Abstract
Agrin (AGRN) is a matricellular glycoprotein involved in extracellular signal transduction. AGRN is involved in tumorigenesis and cancer progression; however, the role of AGRN in thyroid cancer (TC) remains unclear. In the present study, using cell lines derived from various subtypes of TC including CGTH, FTC-133 and BcPAP and transcriptomic data from patients with TC, the role of AGRN in TC was analyzed by migration, invasion, viability and proliferation assays as well as Western blot with EMT markers. AGRN expression was significantly increased in thyroid tumors and cell lines derived from various TC subtypes. The highest AGRN expression was found in follicular and papillary thyroid carcinoma subtypes. Immunocytochemistry revealed nuclear AGRN localization in normal (NTHY) and TC cells. Silencing of AGRN decreased viability, proliferation, migration and invasion of TC cell lines by upregulating vimentin and downregulating N-cadherin and E-cadherin. Furthermore, the expression of AGRN was associated with neutrophil infiltration in thyroid tumors. In conclusion, the present results indicated that increased AGRN expression promoted tumorigenic phenotypes of TC cells, while AGRN expression was associated with immune infiltration in thyroid tumors. AGRN may represent a target for future cancer therapy and requires further evaluation.
Keywords: agrin, thyroid cancer, neutrophil infiltration, migration, proliferation, invasion, viability
Introduction
Thyroid cancer (TC), although accounting for only 2% of all malignancies, is the most common type of endocrine cancer (1). The occurrence of TC is rising, mainly because of improved diagnosis due to widespread ultrasound examination (2). It was estimated that TC would lead to >2,200 deaths in the US in 2022 (3). TC affects mostly women, while in men, although less prevalent, it leads to a more aggressive disease and poor prognosis (1). According to the fifth World Health Organization classification system, histological types of TC include papillary TC (PTC), follicular TC (FTC), oncocytic carcinoma, poorly differentiated TC, anaplastic follicular cell-derived TC (ATC) and medullary TC, as well as several other, less prominent subtypes including mucoepidermoid carcinoma, ectopic thymoma or spindle epithelial tumor with thymus-like differentiation (4,5). PTC is the most common TC subtype, contributing to 80–90% of all diagnosed thyroid malignancies (1,6). The incidence of PTC has increased rapidly, making it the fastest-growing cancer in East Asia (prevalence of 76%) (1,7). A total of 40–80% of PTC cases bear the BRAF V600E mutation that contributes to increased cell proliferation (1,7,8). The prognosis for patients with TC depends on the subtype of cancer (9). PTC and FTC are usually associated with good prognosis (91.1 and 79.9% 5-year survival, respectively), while ATC has an extremely poor outcome (7–12.2% 5-year survival) (10,11). PTC spreads mostly (99%) regionally to the neck, while distant metastases (lung and bones) affect only 1–7% of patients (12–15). FTC is the second most frequently diagnosed type of TC, accounting for 10–30% of cases (16). Compared with PTC, FTC is characterized by worse prognosis and more frequent metastasis to distant organs, affecting 6–23% of patients (16–18). ATC is an undifferentiated tumor, with disease-specific mortality approaching 100%. The metastatic disease most commonly (≤90%) affects lungs and pleura, with less frequent involvement of bone (5–15%) and brain (5% of patients with distant metastasis) (3). ATC responds poorly to conventional therapy including surgery, radiation therapy, radioactive iodine therapy, chemotherapy and hormone therapy (3). Palliative and supportive care should be introduced early in the disease (3).
Agrin (AGRN) is a matricellular glycoprotein involved in multiple physiological and pathological processes, including neuromuscular signaling (19–21), cardiac regeneration (22) and autoimmunity (23,24). Several studies reported enhanced AGRN expression in cancers including glioblastoma (25), non-small cell lung cancer (NSCLC) (26), oral squamous cell carcinoma (27) as well as its involvement in tumor-promoting signaling pathways (25–28). AGRN stimulates tumorigenesis and metastasis via focal adhesion and mitogen-activated protein kinase activation (26); to the best of our knowledge, however, the role of AGRN in the pathobiology of TC has not been analyzed and the present study aimed to explore the potential roles of AGRN in the functioning of TC cells.
Materials and methods
Cell lines
Thyroid follicular epithelial cells (Nthy-ori 3-1; NTHY) and FTC cells (FTC-133) were obtained from the European Collection of Authenticated Cell Cultures. Thyroid gland squamous cell carcinoma (CGTH-W-1), a cell line derived from SW-579 (CGTH; cat. no. ACC 360) and TC cells (BcPAP) were obtained from the Leibniz Institute (DSMZ-German Collection of Microorganisms and Cell Cultures GmbH). A cell line derived from anaplastic carcinoma (8505C) was generously provided by Dr Cuong Hoang-Vu (Martin Luther University; Halle, Germany). All cell lines were authenticated by immunofluorescence with tetraspanins including CD9, CD63, CD81, CD82 and CD151 (29). Analyzed cell lines were cultured according to the supplier's protocols as follows: i) NTHY and CGTH cell lines cultured in complete RPMI-1640 medium; ii) FTC-133 and 8505C cell lines in DMEM/F12 and iii) BcPAP cell line in complete DMEM/GlutaMAX™ (all Thermo Fisher Scientific, Inc.). All analyzed cell lines were cultured in medium supplemented with 10% heat-inactivated fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.), at 37°C in a humidified 5% CO2 atmosphere. The number of cells was estimated using Trypan Blue exclusion test with automatic counting on an EVA Automatic Cell counter (NanoEnTek, Inc.). All cell lines were tested for presence of mycoplasma.
Small interfering (si)RNA transfection
AGRN silencing was performed using the following siRNA to target AGRN (siAGRN): Sense, 5′-CGUAUGACAGUGAUUGCUGTT-3′; antisense, 3′-CAGCAAUCACUGUCAUACGTG-5′ (Thermo Fisher Scientific, Inc.). Briefly, following trypsinization, cells were diluted in RPMI-1640 or DMEM/GlutaMAX™ (both Thermo Fisher Scientific, Inc.). The transfection mixture comprised 30 nM siRNA in OptiMem and Lipofectamine™ 2000 (both Thermo Fisher Scientific, Inc.). The mixture was added to the cells for 10 min incubation at room temperature. The cells were seeded on a 6 (1.25×105/well)-, 12 (5×104/well)- or 96 (1,200 cells/well)-well plate and incubated at 37°C for 48 h prior to RNA isolation and 72 h for protein isolation. As a control, MISSION siRNA Universal Negative Control (siNEG; cat. no. SIC001; Sigma-Aldrich; Merck KGaA) was used for transfection. The experiments were performed in triplicate.
Reverse transcritpion-quantitative (RT-q)PCR
Total RNA from TC cell lines was isolated using a Universal RNA Purification kit (EURx, Ltd.), according to the manufacturer's protocol. Both concentration and purity were evaluated by measuring absorbance at 260 and 280 nm with a Synergy 2 Multi-Mode Reader (BioTek Instruments, Inc.). PrimeScript™ RT reagent kit (Takara Bio, Inc.) with oligo dT primers and random hexamers were used to transcribe 200 ng RNA into cDNA. RT was performed on a T100™ Thermal Cycler (Bio-Rad Laboratories, Inc.), according to the manufacturer's protocol. Subsequently, gene expression was analyzed by RT-qPCR using Maxima SYBR Green/Fluorescein qPCR Master Mix (Thermo Fisher Scientific, Inc.), 5 nM specific oligonucleotide primers (Genomed, Ltd.) and 5-fold diluted cDNA samples. Amplification and data analysis were performed using CFX96 Detection System (Bio-Rad Laboratories, Inc.) under the following thermocycling conditions: Initial denaturation at 95°C for 30 sec; 95°C for 5 sec (40 cycles); 58°C for 15 sec and 72°C for 10 sec. The expression of AGRN was normalized to that of β-actin and 18S and quantified using the 2−ΔΔCq method (30). Primer sequences were as follows: 18S forward, 5′ CCAGTAAGTGCGGGTCATAAG3′ and reverse, 5′ CCATCCAATCGGTAGTAGCG3′; β-actin forward, 5′ GCCGAGGACTTTGATTGC3′ and reverse, 5′ CTGTGTGGACTTGGGAGAG3′ and AGRN forward, 5′ACACCGTCCTCAACCTGAAG3′ and reverse, 5′ CCAGGTTGTAGCTCAGTTGC-3′ (31).
Western blotting
Total protein was extracted using RIPA cell lysis buffer (Pierce RIPA Buffer; Thermo Fisher Scientific, Inc.) that contained phosphatase and protease inhibitors. Following 30 min incubation on ice and centrifugation at 12,092 × g at 4°C for 10 min, supernatant was boiled in 5X SDS loading buffer for 5 min. Pierce BCA Protein Assay kit (Thermo Fisher Scientific, Inc.) was used to quantify the protein concentration. Subsequently, 30 µg protein/lane was separated by SDS-PAGE on a 10 or 8% gel and transferred onto a PVDF membrane. The membrane was blocked with 5% non-fat milk in TBST (TBS with 0.1% Tween) overnight at 4°C and incubated with monoclonal mouse anti-human AGRN antibody (1:100; cat. no. sc-374117; Santa Cruz Biotechnology, Inc.) at room temperature for 1 h. Rabbit polyclonal vimentin (1:500; cat. no. sc-7558, Santa Cruz Biotechnology, Inc.) and N-cadherin (1:3,000; cat. no. GTX127345; GeneTex, Inc.) and mouse monoclonal E-cadherin (1:3,000; cat. no. GTX629691, GeneTex, Inc.) antibodies were incubated overnight at 4°C after blocking membranes in 5% non-fat milk in TBST for 1 h at room temperature. After washing in TBST, the membranes were incubated with HRP-conjugated mouse (1:10,000; cat. no. 115-035-146; Jackson ImmunoResearch Laboratoies, Inc.) or rabbit antibody (1:10,000; cat. no. P0448; Dako; Agilent Technologies, Inc.) at room temperature for 1 h. The expression of proteins was normalized to β-actin (1:10,000; cat. no. ab6276; Abcam) and GAPDH (1:5,000; cat. no. MAB374; Merck KGaA.). Immunoreactive bands were detected using the Super-Signal™ West Dura Extended Duration Substrate kit (Thermo Fisher Scientific, Inc.) on Carestream membranes. Quantification of scanned images was performed with ImageJ version 1.53k software (National Institute of Health).
Immunofluorescence
The cells were fixed on 24×24 mm glass slides with methanol for 10 min at −20°C. Following washing and blocking with 2% BSA (cat. no. A9418; Sigma-Aldrich; Merk KGaA), in TBS + 0.1% Tween-20 at room temperature for 1 h, the cells were incubated with monoclonal mouse anti-human AGRN antibody (1:150; cat. no. sc-374117; Santa Cruz Biotechnology, Inc.) at 4°C overnight. Nuclei were stained with DAPI (1:50,000) and F-actin was stained with phalloidin-FITC (1:500; Sigma-Aldrich; Merk KGaA) for 30 min at room temperature. The images were obtained using a scanning confocal microscope LSM 800 AxioObserver Z.1 using ZEN 2.6 software (both Zeiss AG).
Viability assay
Cell viability was analyzed using CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega Corporation). MTS reagent was added to cells seeded in a 96-well plate at ~1,200 cells/well and incubated at 37°C for 1 h. The quantity of formazan was assessed by measuring absorbance at 490 nm on an L Synergy 2 Multi-Mode Reader (BioTek Instruments, Inc.).
Proliferation assay
Cell proliferation was analyzed using a bromodeoxyuridine (BrdU; colorimetric) assay kit (cat. no. 11647229001, Roche Diagnostics GmbH), according to the manufacturer's instructions. Briefly, cells (1,200 cells/well) were labeled at 37°C with BrdU reagent for 2 h, then dried at 65°C for 1 h and stored for 2 days in the fridge. Subsequently, cells were incubated for 30 min with fixing solution (70% ethanol) at room temperature followed by a mouse anti-BrdU antibody (1:100; cat. no. 11647229001; Roche Diagnostics GmbH) incubation for 1.5 h at room temperature. Following brief washing, cells were stained at room temperature with peroxidase substrate (100 µl/well) for 30 min. The absorbance was measured according to the manufacturer's instructions at 450 and 550 nm on the Synergy 2 Multi-Mode Reader (BioTek Instruments, Inc.).
Modified Boyden chamber assay
NTHY or BcPAP cells (2×104 cells/well) were suspended in RPMI-1640 medium or DMEM/GlutaMAX (both from Thermo Fisher Scientific, Inc.), respectively, without 5% FBS and plated into the upper chambers of a Transwell with a membrane with 8-µm pores (cat. no. 353097; Falcon; Corning Life Sciences) for migration or Transwell with a membrane with 8-µm pores coated with Matrigel (cat. no. 354480; Corning Life Sciences) for invasion assay. RPMI-1640 or DMEM/GlutaMAX with 5% FBS was added to the lower chamber. Following incubation for 24 h at 37°C, cells in the upper surface of the chamber were wiped using a cotton swab. The membranes were stained at room temperature with Diff-Quick reagent (Medion Diagnostics, Ltd.) for 2 min for invasion assay and 30 sec for the migration assay. Cells were counted manually and photographed at ×200 magnification using a light microscope Olympus BX41 (Olympus Corporation).
Bioinformatics analysis
AGRN expression was evaluated using The Cancer Genome Atlas (TCGA) transcriptomic data of TC cohort (THCA; n=505) and normal tissue controls (n=59) provided by The University of Alabama at Birmingham Cancer Data Analysis Portal (UALCAN; ualcan.path.uab.edu/analysis.html) (32–34). The association between AGRN expression and the survival of patients with TC was analyzed using the THCA cohort (n=412) provided by UALCAN. Immune infiltration was evaluated using TIMER 2.0 (timer.cistrome.org/) and TCGA data of the THCA cohort (35–37).
Statistical analysis
Statistical analysis was performed using unpaired t test or one-way ANOVA followed by Dunnett's post hoc test (GraphPad Prism 6.00 for Windows; GraphPad Software, Inc.; Dotmatics). Data are presented as the mean ± SD from at least three independent experiments. P<0.05 was considered to indicate a statistically significant difference.
Results
AGRN is overexpressed in TC
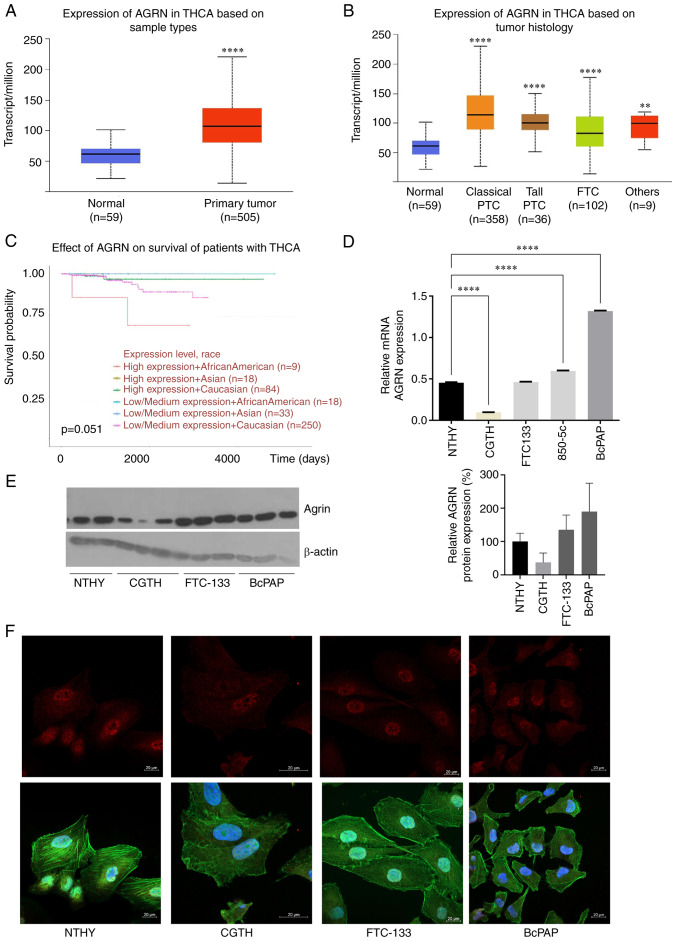
Analysis of TCGA data revealed AGRN upregulation in TC samples (n=505) compared with that in the adjacent normal thyroid tissues (n=59; Fig. 1A; P<0.0001). AGRN upregulation was observed in classical (n=358) and tall PTC (n=36). In FTC (n=102), AGRN expression was lower than in PTC (Fig. 1B; P<0.0001 vs. Classical and Tall PTC and FTC). Patients of African-American descent with higher AGRN expression had lower long-term survival rates (Fig. 1C; P=0.051). Consistent with clinical samples, RT-qPCR (Fig. 1D; P<0.0001), immunoblots (Fig. 1E) and immunofluorescence (Fig. 1F) confirmed high AGRN levels in the BcPAP TC cell line. At the protein level, AGRN was increased in the cells of FTC (FTC-133). In the CGTH cell line, AGRN expression was lower compared with that in the NTHY cell line (Fig. 1E and F). The nuclear localization of AGRN was confirmed by immunofluorescence assay (Fig. 1F).
Figure 1.
AGRN is overexpressed in TC. (A) AGRN expression in TC (n=505) vs. normal thyroid tissue (n=59). (B) AGRN expression in THCA subtypes. The plot shows analysis of the TCGA THCA cohort data performed using UALCAN. **P<0.005, ****P<0.0001 vs. normal. (C) Expression of AGRN is associated with survival of patients with TC. The analysis was performed using UALCAN. (D) Reverse transcription-quantitative PCR analysis of AGRN mRNA expression in cell lines derived from NTHY and TC (CGTH, FTC-133 and BcPAP). Expression was analyzed in RNA isolated from one cell culture plate/cell line and three technical replicates ****P<0.0001. (E) Western blotting of AGRN protein expression in cell line derived from NTHY and TC (CGTH, FTC-133, BcPAP). Western blotting was performed on three biological replicates using monoclonal mouse anti-human AGRN antibody. The plot shows changes in AGRN protein expression normalized to β-actin. (F) Representative images of AGRN immunostaining in cell lines derived from TC (AGRN, red; phalloidin-FITC, green and DAPI, blue). Scale bar, 20 µm. TC, thyroid cancer; TCGA, The Cancer Genome Atlas; AGRN, agrin; THCA, thyroid carcinoma; UALCAN, University of Alabama at Birmingham Cancer Data Analysis Portal; PTC, papillary thyroid carcinoma; FTC, follicular thyroid carcinoma.
AGRN contributes to tumorigenesis of TC
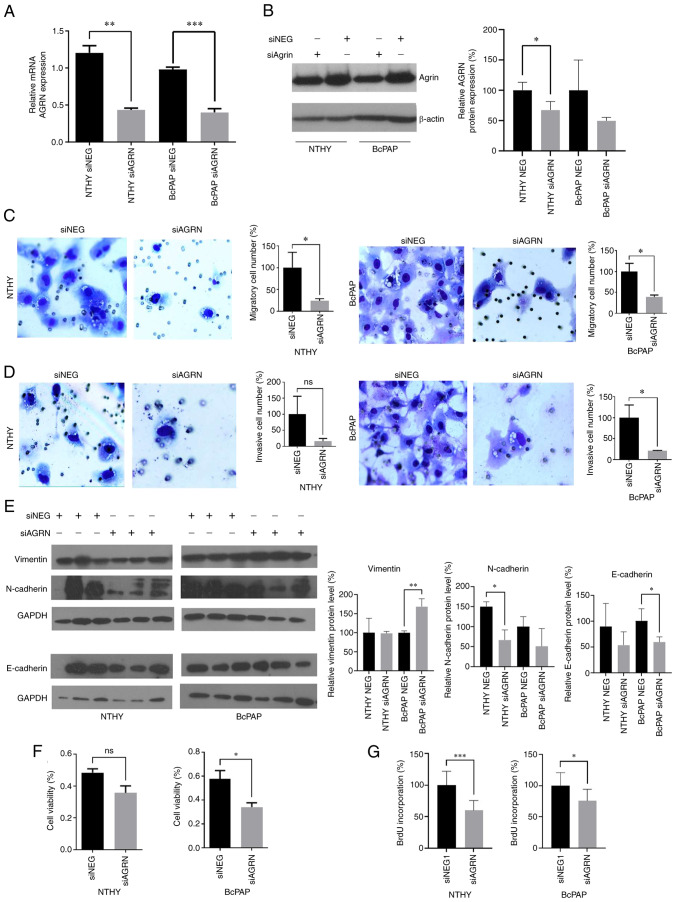
To analyze the impact of AGRN on TC cells, AGRN expression was silenced in normal thyroid cells and BcPAP cell line in which AGRN was most highly expressed. Knockdown of AGRN expression was confirmed by RT-qPCR (Fig. 2A; P<0.0016 and P<0.0006) and immunoblotting (Fig. 2B). Suppression of AGRN expression attenuated migration, invasion, viability and proliferation of BcPAP cells (Fig. 2C, D, F and G). By contrast, in NTHY cells only migration and proliferation were significantly decreased following AGRN silencing (Fig. 2C and G). To investigate the involvement of AGRN in cell migration and invasion, expression of epithelial-mesenchymal transition markers, including vimentin, N-cadherin and E-cadherin, were analyzed. Increased protein levels of vimentin (mesenchymal cell marker) and decreased levels of E-cadherin (epithelial cell marker) in siAGRN-transfected BcPAP cells were observed (Fig. 2; P<0.005 and P<0.05, respectively). N-cadherin levels (mesenchymal cell marker) decreased in siAGRN-transfected NTHY cell line (Fig. 2E; P<0.025). These results suggested that AGRN served an important role in TC tumorigenesis by stimulating key cellular processes that contribute to cancer progression.
Figure 2.
AGRN silencing suppresses migration, invasion, viability and proliferation of TC cells. (A) mRNA and (B) protein expression of AGRN in NTHY and BcPAP cells transfected with siAGRN or NEG were determined using reverse transcription-quantitative PCR and western blotting, respectively. *P<0.05, **P<0.0016 and ***P<0.0006. The plot shows changes in protein expression that were normalized to β-actin. The effects of siAGRN on (C) migration and (D) invasion of NTHY and BcPAP cells. The plots show modified Boyden chamber migration and invasion assays performed in three independent biological experiments. Magnification, ×200. (E) Effects of siAGRN transfection on vimentin, N-cadherin and E-cadherin protein levels in NTHY and BcPAP cells. *P<0.05 and **P<0.005. Representative western blotting of protein expression normalized GAPDH. (F) Effects of siAGRN transfection on the viability of NTHY and BcPAP cells. The plots show the results of the MTS assay performed in three independent biological experiments. *P<0.05. (G) Effects of siAGRN on the proliferation of NTHY and BcPAP cells. The plots show BrdU assay performed in three independent biological experiments. ***P<0.0002 and *P<0.01. TC, thyroid cancer; siRNA, small interfering RNA; NEG, negative control; AGRN, agrin.
AGRN expression is associates with neutrophil infiltration in thyroid tumors
The present study analyzed the association between AGRN expression and the presence of immune infiltrates in thyroid tumors (Table SI). Analysis revealed variable weak-to-moderate correlations between the expression of agrin and various immune cell types, including T cells, plasma or macrophages. AGRN expression was significantly correlated with the infiltration of neutrophils (ρ=0.566 and P=1.02×10−42; Fig. 3).
Figure 3.
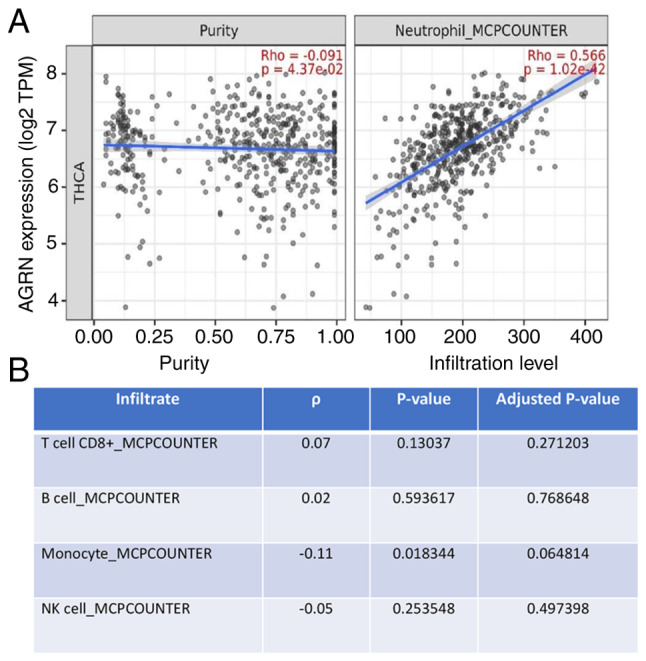
Expression of AGRN is associated with neutrophil infiltration in THCA. (A) Analysis performed using Timer. (B) Lack of a correlation between the expression of AGRN and T cells or inflammatory cells in THCA (n=509). AGRN, agrin; THCA, thyroid carcinoma; TPM, transcripts per million.
Discussion
The present study showed that AGRN was overexpressed in TC and promoted viability, proliferation, migration and invasion of TC cells. For functional analysis of AGRN involvement in TC, BcPAP cell line was selected, which is characterized by a mutation in the BRAF gene (V600E) that occurs in the majority of cases of PTC (up to 80%) (1). Moreover, AGRN expression was associated with neutrophil infiltration in thyroid tumors.
The results of the present study are consistent with the established AGRN roles in other types of cancer (25–28). AGRN is a proteoglycan component of the extracellular matrix (ECM) that serves as a ligand for the co-receptors lipoprotein-related receptor 4 (Lrp4) and muscle-specific kinase (MuSK) (38). The tumor-promoting effects of AGRN are primarily mediated by the activation of the integrin/focal adhesion/Lrp4/MuSK receptor pathway (39) In liver and breast cancer, AGRN triggers this pathway to activate transcriptional coactivator Yes-associated protein, thereby contributing to tumorigenesis (38,39). In hepatocellular carcinoma (HCC), AGRN promotes proliferation, migration and invadopodia formation by stimulating epithelial-mesenchymal transition via Lrp4/MuSK/focal adhesion kinase (FAK) signaling (39). Furthermore, AGRN activates Lrp4/MuSK/FAK signaling to promote the VEGFR2 pathway to recruit endothelial cells and facilitate adhesion to cancer cells to promote liver tumor angiogenesis (40). Other AGRN-stimulated signaling pathways include PI3K/AKT-mediated stimulation of IL-6 secretion to promote growth and immune infiltration of non-small cell lung cancer (26) and Wnt-dependent promotion of proliferation, migration and invasion of rectal cancer (41). AGRN promotes cancer progression and in vivo tumor formation in cholangiocarcinoma (42), pancreatic ductal adenocarcinoma (28) and oral squamous cell carcinoma (27,43). Finally, in HCC, AGRN depletion decreases expression of mesenchymal markers including N-cadherin, vimentin and snail and increases the expression of epithelial marker E-cadherin (39). To the best of our knowledge, the role of AGRN in non-cancerous thyroid tissue has not been studied. Studies have showed that AGRN is highly expressed in normal thyroid tissue (44,45); however, its role is unknown. It is hypothesized that AGRN serves a role in normal thyroid tissue by interacting with dystroglycan (DC), a thyroid-stimulating hormone-regulated transmembrane glycoprotein, providing interactions with the ECM (45). Furthermore, endodermal cells expressing thyroid transcription factor 1, competent to form thyroid epithelial lineages, are enriched in AGRN interactions at neuromuscular junction pathways, suggesting a potential role in thyroid development (46). Furthermore, it is hypothesized that the thyroid hormone controls the expression of highly glycosylated proteoglycans, including the family of heparan sulfate, which AGRN belongs to (47). Heparan sulfate proteoglycans (HSPG) participate in Indian hedgehog and fibroblast growth factor signaling by regulating degradation, sequestration and diffusion of growth factors and morphogens (47). HSPG role is essential for bone development when thyroid hormones coordinate the progression of endochondral ossification (48). Basset et al (47) showed that HSPG expression is upregulated in hypothyroidism. Therefore, homeostasis of thyroid hormone is key to maintain the proper expression of HSPGs such as AGRN.
Previous studies have reported involvement of AGRN in immune regulation or autoimmunity (23,24). AGRN autoantibodies are detected in patients with myasthenia gravis (23). In T cells, T cell receptor activation triggers expression of AGRN, which acts as a receptor activator and leads to intracellular signaling, resulting in actin polymerization and changes in T cell responsiveness (24). This mechanism is pronounced in patients with lupus in whom AGRN is overexpressed in T lymphocytes (24). In non-small cell lung cancer, AGRN expression is correlated with regulatory T cell (Treg) infiltration (26). AGRN is required for proper maturation and viability of monocytes and macrophages, with α-DC acting as a receptor (49). When activated by AGRN, α-DC triggers intracellular signaling involving Erk1/2 kinase, which results in changes in actin polymerization, contributing to proper conduction of phagocytosis (49). TCs are infiltrated by at least 22 types of immune cells that contribute to tumor progression (50). The aforementioned study showed that a high stroma score, low CD8+ T cell infiltration and increased presence of static memory CD4+ T cells, as well as active dendritic cells, are associated with poor prognosis for patients with TC (50). The presence of tumor-associated macrophages is associated with lymph node metastasis and larger tumor size, as well as poor survival of patients with TC (51,52). PTC progression is associated with higher tumor infiltration by Tregs and decreased presence of NK cells (53). The present study found that AGRN expression was correlated with neutrophil infiltration in thyroid tumors but not other types of immune cell. Several studies described the presence of a correlation between tumor-associated neutrophils (TANs) in cancer and clinical outcomes of patients (54–56). In some studies, TANs are shown to be involved in the promotion of cancer cell proliferation, invasive behavior or angiogenesis (57,58). Furthermore, in TC, neutrophils serve tumor-promoting roles by modulating immune and inflammatory responses (59–61). For example, the conditioned media derived from TC induces neutrophil chemotaxis by releasing CXCL8 and IL-8, which are ligands of neutrophil receptors, such as C-X-C Motif Chemokine Receptor (CXCR) 1 and CXCR2, and induces the production of reactive oxygen species, the release of MMP-9 and the expression of proinflammatory and angiogenic factors (62). Therefore, TC cells release soluble factors that induce neutrophil chemotaxis and survival (62). These correlations suggest that AGRN may be involved in the regulation of the immune environment of thyroid tumors by inducing neutrophil recruitment. This hypothesis and the mechanisms by which AGRN contribute to immune infiltration require experimental verification. AGRN localizes to the nuclei of thyroid cells, suggesting that its role in TC may not rely on extracellular-mediated signaling (63). Furthermore, AGRN nuclear localization in lung cancer with high nuclear AGRN localization is associated not only with the clinical stage and poor differentiation of lung adenocarcinoma, but also with lymph node metastases (63). These data suggest possible mechanisms of actions of AGRN in the nuclei which should be evaluated in future studies.
The present study did not identify the mechanism of action used by AGRN to modulate cancer progression and the immune environment. However, the present study showed that AGRN was overexpressed in thyroid tumors and contributed to the proliferation, viability, migration and invasion of TC cells. Moreover, AGRN silencing upregulated epithelial-mesenchymal transition marker and downregulated N-cadherin and E-cadherin. The association of AGRN expression with presence of neutrophil infiltration in thyroid tumors suggested that AGRN may contribute to the cancer immune environment.
Supplementary Material
Acknowledgements
The authors would like to thank Dr Cuong Hoang-Vu (Martin Luther University (Halle, Germany) for the 8505C cell line and Professor Marlena Godlewska (Department of Cell Biology and Immunology, Centre of Postgraduate Medical Education, Warsaw, Poland) for N-cadherin and E-cadherin antibodies (purchased from GeneTex, Inc.).
Funding Statement
The present study was supported by The National Science Center, Poland (grant no. 2016/21/B/NZ5/00063) and The Centre of Postgraduate Medical Education, Poland (grant no. 501-1-025-01-23).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
BC conceived and supervised the study, and wrote the manuscript. AAO and MG perfomed the experiments and wrote the manuscript. AAO and MG confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Scheffel RS, Dora JM, Maia AL. BRAF mutations in thyroid cancer. Curr Opin Oncol. 2022;34:9–18. doi: 10.1097/CCO.0000000000000797. [DOI] [PubMed] [Google Scholar]
- 2.European Network of Cancer Registries, corp-author. Thyroid cancer (TC) Factsheet, January 2017. https://www.encr.eu/sites/default/files/factsheets/ENCR_Factsheet_Thyroid_2017-2.pdf. [ December 4; 2022 ]; [Google Scholar]
- 3.Haddad RI, Bischoff L, Ball D, Bernet V, Blomain E, Busaidy NL, Campbell M, Dickson P, Duh QY, Ehya H, et al. Thyroid carcinoma, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20:925–951. doi: 10.6004/jnccn.2022.0040. [DOI] [PubMed] [Google Scholar]
- 4.Bai Y, Kakudo K, Jung CK. Updates in the pathologic classification of thyroid neoplasms: A review of the World Health Organization Classification. Endocrinol Metab (Seoul) 2020;35:696–715. doi: 10.3803/EnM.2020.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jung CK, Bychkov A, Kakudo K. Update from the 2022 World Health Organization Classification of Thyroid Tumors: A standardized diagnostic approach. Endocrinol Metab (Seoul) 2022;37:703–718. doi: 10.3803/EnM.2022.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantwell-Dorris ER, O'Leary JJ, Sheils OM. BRAFV600E: Implications for carcinogenesis and molecular therapy. Mol Cancer Ther. 2011;10:385–394. doi: 10.1158/1535-7163.MCT-10-0799. [DOI] [PubMed] [Google Scholar]
- 7.Zhang P, Guan H, Yuan S, Cheng H, Zheng J, Zhang Z, Liu Y, Yu Y, Meng Z, Zheng X, Zhao L. Author Correction: Targeting myeloid derived suppressor cells reverts immune suppression and sensitizes BRAF-mutant papillary thyroid cancer to MAPK inhibitors. Nat Commun. 2022;13:7025. doi: 10.1038/s41467-022-34545-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longheu A, Canu GL, Cappellacci F, Erdas E, Medas F, Calò PG. Tall cell variant versus conventional papillary thyroid carcinoma: A retrospective analysis in 351 consecutive patients. J Clin Med. 2020;10:70. doi: 10.3390/jcm10010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Śmiech M, Leszczyński P, Kono H, Wardell C, Taniguchi H. Emerging BRAF mutations in cancer progression and their possible effects on transcriptional networks. Genes (Basel) 2020;11:1342. doi: 10.3390/genes11111342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prete A, Matrone A, Gambale C, Torregrossa L, Minaldi E, Romei C, Ciampi R, Molinaro E, Elisei R. Poorly differentiated and anaplastic thyroid cancer: Insights into genomics, microenvironment and new drugs. Cancers (Basel) 2021;13:3200. doi: 10.3390/cancers13133200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, Zhao Y, Ding X, Liang J, Xu H, Lin Y, Khan HH, Shi B. A new way out of the predicament of anaplastic thyroid carcinoma from existing data analysis. Front Endocrinol (Lausanne) 2022;13:887906. doi: 10.3389/fendo.2022.887906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iñiguez-Ariza NM, Bible KC, Clarke BL. Bone metastases in thyroid cancer. J Bone Oncol. 2020;21:100282. doi: 10.1016/j.jbo.2020.100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erden ES, Babayigit C, Davran R, Akin M, Karazincir S, Isaogullari N, Demirkose M, Genc S. Papillary thyroid carcinoma with lung metastasis arising from dyshormonogenetic goiter: A case report. Case Rep Med. 2013;2013:813167. doi: 10.1155/2013/813167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basnet A, Pandita A, Fullmer J, Sivapiragasam A. Squamous cell carcinoma of the thyroid as a result of anaplastic transformation from BRAF-positive papillary thyroid cancer. Case Rep Oncol Med. 2017;2017:4276435. doi: 10.1155/2017/4276435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toraih EA, Hussein MH, Zerfaoui M, Attia AS, Marzouk Ellythy A, Mostafa A, Ruiz EML, Shama MA, Russell JO, Randolph GW, Kandil E. Site-Specific metastasis and survival in papillary thyroid cancer: The importance of brain and multi-organ disease. Cancers (Basel) 2021;13:1625. doi: 10.3390/cancers13071625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varadarajan VV, Pace EK, Patel V, Sawhney R, Amdur RJ, Dziegielewski PT. Follicular thyroid carcinoma metastasis to the facial skeleton: A systematic review. BMC Cancer. 2017;17:225. doi: 10.1186/s12885-017-3199-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parameswaran R, Shulin Hu J, Min En N, Tan WB, Yuan NK. Patterns of metastasis in follicular thyroid carcinoma and the difference between early and delayed presentation. Ann R Coll Surg Engl. 2017;99:151–154. doi: 10.1308/rcsann.2016.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu MH, Lee YY, Lu YL, Lin SF. Risk factors and prognosis for metastatic follicular thyroid cancer. Front Endocrinol (Lausanne) 2022;13:791826. doi: 10.3389/fendo.2022.791826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim N, Stiegler AL, Cameron TO, Hallock PT, Gomez AM, Huang JH, Hubbard SR, Dustin ML, Burden SJ. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell. 2008;135:334–342. doi: 10.1016/j.cell.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruegg MA, Bixby JL. Agrin orchestrates synaptic differentiation at the vertebrate neuromuscular junction. Trends Neurosci. 1998;21:22–27. doi: 10.1016/S0166-2236(97)01154-5. [DOI] [PubMed] [Google Scholar]
- 21.Zhang B, Luo S, Wang Q, Suzuki T, Xiong WC, Mei L. LRP4 serves as a coreceptor of agrin. Neuron. 2008;60:285–297. doi: 10.1016/j.neuron.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bassat E, Mutlak YE, Genzelinakh A, Shadrin IY, Baruch Umansky K, Yifa O, Kain D, Rajchman D, Leach J, Riabov Bassat D, et al. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature. 2017;547:179–184. doi: 10.1038/nature22978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lazaridis K, Tzartos SJ. Myasthenia Gravis: Autoantibody specificities and their role in MG management. Front Neurol. 2020;11:596981. doi: 10.3389/fneur.2020.596981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jury EC, Eldridge J, Isenberg DA, Kabouridis PS. Agrin signalling contributes to cell activation and is overexpressed in T lymphocytes from lupus patients. J Immunol. 2007;179:7975–7983. doi: 10.4049/jimmunol.179.11.7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sethi MK, Downs M, Shao C, Hackett WE, Phillips JJ, Zaia J. In-Depth matrisome and glycoproteomic analysis of human brain glioblastoma versus control tissue. Mol Cell Proteomics. 2022;21:100216. doi: 10.1016/j.mcpro.2022.100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han L, Shi H, Ma S, Luo Y, Sun W, Li S, Zhang N, Jiang X, Gao Y, Huang Z, et al. Agrin promotes non-small cell lung cancer progression and stimulates regulatory T cells via increasing IL-6 secretion through PI3K/AKT pathway. Front Oncol. 2022;11:804418. doi: 10.3389/fonc.2021.804418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawahara R, Granato DC, Carnielli CM, Cervigne NK, Oliveria CE, Rivera C, Yokoo S, Fonseca FP, Lopes M, Santos-Silva AR, et al. Agrin and perlecan mediate tumorigenic processes in oral squamous cell carcinoma. PLoS One. 2014;9:e115004. doi: 10.1371/journal.pone.0115004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian C, Öhlund D, Rickelt S, Lidström T, Huang Y, Hao L, Zhao RT, Franklin O, Bhatia SN, Tuveson DA, Hynes RO. Cancer cell-derived matrisome proteins promote metastasis in pancreatic ductal adenocarcinoma. Cancer Res. 2020;80:1461–1474. doi: 10.1158/0008-5472.CAN-19-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grzanka M, Stachurska-Skrodzka A, Adamiok-Ostrowska A, Gajda E, Czarnocka B. Extracellular vesicles as signal carriers in malignant thyroid tumors? Int J Mol Sci. 2022;23:3262. doi: 10.3390/ijms23063262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Wang X, Song W, Xu H, Huang R, Wang Y, Zhao W, Xiao Z, Yang X. Oncogenic properties of NEAT1 in prostate cancer cells depend on the CDC5L-AGRN transcriptional regulation circuit. Cancer Res. 2018;78:4138–4149. doi: 10.1158/0008-5472.CAN-18-0688. [DOI] [PubMed] [Google Scholar]
- 32.Chandrashekar DS, Karthikeyan SK, Korla PK, Patel H, Shovon AR, Athar M, Netto GJ, Qin ZS, Kumar S, Manne U, et al. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia. 2022;25:18–27. doi: 10.1016/j.neo.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK, Varambally S. UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19:649–658. doi: 10.1016/j.neo.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen F, Chandrashekar DS, Varambally S, Creighton CJ. Pan-cancer molecular subtypes revealed by mass-spectrometry-based proteomic characterization of more than 500 human cancers. Nat Commun. 2019;10:5679. doi: 10.1038/s41467-019-13528-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS, Li B, Liu XS. TIMER: A web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017;77:e108–e110. doi: 10.1158/1538-7445.AM2017-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q, Li B, Liu XS. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020;48((W1)):W509–W514. doi: 10.1093/nar/gkaa407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li B, Severson E, Pignon JC, Zhao H, Li T, Novak J, Jiang P, Shen H, Aster JC, Rodig S, et al. Comprehensive analyses of tumor immunity: Implications for cancer immunotherapy. Genome Biol. 2016;17:174. doi: 10.1186/s13059-016-1028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chakraborty S, Lakshmanan M, Swa HL, Chen J, Zhang X, Ong YS, Loo LS, Akıncılar SC, Gunaratne J, Tergaonkar V, et al. An oncogenic role of Agrin in regulating focal adhesion integrity in hepatocellular carcinoma. Nat Commun. 2015;6:6184. doi: 10.1038/ncomms7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chakraborty S, Njah K, Pobbati AV, Lim YB, Raju A, Lakshmanan M, Tergaonkar V, Lim CT, Hong W. Agrin as a Mechanotransduction Signal Regulating YAP through the Hippo Pathway. Cell Rep. 2017;18:2464–2479. doi: 10.1016/j.celrep.2017.02.041. [DOI] [PubMed] [Google Scholar]
- 40.Njah K, Chakraborty S, Qiu B, Arumugam S, Raju A, Pobbati AV, Lakshmanan M, Tergaonkar V, Thibault G, Wang X, Hong W. A role of agrin in maintaining the stability of vascular endothelial growth factor receptor-2 during tumor angiogenesis. Cell Rep. 2019;28:949–965.e7. doi: 10.1016/j.celrep.2019.06.036. [DOI] [PubMed] [Google Scholar]
- 41.Wang ZQ, Sun XL, Wang YL, Miao YL. Agrin promotes the proliferation, invasion and migration of rectal cancer cells via the WNT signaling pathway to contribute to rectal cancer progression. J Recept Signal Transduct Res. 2021;41:363–370. doi: 10.1080/10799893.2020.1811325. [DOI] [PubMed] [Google Scholar]
- 42.He M, Cheng C, Tu J, Ji SS, Lou D, Bai B. Agrin expression is correlated with tumor development and poor prognosis in cholangiocarcinoma. J Int Med Res. 2021;49:3000605211009722. doi: 10.1177/03000605211009722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rivera C, Zandonadi FS, Sánchez-Romero C, Soares CD, Granato DC, González-Arriagada WA, Paes Leme AF. Agrin has a pathological role in the progression of oral cancer. Br J Cancer. 2018;118:1628–1638. doi: 10.1038/s41416-018-0135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Groffen AJ, Buskens CA, van Kuppevelt TH, Veerkamp JH, Monnens LA, van den Heuvel LP. Primary structure and high expression of human agrin in basement membranes of adult lung and kidney. Eur J Biochem. 1998;254:123–128. doi: 10.1046/j.1432-1327.1998.2540123.x. [DOI] [PubMed] [Google Scholar]
- 45.Collins BJ, Gorelick G, Schneider AB. Dystroglycan is present in rat thyroid and rat thyroid cells and responds to thyrotropin. Endocrinology. 2001;142:3152–3162. doi: 10.1210/endo.142.7.8251. [DOI] [PubMed] [Google Scholar]
- 46.Kurmann AA, Serra M, Hawkins F, Rankin SA, Mori M, Astapova I, Ullas S, Lin S, Bilodeau M, Rossant J, et al. Regeneration of thyroid function by transplantation of differentiated pluripotent stem cells. Cell Stem Cell. 2015;17:527–542. doi: 10.1016/j.stem.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bassett JH, Swinhoe R, Chassande O, Samarut J, Williams GR. Thyroid hormone regulates heparan sulfate proteoglycan expression in the growth plate. Endocrinology. 2006;147:295–305. doi: 10.1210/en.2005-0485. [DOI] [PubMed] [Google Scholar]
- 48.Bassett JH, Williams GR. Role of thyroid hormones in skeletal development and bone maintenance. Endocr Rev. 2016;37:135–187. doi: 10.1210/er.2015-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mazzon C, Anselmo A, Soldani C, Cibella J, Ploia C, Moalli F, Burden SJ, Dustin ML, Sarukhan A, Viola A. Agrin is required for survival and function of monocytic cells. Blood. 2012;119:5502–5511. doi: 10.1182/blood-2011-09-382812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gong J, Jin B, Shang L, Liu N. Characterization of the immune cell infiltration landscape of thyroid cancer for improved immunotherapy. Front Mol Biosci. 2021;8:714053. doi: 10.3389/fmolb.2021.714053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim S, Cho SW, Min HS, Kim KM, Yeom GJ, Kim EY, Lee KE, Yun YG, Park DJ, Park YJ. The expression of tumor-associated macrophages in papillary thyroid carcinoma. Endocrinol Metab (Seoul) 2013;28:192–198. doi: 10.3803/EnM.2013.28.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ryder M, Ghossein RA, Ricarte-Filho JC, Knauf JA, Fagin JA. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocr Relat Cancer. 2008;15:1069–1074. doi: 10.1677/ERC-08-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gogali F, Paterakis G, Rassidakis GZ, Kaltsas G, Liakou CI, Gousis P, Neonakis E, Manoussakis MN, Liapi C. Phenotypical analysis of lymphocytes with suppressive and regulatory properties (Tregs) and NK cells in the papillary carcinoma of thyroid. J Clin Endocrinol Metab. 2012;97:1474–1482. doi: 10.1210/jc.2011-1838. [DOI] [PubMed] [Google Scholar]
- 54.Galdiero MR, Bianchi P, Grizzi F, Di Caro G, Basso G, Ponzetta A, Bonavita E, Barbagallo M, Tartari S, Polentarutti N, et al. Occurrence and significance of tumor-associated neutrophils in patients with colorectal cancer. Int J Cancer. 2016;139:446–456. doi: 10.1002/ijc.30076. [DOI] [PubMed] [Google Scholar]
- 55.Wikberg ML, Ling A, Li X, Öberg A, Edin S, Palmqvist R. Neutrophil infiltration is a favorable prognostic factor in early stages of colon cancer. Hum Pathol. 2017;68:193–202. doi: 10.1016/j.humpath.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 56.Zhou L, Xu L, Chen L, Fu Q, Liu Z, Chang Y, Lin Z, Xu J. Tumor-infiltrating neutrophils predict benefit from adjuvant chemotherapy in patients with muscle invasive bladder cancer. Oncoimmunology. 2017;6:e1293211. doi: 10.1080/2162402X.2017.1293211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Masucci MT, Minopoli M, Carriero MV. Tumor associated neutrophils. Their role in tumorigenesis, metastasis, prognosis and therapy. Front Oncol. 2019;9:1146. doi: 10.3389/fonc.2019.01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu L, Zhang XH. Tumor-Associated neutrophils and macrophages-heterogenous but not chaotic. Front Immunol. 2020;11:553967. doi: 10.3389/fimmu.2020.553967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest. 2010;120:1151–1164. doi: 10.1172/JCI37223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, Jorcyk CL. Breast cancer cells stimulate neutrophils to produce oncostatin M: Potential implications for tumor progression. Cancer Res. 2005;65:8896–8904. doi: 10.1158/0008-5472.CAN-05-1734. [DOI] [PubMed] [Google Scholar]
- 61.Scapini P, Cassatella MA. Social networking of human neutrophils within the immune system. Blood. 2014;124:710–719. doi: 10.1182/blood-2014-03-453217. [DOI] [PubMed] [Google Scholar]
- 62.Galdiero MR, Varricchi G, Loffredo S, Bellevicine C, Lansione T, Ferrara AL, Iannone R, di Somma S, Borriello F, Clery E, et al. Potential involvement of neutrophils in human thyroid cancer. PLoS One. 2018;13:e0199740. doi: 10.1371/journal.pone.0199740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li D, Gu Q, Xie Z, Shen Q, Li H. Clinical significance of nuclear localisation of agrin in lung adenocarcinoma. Pol J Pathol. 2019;70:198–204. doi: 10.5114/pjp.2019.90396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.