Abstract
Background
Free oxygen radicals have been implicated in the pathogenesis of bronchopulmonary dysplasia (BPD) in preterm infants. Superoxide dismutase (SOD) is a naturally occurring enzyme which provides a defense against such oxidant injury. Providing supplementary SOD has been tested in clinical trials to prevent BPD in preterm infants.
Objectives
To determine the efficacy and safety of SOD in the prevention and treatment of BPD on mortality and other complications of prematurity in infants at risk for, or having BPD.
Search methods
We searched CENTRAL, PubMed, Embase, and three trials registers on 22 September 2022 together with reference checking, citation searching and contact with study authors to identify additional studies.
Selection criteria
Randomized, quasi‐randomized and cluster‐randomized controlled trials (RCTs) where the participants were preterm infants who had developed, or were at risk of developing BPD, and who were randomly allocated to receive either SOD (in any form, by any route, any dose, anytime) or placebo, or no treatment.
Data collection and analysis
We used standard Cochrane methods. Our primary outcomes were BPD defined as an oxygen requirement at 28 days, BPD defined as oxygen at 36 weeks' postmenstrual age, neonatal mortality, mortality prior to discharge, and BPD or death at 36 weeks' postmenstrual age. We reported risk ratio (RR) and risk difference (RD) with 95% confidence intervals (CIs) for the dichotomous outcomes. We used GRADE to assess certainty of evidence for each outcome.
Main results
We included three RCTs (380 infants) on SOD administration in preterm infants at risk for BPD, and no studies in preterm infants with evolving BPD / early respiratory insufficiency.
The evidence is very uncertain about the effect of SOD on BPD defined as an oxygen requirement at 28 days (RR 1.09, 95% CI 0.94 to 1.26; RD 0.06, 95% CI ‐0.05 to 0.16, 1 study, 302 infants; I2 for RR and RD not applicable), BPD defined as oxygen at 36 weeks' postmenstrual age (RR 0.96, 95% CI 0.72 to 1.29; RD ‐0.01, 95% CI ‐0.11 to 0.09, 2 studies, 335 infants; I2 for RR and RD = 0%), neonatal mortality (RR 0.98, 95% CI 0.57 to 1.68; RD ‐0.00, 95% CI ‐0.08 to 0.07, 2 studies, 335 infants; I2 for RR and RD = 0%), and mortality prior to discharge (RR 1.20, 95% CI 0.53 to 2.71; RD 0.04, 95% CI ‐0.14 to 0.23, 2 studies, 78 infants; I2 for RR and RD = 0%). No studies reported BPD or death at 36 weeks' postmenstrual age. The evidence is very uncertain about the effect of SOD on retinopathy of prematurity any stage (RR 0.95, 95% CI 0.78 to 1.15; RD ‐0.03, 95% CI ‐0.15 to 0.08, 2 studies, 335 infants; I2for RR = 0%, I2 for RD = 8%), and severe retinopathy of prematurity (ROP) (RR 0.97, 95% CI 0.57 to 1.65; RD ‐0.01, 95% CI ‐0.10 to 0.09, 1 study, 244 infants; I2 for RR and RD not applicable). No studies reported moderate to severe neurodevelopmental outcome at 18 to 24 months. Certainty of evidence was very low for all outcomes.
We identified no ongoing trials.
Authors' conclusions
The evidence is very uncertain about the effect of SOD on BPD defined as an oxygen requirement at 28 days, BPD defined as oxygen at 36 weeks' postmenstrual age, neonatal mortality and mortality prior to discharge compared to placebo. No studies reported BPD or death at 36 weeks' postmenstrual age and need for supplemental oxygen. The evidence is very uncertain about the effect of SOD on retinopathy of prematurity any stage and severe retinopathy of prematurity. No studies reported moderate to severe neurodevelopmental outcome at 18 to 24 months.
The effects of SOD in preterm infants has not been reported in any trial in the last few decades, considering that the most recent trial on SOD in preterm infants was conducted in 1997/1998, and no new studies are ongoing. In the light of the limited available evidence, new data from preclinical and observational studies are needed to justify the conduction of new RCTs. Observational studies might report how SOD is administered, including indication, dose and association with relevant outcomes such as mortality, BPD and long‐term neurodevelopment.
Keywords: Humans; Infant; Infant, Newborn; Bronchopulmonary Dysplasia; Bronchopulmonary Dysplasia/prevention & control; Infant, Premature; Oxygen; Randomized Controlled Trials as Topic; Retinopathy of Prematurity; Retinopathy of Prematurity/prevention & control; Superoxide Dismutase; Superoxide Dismutase/therapeutic use
Plain language summary
Superoxide dismutase in preterm newborns at risk of lung disease
Key messages
• We did not find enough good‐quality evidence about the use of superoxide dismutase in newborns born too early: only three studies for the prevention of lung disease.
• The evidence is very uncertain about the effect of superoxide dismutase on bronchopulmonary dysplasia, also known as chronic lung disease, death, retinopathy of prematurity (eye damage which might cause blindness) compared to a placebo or no treatment.
• No studies reported bronchopulmonary dysplasia or death at 36 weeks' postmenstrual age, need for giving oxygen, and long‐term development. Postmenstrual age is the combination of gestational age (length of pregnancy) and chronological age the day after the birth of the baby.
What is bronchopulmonary dysplasia?
Newborns born too early ("preterm"), especially babies born before 28 weeks of pregnancy, have a higher risk for death, lung disease and brain impairment than those born at or near term. For instance, some of these babies develop intellectual disabilities, blindness or deafness. Bronchopulmonary dysplasia, also known as chronic lung disease, is a common problem in preterm babies who are mechanically ventilated (machine assisted breathing) and consists of being dependent of oxygen or breathing machines. Free oxygen radicals, i.e. products of chemical reactions that use oxygen, are believed to cause bronchopulmonary dysplasia because they are very unstable, so they can damage other cells.
What is superoxide dismutase?
Superoxide dismutase is a protein normally present in the body to provide a defense against free radicals, but preterm infants do not have a sufficient supply to provide natural resistance. Giving superoxide dismutase to preterm infants may therefore prevent bronchopulmonary dysplasia.
What did we want to find out?
We wanted to find out if superoxide dismutase could reduce the risk of:
• bronchopulmonary dysplasia
• death
• the combination of bronchopulmonary dysplasia and death
What did we do?
We searched for studies that looked at superoxide dismutase in babies born too early. We compared and summarized the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We included three studies in our review, with a total of 380 preterm newborns at risk of bronchopulmonary dysplasia. It is unclear whether superoxide dismutase reduces bronchopulmonary dysplasia, death, or the combination of bronchopulmonary dysplasia and death. The dose ranged from 0.25 mg/kg to 5.0 mg/kg. Superoxide dismutase was given to the babies by injection under the skin or directly into the trachea. There are no ongoing studies.
What are the limitations of the evidence?
We are not confident in the evidence on bronchopulmonary dysplasia and death because the studies were small and used methods likely to introduce errors in their results. Overall, the results of the studies are unlikely to reflect the results of all the studies that have been conducted in this area, some of which have not made their results public yet.
How up to date is this evidence?
The evidence is up‐to‐date to September 2022.
Summary of findings
Summary of findings 1. Superoxide dismutase (SOD) compared to placebo for preventing bronchopulmonary dysplasia (BPD) in preterm infants.
| Superoxide dismutase (SOD) compared to placebo for preventing bronchopulmonary dysplasia (BPD) in preterm infants | ||||||
| Patient or population: preterm infants at risk of developing BPD Setting: neonatal intensive care units Intervention: SOD Comparison: placebo | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | What happens | ||
| Without SOD | With SOD | Difference | ||||
| BPD defined as an oxygen requirement at 28 days № of participants: 302 (1 RCT) | RR 1.09 (0.94 to 1.26) | Study population | ⊕⊝⊝⊝ Very low 1 | The evidence is very uncertain about the effect of superoxide dismutase on BPD defined as an oxygen requirement at 28 days compared to placebo. | ||
| 66.9% | 72.9% (62.9 to 84.3) | 6.0% more (4 fewer to 17.4 more) | ||||
| BPD defined as oxygen at 36 weeks' postmenstrual age № of participants: 335 (2 RCTs) | RR 0.96 (0.72 to 1.29) | Study population | ⊕⊝⊝⊝ Very low 1 | The evidence is very uncertain about the effect of superoxide dismutase on BPD defined as an oxygen at 36 weeks' postmenstrual age compared to placebo. | ||
| 35.2% | 33.8% (25.4 to 45.4) | 1.4% fewer (9.9 fewer to 10.2 more) | ||||
| Neonatal mortality (i.e. within 28 days) № of participants: 335 (2 RCTs) | RR 0.98 (0.57 to 1.68) | Study population | ⊕⊝⊝⊝ Very low 1 | The evidence is very uncertain about the effect of superoxide dismutase on neonatal mortality compared to placebo. | ||
| 13.8% | 13.6% (7.9 to 23.2) | 0.3% fewer (5.9 fewer to 9.4 more) | ||||
| Mortality prior to discharge № of participants: 78 (2 RCTs) | RR 1.20 (0.53 to 2.71) | Study population | ⊕⊝⊝⊝ Very low 1 | The evidence is very uncertain about the effect of superoxide dismutase on mortality prior to discharge compared to placebo. | ||
| 22.9% | 27.4% (12.1 to 61.9) | 4.6% more (10.7 fewer to 39.1 more) | ||||
| BPD or death at 36 weeks' postmenstrual age № of participants: 0 (0 RCTs) | Not reported | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome. |
| Need for supplemental oxygen (days) № of participants: 0 (0 RCTs) | Not reported | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome. |
| Retinopathy of prematurity № of participants: 277 (2 RCTs) | RR 0.95 (0.78 to 1.15) | Study population | ⊕⊝⊝⊝ Very low 1 | The evidence is very uncertain about the effect of superoxide dismutase on retinopathy of prematurity (any stage) compared to placebo. | ||
| 61.1% | 58.0% (47.6 to 70.2) | 3.1% fewer (13.4 fewer to 9.2 more) | ||||
| Severe retinopathy of prematurity (stage II or greater) № of participants: 244 (1 RCT) | RR 0.97 (0.57 to 1.65) | Study population | ⊕⊝⊝⊝ Very low 1 | The evidence is very uncertain about the effect of superoxide dismutase on severe retinopathy of prematurity (stage II or greater) compared to placebo. | ||
| 18.3% | 17.7% (10.1 to 29.3) | 0.6% fewer (8.2 fewer to 11 more) | ||||
| Moderate to severe neurodevelopmental outcome at 18 to 24 months № of participants: 0 (0 RCTs) | Not reported | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomized controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 downgrade one level for risk of bias: unclear risk in multiple domains; downgrade two levels for imprecision: few events, small sample size, confidence interval overlapping the no difference line
Background
Description of the condition
Survival of preterm infants has increased in the previous 30 years due to a variety of antenatal and postnatal interventions (Horbar 2012; Soll 2013). Despite these significant advances in neonatal intensive care, bronchopulmonary dysplasia (BPD) still occurs in a significant proportion of preterm infants (Horbar 2012). BPD is a common morbidity in preterm infants, affecting 22% to 38% of extremely low gestational age neonates (gestational age < 28 weeks) (Poets 2018). Annually in the USA, there are approximately 10,000 to 15,000 new cases of BPD each year; BPD is typically more prevalent in infants that weigh less than 1250 g (Balany 2015). BPD has long‐lasting adverse effects including chronic respiratory difficulties, recurrent infection, prolonged and recurrent hospitalizations, increased evidence of neurodevelopmental disabilities, growth restriction and death (Anderson 2006; Bhandari 2006; O'Brodovich 1985).
The history of BPD is ever evolving and elucidation of its pathophysiology has great potential to provide life‐saving treatments for preterm infants (Bancalari 1979; Farrell 1997; Northway 1967). Northway was the first to describe this disease in infants who had used mechanical ventilation or had high oxygen requirements (Northway 1967). In the decades that followed, multiple definitions of BPD were proposed, many focusing on clinical and radiographic characteristics that were present at one month of age (Ehrenkranz 2005). In 1988, Shennan and colleagues proposed a new definition, which is widely used now, of an oxygen requirement at 36 weeks' postmenstrual age (PMA) because it is more accurate in predicting the long‐term pulmonary consequences of BPD (Isayama 2017; Shennan 1988).
The etiology of chronic lung disease in preterm infants is thought to be multifactorial. In his original report in 1967, Northway hypothesized that oxygen toxicity, pulmonary healing in the setting of severe respiratory distress, and poor ventilation were implicated in BPD (Northway 1967). Currently, pathogenesis of BPD is also thought to be a multifactorial event that encompasses prenatal, postnatal, genetic, and environmental factors that act on immature lungs (Sampath 2015). Events such as infection can cause localized inflammation that can persist and are exacerbated by the use of mechanical ventilation or assistance with oxygen (Balany 2015). Increased ventilation and oxygen generate production of free radicals, which further elicit inflammation and injury to the mucosal surface of the lung (Sampath 2015). Balany postulated that these injuries cause immune dysregulation, and subsequent remodeling of the premature lung, which later progress to BPD (Balany 2015).
Given the multifaceted nature of the pathogenesis of BPD, it is not surprising that multiple interventions have been tested to prevent or treat BPD, and met with variable success. Antenatal infections can cause chorioamnionitis from organisms, such as Ureaplasma species, which can cause dysregulation of lung growth through inflammatory effects (Kallapur 2013). Antenatal corticosteroids work by stimulating growth of the immature lung (McGoldrick 2020). It has been shown that antenatal steroids reduce respiratory distress syndrome and mortality; however there seems to be no significant statistical reduction in BPD alone (Goldstein 2017; Jain 2014). In contrast, postnatal steroids administered in the first few weeks of life to infants at risk of BPD have reduced the use of assisted ventilation and BPD (Doyle 2021a; Doyle 2021b). However, both the short‐term and longer‐term complications of postnatal steroid exposure (including increased risk of cerebral palsy) have led to curtailed use in very low birth weight infants (AAP 2010).
Other potential strategies to reduce BPD in preterm infants include managing respiratory support. Trials have shown that use of high frequency ventilation, non‐invasive respiratory support (continuous positive airway pressure [CPAP] or non‐invasive positive pressure ventilation pressure [NIPPV]), permissive hypercapnia, and reduced oxygen support do not produce any statistically significant reductions in the incidence of BPD (Jain 2014; Ma 2016). However, volume‐controlled ventilation, as well as exogenous surfactant, have been shown to reduce rates of BPD and mortality of preterm infants (Klingenberg 2017; Seger 2009).
Multiple pharmacologic agents have been proposed to treat BPD with varying degrees of success. Current pharmacologic interventions include systemic corticosteroids (Doyle 2021a; Doyle 2021b), caffeine (Schmidt 2006), and vitamin A (Darlow 2016; Ghanta 2013). Interestingly, caffeine has been used for apnea of prematurity; however studies have found that it decreases rates of BPD and reduces the need for ventilation in the first seven days of life (Schmidt 2006). Intramuscular vitamin A is another agent that has shown efficacy in reducing the rates of chronic lung disease and mortality, with no known long‐term effects on neurodevelopmental disease (Darlow 2016). Inhaled nitric oxide (iNO) is another pharmacological treatment that has been suggested for preventing BPD; however, a meta‐analysis of trials in various preterm populations found that there is no significant reduction in mortality or incidence of BPD (Barrington 2017).
Antioxidant therapy has also been a suggested for treatment of BPD. Vitamin E has been shown to function as a scavenger for free radicals protecting cells from oxidant injury (Biniwale 2006). Consequently, other antioxidant therapies, such as superoxide dismutase (SOD), have great potential to mitigate or reduce BPD by blocking the effects of free radicals.
Description of the intervention
SOD is an intracellular enzyme that converts the extremely toxic superoxide radical into potentially less toxic hydrogen peroxide (Pham‐Huy 2008). SOD appears in two forms: one in the cytoplasm of the cell or in the extracellular spaces with two subunits, each with one equivalent of Cu2+ and Zn2+; the other in the mitochondria with Mn2+ as its subunit.
How the intervention might work
Arguably, oxygen is the most essential element in the human body. It is involved in maintaining basic life process, but if unregulated can cause severe damage in the form of free radicals. A free radical is an atom or molecule that contains an unpaired electron. Radicals produced within the body include the superoxide and hydroxyl radicals, hydrogen peroxide, hypochlorous acid, peroxynitrite, and nitric oxide. Free radicals are produced in abundance in all cells. This type of oxidative stress causes cell damage and at a molecular level, DNA and RNA damage (Wojtunik‐Kulesza 2016).
In healthy humans a balance exists between oxygen‐derived free‐radical production and their inactivation by antioxidant defenses. Typically, the body can counteract free radicals; however if there is an inability to reduce them via anti‐oxidative enzymes, then catastrophic tissue damage can occur (Wojtunik‐Kulesza 2016). In adults, oxidative stress is implicated in aging, cardiovascular disease, cataracts, neurodegenerative disorders, neoplasia, and other disorders (Knight 1998; Pham‐Huy 2008).
Disturbances in this balance may contribute to the pathogenesis of certain disease processes seen in the preterm infant such as chronic lung disease (Fardy 1995; Kelly 1993; Saugstad 1990), retinopathy of prematurity (ROP) (Kelly 1993; Saugstad 1990), intraventricular hemorrhage (IVH) (Kelly 1993), and periventricular leukomalacia (PVL) (Volpe 1997). Preterm infants are often exposed to excessive oxidative stress because they are exposed to high oxygen concentrations due to surfactant deficiency and immature lungs. In addition, preterm infants have inadequate antioxidant defenses and are not able to induce antioxidant enzymes in response to oxidative stress (Davis 1998; Saugstad 1998). Also, inflammation and infection, which are closely linked to oxidative stress, are more common in preterm infants (Saugstad 1998).
However, numerous natural defenses exist either to prevent the formation of free radicals, or to neutralize them once they are produced. Antioxidants, including SOD, play a key role in mitigating the damage caused by free radicals. It is thought that infants have a reduction or a deficiency in these enzymes due to prematurity, which results in increased susceptibility to oxidative damage to growing tissue. It has been hypothesized that providing exogenous antioxidants in the form of SOD can potentially prevent BPD and other secondary outcomes by reducing the increased oxidative stress experienced by preterm infants born to a hyperoxic environment.
Why it is important to do this review
BPD remains an ongoing problem in preterm infants with few safe treatments available. Ongoing research into antioxidant therapies, including SOD, hold great promise for future treatment. This systematic review will review all randomized trials of exogenously administered SOD for the prevention of chronic lung disease in preterm infants. It is an update of the original Suresh 2001 review, which however included only ventilated infants.
Objectives
To determine the efficacy and safety of superoxide dismutase (SOD) in the prevention and treatment of bronchopulmonary dysplasia (BPD) on mortality and other complications of prematurity in infants at risk for or having BPD.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomized controlled trials (RCTs) where participants were randomly allocated to receive SOD versus placebo or no treatment. We did not find quasi‐randomized controlled trials(QRCTs) or cluster‐RCTs to include. Non‐randomized cohort studies were deemed not eligible for this review, given the fact of potential bias of confounding by indication or residual confounding influencing the results of studies with such designs (Fewell 2007; Kyriacou 2016).
Types of participants
We included studies conducted in preterm infants of 32 weeks' gestation or less, or very low birth weight (VLBW) infants weighing less than 1500 g who were at risk of BPD (regardless of respiratory support), or infants with evolving BPD / early respiratory insufficiency who required respiratory support, including conventional ventilation, high frequency ventilation, non‐invasive positive pressure ventilation pressure (NIPPV), Nasal continuous positive airway pressure (NCPAP) or supplemental oxygen.
Types of interventions
We included studies in which SOD was administered in any form, by any route, any dose, and at any time in the first six months of life compared to placebo or no treatment in the control group.
Comparison 1: SOD versus no treatment or placebo in preterm infants at risk for BPD.
Comparison 2: SOD versus no treatment or placebo in preterm infants with evolving BPD / early respiratory insufficiency.
Types of outcome measures
Primary outcomes
BPD defined as an oxygen requirement at 28 days.
BPD defined as oxygen at 36 weeks' postmenstrual age (Jobe 2001).
Neonatal mortality.
Mortality prior to discharge.
BPD or death at 36 weeks' postmenstrual age.
Secondary outcomes
Hemodynamically significant patent ductus arteriosus (PDA) (Arlettaz 2017).
PDA requiring treatment.
Late onset sepsis (with proven culture).
Necrotizing enterocolitis (NEC) (Bell ≥ stage 2) (Bell 1978).
IVH (any) (Papile 1978).
Intraventricular hemorrhage (IVH) (grades III to IV) (Papile 1978).
Periventricular leukomalacia (PVL).
ROP (any stage) (International Committee 2005).
Severe retinopathy of prematurity (ROP) (stage II or greater).
Duration of assisted ventilation (days).
Duration of oxygen dependence (days).
Duration of hospital stay (days).
-
Moderate to severe neurodevelopmental outcome at 18 to 24 months (any of the following complications):
cerebral palsy, developmental delay (Bayley or Griffith assessment > 2 standard deviations (SD) below the mean) (Bayley 2006; Griffiths 1954);
intellectual impairment (intelligence quotient [IQ] > 2 SD below the mean);
blindness (vision < 6/60 in both eyes);
sensorineural deafness (requiring amplification)
-
Components of moderate to severe neurodevelopmental outcomes at 18 to 24 months, including:
cerebral palsy;
developmental delay (Bayley or Griffith assessment > 2 SD below the mean);
intellectual impairment (IQ > 2 SD below the mean);
blindness (vision < 6/60 in both eyes);
sensorineural deafness requiring amplification.
Search methods for identification of studies
The Cochrane Sweden Information Specialist developed a draft search strategy for PubMed (National Library of Medicine) in consultation with the authors (Appendix 1). This strategy has been peer‐reviewed by an Information Specialist using the PRESS Checklist (McGowan 2016a; McGowan 2016b). The PubMed strategy has been translated, using appropriate syntax, for other databases.
A population filter developed by Cochrane Neonatal has been used. The RCT search filter for Ovid MEDLINE as recommended by Cochrane Neonatal was adapted to the syntax of PubMed (NLM) and used to identify randomized and quasi‐randomized studies. Searches for eligible trials have been conducted without language, publication year, publication type, or publication status restrictions.
Electronic searches
The following databases have been searched:
Cochrane CENTRAL Register of Controlled Trials (CENTRAL), via Wiley;
PubMed (National Library of Medicine) (1946 to September 2022);
Embase.com, (Elsevier) (1974 to September 2022).
Searching other resources
Trial registration records have been identified using CENTRAL and by independent searches of:
ISRCTN registry (https://www.isrctn.com) (October 2022);
US NIH 1979 Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov), (October 2022);
ICTRP‐‐World Health Organization International Clinical Trials Registry Platform (https://trialsearch.who.int/Default.aspx), (October 2022);
The reference lists of included studies and related systematic reviews have been screened for studies not identified by the database searches.
We searched for errata or retractions for included studies published on PubMed (www.ncbi.nlm.nih.gov/pubmed), (October 2022).
Data collection and analysis
We collected information regarding the method of randomization, blinding, intervention, stratification, and whether the trial was single or multicenter for each included study. We noted information regarding trial participants including gestational age, birthweight, sex. We analyzed the clinical outcomes noted above in Types of outcome measures.
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to a reference management software and removed duplicates. The remaining title/abstracts have been screened independently by two review authors (MA, TP). The full‐text of references included after title/abstract review have been assessed independently by two review authors. At any point in the screening process, disagreements between review authors have been resolved by discussion or a third review author. The reasons for excluding studies during review of full‐texts are documented in the review; reasons for exclusion were the absence of one or more PICO‐S elements; where a study omitted more than one PICO‐S element, we documented only one. We collated multiple reports of the same study so that each study, rather than each report, is the unit of interest in the review. We did not find any ongoing studies. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Liberati 2009).
Data extraction and management
Two review authors (MA, TP) independently extracted data using a data extraction form integrated with a modified version of the Cochrane Effective Practice and Organization of Care Group data collection checklist (Cochrane EPOC Group 2017). We planned to pilot the form within the review team using a sample of included studies, however this was not performed as we included only three studies.
We extracted the following characteristics from each included study.
Administrative details: study author(s); published or unpublished; year of publication; year in which study was conducted; presence of vested interest; details of other relevant papers cited.
Study characteristics: study registration, study design type, study setting, completeness of follow‐up (e.g. greater than 80%).
Participants: number randomized, number lost to follow‐up/withdrawn, number analyzed, mean gestational age (GA), GA age range, severity of condition, diagnostic criteria, inclusion criteria and exclusion criteria.
Interventions: initiation, dose, way of administration and duration of administration.
Outcomes as mentioned above under Types of outcome measures.
We resolved any disagreements by discussion.
We planned to describe ongoing studies identified by our search and document available information however, we did not find any ongoing studies.
Two review authors (MA, TP) used Cochrane statistical software for data entry (Review Manager 2020). We planned to replace any standard error of the mean (SEM) by the corresponding SD, but it was not necessary because we did not find any studies reporting the SEM.
Assessment of risk of bias in included studies
Independently, two review authors (MA, TP) assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane risk of bias’ tool for the following domains (Higgins 2022a).
Sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Any other bias
We resolved any disagreements by discussion or with the input of a third assessor. See Appendix 2 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
Dichotomous data
For dichotomous data we presented results using risk ratios (RRs) and risk differences (RDs) with 95% confidence intervals (CIs). We planned to calculate the number needed to treat for an additional beneficial outcome (NNTB), or the number needed to treat for an additional harmful outcome (NNTH) with 95% CIs, however there was no a statistically significant reduction (or increase) in RD.
Continuous data
For continuous data we planned to use the mean difference (MD) when outcomes were measured in the same way between trials; however, no studies reported means and standard deviation (SD). We planned to use the standardized mean difference (SMD) to combine trials that measured the same outcome but used different methods. Where trials reported continuous data as median and interquartile range (IQR), and data passed the test of skewness, we planned to convert median to mean and estimate the SD as IQR/1.35, however we could not ensure that data were normally distributed.
If data were not reported in an RCT in a format that could be entered directly into a meta‐analysis, we planned to convert them to the required format using the information inChapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022b).
Unit of analysis issues
We performed the primary analysis per individual randomized.
For cluster‐randomized trials, we planned to abstract information on the study design and unit of analysis for each study, indicating whether clustering of observations was present due to allocation to the intervention at the group level or clustering of individually randomized observations (e.g. patients within clinics). We planned to abstract available statistical information needed to account for the implications of clustering on the estimation of outcome variances, such as design effects or intra‐cluster correlations, and whether the study adjusted results for the correlations in the data. In cases where the study did not account for clustering, we planned to ensure that appropriate adjustments were made to the effective sample size following Cochrane guidance (Higgins 2022b). Where possible, we planned to derive the intra‐cluster correlation (ICC) for these adjustments from the trial itself, or from a similar trial. If an appropriate ICC was unavailable, we planned to conduct sensitivity analyses to investigate the potential effect of clustering by imputing a range of values of ICC. However, we did not include any cluster‐randomized trials in our review.
If any trials had multiple arms that were compared against the same control condition that was included in the same meta‐analysis, we would have combined the data to compare the intervention arms together against the control arm, and we specified it in a footnote in the forest plot.
Dealing with missing data
Where feasible, we intended to carry out analysis on an intention‐to‐treat basis for all outcomes. We analyzed all participants in the treatment group to which they were randomized, regardless of the actual treatment received, whenever possible. If important missing data (in the outcomes) or unclear data were identified, we requested the missing data by contacting the original investigators. We made explicit the assumptions of any methods we used to cope with missing data. We planned to perform sensitivity analyses to assess how sensitive results were to reasonable changes in the assumptions that were made. We addressed the potential impact of missing data on the findings of the review in the Discussion section.
Assessment of heterogeneity
We described the clinical diversity and methodological variability of the evidence narratively and in tables. Tables (Characteristics of included studies), include data on study characteristics such as design features, population characteristics, and intervention details.
To assess statistical heterogeneity, we visually inspected forest plots and described the direction and magnitude of effects and the degree of overlap between confidence intervals. We also considered the statistics generated in forest plots that measure statistical heterogeneity. We used the I² statistic to quantify inconsistency among the trials in each analysis. We also considered the P value from the Chi² test to assess if this heterogeneity is significant (P < 0.1). If we identified substantial heterogeneity, we planned to report the finding and explore possible explanatory factors using prespecified subgroup analysis, however, no substantial heterogeneity was identified.
We graded the degree of heterogeneity as:
0% to 40% might not represent important heterogeneity;
30% to 60% may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity;
more than 75% may represent considerable heterogeneity
A rough guideline has been used to interpret the I2 value rather than a simple threshold, and our interpretation took into account an understanding that measures of heterogeneity (I2 and Tau) have been estimated with high uncertainty because the number of studies was small (Deeks 2022).
Assessment of reporting biases
We assessed reporting bias by comparing the stated primary outcomes and secondary outcomes and reported outcomes. If study protocols had been available, we would have compared these to the full publications to determine the likelihood of reporting bias; however, study protocols were not available. Studies using the interventions in a potentially eligible infant population, but not reporting on any of the primary and secondary outcomes were planned to be documented in the'Characteristics of included studies” tables' however, this did not occur.
We planned to use the funnel plots to screen for publication bias if there was a sufficient number of studies (> 10) reporting the same outcome. If publication bias had been suggested by a significant asymmetry of the funnel plot on visual assessment, we would have incorporated this in our assessment of certainty of evidence (Egger 1997). Since our review included few studies eligible for meta‐analysis, the ability to detect publication bias was largely diminished, and we simply noted our inability to rule out possible publication bias or smalls‐study effects.
Data synthesis
As we considered the three trials to be sufficiently similar, we performed meta‐analysis using Review Manager 5 (Review Manager 2020). For categorical outcomes, we calculated the typical estimates of RR and RD, each with its 95% CI; for continuous outcomes. We planned to calculate the MD or the SMD, each with its 95% CI. We used a fixed‐effect model to combine data where it was reasonable to assume that studies were estimating the same underlying treatment effect.
Subgroup analysis and investigation of heterogeneity
We planned to interpret tests for subgroup differences in effects with caution given the potential for confounding with other study characteristics and the observational nature of the comparisons. See Section 10.11.2 Cochrane handbook version six. In particular, subgroup analyses with fewer than five studies per category are unlikely to be adequate to ascertain valid differences in effects and were not highlighted in our results. When subgroup comparisons should be possible, stratified meta‐analysis and a formal statistical test for interaction would be conducted to examine subgroup differences that could account for effect heterogeneity (e.g., Cochran’s Q test, meta‐regression) (Borenstein 2013; Higgins 2022b).
Given the potential differences in the intervention effectiveness related to gestational age, type and dose of SOD, respiratory status and age at treatment discussed in the Background section, we planned to conduct subgroup comparisons to see if SOD is more effective for BPD in preterm infants.
We planned to carry out the following subgroup analyses of factors that may contribute to heterogeneity in the effects of the intervention:
gestational age (very preterm infants, i.e. less than 32 weeks' gestation; extremely preterm infants, i.e. less than 28 weeks' gestation);
type and dose of SOD used;
respiratory status (assisted ventilation, CPAP, NIPPV);
age at treatment (less than 32 weeks' gestation).
However, we did not perform any subgroup analyses because we only included three studies.
We would have used the main outcomes in subgroup analyses if there had been enough studies reporting to support valid subgroup comparisons (at least five studies per subgroup).
Sensitivity analysis
If we had identified substantial heterogeneity, we would have conducted sensitivity analysis to determine if the findings were affected by the inclusion of only those trials:
considered to have used adequate methodology with a low risk of bias (selection, performance and reporting bias);
with the right characteristics of participants (e.g. infants in some RCTs meet the age range criteria of the review).
We would have only reported the results of sensitivity analyses for primary outcomes.
Given that there is no formal statistical test that can be used for sensitivity analysis, we would have provided informal comparisons between the different ways of estimating the effect under different assumptions. Changes in the P values should not be used to judge whether there is a difference between the main analysis and sensitivity analysis, since statistical significance may be lost with fewer studies included.
We planned to report sensitivity analysis results in tables rather than forest plots.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the certainty of evidence for the following outcomes.
BPD defined by an oxygen requirement at 28 days or an oxygen at 36 weeks' postmenstrual age.
Mortality (neonatal mortality at 28 days and death prior to discharge).
BPD or death at 36 weeks' postmenstrual age.
Need for supplemental oxygen (days).
ROP defined by any stages or zones reported (stage 2 or greater)(International Committee 2005).
Moderate to severe neurodevelopmental outcome at 18 to 24 months (any of the following complications): cerebral palsy, developmental delay (Bayley or Griffith assessment > 2 SDs below the mean); intellectual impairment (intelligence quotient (IQ) > 2 SD below the mean); blindness (vision < 6/60 in both eyes); sensorineural deafness requiring amplification.
Two review authors (MA, MB) independently assessed the certainty of the evidence for each of the outcomes above. We considered evidence from randomized controlled trials (RCTs) as high certainty, downgrading the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used GRADEpro GDT Guideline Development Tool to create a summary of findings table to report the certainty of the evidence.
The GRADE approach results in an assessment of the certainty of a body of evidence in one of the following four grades.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
We have provided results of the search for this review in the study flow diagram (Figure 1).
1.
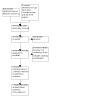
Study flow diagram
For the included studies, see Table 1; Characteristics of included studies.
For the other studies, see Characteristics of excluded studies; Characteristics of studies awaiting classification.
Results of the search
We searched the databases in September 2022 and identified 850 references. After screening, we assessed 14 full‐text articles for eligibility and included six articles, corresponding to three trials, (Davis 1997; Davis 2003; Rosenfeld 1984). We excluded three studies (Davis 2000; Jobe 2003; Rosenfeld 1996), and classified five as awaiting classification (Davis 1999; Davis 2000a; Davis 2001; Michele 2001; Parad 2006). We found no relevant ongoing studies by searching clinical trial registries.
Included studies
Davis 1997: dose‐ranging multi‐center RCT from six different hospitals that enrolled 33 infants (700g to 1300 g) who were less than 24 hours old, intubated and mechanically ventilated for treatment of respiratory distress. Participants had a mean gestational age of 27 weeks. Infants were given surfactant within the first 24 hours of life. Major exclusion criteria were congenital anomalies, infections, and perinatal asphyxia. This study contained three arms with drug or placebo administered intratracheally. In one experimental arm, infants received 2.5 mg/kg dose of recombinant human superoxide dismutase (rh)SOD (n = 11). Another experimental group received a 5 mg/kg dose of rhSOD (n = 11). The control group was given an equal volume of saline. Experimental drug and saline were administered every 48 hours until extubated or until seven doses were completed, whichever came first. The outcomes of this study are as follows: BPD defined as oxygen dependency with abnormal chest radiograph at 28 days, BPD defined as oxygen dependency at 36 weeks' postmenstrual age (PMA), IVH all grades, IVH grades 3 and 4, PDA, ROP, NEC, sepsis, mortality at 28 days, overall mortality, plasma SOD levels, urine SOD levels, neutrophil chemotactic activity and albumin levels in tracheal aspirates, anti rhSOD antibodies, severity of respiratory distress syndrome(RDS), apnea, renal failure, total days on oxygen, duration of mechanical ventilation.
Davis 2003: multi‐center RCT from 15 centers around the world that enrolled 302 infants. Eligibility criteria included: weighing 600 g to 1200 g at birth at or > 24 weeks gestation, ≤ 24 hours of age, clinical or radiographic evidence of RDS, being treated with supplemental oxygen mechanical ventilation or exogenous surfactants. Infants were excluded if there was evidence of any major congenital abnormalities, overwhelming congenital infection, or severe perinatal asphyxia. The experimental group received rh CuZnSOD intratracheally (5 mg/kg in 2 mL/kg saline) in two divided doses, which occurred 0.5 mL to 4.0 hours after exogenous surfactant. Further doses were administered every 48 hours until 28 days or until extubation. The control group was given 2mL/kg of saline after initial exogenous surfactant treatment. The outcomes reported by this study were as follows: mortality during the first month, development of BPD at 28 days of life, Edwards Score at 28 days of life, oxygen requirement at 36 weeks’ PMA, number of days in oxygen, number of days of respiratory support, number of days in the hospital, NEC, pneumonia, IVH (all grades, grades 3 to 4), ROP (any stage of ROP; severe ROP, i.e. > stage 2), PVL, number of episodes of significant pulmonary illness requiring treatment with asthma medications such as bronchodilators and corticosteroids, number and type of doctor’s visits, emergency department visits, hospital admissions, any abnormalities in growth parameters or physical examination. This study was reported in multiple study records, including: an economic evaluation determining the cost‐effectiveness of rhSOD in the prevention of chronic respiratory morbidity in preterm infants; a post hoc analysis comparing ROP outcomes in infants in relation to the gestational age (GA).
Rosenfeld 1984: single‐center study enrolling 45 infants (560 g to 2260 g) who were admitted to the neonatal intensive car unit (NICU) (Jewish Hospital Division, Interfaith Medical Center) between April 1981 and March 1983 with severe RDS who were on mechanical ventilation with Fio2 > 0.70 at 24 hours of age to maintain Pao2 ≥ 50 torr. Infants with major congenital anomalies, other causes of respiratory distress, including aspiration, sepsis, pneumonia, and drug withdrawal were excluded. This study was done in the pre‐surfactant era. The experimental group (n = 21) was given 0.25 mg/kg of bovine SOD by subcutaneous injection every 12 hours until patients no longer needed the ventilator or CPAP and maintained on room air. Test dose of 0.1 mg/kg administered intradermally prior to therapeutic dose to ensure safety. The control group (n = 24) was given equal volumes of saline every 12 hours until patients no longer needed ventilator and maintained saturations on room air. Outcomes reported by Rosenfeld 1984 included mortality, severity of RDS, total days of oxygen therapy, days of mechanical ventilation at various rates, mean peak Fio2 and distribution of days at various oxygen concentrations, mean peak inspiratory pressures during the first week, duration of total CPAP, incidence of PDA, congestive heart failure, need for indomethacin, incidence and severity of IVH, respiratory signs and clinical findings associated with BPD after discharge from NICU, radiological evidence of BPD, clinical problems associated with BPD, clinical diagnosis of BPD, pneumonia, hospitalization.
Excluded studies
We excluded three studies at full‐text screening as they were not RCTs (Davis 2000; Jobe 2003; Rosenfeld 1996).
Davis 2000 aims at examining the long‐term effects, including neurodevelopmental abnormalities, of treatment with rhCuZnSOD in infants enrolled previously in two placebo‐controlled trials. The first study was conducted at two participating hospitals from April to December 1993 and enrolled 26 infants with RDS receiving a single intratracheally dose of placebo (n = 11), a very low (test) dose of rhSOD (0.5 mg/kg per dose; n = 8), or a therapeutic dose of rhSOD (5.0 mg/kg per dose; n = 7) within two hours of surfactant administration. The groups were studied sequentially, i.e. not following a randomization process. The second study was conducted at six participating hospitals from November 1994 to June 1995; 33 infants received multiple intratracheal doses of placebo or rhSOD (2.5 mg or 5.0 mg/kg per dose) within two hours of surfactant therapy, and every 48 hours thereafter (while requiring intubation and mechanical ventilation) for up to seven doses.
Jobe 2003 is a commentary of Davis 2003, speculating on CuZnSOD function mechanisms and effects on outcomes.
Rosenfeld 1996 is a placebo‐controlled, unblinded, dose‐ranging study, conducted at two participating hospitals (Winthrop‐University Hospital and University Hospital at Stony Brook) from April to December 1993. A total of 28 infants were studied in three sequential groups. Patients were considered eligible if they were less than or equal to 24 hours of age, weighed 750 g to 1250 g at birth, required intubation and mechanical ventilation for treatment of RDS, and had received surfactant therapy within the first 24 hours of life. The first group (n = 12) received placebo in an unblinded fashion. The second (n = 8), and third (n = 8) groups received 0.5 mg/kg and 5 mg/kg of rhSOD, respectively. The groups were studied sequentially and not randomized.
Studies awaiting classification
Abstracts and full text were not available for four studies (Davis 2000a; Davis 2001; Michele 2001; Parad 2006). An abstract was available in Davis 1999, a multicenter blinded RCT that enrolled 301 infants with birthweight 600 g to 1200 g, receiving exogenous surfactant for the treatment of RDS. The experimental arm received rhSOD (5 mg/kg suspended in 2 ml/kg of saline) every 48 hours, as long as intubation was required, up to 28 days of life. The control group received placebo. It is unclear whether Davis 1999 is the same cohort of 302 infants reported by the same author (Davis 2003).
Risk of bias in included studies
Specific methodologic issues are addressed in the following sections and in Figure 2; Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation was judged to be low risk of bias for Rosenfeld 1984, which used "random selection charts" for randomization. Davis 1997 and Davis 2003 did not describe randomization in detail, so they were judged to be unclear for this domain.
Allocation bias was judged to be unclear in all three studies, as information about allocation concealment was not provided.
Blinding
All three studies were described as double‐blinded by study authors; with intervention and placebo administered with the same route of administration.
In Rosenfeld 1984 the radiologists reading chest radiographs at three and 12 months during outpatient follow‐up were masked to the nature of the therapy. In Davis 1997 and Davis 2003 chest radiographs and cranial ultrasounds were interpreted by a single pediatric radiologist who was also blinded to treatment assignment. Caregivers assessing clinical outcomes were also blinded to intervention.
All three studies were judged to be at low risk regarding performance and detection bias.
Incomplete outcome data
In all three studies there was no relevant attrition regarding short‐term outcomes. However, in Davis 2003 the short‐term outcomes were evaluated on all the enrolled infants, while for the long‐term outcomes; of the 274 surviving, 13 were excluded because of death and 65 because they were lost to follow‐up. For this reason, Davis 2003 was judged to be at unclear risk regarding attrition bias, whereas the other two studies were judged at low risk (Davis 1997; Rosenfeld 1984).
Selective reporting
All studies were judged to be unclear regarding reporting bias, as their protocols were not available.
Other potential sources of bias
Funding sources were only reported in Davis 2003. Baseline imbalances that did not reach statistical significance due to low number of enrolled infants, were found in Davis 1997. All included studies were performed by the same research group.
Effects of interventions
See: Table 1
The three included studies (Davis 1997; Davis 2003; Rosenfeld 1984), were pooled in the Comparison 1, i.e. SOD versus no treatment or placebo in preterm infants at risk for BPD (Table 1).
We found no studies within Comparison 2, i.e. SOD versus no treatment or placebo in preterm infants with evolving BPD / early respiratory insufficiency.
Primary outcomes
BPD defined as an oxygen requirement at 28 days
One study reported this outcome (Davis 2003). We are uncertain whether SOD reduces BPD defined as an oxygen requirement at 28 days compared with placebo (risk ratio (RR) 1.09, 95% confidence interval (CI) 0.94 to 1.26; risk difference (RD) 0.06, 95% CI ‐0.05 to 0.16; 1 study, 302 infants; I2 for RR and RD not applicable; very low‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: Superoxide dismutase versus placebo, Outcome 1: BPD defined as an oxygen requirement at 28 days
BPD defined as oxygen at 36 weeks' postmenstrual age
Two studies reported this outcome (Davis 1997; Davis 2003). We are uncertain whether SOD reduces BPD defined as an oxygen at 36 weeks' postmenstrual age compared with placebo (RR 0.96, 95% CI 0.72 to 1.29; RD ‐0.01, 95% CI ‐0.11 to 0.09; 2 studies, 335 infants; I2 for RR and RD = 0%; very low‐certainty evidence; Analysis 1.2).
1.2. Analysis.

Comparison 1: Superoxide dismutase versus placebo, Outcome 2: BPD defined as oxygen at 36 weeks' postmenstrual age
Neonatal mortality
Two studies reported this outcome (Davis 1997; Davis 2003). We are uncertain whether SOD reduces neonatal mortality compared with placebo (RR 0.98, 95% CI 0.57 to 1.68; RD ‐0.00, 95% CI ‐0.08 to 0.07; 2 studies, 335 infants; I2 for RR and RD = 0%; very low‐certainty evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1: Superoxide dismutase versus placebo, Outcome 3: Neonatal Mortality
Mortality prior to discharge
Two studies reported this outcome (Davis 1997; Rosenfeld 1984). We are uncertain whether SOD reduces mortality prior to discharge compared with placebo (RR 1.20, 95% CI 0.53 to 2.71; RD 0.04, 95% CI ‐0.14 to 0.23; 2 studies, 78 infants; I2for RR and RD = 0%; very low‐certainty evidence; Analysis 1.4,).
1.4. Analysis.

Comparison 1: Superoxide dismutase versus placebo, Outcome 4: Mortality prior to discharge
BPD or death at 36 weeks' postmenstrual age
No studies reported this outcome.
Secondary outcomes
Hemodynamically significant patent ductus arteriosus (PDA)
One study reported this outcome (Davis 1997). We are uncertain whether SOD reduces hemodynamically significantPDA compared with placebo (RR 1.00, 95% CI 0.45 to 2.21; RD 0.00, 95% CI ‐0.36 to 0.36; 1 study, 33 infants, I2 not applicable, Analysis 1.5).
1.5. Analysis.

Comparison 1: Superoxide dismutase versus placebo, Outcome 5: Hemodynamically significant patent ductus arteriosus
Patent ductus arteriosus (PDA) requiring treatment
No studies reported this outcome.
Late onset sepsis (with proven culture)
Two studies reported this outcome (Davis 1997; Davis 2003). We are uncertain whether SOD reduces late onset sepsis (with proven culture) compared with placebo (RR 1.06, 95% CI 0.78 to 1.43; RD 0.02, 95% CI ‐0.08 to 0.12; 2 studies, 335 infants; I2 for RR = 36%, I2 for RD = 62%; Analysis 1.6).
1.6. Analysis.

Comparison 1: Superoxide dismutase versus placebo, Outcome 6: Late onset sepsis (with proven culture)
Necrotizing enterocolitis (NEC) (Bell ≥ stage 2)
Two studies reported this outcome (Davis 1997; Davis 2003). We are uncertain whether SOD reduces NEC (Bell ≥ stage 2) compared with placebo (RR 0.80, 95% CI 0.34 to 1.84; RD 0.01, 95% CI ‐0.07 to 0.04; 2 studies, 335 infants; I2 for RR and RD = 0%; Analysis 1.7).
1.7. Analysis.

Comparison 1: Superoxide dismutase versus placebo, Outcome 7: Necrotizing enterocolitis
Intraventricular hemorrhage (IVH) (any)
Two studies reported this outcome (Davis 1997; Davis 2003). We are uncertain whether SOD reduces IVH (any) compared with placebo (RR 0.98, 95% CI 0.65 to 1.47; RD ‐0.00, 95% CI ‐0.09 to 0.08; 2 studies, 335 infants; I2 for RR and RD = 0%; Analysis 1.8).
1.8. Analysis.

Comparison 1: Superoxide dismutase versus placebo, Outcome 8: Intraventricular hemorrhage (any grade)
Severe intraventricular hemorrhage (IVH) (grades III to IV)
Two studies reported this outcome (Davis 1997; Davis 2003). We are uncertain whether SOD reduces severe IVH (grades III to IV) compared with placebo (RR 0.71, 95% CI 0.39 to 1.31; RD 0.04, 95% CI ‐0.10 to 0.03; 2 studies, 335 infants; I2 for RR = 0%, I2 for RD = 15%; Analysis 1.9).
1.9. Analysis.

Comparison 1: Superoxide dismutase versus placebo, Outcome 9: Severe Intraventricular hemorrhage (Grades III / IV)
Periventricular leukomalacia (PVL)
One study reported this outcome (Davis 2003). We are uncertain whether SOD reduces PVL compared with placebo (RR 0.64, 95% CI 0.11 to 3.78; RD ‐0.01, 95% CI ‐0.04 to 0.02; 1 study, 302 infants; I2 for RR and RD not applicable; Analysis 1.10).
1.10. Analysis.

Comparison 1: Superoxide dismutase versus placebo, Outcome 10: Periventricular leukomalacia (PVL)
Retinopathy of prematurity (ROP) (any stage)
Two studies reported this outcome (Davis 1997; Davis 2003). We are uncertain whether SOD reduces ROP (any stage) compared with placebo (RR 0.95, 95% CI 0.78 to 1.15; RD ‐0.03, 95% CI ‐0.15 to 0.08; 2 studies, 335 infants; I2for RR = 0%, I2 for RD = 8%; very low‐certainty evidence; Analysis 1.11).
1.11. Analysis.

Comparison 1: Superoxide dismutase versus placebo, Outcome 11: Retinopathy of prematurity (any stage)
Severe retinopathy of prematurity (ROP) (stage II or greater)
One study reported this outcome (Davis 2003). We are uncertain whether SOD reduces severe ROP (stage II or greater) compared with placebo(RR 0.97, 95% CI 0.57 to 1.65; RD ‐0.01, 95% CI ‐0.10 to 0.09; 1 study, 244 infants; I2 for RR and RD not applicable; very low‐certainty evidence; Analysis 1.12).
1.12. Analysis.

Comparison 1: Superoxide dismutase versus placebo, Outcome 12: Severe retinopathy of prematurity (stage II or greater)
Duration of assisted ventilation (days)
No studies reported this outcome.
Duration of oxygen dependence (days)
No studies reported this outcome.
Duration of hospital stay (days)
No studies reported this outcome.
Moderate to severe neurodevelopmental outcome at 18 to 24 months
No studies reported this outcome.
Subgroup analysis
We did not conduct subgroup analysis because no more than two studies were pooled in the same analysis.
Post hoc outcomes
Davis 1997 reported that apnea occurred in 10 of 22 infants who received SOD and in six of the 11 infants in the placebo group (RR 0.83, 95% CI 0.41 to 1.69).
Rosenfeld 1984 reported that chest radiograph abnormalities occurred in three of 14 surviving infants who received SOD and in 12 of 17 surviving infants who received placebo (RR 0.30, 95% CI 0.11 to 0.87).
Rosenfeld 1984 reported that respiratory problems after discharge from the neonatal intensive care unit (NICU) occurred in three of 14 surviving infants who had received SOD and in 11 of 17 survivors in the placebo group (RR 0.33, 95% CI 0.11 to 0.96).
Rosenfeld 1984 reported that respiratory problems after discharge or death before discharge occurred in 10 of the 21 infants who received SOD, and in 18 of 24 infants who received placebo (RR 0.63, 95% CI 0.38 to 1.05). We derived this outcome from the data of Rosenfeld 1984 by combining the numbers of the infants who died with those survivors who subsequently developed clinical findings of bronchopulmonary dysplasia after discharge from theNICU.
Discussion
Summary of main results
We included three studies enrolling a total of 380 infants (Davis 1997; Davis 2003; Rosenfeld 1984), comparing v (SOD) with no treatment or placebo in preterm infants at risk for bronchopulmonary dysplasia (BPD). We found no studies in preterm infants with early respiratory insufficiency.
Compared to placebo, the evidence is very uncertain about the effect of SOD on BPD, defined as an oxygen requirement at 28 days, BPD defined as an oxygen requirement at 36 weeks' postmenstrual age, neonatal mortality, death prior to discharge. No studies reported the composite outcome BPD or death at 36 weeks' postmenstrual age and the need for supplemental oxygen. The evidence is very uncertain about the effect of SOD on retinopathy of prematurity( ROP) defined by any stages or zones reported (stage 2 or greater). No studies reported moderate to severe neurodevelopmental outcome at 18 to 24 months.
We identified no ongoing studies matching the inclusion criteria of this review.
Overall completeness and applicability of evidence
To date, three studies comparing SOD versus placebo in very preterm infants have enrolled 380 newborns. In all studies infants were treated in the first hours of life, i.e. SOD administration was aimed to prevent BPD. Studies on the role of SOD for treating an evolving or established BPD are lacking. In the oldest study, conducted in the pre‐surfactant era, a dose of 0.25 mg/kg of bovine SOD was administered by subcutaneous injection every 12 hours (Rosenfeld 1984). In the other studies, SOD was administered intratracheally with doses ranging 2.5 mg/kg to 5 mg/kg dose. Study authors reported extremely limited data on critical outcomes such as long‐term neurodevelopmental assessment. We could not perform an appropriate a priori subgroup analysis to detect differential effects because of the paucity of the included studies.
Certainty of the evidence
According to the GRADE approach, the overall certainty of evidence for critical outcomes for SOD administration was very low (see Table 1). All outcomes were downgraded one level because of limitations in study design, i.e. unclear risk of bias in different domains, mainly selection and reporting bias. Moreover, outcomes were downgraded two levels for imprecision due to the small sample size, few events, and CIs overlapping the no difference line.
We did not explore publication bias using funnel plots because fewer than 10 studies met the inclusion criteria of this Cochrane Review.
Potential biases in the review process
We updated the methods section of this review to the latest template used by Cochrane Neonatal, to ensure the optimal methodology. It is unlikely that the literature search applied to this review may have missed relevant trials. However, we could not retrieve abstract or full text of four studies currently awaiting classification (Davis 2000a; Davis 2001; Michele 2001; Parad 2006) An abstract was available in Davis 1999, a multicenter blinded RCT that enrolled 301 infants, but it is unclear whether it is the same cohort of 302 infants reported by the same author (Davis 2003). We attempted to obtain data from researchers for studies that did not clearly mention our eligibility criteria, but we received no response. Some continuous outcomes were reported as medians; as we could not ascertain if data were normally distributed, we did not transform these values to means and could not pool them in meta‐analyses.
Agreements and disagreements with other studies or reviews
We did not find any other completed or ongoing systematic reviews on the role of SOD administration in preterm infants.
In an observational cohort study (Bhunwal 2018), serum SOD levels were comparable between preterm neonates, with or without BPD. Similarly, no significant correlation between SOD dynamics within the first week of life and the risk of BPD development were found in another study (Kicinski 2019). In one of the excluded studies of this review (Davis 2000), a trend towards lower long‐term neurodevelopmental and chronic pulmonary abnormalities in infants exposed to SOD was suggested, however this finding should be interpreted with caution due to potential confounding, lack of randomization and small number of infants.
Authors' conclusions
Implications for practice.
The evidence is very uncertain about the effect of SOD on BPD defined as an oxygen requirement at 28 days, BPD defined as oxygen at 36 weeks' postmenstrual age, neonatal mortality and mortality prior to discharge compared to placebo. No studies reported BPD or death at 36 weeks' PMA and need for supplemental oxygen. The evidence is very uncertain about the effect of SOD on retinopathy of prematurity any stage and severe retinopathy of prematurity. No studies reported moderate to severe neurodevelopmental outcome at 18 to 24 months.
Implications for research.
The effects of SOD in preterm infants has not been reported in any trial in the last decades, considering that the most recent trial on = superoxide dismutase (SOD) in preterm infants was conducted 1997/1998, and no new studies are ongoing. In the light of the limited available evidence, new data from preclinical and observational studies are needed to justify the conduction of new RCTs. Observational studies might report how SOD is administered, including indication, dose and association with relevant outcomes such as mortality, BPD and long‐term neurodevelopment.
History
Protocol first published: Issue 1, 2019
Acknowledgements
The methods section of this review is based on a standard template used by Cochrane Neonatal.
We thank Matthias Bank (Library and ICT services, Lund University) for designing and running the search strategy; Michelle Fiander (Cochrane Neonatal) for peer‐reviewing the searches.
We thank Dr Manoj Malviya (Senior Consultant neonatologist, Khoula Hospital, Muscat, Oman) and Prof Jan Miletin (MD, PhD, ‐ The Coombe Hospital, Dublin, Ireland; UCD Dublin, Ireland; 2nd Faculty of Medicine, Prague, Czechia; Institute for the Care of Mother and Child, Prague, Czechia) for peer‐review.
We would like to thank Cochrane Neonatal: Jane Cracknell and Michelle Fiander, Managing Editors; and Bill McGuire, Co‐coordinating Editor, who provided editorial and administrative support.
We would also like to thank Heather Maxwell, Cochrane Central Production Service, for copy editing the review.
Appendices
Appendix 1. Search strategy
Information specialist: Matthias Bank
Affiliation: Lund University, Faculty of Medicine, Library & ICT, Sweden
PICO(s)
Patients: Preterm infants who are mechanically ventilated.
Intervention: Exogenously administered superoxide dismutase
Control
Outcomes: Decrease of bronchopulmonary dysplasia, intraventricular hemorrhage, periventricular leukomalacia, retinopathy of prematurity, necrotizing enterocolitis, patent ductus arteriosus, mortality.
Study design(s): Randomized controlled trials.
Search strategies
Databases searched: PubMed (National Library of Medicine), Embase.com (Elsevier), CENTRAL via Cochrane Library Online
PubMed (National Library of Medicine)
Date of search: 21 September 2022
No publication date limitations or language limitations were used.
Search filters: The search filter for RCT was converted from the Ovid Medline RCT filter designed by Cochrane Neonatal, see https://neonatal.cochrane.org/Literature-Search-Filters-for-Neonatal-Reviews. The search filter for neonates was converted from the Ovid Medline Neonatal filter designed by Cochrane Neonatal.
#1 superoxide dismutase[Mesh] 55,726
#2 “superoxide dismutase*”[TW] OR cytocuprein[TW] OR dismuzyme[TW] OR pegorgotein[TW] OR erythrocuprein[TW] OR hemocuprein[TW] OR lipsod[TW] OR ontocin[TW] OR ontosein[TW] OR orgotein[TW] OR “orgotein superoxide”[TW] OR orgoteine[TW] OR ormetein[TW] OR oxinorm[TW] OR palosein[TW] OR peroxinorm[TW] OR “rh‐sod”[TW] OR rhSOD[TW] OR “s 8524”[TW] OR “superoxide oxidoreductase”[TW] OR “copper‐zinc‐SOD”[TW] OR “CuZn SOD”[TW] OR “Cu‐Zn SOD”[TW] OR “Zn‐SOD”[TW] OR “iron SOD”[TW] OR “Fe‐SOD”[TW] OR “manganese SOD”[TW] OR “Mn‐SOD”[TW] OR “nickel SOD”[TW] OR “Ni‐SOD”[TW] OR “bovine SOD”[TW] OR “Cu‐SOD”[TW] OR “Ag‐Zn SOD”[TW] OR “cobalt SOD”[TW] OR “PEG SOD”[TW] OR SOD1[TW] OR SOD2[TW] OR SOD3[TW] OR SODs[TW] 102,005
#3 #1 OR #2 102,005
SUPEROXIDE DISMUTASE AND SYNONYMS
#4 randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab] 5,537,617
#5 quasirandom*[tw] or quasi‐random*[tw] or randomi*[tw] or randomly[tw] 1,265,298
#6 control*[tw] AND (group[tw] OR groups[tw] OR random[tw] OR trial[tw] OR trials[tw] OR study[tw]) 3,736,879
#7 #4 OR #5 OR #6 7,352,353
#8 (animals [mh] NOT humans [mh]) 5,045,521
#9 #7 NOT #8 6,296,455
RANDOMIZED CONTROLLED TRIALS
#10 (infant, newborn[Mesh]) OR (intensive care, neonatal[Mesh]) OR (intensive care units, neonatal[Mesh]) OR (gestational age[Mesh]) 709,054
#11 babe[TW] OR babes[TW] baby*[TW] OR babies[TW] OR gestational age[TW] OR gestational ages[TW] OR infant[TW] OR infants[TW] OR infant's[TW] OR infantile[TW] OR infancy[TW] OR low birth weight[TW] OR low birthweight[TW] OR neonat*[TW] OR neo‐nat*[TW] OR newborn*[TW] OR new born[TW] OR new borns[TW] OR newly born[TW] OR premature[TW] OR pre‐mature*[TW] OR prematures[TW] OR prematurity[TW] OR pre‐maturity[TW] OR preterm[TW] OR preterms[TW] OR pre term[TW] OR pre terms[TW] OR preemie[TW] OR preemies[TW] OR premies[TW] OR premie[TW] OR VLBW[TW] OR VLBWI[TW] OR VLBW‐I[TW] OR VLBWs[TW] OR LBW[TW] OR LBWI[TW] OR LBWs[TW] OR ELBW[TW] OR ELBWI[TW] OR ELBWs[TW] OR NICU[TW] OR NICUs[TW] 1,767,609
#12 #10 OR #11 1,767,609
NEONATES
#13 #3 AND #9 AND #12 617
INTERVENTION, RCT AND NEONATES
Embase.com (Elsevier, 1947‐present)
Date of search: 21 September 2022
No publication date limitations or language limitations were used.
Search filters: The Cochrane Neonatal filter for RCTs for OVID Embase was adapted to the syntax of Embase.com. The Cochrane Neonatal filter for neonatal populations for OVID Embase was adapted to the syntax of Embase.com.
#1. 'superoxide dismutase'/exp OR 'copper zinc superoxide dismutase'/exp OR 'manganese superoxide dismutase'/exp OR 'extracellular superoxide dismutase'/exp OR 'iron superoxide dismutase'/exp OR 'pegorgotein'/exp 132,093
#2. 'superoxide dismutase*':ti,ab,kw OR cytocuprein:ti,ab,kw OR dismuzyme:ti,ab,kw OR pegorgotein:ti,ab,kw OR erythrocuprein:ti,ab,kw OR hemocuprein:ti,ab,kw OR lipsod:ti,ab,kw OR ontocin:ti,ab,kw OR ontosein:ti,ab,kw OR orgotein:ti,ab,kw OR 'orgotein superoxide':ti,ab,kw OR orgoteine:ti,ab,kw OR ormetein:ti,ab,kw OR oxinorm:ti,ab,kw OR palosein:ti,ab,kw OR peroxinorm:ti,ab,kw OR 'rh‐sod':ti,ab,kw OR rhsod:ti,ab,kw OR 's 8524':ti,ab,kw OR 'superoxide oxidoreductase':ti,ab,kw OR 'copper‐zinc‐sod':ti,ab,kw OR 'cuzn sod':ti,ab,kw OR 'cu‐zn sod':ti,ab,kw OR 'zn‐sod':ti,ab,kw OR 'iron sod':ti,ab,kw OR 'fe‐sod':ti,ab,kw OR 'manganese sod':ti,ab,kw OR 'mn‐sod':ti,ab,kw OR 'nickel sod':ti,ab,kw OR 'ni‐sod':ti,ab,kw OR 'bovine sod':ti,ab,kw OR 'cu‐sod':ti,ab,kw OR 'ag‐zn sod':ti,ab,kw OR 'cobalt sod':ti,ab,kw OR 'peg sod':ti,ab,kw OR sod1:ti,ab,kw OR sod2:ti,ab,kw OR sod3:ti,ab,kw OR sods:ti,ab,kw 109,621
#3 #1 OR #2 151,586
SUPEROXIDE DISMUTASE AND SYNONYMS
#4. 'randomized controlled trial'/de OR 'controlled clinical trial'/de 907,837
#5. random*:ti,ab,kw 1,842,002
#6. 'randomization'/de 95,009
#7. placebo:ti,ab,kw 348,486
#8. ((double OR single OR doubly OR singly) NEAR/2 (blind OR blinded OR blindly)):ti,ab,kw 264,542
#9. 'double blind procedure'/de 199,569
#10. (controlled NEAR/7 (study OR design OR trial)):ti,ab,kw 428,384
#11. 'parallel group$':ti,ab 30,024
#12. crossover:ti,ab OR 'cross over':ti,ab 118,554
#13. ((assign* OR match OR matched OR allocation) NEAR/5 (alternate OR group$ OR intervention$ OR patient$ OR subject$ OR participant$)):ti,ab 387,867
#14. (open NEAR/2 label):ti,ab 100,203
#15. (quasirandom* OR quasi‐random* OR randomi* OR randomly):ti,ab,kw 1,500,514
#16. (control* NEAR/2 (group$ OR random*)):ti,ab,kw 1,222,639
#17. #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 3,143,799
#18. ('animal'/exp OR 'invertebrate'/exp OR 'animal experiment'/de OR 'animal model'/de OR 'animal tissue'/de OR 'animal cell'/de OR 'nonhuman'/de) AND ('human'/de OR 'normal human'/de OR 'human cell'/de) 25,269,619
#19. 'animal'/exp OR 'invertebrate'/exp OR 'animal experiment'/de OR 'animal model'/de OR 'animal tissue'/de OR 'animal cell'/de OR 'nonhuman'/de 32,850,952
#20. #19 NOT #18 7,581,333
#21. #17 NOT #20 2,699,536
RANDOMIZED CONTROLLED TRIALS
#22. 'newborn'/de OR 'prematurity'/de OR 'newborn intensive care'/de OR 'newborn care'/de OR ‘gestational age’/de 838,655
#23. (babe or babes or baby* or babies or ‘gestational age$’ or infant$ or infantile or infancy or ‘low birth weight’ or ‘low birthweight’ or neonat* or ‘neo‐nat*’ or newborn* or ‘new born$’ or ‘newly born’ or premature or pre‐mature or pre‐matures or prematures or prematurity or pre‐maturity or preterm or preterms or ‘pre term$’ or preemie or preemies or premies or premie or VLBW or VLBWI or VLBW‐I or VLBWs or LBW or LBWI or LBWs or ELBW or ELBWI or ELBWs or NICU or NICUs):ti,ab,kw 1,264,441
#24. #22 OR #23 1,575,876
NEONATES
#25. #3 AND #21 AND #24 272
INTERVENTION, RCT AND NEONATES
CENTRAL via Cochrane Library Online (Issue 9 of 12, September 2022)
Date of search: 21 September 2022
No publication date limitations or language limitations were used.
Search filters: The Cochrane Neonatal filter for Cochrane Library, neonatal populations, was used.
#1 MeSH descriptor: [Superoxide Dismutase] explode all trees 830
#2 (“superoxide dismutase*” OR cytocuprein OR dismuzyme OR pegorgotein OR erythrocuprein OR hemocuprein OR lipsod OR ontocin OR ontosein OR orgotein OR “orgotein superoxide” OR orgoteine OR ormetein OR oxinorm OR palosein OR peroxinorm OR “rh‐sod” OR rhSOD OR “s 8524” OR “superoxide oxidoreductase” OR “copper‐zinc‐SOD” OR “CuZn SOD” OR “Cu‐Zn SOD” OR “Zn‐SOD” OR “iron SOD” OR “Fe‐SOD” OR “manganese SOD” OR “Mn‐SOD” OR “nickel SOD” OR “Ni‐SOD” OR “bovine SOD” OR “Cu‐SOD” OR “Ag‐Zn SOD” OR “cobalt SOD” OR “PEG SOD” OR SOD1 OR SOD2 OR SOD3 OR SODs):ti,ab,kw (Word variations have been searched) 3,473
#3 #1 OR #2 3,473
SUPEROXIDE DISMUTASE AND SYNONYMS
#4 MeSH descriptor: [Infant, Newborn] explode all trees 17,651
#5 MeSH descriptor: [Intensive Care, Neonatal] this term only 353
#6 MeSH descriptor: [Intensive Care Units, Neonatal] this term only 867
#7 MeSH descriptor: [Gestational Age] this term only 2,784
#8 #4 OR #5 OR #6 OR #7 17,365
#9 ("babe" or "babes" or baby* or "babies" or "gestational age" or "gestational ages" or infant? or "infantile" or infancy or "low birth weight" OR "low birth weights" or "low birthweight" or "low birthweights" or neonat* or "neo‐nat*" or newborn* or "new born?" or "newly born" or "premature" or "pre‐mature" or "pre‐matures" or prematures or prematurity or "pre‐maturity" or "preterm" or "preterms" or "pre term?" or "preemie" or "preemies" or "premies" or "premie" or "VLBW" or "VLBWI" or "VLBW‐I" or "VLBWs" or "LBW" or "LBWI" or "LBWs" or "ELBW" or "ELBWI" or "ELBWs" or "NICU" or "NICUs"):ti,ab,kw [Line updated August 2022] 102,920
#10 #8 OR #9 102,920
NEONATES
#11 #3 AND #10 138
INTERVENTION AND NEONATES
Trial Registries
ClinicalTrials.gov (US National Library of Medicine)
Date of search: 14 October 2022
Advanced search
Intervention: “superoxide dismutase” OR “superoxide dismutases” OR cytocuprein OR dismuzyme OR pegorgotein OR erythrocuprein OR hemocuprein OR lipsod OR ontocin OR ontosein OR orgotein OR “orgotein superoxide” OR orgoteine OR ormetein OR oxinorm OR palosein OR peroxinorm OR “rh‐sod” OR rhSOD OR “s 8524” OR “superoxide oxidoreductase” OR “copper‐zinc‐SOD” OR “CuZn SOD” OR “Cu‐Zn SOD” OR “Zn‐SOD” OR “iron SOD” OR “Fe‐SOD” OR “manganese SOD” OR “Mn‐SOD” OR “nickel SOD” OR “Ni‐SOD” OR “bovine SOD” OR “Cu‐SOD” OR “Ag‐Zn SOD” OR “cobalt SOD” OR “PEG SOD” OR SOD1 OR SOD2 OR SOD3 OR SODs
Other terms: premature OR prematurity OR preterms OR preterm OR very low birth OR low birth weight OR newborn OR newborns OR neonate OR neonates OR infant OR infants No further limits applied. 3 records
International Clinical Trials Registries Platform Search Portal, ICTRP (World Health Organization)
Date of search: 14 October 2022
Advanced search
Intervention: “superoxide dismutase” OR “superoxide dismutases” OR cytocuprein OR dismuzyme OR pegorgotein OR erythrocuprein Ticked box: search for clinical trials in children Recruitment status: ALL, Phases: ALL 2 records
Number of records from trial registries 5 records
Appendix 2. Risk of bias tool
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorized the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table; computer random number generator);
high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorized the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorized the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorize d the methods as:
low risk, high risk or unclear risk for participants; and
low risk, high risk or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorized the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorize d the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we will describe the completeness of data including attrition and exclusions from the analysis. We will note whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we will re‐include missing data in the analyses. We we will categorize the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
6. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we describe d how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compare d prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contact ed study authors to gain access to the study protocol. We assess ed the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assess ed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk;
unclear risk
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Superoxide dismutase versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 BPD defined as an oxygen requirement at 28 days | 1 | 302 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.94, 1.26] |
| 1.2 BPD defined as oxygen at 36 weeks' postmenstrual age | 2 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.72, 1.29] |
| 1.3 Neonatal Mortality | 2 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.57, 1.68] |
| 1.4 Mortality prior to discharge | 2 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.53, 2.71] |
| 1.5 Hemodynamically significant patent ductus arteriosus | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.45, 2.21] |
| 1.6 Late onset sepsis (with proven culture) | 2 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.78, 1.43] |
| 1.7 Necrotizing enterocolitis | 2 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.34, 1.84] |
| 1.8 Intraventricular hemorrhage (any grade) | 2 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.65, 1.47] |
| 1.9 Severe Intraventricular hemorrhage (Grades III / IV) | 2 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.39, 1.31] |
| 1.10 Periventricular leukomalacia (PVL) | 1 | 302 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.11, 3.78] |
| 1.11 Retinopathy of prematurity (any stage) | 2 | 277 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.78, 1.15] |
| 1.12 Severe retinopathy of prematurity (stage II or greater) | 1 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.57, 1.65] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Davis 1997.
| Study characteristics | ||
| Methods | Dose‐ranging double‐blinded RCT | |
| Participants | 33 preterm infants in the USA enrolled in 1994 ‐ 1995 with birth weight 700 g to 1300 g, less than 24 hours old, intubated and on mechanical ventilation for treatment of respiratory distress syndrome. Had received surfactant within first 24 hours of life. | |
| Interventions | Three groups: Experimental group 1: SOD 2.5 mg/kg (n = 11) Experimental group 2: SOD 5 mg/kg (n = 11) In both these groups the drug was given intratracheally, repeated every 48 hours until extubated or until seven doses completed, whichever was earlier. Control group: equal volume of saline intratracheally, repeated every 48 hours until extubated or until seven doses completed, whichever was earlier (n = 11). The initial drug or placebo was administered in 2 aliquots over a 1‐minute period within 30 to 120 minutes after surfactant administration. If infants were extubated before 14‐days of age, then the study drug was discontinued. However, if infants were re‐intubated before 14‐days of age, additional doses were administered every 48 hours until 14‐days of life. rhSOD or placebo was never administered after 14‐days of life. |
|
| Outcomes | SOD concentration in serum, TA and urine, neutrophil chemotactic activities, anti rhSOD antibodies, severity of RDS, IVH all grades, IVH grade 3‐4, NEC, apnea, PDA, ROP, renal failure, BPD, sepsis, mortality at 28 days, overall mortality, total days of oxygen, duration of mechanical ventilation. | |
| Funding sources / declarations of interest | Funding and conflicts of interest not reported. | |
| Notes | Small sample size. In this review we combined the data from patients from both dosage regimens as no dose‐response relationship was evident in the results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in sufficient detail in text. |
| Allocation concealment (selection bias) | Unclear risk | Not described in text. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded study and placebo was used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded study. Additionally, (quote;) "Chest radiographs and cranial ultrasounds were interpreted by a single pediatric radiologist who was also blinded to treatment assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients lost to follow up. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Unclear risk | Imbalances in group characteristics. |
Davis 2003.
| Study characteristics | ||
| Methods | Double blinded RCT | |
| Participants | 302 infants enrolled in the USA in 1997/1998 ≤ 24 hours of age, with evidence of RDS, between 600 g and 1200 g birth weight, ≥ 24 weeks of gestation receiving supplemental oxygen via mechanical ventilation and exogenous surfactant. | |
| Interventions | Experimental (n = 154): intratracheal r‐h CuZnSOD (5 mg/kg in 2 mL/kg saline) Doses occurred 0.5 to 4.0 hours after exogenous surfactant. Further doses every 48 hours until 28 days of age or until extubation. Control (n = 148): 2 mL/kg of saline intratracheally was administered after exogenous surfactant treatment. Intervention was administered in 2 divided doses with the infant placed in the lateral decubitus position in 30° Trendelenburg to enhance distribution. If the infant was subsequently re‐intubated, the study medication was continued every 48 hours through 28 days of age. |
|
| Outcomes | Mortality during the 1st month, development of BPD at 28 days of life, Edwards Score at 28 days of life, oxygen requirement at 36 weeks’ PMA, number of days in oxygen, number of days of respiratory support, number of days in the hospital, and short‐term, NEC, pneumonia, IVH, ROP, PVL, number of episodes of significant pulmonary illness requiring treatment with asthma medications such as bronchodilators and corticosteroids, number and type of doctor’s visits, emergency department visits, and hospital admissions, any abnormalities in growth parameters or physical examination, economic evaluation (cost‐effectiveness, cost comparison). | |
| Funding sources / declarations of interest | This study was supported by funds from Bio‐Technology General Corporation. The company also provided funds to some authors for preparation of materials for submission to the Food and Drug Administration. | |
| Notes | Quote: "Study was cut short by DSMC due to little possibility of significant efficacy of r‐h CuZnSOD w/respect to death or incidence of death or BPD at 28 days of life." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization process not described. No baseline imbalances. |
| Allocation concealment (selection bias) | Unclear risk | Not enough details provided in the text. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double‐blind by study authors. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Personnel was blinded to intervention. Additionally, (quote:) "all radiographs and sonograms were evaluated by a single pediatric radiologist who was blinded to treatment assignment". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Health assessment and physical examination were performed on 209 surviving infants (80%) with complete data available on 189 infants; 65 infants were lost to follow up". Relevant rate of lost to follow up. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Low risk | None. |
Rosenfeld 1984.
| Study characteristics | ||
| Methods | Double‐blinded RCT | |
| Participants | 45 neonates admitted to NICU (Jewish Hospital Division, Interfaith Medical Center, New York, USA) between April 1981 and March 1983 with severe RDS. Inclusion requirements were ventilator dependent, with FiO2 > 0.7 at 24 hours of age to maintain PaO2 ≥ 50 torr. | |
| Interventions | Experimental: bovine SOD 0.25 mg/kg subcutaneously every 12 hours until no longer needing ventilator or CPAP and maintained on room air (n = 21). Control (Placebo): saline 0.25 mg/kg subcutaneously (n = 24). |
|
| Outcomes | Mortality, severity of RDS, total days of oxygen therapy, days of mechanical ventilation at various rates, mean peak FiO2 and distribution of days at various oxygen concentrations, and mean peak inspiratory pressures during the first week were comparable in the 2 groups, duration of total CPAP, incidence of PDA, congestive heart failure need for indomethacin, incidence and severity of IVH, respiratory signs and clinical findings associated with BPD after discharge from NICU, radiological evidence of BPD, clinical problems associated with BPD, clinical diagnosis of BPD, pneumonia, hospitalization. | |
| Funding sources / declarations of interest | Funding and potential conflicts of interest not reported. | |
| Notes | This study had a small population size and was done in the pre‐surfactant era where the definition of bronchopulmonary dysplasia was different from what it is now. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned to SOD or placebo (saline diluent for SOD) groups according to previously constructed random selection charts. Patients were matched according to sex and birth weight (less than or equal to 1200 or greater than 1200 grams)". |
| Allocation concealment (selection bias) | Unclear risk | Not described in text |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study. (Quote:) "Physicians caring for the patients were blind as to the medication being given". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind study. Additionally, (quote:) "Radiographs were taken at 3 and 12 months and were evaluated by a pediatric radiologist who was blind regarding therapy" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Unclear risk | None. |
BPD = bronchopulmonary dysplasia; CPAP = continuous positive airway pressure; DSMC = data and safety monitoring committee; FiO2 = fraction of inspired oxygen; IVH = intraventricular hemorrhage; NEC = necrotizing enterocolitis; NICU = neonatal intensive care unit; PaO2 = partial pressure of oxygen; PDA = patent ductus arteriosus; PMA = postmenstrual age; PVL = proliferative verrucous leukoplakia; RCT = randomized controlled trial; RDS = respiratory distress syndrome; rhSOD = recombinant human superoxide dismutase; ROP = retinopathy of prematurity; SOD = superoxide dismutase; TA = tracheal aspirate.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Davis 2000 | Not an RCT. Neurodevelopmental abnormalities were compared between patients who received superoxide dismutase and those who received placebo. This was done by combining patients who received superoxide dismutase in two different studies and by combining patients in the placebo groups from those studies. One of the two studies had random allocation of patients to treatment/placebo and the other had non‐random allocation of treatment and placebo (groups were studied sequentially). |
| Jobe 2003 | Not a RCT |
| Rosenfeld 1996 | Non‐random allocation of treatment and placebo (groups studied sequentially). |
RCT = randomized controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
Davis 1999.
| Methods | Multicenter blinded RCT |
| Participants | 301 infants with birthweight 600g to 200 g, receiving exogenous surfactant for the treatment of RDS. |
| Interventions | rhSOD (5 mg/kg suspended in 2 mL/kg saline) or placebo, instilled intratracheally, every 48 hours (as long as intubation was required) up to 28 days of life. |
| Outcomes | Death and/or the development of bronchopulmonary dysplasia; days in oxygen; days of ventilation; total days in hospital; intraventricular hemorrhage; periventricular leukomalacia. |
| Notes | Only abstract available. |
Davis 2000a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | No abstract nor full text available. |
Davis 2001.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | No abstract nor full text available. |
Michele 2001.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | No abstract nor full text available. |
Parad 2006.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | No abstract nor full text available. |
RCT = randomized controlled trial; RDS = respiratory distress syndrome; rhSOD = recombinant human superoxide dismutase.
Differences between protocol and review
We updated the methods section of this review (Gentyala 2019), to the latest template used by Cochrane Neonatal, to ensure the optimal methodology.
Following peer review advice, the title was changed from Superoxide dismutase for preventing bronchopulmonary dysplasia (BPD) in preterm infants', to better match the objectives. It is now: 'Superoxide dismutase for bronchopulmonary dysplasia in preterm infants'.
Contributions of authors
MA: contributed to writing and editing, made an intellectual contribution to, advised on, approved the final version prior to submission.
RRG and DE: drafted the review.
TP: developed, contributed to writing and editing, made an intellectual contribution to, advised on, approved the final version prior to submission.
MB and RS: made an intellectual contribution to; advised on; approved the final version of the review prior to submission; RS is a guarantor of the review.
Sources of support
Internal sources
-
Institute for Clinical Sciences, Lund University, Lund, Sweden
MB is employed by this organization
External sources
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
-
Region Skåne, Skåne University Hospital, Lund University and Region Västra Götaland, Sweden
Cochrane Sweden is supported from Region Skåne, Skåne University Hospital Lund University and Region Västra Götaland
Declarations of interest
MA has no relevant interests to declare.
RRG has no relevant interests to declare.
TP has no relevant interests to declare.
DE is an Associate editor of Cochrane Neonatal Review Group. However, her participation in the editorial group has not impacted this review.
MB is an Associate Editor for the Cochrane Neonatal Group. However, his participation in the editorial group has not impacted this review.
RS is the Co‐ordinating Editor of Cochrane Neonatal (therefore the review was seen and edited by other members of the editorial team).
New
References
References to studies included in this review
Davis 1997 {published data only}
- Davis JM, Richter SE, Parad R, Gewolb IH, Pitzer A, Arlo WA, et al. Safety and pharmacokinetics of multiple intratracheal (IT) doses of recombinant human Cu/Zn superoxide dismutase (rhSOD) to premature infants with respiratory distress syndrome (RDS). In: Pediatric Research. Vol. 39(4):330A. 1996. [DOI: 10.1203/00006450-199604001-01989] [DOI]
- Davis JM, Rosenfeld WN, Richter SE, Parad R, Gewolb IH, Spitzer AR, et al. Safety and pharmacokinetics of multiple doses of recombinant human CuZn superoxide dismutase administered intratracheally to premature neonates with respiratory distress syndrome. Pediatrics 1997;100:24-30. [DOI: 10.1542/peds.100.1.24] [PMID: ] [DOI] [PubMed] [Google Scholar]
Davis 2003 {published data only}
- Davis JM, Parad RB, Michele T, Allred E, Price A, Rosenfeld W. Pulmonary outcome at 1 year corrected age in premature infants treated at birth with recombinant human CuZn superoxide dismutase. Pediatrics 2003;111(3):469-76. [DOI: 10.1542/peds.111.3.469] [PMID: ] [DOI] [PubMed] [Google Scholar]
- McBride JA, Parad RB, Davis JM, Zheng Z, Zupancic JA. Economic evaluation of recombinant human copper zinc superoxide dismutase administered at birth to premature infants. Journal of Perinatology 2009;29(5):364-71. [DOI: 10.1038/jp.2008.225] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Parad RB, Allred EN, Rosenfeld WN, Davis JM. Reduction of retinopathy of prematurity in extremely low gestational age newborns treated with recombinant human Cu/Zn superoxide dismutase. Neonatology 2012;102(2):139-44. [DOI: 10.1159/000336639] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rosenfeld 1984 {published data only}
- Rosenfeld W, Evans H, Concepcion L, Jhaveri R, Schaeffer H, Friedman A. Prevention of bronchopulmonary dysplasia by administration of bovine superoxide dismutase in preterm infants with respiratory distress syndrome. Journal of Pediatrics 1984;105:781-5. [DOI: 10.1016/s0022-3476(84)80307-8] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Davis 2000 {published data only}
- Davis JM, Richter SE, Biswas S, Rosenfeld WN, Parton L, Gewolb IH, et al. Long-term follow-up of premature infants treated with prophylactic, intratracheal recombinant human CuZn superoxide dismutase. Journal of Perinatology 2000;20(4):213-6. [DOI: 10.1038/sj.jp.7200363] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jobe 2003 {published data only}
- Jobe AH. An unanticipated benefit of the treatment of preterm infants with CuZn superoxide dismutase. Pediatrics 2003;111(3):680. [DOI: 10.1542/peds.111.3.680] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rosenfeld 1996 {published data only}
- Rosenfeld WN, Davis JM, Parton L, Richter SE, Price A, Flaster E, et al. Safety and pharmacokinetics of recombinant human superoxide dismutase administered intratracheally to premature neonates with respiratory distress syndrome. Pediatrics 1996;97(6 Pt 1):811-7. [PMID: ] [PubMed] [Google Scholar]
References to studies awaiting assessment
Davis 1999 {published data only}
- Davis JM, Rosenfeld WN, Richter SE, Parad R, Gewolb IH, Couser R, et al. The effects of multiple doses of recombinant human CuZn superoxide dismutase (rhSOD) in premature infants with respiratory distress syndrome (RDS). In: Pediatric Research. Vol. 45. 1999:193A (Abstract no.1129).
Davis 2000a {published data only}
- Davis JM, Rosenfeld WN, Parad R, Richter S, Gewolb I, Couser R, et al. Improved pulmonary outcome at one year corrected age in premature neonates treated with recombinant human superoxide dismutase. In: Pediatric Research. Vol. 47. 2000:395A (Abstract no. 2333).
Davis 2001 {published data only}
- Davis JM, Couser R, Parad R, Gewolb I, Huang W, Salerno LM, et al. Safety of recombinant human cu/zn superoxide dismutase in premature infants with respiratory distress syndrome. In: Annual Thoracic Society 97th International Conference; San Francisco CA. Vol. C452001. May 18-23 2001.
Michele 2001 {published data only}
- Michele TM, Couser R, Parad R, Gewolb IH, Huang W, Davis JM. Recombinant human Cu/Zn superoxide dismutase decreases asthma medication use at 1-year follow-up in premature infants with respiratory distress syndrome. Journal of Allergy and Clinical Immunology 2001;107(5):933. [Google Scholar]
Parad 2006 {published data only}
- Parad RB. Incidence and severity of retinopathy of prematurity (ROP) is reduced in extremely low gestational age (GA) newborns (ELGANS) trated with recombinant human CuZn superoxide dismutase (rhSOD). European Journal of Pediatrics 2006;165(not available):not available. [Google Scholar]
Additional references
AAP 2010
- Watterberg KL, American Academy of Pediatrics Committee on Fetus and Newborn. Postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Pediatrics 2010;126(4):800-8. [DOI: 10.1542/peds.2010-1534] [PMID: ] [DOI] [PubMed] [Google Scholar]
Anderson 2006
- Anderson PJ, Doyle LW. Neurodevelopmental outcome of bronchopulmonary dysplasia. Seminars in Perinatology 2006;30(4):227–32. [DOI: 10.1053/j.semperi.2006.05.010] [PMID: ] [DOI] [PubMed] [Google Scholar]
Arlettaz 2017
- Arlettaz R. Echocardiographic evaluation of patent ductus arteriosus in preterm infants. Frontiers in Pediatrics 2017;5:147. [DOI: 10.3389/fped.2017.00147] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Balany 2015
- Balany J, Bhandari V. Understanding the impact of infection, inflammation, and their persistence in the pathogenesis of bronchopulmonary dysplasia. Frontiers in Medicine 2015;2(90):1-10. [DOI: 10.3389/fmed.2015.00090] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bancalari 1979
- Bancalari E, Abdenour GE, Feller R, Gannon J. Bronchopulmonary dysplasia: clinical presentation. Journal of Pediatrics 1979;95(5 Pt 2):819-23. [DOI: 10.1016/s0022-3476(79)80442-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Barrington 2017
- Barrington KJ, Finer N, Pennaforte T. Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane Database of Systematic Reviews 2017, Issue 1. Art. No: CD000509. [DOI: 10.1002/14651858.CD000509.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bayley 2006
- Bayley N. Bayley Scales of Infant and Toddler Development. San Antonio (TX): Harcourt Assessment, 2006. [Google Scholar]
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187(1):1-7. [DOI: 10.1097/00000658-197801000-00001] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhandari 2006
- Bhandari A, Panitch HB. Pulmonary outcomes in bronchopulmonary dysplasia. Seminars in Perinatology 2006;30(4):219–26. [DOI: 10.1053/j.semperi.2006.05.009] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bhunwal 2018
- Bhunwal S, Mukhopadhyay K, Bhattacharya S, Dey P, Dhaliwal LK. Bronchopulmonary dysplasia in preterm neonates in a level III neonatal unit in India. Indian Pediatrics 2018;55(3):211-5. [PMID: ] [PubMed] [Google Scholar]
Biniwale 2006
- Biniwale MA, Ehrenkranz RA. The role of nutrition in the prevention and management of bronchopulmonary dysplasia. Seminars in Perinatology 2006;30(4):200-8. [DOI: 10.1053/j.semperi.2006.05.007] [PMID: ] [DOI] [PubMed] [Google Scholar]
Borenstein 2013
- Borenstein M, Higgins JP. Meta-analysis and subgroups. Prevention Science 2013;14:134-43. [DOI: 10.1007/s11121-013-0377-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cochrane EPOC Group 2017
- Cochrane Effective Practice and Organisation of Care (EPOC). Data extraction and management. EPOC resources for review authors; 2017. epoc.cochrane.org/resources/epoc-resources-review-authors.
Darlow 2016
- Darlow BA, Graham PJ, Rojas-Reyes MX. Vitamin A supplementation to prevent mortality and short- and long-term morbidity in very low birth weight infants. Cochrane Database of Systematic Reviews 2016, Issue 8. Art. No: CD000501. [DOI: 10.1002/14651858.CD000501.pub4] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davis 1998
- Davis JM. Superoxide dismutase: a role in the prevention of chronic lung disease. Biology of the Neonate 1998;74(Supple 1):29-34. [DOI: 10.1159/000047032] [PMID: ] [DOI] [PubMed] [Google Scholar]
Deeks 2022
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Doyle 2021a
- Doyle LW, Cheong JL, Hay S, Manley BJ, Halliday HL, Soll R. Early (< 7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants.. Cochrane Database of Systematic Reviews 2021, Issue 10. Art. No: CD001146. [DOI: 10.1002/14651858.CD001146.pub6] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Doyle 2021b
- Doyle LW, Cheong JL, Hay S, Manley BJ, Halliday HL. Late (≥ 7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database of Systematic Reviews 2021, Issue 11. Art. No: CD001145. [DOI: 10.1002/14651858.CD001145.pub5] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI: 10.1136/bmj.315.7109.629] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ehrenkranz 2005
- Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005;116(6):1353-60. [DOI: 10.1542/peds.2005-0249] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fardy 1995
- Fardy C, Silverman M. Antioxidants in neonatal lung disease. Archives of Disease in Childhood. Fetal and Neonatal Edition 1995;73(2):F112-7. [DOI: 10.1136/fn.73.2.f112] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Farrell 1997
- Farrell PA, Fiascone JM. Bronchopulmonary dysplasia in the 1990s: a review for the pediatrician. Current Problems in Pediatrics 1997;27(4):129-63. [DOI: 10.1016/s0045-9380(97)80017-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fewell 2007
- Fewell Z, Davey Smith G, Sterne JA. The impact of residual and unmeasured confounding in epidemiologic studies: a simulation study. American Journal of Epidemiology 2007;166(6):646-55. [DOI: 10.1093/aje/kwm165] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ghanta 2013
- Ghanta S, Leeman KT, Christou H. An update on pharmacologic approaches to bronchopulmonary dysplasia. Seminars in Perinatology 2013;37(2):115-23. [DOI: 10.1053/j.semperi.2013.01.008] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldstein 2017
- Goldstein ND, Kenaley KM, Locke R, Paul DA. The joint effects of antenatal steroids and gestational age on improved outcomes in neonates. Maternal and Child Health Journal 2017;22(3):384-90. [DOI: 10.1007/s10995-017-2403-z] [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 1 October 2022. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Griffiths 1954
- Griffiths R. The Abilities of Babies: a Study in Mental Measurement. New York (NY): McGraw-Hill Book Co. Inc., 1954. [Google Scholar]
Higgins 2022a
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022), Cochrane, 2022. Available from training.cochrane.org/handbook/archive/v5.2.
Higgins 2022b
- Higgins JP, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Horbar 2012
- Horbar JD, Carpenter JH, Badger GJ, Kenny MJ, Soll RF, Morrow KA, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics 2012;129(6):1019-26. [DOI: 10.1542/peds.2011-3028] [PMID: ] [DOI] [PubMed] [Google Scholar]
International Committee 2005
- International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Archives of Ophthalmology 2005;123(7):991-9. [DOI: 10.1001/archopht.123.7.991] [PMID: ] [DOI] [PubMed] [Google Scholar]
Isayama 2017
- Isayama T, Lee SK, Yang J, Lee D, Daspal S, Dunn M, et al, Canadian Neonatal Network and Canadian Neonatal Follow-Up Network Investigators. Revisiting the definition of bronchopulmonary dysplasia: effect of changing panoply of respiratory support for preterm neonates. JAMA Pediatrics 2017;171(3):271-9. [DOI: 10.1001/jamapediatrics.2016.4141] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jain 2014
- Jain D, Bancalari E. Bronchopulmonary dysplasia: clinical perspective. Birth Defects Research. Part A, Clinical and Molecular Teratology 2014;100(3):134-44. [DOI: 10.1002/bdra.23229] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jobe 2001
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. American Journal of Respiratory and Critical Care Medicine 2001;163(7):1723-9. [DOI: 10.1164/ajrccm.163.7.2011060] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kallapur 2013
- Kallapur SG, Kramer BW, Jobe AH. Ureaplasma and BPD. Seminars in Perinatology 2013;37(2):94-101. [DOI: 10.1053/j.semperi.2013.01.005] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 1993
- Kelly FJ. Free radical disorders of preterm infants. British Medical Bulletin 1993;49(3):668-78. [DOI: 10.1093/oxfordjournals.bmb.a072639] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kicinski 2019
- Kicinski P, Malachowska B, Wyka K, Gach A, Jakubowski L, Gulczynska E. The level of extracellular superoxide dismutase in the first week of life in very and extremely low birth weight infants and the risk of developing bronchopulmonary dysplasia. Journal of Perinatal Medicine 2019;47(6):671-6. [DOI: 10.1515/jpm-2018-0418] [PMID: ] [DOI] [PubMed] [Google Scholar]
Klingenberg 2017
- Klingenberg C, Wheeler KI, McCallion N, Morley CJ, Davis PG. Volume-targeted versus pressure-limited ventilation in neonates. Cochrane Database of Systematic Reviews 2017, Issue 10. Art. No: CD003666. [DOI: 10.1002/14651858.CD003666.pub4] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Knight 1998
- Knight JA. Free radicals: their history and current status in aging and disease. Annals of Clinical and Laboratory Science 1998;28(6):331-46. [PMID: ] [PubMed] [Google Scholar]
Kyriacou 2016
- Kyriacou DN, Lewis RJ. Confounding by indication in clinical research. JAMA 2016;316(17):1818-9. [DOI: 10.1001/jama.2016.16435] [PMID: ] [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Medicine 6;7:e1000100. [DOI: 10.1136/bmj.b2700] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ma 2016
- Ma J, Ye H. Effects of permissive hypercapnia on pulmonary and neurodevelopmental sequelae in extremely low birth weight infants: a meta-analysis. SpringerPlus 2016;5(1):764. [DOI: 10.1186/s40064-016-2437-5] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McGoldrick 2020
- McGoldrick E, Stewart F, Parker R, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews 2020, Issue 12. Art. No: CD004454. [DOI: 10.1002/14651858.CD004454.pub4] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McGowan 2016a
McGowan 2016b
- McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. Journal of Clinical Epidemiology 2016;75:40-6. [DOI: 10.1016/j.jclinepi.2016.01.021] [PMID: ] [DOI] [PubMed] [Google Scholar]
Northway 1967
- Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. New England Journal of Medicine 1967;276(7):357-68. [DOI: 10.1056/NEJM196702162760701] [PMID: ] [DOI] [PubMed] [Google Scholar]
O'Brodovich 1985
- O'Brodovich HM, Mellins RB. Bronchopulmonary dysplasia. Unresolved neonatal acute lung injury. American Review of Respiratory Disease 1985;132(3):694-709. [DOI: 10.1164/arrd.1985.132.3.694] [PMID: ] [DOI] [PubMed] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529-34. [DOI: 10.1016/s0022-3476(78)80282-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pham‐Huy 2008
- Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. International Journal of Biomedical Science 2008;4(2):89-96. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Poets 2018
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Sampath 2015
- Sampath V, Garland JS, Helbling D, Dimmock D, Mulrooney NP, Simpson PM, et al. Antioxidant response genes sequence variants and BPD susceptibility in VLBW infants. Pediatric Research 2015;77(3):477-83. [DOI: 10.1038/pr.2014.200] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saugstad 1990
- Saugstad O. Oxygen toxicity in the neonatal period. Acta Paediatrica Scandinavica 1990;79(10):881-92. [DOI: 10.1111/j.1651-2227.1990.tb11348.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Saugstad 1998
- Saugstad OD. Chronic lung disease: the role of oxidative stress. Biology of the Neonate 1998;74(Suppl 1):21-8. [DOI: 10.1159/000047031] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schmidt 2006
- Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al, Caffeine for Apnea of Prematurity Trial Group. Caffeine therapy for apnea of prematurity. New England Journal of Medicine 2006;354(20):2112-21. [DOI: 10.1056/NEJMoa054065] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Seger 2009
- Seger N, Soll R. Animal derived surfactant extract for treatment of respiratory distress syndrome. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No: CD007836. [DOI: 10.1002/14651858.CD007836] [PMID: ] [DOI] [PubMed] [Google Scholar]
Shennan 1988
- Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirements in the neonatal period. Pediatrics 1988;82(4):527-32. [PMID: ] [PubMed] [Google Scholar]
Soll 2013
- Soll RF, Edwards EM, Badger GJ, Kenny MJ, Morrow KA, Buzas JS, et al. Obstetric and neonatal care practices for infants 501 to 1500 g from 2000 to 2009. Pediatrics 2013;132(2):222-8. [DOI: 10.1542/peds.2013-0501] [PMID: ] [DOI] [PubMed] [Google Scholar]
Volpe 1997
- Volpe JJ. Brain injury in the premature infant. Neuropathology, clinical aspects, pathogenesis and prevention. Clinics in Perinatology 1997;24(3):567-87. [PMID: ] [PubMed] [Google Scholar]
Wojtunik‐Kulesza 2016
- Wojtunik-Kulesza KA, Oniszczuk A, Oniszczuk T, Waksmundzka-Hajnos M. The influence of common free radicals and antioxidants on development of Alzheimer's Disease. Biomedecine & Pharmacotherapie [Biomedicine & Pharmacotherapy] 2016;78:39-49. [DOI: 10.1016/j.biopha.2015.12.024] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gentyala 2019
- Gentyala RR, Ehret D, Suresh G, Soll R. Superoxide dismutase for preventing bronchopulmonary dysplasia (BPD) in preterm infants. Cochrane Database of Systematic Reviews 2019, Issue 1. Art. No: CD013232. [DOI: 10.1002/14651858.CD013232] [DOI] [PMC free article] [PubMed] [Google Scholar]
Suresh 2001
- Suresh GK, Davis JM, Soll RF. Superoxide dismutase for preventing chronic lung disease in mechanically ventilated preterm infants. Cochrane Database of Systematic Reviews 2001, Issue 1. Art. No: CD001968. [DOI: 10.1002/14651858.CD001968] [DOI] [PMC free article] [PubMed] [Google Scholar]


