControl) versus standard care in women with urinary incontinence in primary care.]. trialregister.nl/trialreg/admin/rctview.asp?TC=5052 (first received 22 January 2015). [NTR5052]
Macaulay 1988 {published data only}
- Macaulay AJ, Holmes D, Stanton SL, Stern RS. A prospective, randomised, controlled trial of bladder retaining and brief psychotherapy for urinary urgency and frequency (Abstract). In: International Continence Society (ICS), 15th Annual Meeting; 1985 Sept 3-6; London, UK. 1985:184-5.
- Macaulay AJ, Stern RS, Holmes DM, Stanton SL. Micturition and the mind: psychological factors in the aetiology and treatment of urinary symptoms in women. British Medical Journal (Clinical Research Ed.) 1987;294(6571):540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaulay AJ. Psychological Factors in the Aetiology and Treatment of Urinary Symptoms in Women [MD thesis]. London (UK): Charing Cross and Westminster Medical School, 1988. [Google Scholar]
Madersbacher 2004 {published data only}
- Madersbacher H, Pilloni S. Efficacy of extracorporeal magnetic innervation therapy (EXMI) in comparison to standard therapy for stress, urge and mixed incontinence: a randomised prospective trial (Abstract number 185). European Urology Supplements 2004;3(2):49. [Google Scholar]
Margolis 2009 {published data only}
- Margolis MK, Fox KM, Cerulli A, Ariely R, Kahler KH, Coyne KS. Psychometric validation of the overactive bladder satisfaction with treatment questionnaire (OAB-SAT-q). Neurourology and Urodynamics 2009;28(5):416-22. [NCT00127270] [sr-incont32111] [DOI] [PubMed] [Google Scholar]
- NCT00127270. Using behavioral therapy in combination with drug-darifenacin for symptoms of overactive bladder. clinicaltrials.gov/show/NCT00127270 (first received 5 August 2005). [NCT00127270] [sr-incont61769]
McAdam 2013 {published data only}
- McAdam J, French B, Thomas LH, Watkins CL, ICONS Management Team. Implementation of a systematic voiding programme for incontinence after stroke: identifying mechanisms of action for success (Abstract number OG36). In: 8th UK Stroke Forum Conference; 2013 Dec 3-5; Harrogate, UK. 2013:76.
McDowell 1996 {published data only}
- McDowell BJ, Engberg SJ, Rodriguez E, Engberg R, Sereika S. Characteristics of urinary incontinence in homebound older adults. Journal of the American Geriatrics Society 1996;44(8):963-8. [DOI] [PubMed] [Google Scholar]
McFall 2000 {published data only}
- McFall SL, Yerkes AM, Belzer JA, Cowan LD. Urinary incontinence and quality of life in older women: a community demonstration in Oklahoma. Family and Community Health 1994;17(1):64-75. [Google Scholar]
- McFall SL, Yerkes AM, Cowan LD. Outcomes of a small group educational intervention for urinary incontinence: episodes of incontinence and other urinary symptoms. Journal of Aging and Health 2000;12(2):250-67. [DOI] [PubMed] [Google Scholar]
- McFall SL, Yerkes AM, Cowan LD. Outcomes of a small group educational intervention for urinary incontinence: health-related quality of life. Journal of Aging and Health 2000;12(3):301-17. [DOI] [PubMed] [Google Scholar]
Messer 2006 {published data only}
- Messer KL, Herzog AR, Seng JS, Sampselle CM, Diokno AC, Raghunathan TE, et al. Evaluation of a mass mailing recruitment strategy to obtain a community sample of women for a clinical trial of an incontinence prevention intervention. International Urology and Nephrology 2006;38(2):255-61. [DOI] [PubMed] [Google Scholar]
NCT00821184 {published data only}
- NCT00821184. Behavioral modification and Vesicare versus Vesicare alone for urge incontinence in patients with overactive bladder [A prospective randomized trial of behavioral modification and solifenacin (Vesicare) vs solifenacin (Vesicare) alone for the treatment of urge incontinence in patients with an overactive bladder]. clinicaltrials.gov/show/NCT00821184 (first received 13 January 2006).
NCT01032265 {published data only}
- NCT01032265. Web-based management of female stress urinary incontinence [Web-based management of female stress urinary incontinence. Evaluation of a treatment programme with pelvic floor muscle training and elements of cognitive behavioural therapy]. clinicaltrials.gov/show/NCT01032265 (first received 15 December 2009).
NCT01187082 {published data only}
- NCT01187082. Group training for overactive bladder in adults [A clinical, randomized, comparative, non-blinded study on the effect of bladder training in groups compared to individual bladder training for female patients with overactive bladder]. clinicaltrials.gov/show/NCT01187082 (first received 23 August 2010). [NCT01187082]
NCT02107820 {published data only}
- NCT02107820. Does bladder training improve the efficacy of nerve stimulation in women with refractory overactive bladders [Does bladder training (BT) improve the efficacy of percutaneous tibial nerve stimulation (PTNS) in women with refractory overactive bladder (OAB) – a randomised controlled study]. clinicaltrials.gov/show/NCT02107820 (first received 8 April 2014).
NCT02202031 {published data only}
- NCT02202031. Controlling urgency through relaxation exercises (CURE). clinicaltrials.gov/show/NCT02202031 (first received 28 July 2014).
NCT02206958 {published data only}
- NCT02206958. Defeating urinary incontinence with exercise training (DUET) feasibility study (DUET) [Preventing toileting disability in frail older women]. clinicaltrials.gov/show/NCT02206958 (first received 1 August 2014).
NCT02505607 {published data only}
- NCT02505607. Overactive bladder education. clinicaltrials.gov/show/NCT02505607 (first received 22 July 2015).
NCT02511314 {published data only}
- NCT02511314. A trial of effectiveness of a smart sensor for continence care: the ARCTICC study (ARCTICC) [A randomised, controlled trial of effectiveness of a smart sensor for continence care: the ARCTICC study]. clinicaltrials.gov/show/NCT02511314 (first received 12 May 2015).
NCT03176901 {published data only}
- NCT03176901. Comparing approaches to treat older adult women's urge incontinence: pilot feasibility and randomized controlled trial (SHUW) [Comparing mindfulness-based stress reduction with the health enhancement program in the treatment of urinary urge incontinence in older adult women: a pilot feasibility and randomized controlled trial]. clinicaltrials.gov/show/NCT03176901 (first received 6 June 2017).
NCT03797365 {published data only}
- NCT03797365. French study to evaluate the impact of a cognitive therapy on urinary incontinent women of all age's perineal settings [Randomized trial to evaluate the impact of cognitive therapy added to normal perineal rehabilitation on pelvic floor muscle contraction for urinary incontinent women]. clinicaltrials.gov/show/NCT03797365 (first received 9 January 2019).
NCT04068025 {published data only}
- NCT04068025. Information, motivation, behavioral skills model on urinary incontinence and quality of life in men [The effect of information, motivation, behavioral skills model on urinary incontinence and quality of life in men with overactive bladder: a randomized controlled trial]. clinicaltrials.gov/show/NCT04068025 (first received 28 August 2019).
NCT04237753 {published data only}
- NCT04237753. Remote access to urinary incontinence treatment for women veterans (PRACTICAL) [Optimizing remote access to urinary incontinence treatment for women veterans]. clinicaltrials.gov/show/NCT04237753 (first received 23 January 2020). [NCT04237753]
Nikoletti 2004 {published data only}
- Nikoletti S, Young J, King M. Evaluation of an electronic monitoring device for urinary incontinence in elderly patients in an acute care setting. Journal of Wound, Ostomy and Continence Nursing 2004;31(3):138-49. [DOI] [PubMed] [Google Scholar]
NL2075 {published data only}
- NL2075. Blaastraining en PTNS bij overactieve blaas (PTOAB study) [Bladder training with or without PTNS (posterior tibial nerve stimulation) in the treatment of overactive bladder]. trialregister.nl/trialreg/admin/rctview.asp?TC=2192 (first received 1 April 2009). [NTR2192]
O'Brien 1991 {published data only}
- O'Brien J, Austin M, Sethi P, O'Boyle P. Urinary incontinence: prevalence, need for treatment, and effectiveness of intervention by nurse. BMJ (Clinical Research Ed.) 1991;303(6813):1308-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Sullivan 2000 {published data only}
- O'Sullivan R, Anderson P, Louey M, Prashar S, Simons A, Bower W, et al. Long term results of a randomised controlled trial of the nurse continence advisor versus the urogynaecologist in conservative therapy (Abstract number 212). In: International Continence Society (ICS), 30th Annual Meeting; 2000 Aug 28-31; Tampere, Finland. 2000.
- Prashar S, Moore K, Anderson P, Louey M, Cragg S, Simons AM, et al. A randomized controlled trial of nurse continence advisor management versus urogynaecology management of conservative continence therapy: benefits and costs (Abstract). Neurourology and Urodynamics 1998;17(4):423-4. [Google Scholar]
Oh‐Oka 2007 {published data only}
- Oh-Oka H, Fujisawa M. Propiverine/behavioral-therapy combination therapy in over-active-bladder patients, and trial to evaluate urgency (Abstract number 316). In: 37th Annual Meeting of the International Continence Society (ICS); 2007 Aug 20-24; Rotterdam, Netherlands. 2007.
Ouslander 1988 {published data only}
- Ouslander JG, Blaustein J, Connor A, Pitt A. Habit training and oxybutynin for incontinence in nursing home patients: a placebo-controlled trial. Journal of the American Geriatrics Society 1988;36(1):40-6. [DOI] [PubMed] [Google Scholar]
Ouslander 1995 {published data only}
- Ouslander JG, Schnelle JF, Uman G, Fingold S, Nigam JG, Tuico E, et al. Does oxybutynin add to the effectiveness of prompted voiding for urinary incontinence among nursing home residents? A placebo-controlled trial. Journal of the American Geriatrics Society 1995;43(6):610-7. [DOI] [PubMed] [Google Scholar]
Pahwa 2016 {published data only}
- Pahwa A, Schmitz KH, Andy UU, Newman DK, Arya LA. An exercise intervention program in older community dwelling women with urinary incontinence: a mixed methods feasibility study. American Journal of Obstetrics and Gynecology 2016;214(4 Suppl 1):S505. [Google Scholar]
Ramsay 1996 {published data only}
- Ramsay IN, Ali HM, Hunter M, Stark D, McKenzie S, Donaldson K, et al. A prospective, randomized controlled trial of inpatient versus outpatient continence programs in the treatment of urinary incontinence in the female. International Urogynecology Journal and Pelvic Floor Dysfunction 1996;7(5):260-3. [DOI] [PubMed] [Google Scholar]
RBR‐64wczh {published data only}
- RBR-64wczh. Imipramine compared to conservative treatment of urinary incontinence in women urgency [Imipramine versus conservative treatment in women with overactive bladder syndrome [Imipramina versus tratamento conservador em mulheres com Síndrome da Bexiga Hiperativa]]. ensaiosclinicos.gov.br/rg/RBR-64wczh/ (first received 2 January 2012).
Sackley 2008 {published data only}
- Sackley CM, Rodriguez NA, den Berg M, Badger F, Wright C, Besemer J, et al. A phase II exploratory cluster randomized controlled trial of a group mobility training and staff education intervention to promote urinary continence in UK care homes. Clinical Rehabilitation 2008;22(8):714-21. [DOI] [PubMed] [Google Scholar]
Sale 1994 {published data only}
- Sale PG, Wyman JF. Achievement of goals associated with bladder training by older incontinent women. Applied Nursing Research 1994;7(2):93-6. [DOI] [PubMed] [Google Scholar]
Saltmarche 1991 {published data only}
- Saltmarche A, Pringle D, Reid DW, Zorzitto M. Habit retraining: an incontinence study that leaked (Abstract). Neurourology and Urodynamics 1991;10(4):413-4. [Google Scholar]
Sampselle 2017 {published data only}
- Sampselle CM, Newman DK, Miller JM, Kirk K, DiCamillo MA, Wagner TH, et al. A randomized controlled trial to compare 2 scalable interventions for lower urinary tract symptom prevention: main outcomes of the TULIP study. Journal of Urology 2017;197(6):1480-6. [DOI] [PubMed] [Google Scholar]
Schnelle 1983 {published data only}
- Schnelle JF, Traughber B, Morgan DB, Embry JE, Binion AF, Coleman A. Management of geriatric incontinence in nursing homes. Journal of Applied Behavior Analysis 1983;16(2):235-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schnelle 1990 {published data only}
- Schnelle JF. Treatment of urinary incontinence in nursing home patients by prompted voiding. Journal of the American Geriatrics Society 1990;38(3):356-60. [DOI] [PubMed] [Google Scholar]
Schnelle 1995 {published data only}
- Schnelle JF, Keeler E, Hays RD, Simmons S, Ouslander JG, Siu AL. A cost and value analysis of two interventions with incontinent nursing home residents. Journal of the American Geriatrics Society 1995;43(10):1112-7. [DOI] [PubMed] [Google Scholar]
Schnelle 2003 {published data only}
- Schnelle JF, Kapur K, Alessi C, Osterweil D, Beck JG, Al-Samarrai NR, et al. Does an exercise and incontinence intervention save healthcare costs in a nursing home population? Journal of the American Geriatrics Society 2003;51(2):161-8. [DOI] [PubMed] [Google Scholar]
Seers 2018 {published data only}
- Seers K, Rycroft-Malone J, Cox K, Crichton N, Edwards RT, Eldh AC, et al. Facilitating Implementation of Research Evidence (FIRE): an international cluster randomised controlled trial to evaluate two models of facilitation informed by the Promoting Action on Research Implementation in Health Services (PARIHS) framework. Implementation Science 2018;13(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sereika 2003 {published data only}
- Sereika S, Engberg S, Engberg R. Predictors of general health-related quality of life in older adults with urinary incontinence (Abstract). Neurourology and Urodynamics 2003;22(5):392-4. [Google Scholar]
Sherburn 2011 {published data only}
- Sherburn M, Bird M, Carey M, Bø K, Galea MP. Incontinence improves in older women after intensive pelvic floor muscle training: an assessor-blinded randomized controlled trial. Neurourology and Urodynamics 2011;30(3):317-24. [DOI] [PubMed] [Google Scholar]
Shirreff 2020 {published data only}
- Shirreff L, Anderson M, McDermott C. Comparing written and verbal delivery of a treatment regimen to women with overactive bladder: a randomized controlled trial. Menopause 2020;27(1):76-81. [DOI] [PubMed] [Google Scholar]
Sran 2016 {published data only}
- Sran M, Mercier J, Wilson P, Lieblich P, Dumoulin C. Physical therapy for urinary incontinence in postmenopausal women with osteoporosis or low bone density: a randomized controlled trial. Menopause 2016;23(3):286-93. [DOI] [PubMed] [Google Scholar]
Subak 2002 {published data only}
- Subak LL, Quesenberry CP, Posner SF, Cattolica E, Soghikian K. The effect of behavioral therapy on urinary incontinence: a randomized controlled trial. Obstetrics and Gynecology 2002;100(1):72-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sung 2015 {published data only}
- NCT01515722. Interventions to enhance medication persistence and compliance in patients with overactive bladder [Interventions to enhance medication persistence and compliance in patients with overactive bladder: 6-month, randomized, open-label, multi-center trial]. clinicaltrials.gov/show/NCT01515722 (first received 24 January 2010). [NCT01515722]
- Sung HH, Han DH, Kim TH, Lee YS, Lee HN, Seo JT, et al. Interventions do not enhance medication persistence and compliance in patients with overactive bladder: a 24 weeks, randomised, open-label, multi-center trial. International Journal of Clinical Practice 2015;69(11):1309-15. [NCT01515722] [DOI] [PubMed] [Google Scholar]
Surdy 1992 {published data only}
- Surdy TM. Rehabilitation of Urinary Incontinent Nursing Home Patients [PhD thesis]. Milwaukee (WI): University of Wisconsin-Milwaukee, 1992. [Google Scholar]
Suzuki 2019 {published data only}
- Suzuki M, Miyazaki H, Kamei J, Yoshida M, Taniguchi T, Nishimura K, et al. Ultrasound-assisted prompted voiding care for managing urinary incontinence in nursing homes: a randomized clinical trial. Neurourology and Urodynamics 2019;38(2):757-63. [JPRN-UMIN000017963] [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMIN000017963. Ultrasound-assisted prompted voiding for incontinent elderly living in geriatric health services facilities. umin.ac.jp/ctr/index.htm (first received 22 June 2015). [JPRN-UMIN000017963]
Szonyi 1995 {published data only}
- Szonyi G, Collas DM, Ding YY, Malone-Lee JG. Oxybutynin with bladder retraining for detrusor instability in elderly people: a randomized controlled trial. Age and Ageing 1995;24(4):287-91. [DOI] [PubMed] [Google Scholar]
Tak 2012 {published data only}ISRCTN63368283
- ISRCTN63368283. Incondtion [Incondtion: a RCT into the effectiveness of a group based exercise program to reduce urinary incontinence in institutionalized older women]. isrctn.com/ISRCTN63368283 (first received 26 March 2012). [DOI: ] [ISRCTN63368283]
- Tak E, Hespen A, Dommelen P, Hopman-Rock M. Incondition; a multi-level randomized controlled trial of a programme to reduce and prevent urinary incontinence in women in homes for the elderly (Abstract number 190). In: Joint Meeting of the International Continence Society (ICS), 34th Annual Meeting, and the International UroGynecological Association (IUGA); 2004 Aug 23-27; Paris, France. 2004. [sr-incont19035]
- Tak EC, Hespen A, Dommelen P, Hopman-Rock M. Does improved functional performance help to reduce urinary incontinence in institutionalized older women? A multicenter randomized clinical trial. BMC Geriatrics 2012;12:51. [ISRCTN63368283] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hespen AT, Tak EC, Dommelen P, Hopman-Rock M. Evaluation of the urinary incontinence training programme, INCondition, for women living in homes for the elderly. Nederlands Tijdschrift voor Fysiotherapie 2006;116(6):136-42. [Google Scholar]
Tobin 1986 {published data only}
- Tobin GW, Brocklehurst JC. The management of urinary incontinence in local authority residential homes for the elderly. Age and Ageing 1986;15(5):292-8. [DOI] [PubMed] [Google Scholar]
Tomlinson 1999 {published data only}
- Tomlinson BU, Dougherty MC, Pendergast JF, Boyington AR, Coffman MA, Pickens SM. Dietary caffeine, fluid intake and urinary incontinence in older rural women. International Urogynecology Journal and Pelvic Floor Dysfunction 1999;10(1):22-8. [DOI] [PubMed] [Google Scholar]
Voorham 2017 {published data only}
- Voorham JC, De Wachter S, den Bos TW, Putter H, Lycklama a Nijeholt GA, Voorham-van der Zalm PJ. The effect of EMG biofeedback assisted pelvic floor muscle therapy on symptoms of the overactive bladder syndrome in women: a randomized controlled trial. Neurourology and Urodynamics 2017;36(7):1796-803. [DOI] [PubMed] [Google Scholar]
Wadensten 2019 {published data only}
- NCT03097549. Mobile app-treatment of mixed and urgency urinary incontinence in women [Mobile app-treatment of mixed and urgency urinary incontinence in women – a randomized controlled study]. clinicaltrials.gov/show/NCT03097549 (first received 31 March 2017).
- Wadensten T, Nystrom E, Franzen K, Stenzelius K, Lindam A, Samuelsson E. A smartphone app for self-management of urgency and mixed urinary incontinence: a randomized controlled trial (Abstract number 487). Neurourology and Urodynamics 2019;38:S361-3. [Google Scholar]
Wagg 2007 {published data only}
- Wagg AR, Barron D, Kirby M, Stott D, Corlett K. A randomised partially controlled trial to assess the impact of self-help vs. structured help from a continence nurse specialist in women with undiagnosed urinary problems in primary care. International Journal of Clinical Practice 2007;61(11):1863-73. [DOI] [PubMed] [Google Scholar]
Williams 2005 {published data only}
- Williams KS, Assassa RP, Cooper NJ, Turner DA, Shaw C, Abrams KR, et al, Leicestershire MRC Incontinence Study Team. Clinical and cost-effectiveness of a new nurse-led continence service. British Journal of General Practice 2005;55(518):696-703. [PMC free article] [PubMed] [Google Scholar]
Wiseman 1991 {published data only}
- Wiseman PA, Malone-Lee J, Rai GS. Terodiline with bladder retraining for treating detrusor instability in elderly people. BMJ (Clinical Research Ed.) 1991;302(6783):994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wyman 1998 {published data only}
- Elser DM, Wyman JF, McClish DK, Robinson D, Fantl JA, Bump RC, on behalf of the Continence Program for Women Research Group. The effect of bladder training, pelvic floor muscle training, or combination training on urodynamic parameters in women with urinary incontinence. Neurourology and Urodynamics 1999;18(5):427-36. [sr-incont8810] [DOI] [PubMed] [Google Scholar]
- Theofrastous JP, Wyman JF, Bump RC, McClish DK, Elser DM, Bland DR, et al, on behalf of the Continence Program for Women Research Group. Effects of pelvic floor muscle training on strength and predictors of response in the treatment of urinary incontinence. Neurourology and Urodynamics 2002;21(5):486-90. [sr-incont14824] [DOI] [PubMed] [Google Scholar]
- Wyman JF, Fantl JA, McClish DK, Bump RC, Continence Program for Women Research Group. Comparative efficacy of behavioral interventions in the management of female urinary incontinence. American Journal of Obstetrics and Gynecology 1998;179(4):999-1007. [sr-incont6656] [DOI] [PubMed] [Google Scholar]
- Wyman JF, McClish DK, Sale P, Earle B, Camp J. Long-term follow-up of behavioral interventions in incontinent women (Abstract). International Urogynecology Journal and Pelvic Floor Dysfunction 1999;10(Suppl 1):S33. [sr-incont9845] [Google Scholar]
Yoon 2003 {published data only}
- Yoon HS, Song HH, Ro YJ. A comparison of effectiveness of bladder training and pelvic muscle exercise on female urinary incontinence. International Journal of Nursing Studies 2003;40(1):45-50. [DOI] [PubMed] [Google Scholar]
Yu 1991 {published data only}
- Yu LC, Kaltreider L, Hu TW, Craighead WE. Impact of a behavior therapy on the psychological status of incontinent elderly nursing home residents: quantitative and qualitative assessment. In: Myers, WA, editors(s). New Techniques in the Psychotherapy of Older Patients. Arlington (VA): American Psychiatric Press Inc, 1991:181-202. [Google Scholar]
- Yu LC, Rohner TJ, Kaltreider DL, Hu TW, Igou JF, Dennis PJ. Profile of urinary incontinent elderly in long-term care institutions. Journal of the American Geriatrics Society 1990;38(4):433-9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
ChiCTR‐TRC‐14004921 {published data only}
- ChiCTR-TRC-14004921. Effects of cognitive behavioural intervention on overactive bladder patients in China: a randomized controlled trial. www.chictr.org.cn/showproj.aspx?proj=4652 (first received 25 May 2014). [ChiCTR-TRC-14004921] [sr-incont80580]
Lee 1995 {published data only}
- Lee PS, Reid DW, Saltmarche A, Linton L. Measuring the psychosocial impact of urinary incontinence: the York Incontinence Perceptions Scale (YIPS). Journal of the American Geriatrics Society 1995;43(11):1275-8. [sr-incont2911] [DOI] [PubMed] [Google Scholar]
NCT03331081 {published data only}
- NCT03331081. Effects of bladder training and pelvic floor muscle training on the symptomatology of overactive bladder syndrome [Effects of bladder training and pelvic floor muscle training on the symptomatology of overactive bladder syndrome – a randomized controlled clinical trial]. clinicaltrials.gov/show/NCT03331081 (first received 6 November 2017). [NCT03331081] [sr-incont78334]
Rajeev 2017 {published data only}
- Rajeev TP, Silva FD, Suchithra BS. Effect of pelvic floor muscle exercise and bladder re-training program on urinary incontinence [Effectiveness of pelvic floor muscle exercise and bladder re-training program on symptoms and quality of life of women with stress, urge and mixed urinary incontinence in selected tertiary care hospital at Mangalore]. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=19104 (first received 4 August 2017). [CTRI/2017/08/009276] [sr-incont77739]
Teo 2008 {published data only}
- Teo JK, Lim SK. Detrusitol (trademark) and multicomponent behavioral training for overactive bladder syndrome: are they synergistic? (Abstract number 191). In: 38th Annual Meeting of the International Continence Society (ICS); 2008 Oct 20-24; Cairo, Egypt. 2008. [sr-incont29139]
Additional references
Balk 2019
- Balk EM, Rofeberg VN, Adam GP, Kimmel HJ, Trikalinos TA, Jeppson PC. Pharmacologic and Nonpharmacologic Treatments for Urinary Incontinence in Women: a Systematic Review and Network Meta-analysis of Clinical Outcomes. Annals of internal medicine 2019;170(7):465-79. [DOI] [PubMed] [Google Scholar]
Bo 2017
- Bo K, Frawley HC, Haylen BT, Abramov Y, Almeida FG, Berghmans B, et al. An International Urogynecological Association (IUGA)/ International Continence Society (ICS) joint report on the terminology for the conservative and nonpharmacological management of female pelvic floor dysfunction. Neurourology and Urodynamics 2017;36(2):221-44. [DOI: 10.1002/nau.23107] [DOI] [PubMed] [Google Scholar]
Burgio 2008
- Burgio K, Kraus S, Menefee S, Borello-France D, Corton M, Johnson H, et al. Behavioral therapy to enable women with urge incontinence to discontinue drug treatment: a randomized trial. Annals of Internal Medicine 2008;149(3):161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Busner 2007
- Busner J, Targum SD. The Clinical Global Impressions Scale: applying a research tool in clinical practice. Psychiatry 2007;4(7):28-37. [PMC free article] [PubMed] [Google Scholar]
Chancellor 2008
- Chancellor MB, Hasenau DL. Is behavioral therapy plus antimuscarinic better than drug alone to treat overactive bladder? Reviews in Urology 2008;10(4):306-8. [PMC free article] [PubMed] [Google Scholar]
Corcos 2017
- Corcos J, Przydacz M, Campeau L, Gray G, Hickling D, Honeine C, et al. CUA guideline on adult overactive bladder. Canadian Urological Association Journal 2017;11(5):E142-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coyne 2002
- Coyne K, Revicki D, Hunt T, Corey R, Stewart W, Bentkover J, et al. Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: the OAB-q. Quality of Life Research 2002;11(6):563-74. [DOI] [PubMed] [Google Scholar]
Coyne 2011
- Coyne KS, Sexton CC, Vats V, Thompson C, Kopp ZS, Milsom I. National community prevalence of overactive bladder in the United States stratified by sex and age. Urology 2011;77(5):1081-7. [DOI] [PubMed] [Google Scholar]
Coyne 2012
- Coyne KS, Margolis MK, Kopp ZS, Kaplan SA. Racial differences in the prevalence of overactive bladder in the United States from the epidemiology of LUTS (EpiLUTS) study. Urology 2012;79(1):95-101. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Zellweger-Zähner T, Schneider M, Junker C, Lengeler C, Antes G. Language bias in randomised controlled trials published in English and German. Lancet 1997;350(9074):326-9. [DOI] [PubMed] [Google Scholar]
EndNote 2018 [Computer program]
- EndNote. Version X8.2. Philadelphia (PA): Clarivate Analytics, 2018.
Fantl 1996
- Fantl JA, Newman DK, Colling J, DeLancey JOL, Keeys C, Loughery R, et al. Urinary incontinence in adults: acute and chronic management. Clinical practice guideline, no. 2. Rockville: Department of Health and Human Services. Public Health Service, Agency for Health Care Policy and Research; 1996. AHCPR Publication No: 96-0682.
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 17 February 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Guyatt 2011a
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence--imprecision. Journal of Clinical Epidemiology 2011;64(12):1283-93. [DOI] [PubMed] [Google Scholar]
Haylen 2010
- Haylen BT, De Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. International Urogynecology Journal 2010;21(1):5-26. [DOI] [PubMed] [Google Scholar]
Higgins 2022
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook/archive/v6.3.
Irwin 2006
- Irwin DE, Milsom I, Hunskaar S, Reilly K, Kopp Z, Herschorn S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. European Urology 2006;50(6):1306-14; discussion 1314-5. [DOI] [PubMed] [Google Scholar]
Irwin 2009
- Irwin DE, Mungapen L, Milsom I, Kopp Z, Reeves P, Kelleher C. The economic impact of overactive bladder syndrome in six Western countries. BJU International 2009;103(2):202-9. [DOI] [PubMed] [Google Scholar]
Irwin 2011
- Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU International 2011;108(7):1132-8. [DOI] [PubMed] [Google Scholar]
Kelleher 1997
- Kelleher CJ, Cardozo LD, Khullar V, Salvatore S. A new questionnaire to assess the quality of life of urinary incontinent women. British Journal of Obstetrics and Gynaecology 1997;104(12):1374-9. [DOI] [PubMed] [Google Scholar]
Kennelly 2008
- Kennelly MJ, Devoe WB. Overactive bladder: pharmacologic treatments in the neurogenic population. Reviews in Urology 2008;10(3):182-91. [PMC free article] [PubMed] [Google Scholar]
Lightner 2019
- Lightner DJ, Gomelsky A, Souter L, Vasavada SP. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU Guideline Amendment 2019. Journal of Urology 2019;202(3):558-63. [DOI: 10.1097/JU.0000000000000309] [DOI] [PubMed] [Google Scholar]
Milsom 2001
- Milsom I, Abrams P, Cardozo L, Roberts RG, Thüroff J, Wein AJ. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study [erratum appears in BJU Int 2001;88(7):807]. BJU International 2001;87(9):760-6. [DOI] [PubMed] [Google Scholar]
Nambiar 2018
- Nambiar AK, Bosch R, Cruz F, Lemack GE, Thiruchelvam N, Tubaro A, et al. EAU guidelines on assessment and nonsurgical management of urinary incontinence. European Urology 2018;73(4):596-609. [DOI] [PubMed] [Google Scholar]
Nygaard 2010
- Nygaard I. Idiopathic urgency urinary incontinence. New England Journal of Medicine 2010;363(12):1156-62. [DOI] [PubMed] [Google Scholar]
Rai 2012
- Rai BP, Cody JD, Alhasso A, Stewart L. Anticholinergic drugs versus non-drug active therapies for non-neurogenic overactive bladder syndrome in adults. Cochrane Database of Systematic Reviews 2012, Issue 12. Art. No: CD003193. [DOI: 10.1002/14651858.CD003193.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roe 2007
- Roe B, Ostaszkiewicz J, Milne J, Wallace S. Systematic reviews of bladder training and voiding programmes in adults: a synopsis of findings from data analysis and outcomes using metastudy techniques. Journal of Advanced Nursing 2007;57(1):15-31. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Available from gdt.gradepro.org/app/handbook/handbook.html.
Schünemann 2019
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Shamliyan 2008
- Shamliyan TA, Kane RL, Wyman J, Wilt TJ. Systematic review: randomized, controlled trials of nonsurgical treatments for urinary incontinence in women. Annals of Internal Medicine 2008;148(6):459-73. [DOI] [PubMed] [Google Scholar]
Stewart 2003
- Stewart WF, Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, et al. Prevalence and burden of overactive bladder in the United States. World Journal of Urology 2003;20(6):327-36. [DOI] [PubMed] [Google Scholar]
Vaughan 2011
- Vaughan CP, Johnson TM, Ala-Lipasti MA, Cartwright R, Tammela TL, Taari K, et al. The prevalence of clinically meaningful overactive bladder: bother and quality of life results from the population-based FINNO study. European Urology 2011;59(4):629-36. [DOI] [PubMed] [Google Scholar]
Wallace 2004
- Wallace SA, Roe B, Williams K, Palmer M. Bladder training for urinary incontinence in adults. Cochrane Database of Systematic Reviews 2004, Issue 1. Art. No: CD001308. [DOI: 10.1002/14651858.CD001308.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiting 2016
- Whiting P, Savović J, Higgins JP, Caldwell DM, Reeves BC, Shea B, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. Journal of Clinical Epidemiology 2016;69:225-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Funada 2020
- Funada S, Yoshioka T, Luo Y, Sato A, Akamatsu S, Watanabe N. Bladder training for treating overactive bladder in adults. Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD013571. [DOI: 10.1002/14651858.CD013571] [DOI] [PMC free article] [PubMed] [Google Scholar]


