Abstract
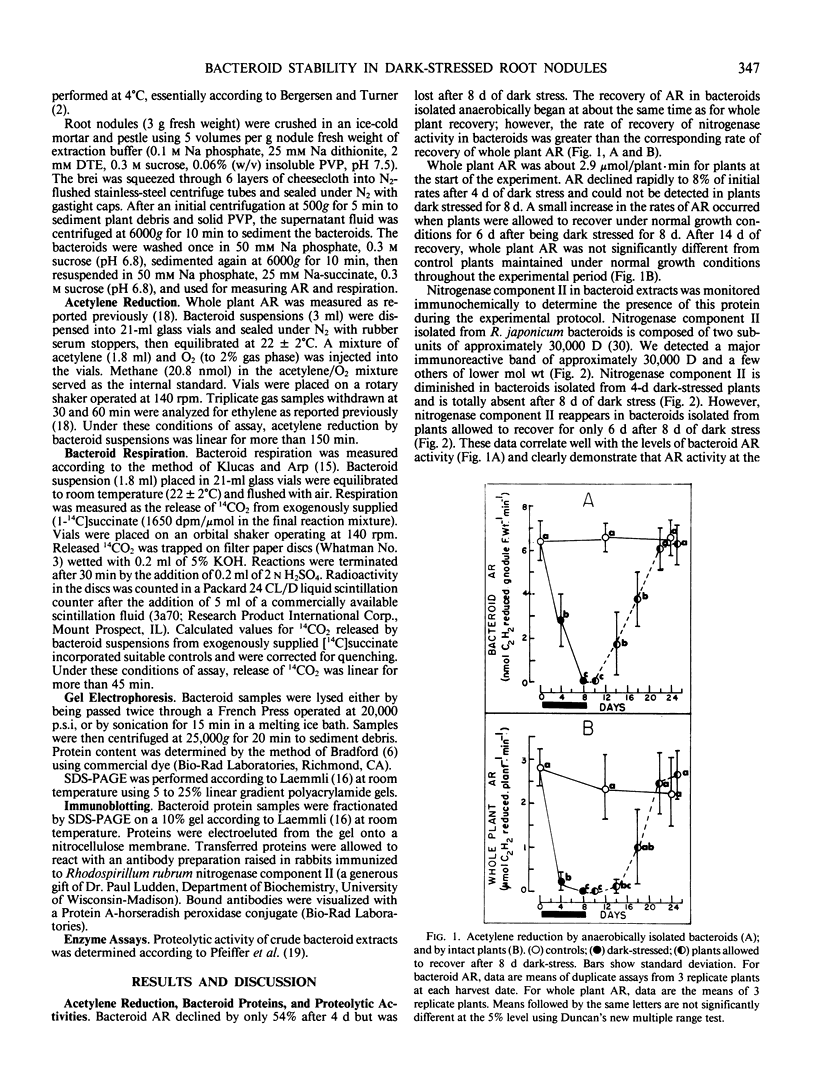
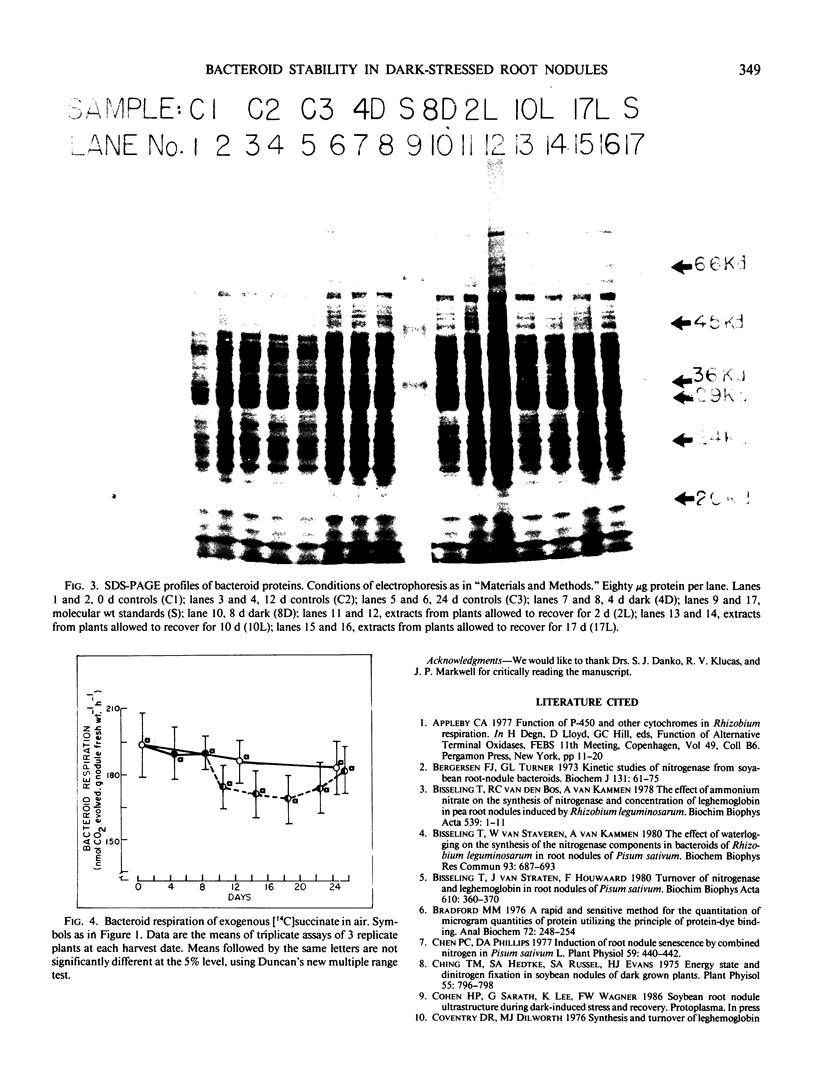
Physiological and biochemical markers of metabolic competence were assayed in bacteroids isolated from root nodules of control, dark-stressed, and recovered plants of Glycine max Merr. cv `Woodworth.' Nitrogenase-dependent acetylene reduction by the whole plant decreased to 8% of control rates after 4 days of dark stress and could not be detected in plants dark stressed for 8 days. However, in bacteroids isolated anaerobically, almost 50% of initial acetylene reduction activity remained after 4 days of dark stress but was totally lost after 8 days of dark stress. Bacteroid acetylene reduction activity recovered faster than whole plant acetylene reduction activity when plants were dark stressed for 8 days and returned to a normal light regimen. Significant changes were not measured in bacteroid respiration, protein content, sodium dodecyl sulfate-polyacrylamide gel electrophoresis protein profiles, or in bacteroid proteolytic activity throughout the experiment. Immunoblots of bacteroid extracts revealed the presence of nitrogenase component II in control, 4-day dark-stressed, and 8-day dark-stressed plants that were allowed to recover under a normal light regimen, but not in 8-day dark-stressed plants. Our data indicate that dark stress does not greatly affect bacteroid metabolism or induce bacteroid senescence.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergersen F. J., Turner G. L. Kinetic studies of nitrogenase from soya-bean root-nodule bacteroids. Biochem J. 1973 Jan;131(1):61–75. doi: 10.1042/bj1310061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisseling T., van Staveren W., van Kammen A. The effect of waterlogging on the synthesis of the nitrogenase components in bacteroids of Rhizobium leguminosarum in root nodules of Pisum sativum. Biochem Biophys Res Commun. 1980 Apr 14;93(3):687–693. doi: 10.1016/0006-291x(80)91132-8. [DOI] [PubMed] [Google Scholar]
- Bisseling T., van Straten J., Houwaard F. Turnover of nitrogenase and leghemoglobin in root nodules of Pisum sativum. Biochim Biophys Acta. 1980 Dec 11;610(2):360–370. doi: 10.1016/0005-2787(80)90017-9. [DOI] [PubMed] [Google Scholar]
- Bisseling T., van den Bos R. C., van Kammen A. The effect of ammonium nitrate on the synthesis of nitrogenase and the concentration of leghemoglobin in pea root nodules induced by Rhizobium leguminosarum. Biochim Biophys Acta. 1978 Feb 13;539(1):1–11. doi: 10.1016/0304-4165(78)90115-0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chen P. C., Phillips D. A. Induction of Root Nodule Senescence by Combined Nitrogen in Pisum sativum L. Plant Physiol. 1977 Mar;59(3):440–442. doi: 10.1104/pp.59.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching T. M., Hedtke S., Russell S. A., Evans H. J. Energy State and Dinitrogen Fixation in Soybean Nodules of Dark-grown Plants. Plant Physiol. 1975 Apr;55(4):796–798. doi: 10.1104/pp.55.4.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coventry D. R., Dilworth M. J. Synthesis and turnover of leghaemoglobin in lupin root nodules. Biochim Biophys Acta. 1976 Sep 20;447(1):1–10. doi: 10.1016/0005-2787(76)90089-7. [DOI] [PubMed] [Google Scholar]
- Houwaard F. Influence of ammonium chloride on the nitrogenase activity of nodulated pea plants (Pisum sativum). Appl Environ Microbiol. 1978 Jun;35(6):1061–1065. doi: 10.1128/aem.35.6.1061-1065.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucas R. V., Arp D. Physiological and biochemical studies on senescing tap root nodules of soybeans. Can J Microbiol. 1977 Oct;23(10):1426–1432. doi: 10.1139/m77-211. [DOI] [PubMed] [Google Scholar]
- Klucas R. V. Studies on soybean nodule senescence. Plant Physiol. 1974 Oct;54(4):612–616. doi: 10.1104/pp.54.4.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Pfeiffer N. E., Malik N. S., Wagner F. W. Reversible dark-induced senescence of soybean root nodules. Plant Physiol. 1983 Feb;71(2):393–399. doi: 10.1104/pp.71.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer N. E., Torres C. M., Wagner F. W. Proteolytic Activity in Soybean Root Nodules : Activity in Host Cell Cytosol and Bacteroids throughout Physiological Development and Senescence. Plant Physiol. 1983 Apr;71(4):797–802. doi: 10.1104/pp.71.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy J. E., Fishbeck K. A., Dejong T. M., Williams L. E., Phillips D. A. Carbon exchange rates of shoots required to utilize available acetylene reduction capacity in soybean and alfalfa root nodules. Plant Physiol. 1980 Jul;66(1):101–104. doi: 10.1104/pp.66.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien H. C., Cain P. S., Schmidt E. L. Viability of Rhizobium bacteroids. Appl Environ Microbiol. 1977 Dec;34(6):854–856. doi: 10.1128/aem.34.6.854-856.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. E., Dejong T. M., Phillips D. A. Effect of changes in shoot carbon-exchange rate on soybean root nodule activity. Plant Physiol. 1982 Feb;69(2):432–436. doi: 10.1104/pp.69.2.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. P., Evans H. J. Poly-beta-hydroxybutyrate Utilization by Soybean (Glycine max Merr.) Nodules and Assessment of Its Role in Maintenance of Nitrogenase Activity. Plant Physiol. 1971 Jun;47(6):750–755. doi: 10.1104/pp.47.6.750. [DOI] [PMC free article] [PubMed] [Google Scholar]




