Molecules of the title complex are centrosymmetric and the Fe—N bond lengths to the N atoms of the porphyrin ring indicate that the FeII atom is in the low-spin state.
Keywords: crystal structure, Hirshfeld surface analysis, iron(II) porphyrin complex, tert butyl isocyanide
Abstract
In the title compound, [FeII(C44H24Cl4N4)(C5H9N)2] or [FeII(TClPP)(t-BuNC)2] [where TClPP and t-BuNC are 5,10,15,20-tetrakis(4-chlorophenyl)porphyrinate and tert-butyl isocyanide ligands, respectively], the metal ion lies on an inversion center and is octahedrally coordinated by the N atoms of the porphyrin ring in the equatorial plane and by carbon atoms of the trans t-BuNC ligands in the axial sites. The Fe—N bond length of 2.0074 (14) Å suggests a low-spin complex (S = 0). The crystal packing of the title compound is sustained by C—H⋯Cl, C—H⋯N and C__H⋯Cg (Cg = the centroid of a pyrrole ring of the TClPP porphyrinate) interactions, leading to a three-dimensional network. The Hirshfeld surface (HS) analysis indicates that 61.4% of the intermolecular interactions are from H⋯H contacts while other contributions are from C⋯H/H⋯C, O⋯H/H⋯O and N⋯H/H⋯N interactions, which comprise 21.3%, 13.3% and 3.6% of the HS, respectively.
1. Chemical context
Since the beginning of the 1960s, hexacoordinated iron(II) metalloporphyrins of type [FeII(Porph)(L)2], where Porph = porphyrin and L is a N-donor neutral axial ligand like pyridine or imidazole, have been widely employed to mimic hemoproteins such as hemoglobin, myoglobin and cytochrome c. These ferrous porphyrin complexes are low-spin 3d
6 systems (S = 0). Such FeII models with π-acceptor axial ligands like CN−, CO and isocyanides (R—NC) are also known. Heme-isocyanide derivatives were studied starting from 1951 (St. George & Pauling, 1951 ▸) as a result of their electronic similarity to CO-hemoproteins. Jameson & Ibers (1979 ▸) studied the IR data and the molecular structure of the [FeII(TPP)(t-BuNC)2] compound. In 1984, the Mössbauer-effect data and the IR of the bis(isocyanide) iron(II) porphyrin [FeII(TPP)(PhCO-NC)2] (PhCO-NC = benzoylisocyanide) and the mixed ligand isocyanide-py ligands [FeII(TPP)(PhCO-NC)(py)] were reported (Le Plouzennec et al., 1984 ▸). Subsequently, Salzmann et al. (1999 ▸) documented the molecular structure, and undertook 13C and 15N NMR studies of the [FeII(TPP)(iPrNC)(1-MeIm)] (iPrNC = 2-isocyanopropane and 1-MeIm = 1- methylimidazole) complex. In 2001, spectroscopic investigations of the FeII and FeIII
n-butyl isocyanide complexes P450cam and P450nor were reported (Lee et al., 2001 ▸). In 2017, we reported the spectroscopic and structural characterization of the [FeII(TPBP)(t-BuNC)2] complex where TPBP is the 5,10,15,20-{tetrakis-[para-(benzoyloxy)phenyl]porphyrinate ligand (Nasri et al., 2017 ▸). In order to obtain more insight into the electronic and structural properties of iron(II) bis(isocyanide) porphyrin complexes, we now report the synthesis, UV/Vis and IR data and the single crystal X-ray structure of the title bis(t-butyl isocyanide)[5,10,15,20-tetra(para-chlorophenyl)porphyrinato]iron(II) complex, [FeII(TClPP)(t-BuNC)2], I.

2. Structural commentary
Complex I forms monoclinic crystals (P21/n space group), wherein the iron(II) atom is positioned on an inversion center. The FeII center atom exhibits an octahedral coordination by four pyrrole N atoms from the porphyrin macrocycle and two trans t-BuNC axial ligands. One-half of the [FeII(TClPP)(t-BuNC)2] molecule comprises the asymmetric unit of complex I (Fig. 1 ▸). Scheidt & Reed (1981 ▸) observed a correlation between the average equatorial Fe—Np (p = porphyrin) bond length and the spin state of iron(II) metalloporphyrins. Consequently, in high-spin (S = 2) complexes, the Fe—Np distances are the longest, exemplified by the [Fe(TpivPP)(N3)]− ion complex (where TpivPP represents α,α,α,α-tetrakis(o-pivalamidophenyl)porphinate, known as the picket-fence porphyrin) where Fe—N = 2.094 (3) Å (Hachem et al., 2009 ▸). In low-spin (S = 0) complexes, the average Fe—Np bond length is reduced. For example, in the [FeII(TMPP)(amp)2] complex [TMPP is 5,10,15,20-tetrakis(4-methoxyphenyl)porphyrinato and amp is the 4-(2-aminoethyl)morpholine], the Fe—N bond length is 1.988 (2) Å (Ben Haj Hassen et al., 2016 ▸). For our ferrous bis(t-BuNC) derivative (I), the Fe—N distance of 2.0074 (14) Å strongly suggests that this species corresponds to an iron(II) low-spin (S = 0) porphyrin. Notably, this value closely resembles those for the related [FeII(TPP)(t-BuNC)2] (Jameson & Ibers, 1979 ▸) and [FeII(TPBP)(t-BuNC)2] (Nasri et al., 2017 ▸) complexes, which are 2.005 (2) and 2.007 (2) Å, respectively.
Figure 1.
The molecular structure of the title compound with displacement ellipsoids drawn at 40%. The H atoms have been omitted for clarity. Symmetry code: (i) 2 − x, −y, 1 − z.
In complex I, the Fe—C distance to the axial ligand measures 1.924 (2) Å, which is very near to those observed in the associated FeII bis(t-BuNC) metalloporphyrins: [FeII(TPP)(t-BuNC)2] (where TPP is 5,10,15,20-tetraphenylporphyrinate) and [FeII(TPBP)(t-BuNC)2] {where TPBP is [4-(benzoyloxy)phenyl]porphyrinate} with FeII—C distances of 1.901 (3) and 1.907 (2) Å, respectively (Jameson & Ibers, 1979 ▸ ; Nasri et al., 2017 ▸). As depicted in Fig. 1 ▸, complex I exhibits a non-linear iron(II)–(t-BuNC) geometry. Specifically, the Fe—C23—N3 and C23—N3—C24 angles measure 165.75 (15) and 163.66 (17)°, respectively, which closely resemble the corresponding angles observed in [FeII(TPP)(t-BuNC)2] (Jameson & Ibers, 1979 ▸), which are 170.58 (19) and 167.4 (2)°, respectively.
In the case of the related iron(III) ion complex [FeIII(TPP)(t-BuCN)2]+, these angles exhibit significantly higher values, measuring 174.2 and 173.5° for the average Fe—C—N and C—N—C angles, respectively. The deviations from linearity, represented by the angles 14.3/16.3° for complex I and 11.0/20.9° for [FeII(TPP)(t-BuNC)2] (Jameson & Ibers, 1979 ▸), are notably greater than those observed in the iron(III) TPP-bis(t-BuNC) derivative, where the average deviation values are 5.8/6.5° (Walker et al., 1996 ▸). The greater deviation from linearity observed in the t-BuCN ligand of ferrous meso-metalloporphyrins, compared to the ferric meso-porphyrin ion complex [FeIII(TPP)(t-BuCN)2]+, is consistent with the dominance of the π-backbonding effect in iron(II) derivatives over iron(III) coordination compounds.
As highlighted in the IR spectroscopy section, the FeII species exhibit significant π-backbonding, which implies that the C—N bond length in the ferrous tert-butyl isocyanide species should be greater than that observed in the ferric tert-butyl isocyanide derivatives. Indeed, in the case of I, the C—N distance measures 1.159 (2) Å, which is quite similar to the C—N distances observed in the related compound [FeII(TPP)(t-BuCN)2] (1.152 and 1.162 Å; Jameson & Ibers, 1979 ▸). Comparatively, for the t-BuNC–iron(III) ion complexes [FeIII(TPP)(t-BuNC)2]+ (Walker et al., 1996 ▸) and [FeIII(OEP)(t-BuNC)2]+ (Walker et al., 1996 ▸), the C—N bond length values for the t-BuNC ligand are 1.13 (3)/1.12 (2) Å and 1.145 (4)/1.144 (4) Å, respectively.
It is certainly true that the iron(II) derivatives display longer C—N distances. Nevertheless, the difference between the longest C—N bond length of an iron(II)–bis(t-BuNC) derivative and the shortest C—N bond length of an iron(III)–bis(t-BuNC) metalloporphyrin is small (0.042 Å).
3. Supramolecular features
Within the crystal structure of I (Figs. 2 ▸ and 3 ▸, Table 1 ▸), the [FeII(TClPP)(t-BuNC)2] complexes are linked to each other via weak non-classical C—H⋯Cl, C—H⋯N hydrogen bonds and C—H⋯Cg intermolecular interactions where Cg is the centroid of a pyrrole ring. It may be noted that Cl1 acts as acceptor for three different C—H groups.
Figure 2.
A portion of the crystal packing of the title complex, viewed down [100].
Figure 3.
A partial view of the crystal packing of I showing the link between the complexes via C__H⋯Cl and C__H⋯N hydrogen bonds and by C—H⋯π interactions.
Table 1. Hydrogen-bond geometry (Å, °).
Cg1 is the centroid of the N1/C1–C4 pyrrole ring.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C25—H25C⋯Cl1i | 0.98 | 2.86 | 3.738 (2) | 149 |
| C27—H27B⋯Cl1i | 0.98 | 2.86 | 3.733 (2) | 149 |
| C7—H7⋯Cl1ii | 0.95 | 2.94 | 3.7953 (18) | 151 |
| C13—H13⋯N2iii | 0.95 | 2.86 | 3.685 (2) | 146 |
| C21—H21⋯Cg1iv | 0.96 | 2.69 | 3.518 (2) | 146 |
Symmetry codes: (i)
 ; (ii)
; (ii)
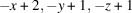 ; (iii)
; (iii)
 ; (iv)
; (iv)
 .
.
4. Database survey
A search in the Cambridge Structural Database (version 5.43, update of September 2022; Groom et al., 2016 ▸), of iron(II) hexacoordinated metalloporphyrin complexes type [FeII(Porph)(L)2] where Porph is a porphyrin and L is a N-donor, O-donor, S-donor or C-donor axial ligand gave 25 hits where L is a neutral N-donor axial ligand, ten hits for neutral O-donor axial ligands, three S-donor neutral S-donor axial ligands and four neutral C-donor axial ligands. In fact, for the latter type of neutral axial ligands, it is the tert-butyl isocyanide corresponding to [FeII(TPP)(t-BuNC)2] (Jameson & Ibers, 1979 ▸), [FeII(OOEP)(t-BuNC)2] (OOEP = octaethyloxophlorinato) (Rath et al., 2004 ▸) and [FeII(TPBP)(t-BuNC)2] (TPBP = 5,10,15,20-(tetrakis-[4-(benzoyloxy)phenyl]porphyrinate]).
5. FT-IR and UV/Vis spectroscopies
The FT–IR spectrum of [FeII(TClPP)(t-Bu-NC)2] (I) (Fig. 4 ▸) was obtained in the 4000–400 cm−1 range by a PerkinElmer Spectrum Two FTIR spectrometer. The spectrum exhibits characteristic IR bands of the TClPP porphyrinate. The C—H stretching frequencies of the porphyrin are shown between 3083 and 2923 cm−1 while ν(CH) of the methyl groups of the t-BuNC axial ligand occurs at 2883 cm−1. The strong band at 996 cm−1 is attributed to the bonding vibration δ(CCH) of the porphyrin core for which a value around 1000 cm−1 is characteristic of a metalled porphyrin while a δ(CCH) value around 960 cm−1 is specific of a free base porphyrin.
Figure 4.
FT–IR spectrum of I.
It has been found that the values of the C≡N stretching frequency for ferric t-BuNC metalloporphyrins are displaced by at least 60 cm−1 to higher frequency compared to those of ferrous t-BuNC porphyrin complexes. Thus for the [FeIII(TPP)(t-BuNC)2]+ ion complex (Simonneaux et al., 1989 ▸), the ν(C≡N) frequency value of the t-BuNC axial ligand is 2222 cm−1 while that of the [FeII(TPP)(t-BuNC)2] compound (Simonneaux et al., 1989 ▸) is 2129 cm−1. Our FeII–TClPP-bis(t-BuNC) species (I), exhibits two bands attributed to the ν(C≡N) frequency value of the t-BuNC axial ligand with the weak one at 2202 cm−1 and the main strong IR band is shown at 2126 cm−1. This indicates clearly that complex I is iron(II) metalloporphyrin.
The UV/Vis spectrum of complex I was obtained in chloroform using a WinASPECT PLUS scanning spectrophotometer (Fig. 5 ▸). The measurements were conducted in 1.0 cm path length cuvettes containing dry degassed chloroform solutions, all under an argon atmosphere. The λmax value of the Soret band for complex I is 436 nm (Gouterman et al., 1963 ▸), which closely resembles the values observed in the related species [FeII(TPP)(t-BuNC)2] and [FeII(TBPPP)(t-BuNC)2], which are 432 nm and 437 nm, respectively (Jameson & Ibers, 1979 ▸; Nasri et al., 2017 ▸). Notably for the bis(t-BuNC) ferric metalloporphyrins, the λmax of the Soret band value is blue shifted compared to those of the ferrous bis(t-BuNC) porphyrin complexes, e.g., for [FeIII(TPP)(t-BuNC)2]+ (Simonneaux et al., 1989 ▸), the λmax of the Soret band is 420 nm.
Figure 5.
UV/Vis spectrum of I recorded in chloroform.
6. Hirshfeld surface analysis
The supramolecular interactions in the title structure have been further investigated and visualized by Hirshfeld surface (HS) analysis performed with Crystal Explorer 17 (Turner et al., 2017 ▸). The Hirshfeld surface of complex I mapped over d norm in the range −0.19 to 1.14 a.u. is represented in Fig. 6 ▸. This study confirms that the crystal packing of complex I is mainly made by C—H⋯Cl, C—H⋯N and C—H⋯Cg intermolecular interactions, as already shown by the PLATON program (Spek, 2020 ▸) (Fig. 3 ▸). According to the two-dimensional fingerprint plots of complex I shown in Fig. 7 ▸, most important intermolecular interactions are H ⋯H contacts (61.4%). The C⋯H/H⋯C, O⋯H/H⋯O and N⋯H/H⋯N interactions comprise 21.3%, 13.3% and 3.6% of the HS, respectively.
Figure 6.
View of the three-dimensional Hirshfeld surface of complex (I) plotted over d norm.
Figure 7.
Two-dimensional fingerprint plots of complex I showing close contacts of (a) all contributions in the crystal and those delineated into (b) H⋯H, (c) C⋯H/H⋯C and (d) Cl⋯H/H⋯Cl interactions.
7. Synthesis and crystallization
The starting materials 5,10,15,20-tetra(para-chlorophenyl)porphyrin (H2TClPP) and [FeIII(TClPP)(SO3CF3)] were prepared as described in the literature (Adler et al., 1967 ▸; Gismelseed et al., 1990 ▸). To a solution of [FeIII(TClPP)(SO3CF3)] (100 mg, 0.105 mmol) in dichloromethane (35 ml) was added an excess of tert-butyl isocyanide (t-BuNC) (1.2 ml, 10.5 mmol). The reaction mixture was stirred at room temperature for 3 h. Crystals of the title complex were obtained by diffusion of the n-hexane and dichloromethane solutions. Elemental analysis calculated (%) for C54H42Cl4FeN6: C 66.68, H 4.35, N 8.64; found: C 66.81, H 4.41, N 8.78,
8. Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. All H atoms attached to C atoms were fixed geometrically and treated as riding with C—H = 0.99 Å (methylene) and 0.95 Å (aromatic) with U iso(H) = 1.2U eq(C).
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | [Fe(C44H24Cl4N4)(C5H9N)2] |
| M r | 972.58 |
| Crystal system, space group | Monoclinic, P21/n |
| Temperature (K) | 115 |
| a, b, c (Å) | 10.9679 (5), 16.9240 (7), 13.3536 (5) |
| β (°) | 114.015 (1) |
| V (Å3) | 2264.15 (16) |
| Z | 2 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.62 |
| Crystal size (mm) | 0.28 × 0.21 × 0.18 |
| Data collection | |
| Diffractometer | Nonius Kappa APEXII |
| Absorption correction | Numerical (SADABS; Krause et al., 2015 ▸) |
| T min, T max | 0.903, 0.982 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 44887, 5201, 4077 |
| R int | 0.052 |
| (sin θ/λ)max (Å−1) | 0.650 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.033, 0.083, 1.05 |
| No. of reflections | 5201 |
| No. of parameters | 295 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.43, −0.35 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989023008083/hb8075sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989023008083/hb8075Isup2.hkl
CCDC reference: 975663
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
The author would like to thank Deanship of Scientific Research at Majmaah University for supporting this work under Project No. R-2023-608.
supplementary crystallographic information
Crystal data
| [Fe(C44H24Cl4N4)(C5H9N)2] | F(000) = 1004 |
| Mr = 972.58 | Dx = 1.427 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| a = 10.9679 (5) Å | Cell parameters from 9956 reflections |
| b = 16.9240 (7) Å | θ = 2.4–27.2° |
| c = 13.3536 (5) Å | µ = 0.62 mm−1 |
| β = 114.015 (1)° | T = 115 K |
| V = 2264.15 (16) Å3 | Prism, dark violet |
| Z = 2 | 0.28 × 0.21 × 0.18 mm |
Data collection
| Nonius Kappa APEXII diffractometer | 5201 independent reflections |
| Radiation source: X-ray tube | 4077 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.052 |
| Detector resolution: 512 x 512 pixels mm-1 | θmax = 27.5°, θmin = 2.4° |
| φ and ω scans | h = −14→14 |
| Absorption correction: numerical (SADABS; Krause et al., 2015) | k = −22→21 |
| Tmin = 0.903, Tmax = 0.982 | l = −17→15 |
| 44887 measured reflections |
Refinement
| Refinement on F2 | 0 restraints |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.033 | H-atom parameters constrained |
| wR(F2) = 0.083 | w = 1/[σ2(Fo2) + (0.0294P)2 + 1.8163P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.05 | (Δ/σ)max < 0.001 |
| 5201 reflections | Δρmax = 0.43 e Å−3 |
| 295 parameters | Δρmin = −0.35 e Å−3 |
Special details
| Experimental. SADABS-2012/1 (Bruker,2012) was used for absorption correction. wR2(int) was 0.0545 before and 0.0502 after correction. The Ratio of minimum to maximum transmission is 0.9200. The λ/2 correction factor is 0.0015. |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Fe | 1.0000 | 0.0000 | 0.5000 | 0.01108 (9) | |
| C23 | 1.02702 (17) | 0.01547 (10) | 0.36771 (14) | 0.0145 (4) | |
| N3 | 1.05491 (15) | 0.03942 (9) | 0.29849 (12) | 0.0170 (3) | |
| C24 | 1.10111 (19) | 0.08937 (11) | 0.23145 (15) | 0.0198 (4) | |
| C25 | 1.0239 (2) | 0.16655 (11) | 0.21248 (19) | 0.0276 (5) | |
| H25A | 1.0520 | 0.2019 | 0.1677 | 0.041* | |
| H25B | 1.0418 | 0.1917 | 0.2832 | 0.041* | |
| H25C | 0.9281 | 0.1558 | 0.1744 | 0.041* | |
| C26 | 1.2495 (2) | 0.10337 (15) | 0.29680 (19) | 0.0349 (5) | |
| H26A | 1.2841 | 0.1367 | 0.2543 | 0.052* | |
| H26B | 1.2966 | 0.0526 | 0.3123 | 0.052* | |
| H26C | 1.2636 | 0.1298 | 0.3660 | 0.052* | |
| C27 | 1.0745 (2) | 0.04753 (13) | 0.12358 (17) | 0.0312 (5) | |
| H27A | 1.1054 | 0.0808 | 0.0785 | 0.047* | |
| H27B | 0.9786 | 0.0378 | 0.0845 | 0.047* | |
| H27C | 1.1226 | −0.0029 | 0.1384 | 0.047* | |
| N1 | 0.82346 (14) | 0.05565 (8) | 0.43209 (11) | 0.0123 (3) | |
| N2 | 1.09274 (14) | 0.10501 (8) | 0.53887 (11) | 0.0123 (3) | |
| C1 | 0.69880 (17) | 0.02084 (10) | 0.38631 (14) | 0.0137 (3) | |
| C2 | 0.59681 (18) | 0.08046 (10) | 0.34686 (14) | 0.0164 (4) | |
| H2 | 0.5034 | 0.0716 | 0.3103 | 0.020* | |
| C3 | 0.65836 (18) | 0.15122 (11) | 0.37137 (15) | 0.0175 (4) | |
| H3 | 0.6166 | 0.2016 | 0.3566 | 0.021* | |
| C4 | 0.79981 (17) | 0.13574 (10) | 0.42436 (14) | 0.0137 (3) | |
| C5 | 0.89792 (17) | 0.19464 (10) | 0.46487 (14) | 0.0138 (3) | |
| C6 | 1.03494 (17) | 0.17860 (10) | 0.51399 (14) | 0.0138 (3) | |
| C7 | 1.13680 (18) | 0.23832 (10) | 0.53796 (15) | 0.0176 (4) | |
| H7 | 1.1230 | 0.2937 | 0.5281 | 0.021* | |
| C8 | 1.25547 (18) | 0.20053 (10) | 0.57700 (15) | 0.0177 (4) | |
| H8 | 1.3410 | 0.2243 | 0.5995 | 0.021* | |
| C9 | 1.22812 (17) | 0.11779 (10) | 0.57818 (14) | 0.0142 (3) | |
| C10 | 0.67285 (17) | −0.05975 (10) | 0.38344 (14) | 0.0137 (3) | |
| C11 | 0.85653 (17) | 0.27954 (10) | 0.45362 (14) | 0.0152 (4) | |
| C12 | 0.78007 (18) | 0.31308 (10) | 0.35164 (15) | 0.0172 (4) | |
| H12 | 0.7471 | 0.2803 | 0.2884 | 0.021* | |
| C13 | 0.75130 (18) | 0.39353 (11) | 0.34085 (15) | 0.0188 (4) | |
| H13 | 0.6975 | 0.4156 | 0.2714 | 0.023* | |
| C14 | 0.80245 (18) | 0.44073 (10) | 0.43308 (16) | 0.0190 (4) | |
| C15 | 0.87414 (19) | 0.40905 (11) | 0.53618 (16) | 0.0217 (4) | |
| H15 | 0.9057 | 0.4420 | 0.5992 | 0.026* | |
| C16 | 0.89919 (19) | 0.32841 (11) | 0.54595 (15) | 0.0196 (4) | |
| H16 | 0.9461 | 0.3060 | 0.6166 | 0.024* | |
| C17 | 0.52953 (17) | −0.08653 (10) | 0.33800 (14) | 0.0148 (4) | |
| C18 | 0.46030 (18) | −0.10376 (12) | 0.22725 (15) | 0.0219 (4) | |
| H18 | 0.5034 | −0.0973 | 0.1788 | 0.026* | |
| C19 | 0.32887 (19) | −0.13036 (12) | 0.18617 (16) | 0.0236 (4) | |
| H19 | 0.2825 | −0.1420 | 0.1104 | 0.028* | |
| C20 | 0.26692 (18) | −0.13963 (11) | 0.25659 (17) | 0.0209 (4) | |
| C21 | 0.3315 (2) | −0.12213 (14) | 0.36586 (17) | 0.0299 (5) | |
| H21 | 0.2873 | −0.1280 | 0.4136 | 0.036* | |
| C22 | 0.4631 (2) | −0.09559 (13) | 0.40590 (16) | 0.0270 (5) | |
| H22 | 0.5083 | −0.0834 | 0.4816 | 0.032* | |
| Cl2 | 0.10157 (5) | −0.17304 (3) | 0.20542 (5) | 0.03464 (14) | |
| Cl1 | 0.78308 (5) | 0.54293 (3) | 0.41772 (5) | 0.03197 (14) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Fe | 0.00822 (17) | 0.01188 (16) | 0.01215 (17) | −0.00047 (13) | 0.00312 (13) | 0.00022 (13) |
| C23 | 0.0088 (8) | 0.0138 (8) | 0.0178 (9) | −0.0010 (6) | 0.0024 (7) | −0.0018 (7) |
| N3 | 0.0138 (7) | 0.0197 (8) | 0.0167 (8) | 0.0018 (6) | 0.0053 (6) | −0.0003 (6) |
| C24 | 0.0179 (9) | 0.0241 (9) | 0.0198 (9) | 0.0024 (8) | 0.0102 (8) | 0.0065 (8) |
| C25 | 0.0253 (11) | 0.0195 (9) | 0.0401 (12) | 0.0018 (8) | 0.0154 (10) | 0.0045 (9) |
| C26 | 0.0185 (11) | 0.0500 (14) | 0.0349 (12) | −0.0002 (10) | 0.0095 (9) | 0.0147 (11) |
| C27 | 0.0425 (13) | 0.0337 (12) | 0.0243 (11) | 0.0067 (10) | 0.0207 (10) | 0.0053 (9) |
| N1 | 0.0107 (7) | 0.0130 (7) | 0.0132 (7) | −0.0004 (5) | 0.0049 (6) | 0.0000 (6) |
| N2 | 0.0084 (7) | 0.0133 (7) | 0.0138 (7) | −0.0003 (5) | 0.0031 (6) | 0.0001 (6) |
| C1 | 0.0104 (8) | 0.0171 (8) | 0.0136 (8) | 0.0003 (7) | 0.0049 (7) | −0.0003 (7) |
| C2 | 0.0101 (8) | 0.0192 (9) | 0.0175 (9) | 0.0013 (7) | 0.0032 (7) | 0.0019 (7) |
| C3 | 0.0145 (9) | 0.0178 (9) | 0.0194 (9) | 0.0024 (7) | 0.0060 (7) | 0.0025 (7) |
| C4 | 0.0123 (8) | 0.0155 (8) | 0.0132 (8) | 0.0025 (7) | 0.0051 (7) | 0.0016 (7) |
| C5 | 0.0151 (9) | 0.0137 (8) | 0.0132 (8) | 0.0009 (7) | 0.0062 (7) | 0.0000 (7) |
| C6 | 0.0149 (9) | 0.0128 (8) | 0.0136 (8) | −0.0002 (7) | 0.0056 (7) | −0.0009 (7) |
| C7 | 0.0163 (9) | 0.0136 (8) | 0.0214 (9) | −0.0022 (7) | 0.0060 (8) | −0.0002 (7) |
| C8 | 0.0135 (9) | 0.0160 (9) | 0.0220 (9) | −0.0043 (7) | 0.0054 (8) | −0.0023 (7) |
| C9 | 0.0124 (8) | 0.0171 (8) | 0.0128 (8) | −0.0026 (7) | 0.0047 (7) | −0.0017 (7) |
| C10 | 0.0105 (8) | 0.0177 (8) | 0.0118 (8) | −0.0012 (7) | 0.0036 (7) | −0.0012 (7) |
| C11 | 0.0131 (9) | 0.0149 (8) | 0.0187 (9) | −0.0001 (7) | 0.0078 (7) | 0.0002 (7) |
| C12 | 0.0162 (9) | 0.0167 (8) | 0.0181 (9) | −0.0008 (7) | 0.0062 (7) | −0.0008 (7) |
| C13 | 0.0147 (9) | 0.0205 (9) | 0.0212 (9) | 0.0029 (7) | 0.0072 (8) | 0.0049 (7) |
| C14 | 0.0157 (9) | 0.0119 (8) | 0.0309 (11) | 0.0018 (7) | 0.0112 (8) | −0.0006 (7) |
| C15 | 0.0194 (10) | 0.0205 (9) | 0.0226 (10) | 0.0020 (8) | 0.0061 (8) | −0.0055 (8) |
| C16 | 0.0191 (9) | 0.0205 (9) | 0.0176 (9) | 0.0029 (7) | 0.0058 (8) | −0.0004 (7) |
| C17 | 0.0103 (8) | 0.0114 (8) | 0.0202 (9) | 0.0009 (6) | 0.0035 (7) | 0.0007 (7) |
| C18 | 0.0137 (9) | 0.0317 (11) | 0.0204 (10) | −0.0021 (8) | 0.0071 (8) | −0.0027 (8) |
| C19 | 0.0150 (9) | 0.0300 (10) | 0.0215 (10) | −0.0030 (8) | 0.0029 (8) | −0.0071 (8) |
| C20 | 0.0088 (8) | 0.0187 (9) | 0.0312 (10) | −0.0037 (7) | 0.0041 (8) | 0.0001 (8) |
| C21 | 0.0173 (10) | 0.0489 (13) | 0.0257 (11) | −0.0065 (9) | 0.0109 (9) | 0.0028 (10) |
| C22 | 0.0163 (10) | 0.0453 (13) | 0.0185 (10) | −0.0059 (9) | 0.0061 (8) | −0.0032 (9) |
| Cl2 | 0.0135 (2) | 0.0395 (3) | 0.0448 (3) | −0.0117 (2) | 0.0056 (2) | −0.0006 (2) |
| Cl1 | 0.0304 (3) | 0.0139 (2) | 0.0455 (3) | 0.00429 (19) | 0.0091 (2) | 0.0003 (2) |
Geometric parameters (Å, º)
| Fe—C23 | 1.9244 (18) | C5—C11 | 1.496 (2) |
| Fe—C23i | 1.9244 (18) | C6—C7 | 1.443 (2) |
| Fe—N1i | 2.0074 (14) | C7—C8 | 1.350 (3) |
| Fe—N1 | 2.0074 (14) | C7—H7 | 0.9500 |
| Fe—N2i | 2.0081 (14) | C8—C9 | 1.434 (2) |
| Fe—N2 | 2.0081 (14) | C8—H8 | 0.9500 |
| C23—N3 | 1.159 (2) | C9—C10i | 1.398 (2) |
| N3—C24 | 1.464 (2) | C10—C9i | 1.398 (2) |
| C24—C26 | 1.520 (3) | C10—C17 | 1.506 (2) |
| C24—C25 | 1.521 (3) | C11—C12 | 1.397 (2) |
| C24—C27 | 1.523 (3) | C11—C16 | 1.398 (2) |
| C25—H25A | 0.9800 | C12—C13 | 1.392 (2) |
| C25—H25B | 0.9800 | C12—H12 | 0.9500 |
| C25—H25C | 0.9800 | C13—C14 | 1.381 (3) |
| C26—H26A | 0.9800 | C13—H13 | 0.9500 |
| C26—H26B | 0.9800 | C14—C15 | 1.386 (3) |
| C26—H26C | 0.9800 | C14—Cl1 | 1.7446 (18) |
| C27—H27A | 0.9800 | C15—C16 | 1.388 (3) |
| C27—H27B | 0.9800 | C15—H15 | 0.9500 |
| C27—H27C | 0.9800 | C16—H16 | 0.9500 |
| N1—C4 | 1.376 (2) | C17—C22 | 1.383 (3) |
| N1—C1 | 1.382 (2) | C17—C18 | 1.391 (3) |
| N2—C6 | 1.376 (2) | C18—C19 | 1.392 (3) |
| N2—C9 | 1.376 (2) | C18—H18 | 0.9500 |
| C1—C10 | 1.391 (2) | C19—C20 | 1.375 (3) |
| C1—C2 | 1.438 (2) | C19—H19 | 0.9500 |
| C2—C3 | 1.348 (2) | C20—C21 | 1.370 (3) |
| C2—H2 | 0.9500 | C20—Cl2 | 1.7513 (18) |
| C3—C4 | 1.443 (2) | C21—C22 | 1.394 (3) |
| C3—H3 | 0.9500 | C21—H21 | 0.9500 |
| C4—C5 | 1.404 (2) | C22—H22 | 0.9500 |
| C5—C6 | 1.400 (2) | ||
| C23—Fe—C23i | 180.0 | N1—C4—C3 | 110.35 (15) |
| C23—Fe—N1i | 89.88 (6) | C5—C4—C3 | 124.23 (16) |
| C23i—Fe—N1i | 90.12 (6) | C6—C5—C4 | 123.44 (16) |
| C23—Fe—N1 | 90.12 (6) | C6—C5—C11 | 117.22 (15) |
| C23i—Fe—N1 | 89.88 (6) | C4—C5—C11 | 119.32 (15) |
| N1i—Fe—N1 | 180.00 (5) | N2—C6—C5 | 126.17 (15) |
| C23—Fe—N2i | 97.71 (6) | N2—C6—C7 | 109.97 (15) |
| C23i—Fe—N2i | 82.29 (6) | C5—C6—C7 | 123.69 (16) |
| N1i—Fe—N2i | 89.75 (6) | C8—C7—C6 | 106.95 (16) |
| N1—Fe—N2i | 90.25 (6) | C8—C7—H7 | 126.5 |
| C23—Fe—N2 | 82.29 (6) | C6—C7—H7 | 126.5 |
| C23i—Fe—N2 | 97.71 (6) | C7—C8—C9 | 107.09 (16) |
| N1i—Fe—N2 | 90.25 (6) | C7—C8—H8 | 126.5 |
| N1—Fe—N2 | 89.75 (6) | C9—C8—H8 | 126.5 |
| N2i—Fe—N2 | 180.00 (4) | N2—C9—C10i | 125.90 (16) |
| N3—C23—Fe | 165.75 (15) | N2—C9—C8 | 110.34 (15) |
| C23—N3—C24 | 163.66 (17) | C10i—C9—C8 | 123.75 (16) |
| N3—C24—C26 | 107.17 (15) | C1—C10—C9i | 124.00 (16) |
| N3—C24—C25 | 106.85 (15) | C1—C10—C17 | 118.33 (15) |
| C26—C24—C25 | 110.81 (17) | C9i—C10—C17 | 117.65 (15) |
| N3—C24—C27 | 109.23 (16) | C12—C11—C16 | 118.18 (16) |
| C26—C24—C27 | 111.31 (17) | C12—C11—C5 | 121.71 (16) |
| C25—C24—C27 | 111.25 (16) | C16—C11—C5 | 120.05 (16) |
| C24—C25—H25A | 109.5 | C13—C12—C11 | 121.30 (17) |
| C24—C25—H25B | 109.5 | C13—C12—H12 | 119.4 |
| H25A—C25—H25B | 109.5 | C11—C12—H12 | 119.4 |
| C24—C25—H25C | 109.5 | C14—C13—C12 | 118.74 (17) |
| H25A—C25—H25C | 109.5 | C14—C13—H13 | 120.6 |
| H25B—C25—H25C | 109.5 | C12—C13—H13 | 120.6 |
| C24—C26—H26A | 109.5 | C13—C14—C15 | 121.51 (17) |
| C24—C26—H26B | 109.5 | C13—C14—Cl1 | 118.87 (14) |
| H26A—C26—H26B | 109.5 | C15—C14—Cl1 | 119.55 (14) |
| C24—C26—H26C | 109.5 | C14—C15—C16 | 118.98 (17) |
| H26A—C26—H26C | 109.5 | C14—C15—H15 | 120.5 |
| H26B—C26—H26C | 109.5 | C16—C15—H15 | 120.5 |
| C24—C27—H27A | 109.5 | C15—C16—C11 | 121.10 (17) |
| C24—C27—H27B | 109.5 | C15—C16—H16 | 119.4 |
| H27A—C27—H27B | 109.5 | C11—C16—H16 | 119.4 |
| C24—C27—H27C | 109.5 | C22—C17—C18 | 117.94 (17) |
| H27A—C27—H27C | 109.5 | C22—C17—C10 | 120.72 (16) |
| H27B—C27—H27C | 109.5 | C18—C17—C10 | 121.34 (16) |
| C4—N1—C1 | 105.33 (14) | C17—C18—C19 | 121.09 (18) |
| C4—N1—Fe | 127.86 (11) | C17—C18—H18 | 119.5 |
| C1—N1—Fe | 126.77 (11) | C19—C18—H18 | 119.5 |
| C6—N2—C9 | 105.64 (14) | C20—C19—C18 | 119.22 (18) |
| C6—N2—Fe | 127.13 (11) | C20—C19—H19 | 120.4 |
| C9—N2—Fe | 126.32 (11) | C18—C19—H19 | 120.4 |
| N1—C1—C10 | 125.79 (16) | C21—C20—C19 | 121.22 (17) |
| N1—C1—C2 | 110.18 (15) | C21—C20—Cl2 | 119.33 (16) |
| C10—C1—C2 | 123.93 (16) | C19—C20—Cl2 | 119.44 (15) |
| C3—C2—C1 | 107.24 (16) | C20—C21—C22 | 118.95 (19) |
| C3—C2—H2 | 126.4 | C20—C21—H21 | 120.5 |
| C1—C2—H2 | 126.4 | C22—C21—H21 | 120.5 |
| C2—C3—C4 | 106.85 (16) | C17—C22—C21 | 121.57 (18) |
| C2—C3—H3 | 126.6 | C17—C22—H22 | 119.2 |
| C4—C3—H3 | 126.6 | C21—C22—H22 | 119.2 |
| N1—C4—C5 | 125.39 (16) |
Symmetry code: (i) −x+2, −y, −z+1.
Hydrogen-bond geometry (Å, º)
Cg1 is the centroid of the N1/C1–C4 pyrrole ring.
| D—H···A | D—H | H···A | D···A | D—H···A |
| C25—H25C···Cl1ii | 0.98 | 2.86 | 3.738 (2) | 149 |
| C27—H27B···Cl1ii | 0.98 | 2.86 | 3.733 (2) | 149 |
| C7—H7···Cl1iii | 0.95 | 2.94 | 3.7953 (18) | 151 |
| C13—H13···N2iv | 0.95 | 2.86 | 3.685 (2) | 146 |
| C21—H21···Cg1v | 0.96 | 2.69 | 3.518 (2) | 146 |
Symmetry codes: (ii) −x+3/2, y−1/2, −z+1/2; (iii) −x+2, −y+1, −z+1; (iv) x−1/2, −y+1/2, z−1/2; (v) −x+1, −y, −z.
References
- Adler, A. D., Longo, F. R., Finarelli, J. D., Goldmacher, J., Assour, J. & Korsakoff, L. (1967). J. Org. Chem. 32, 476–476.
- Agilent (2014). CrysAlis PRO. Agilent Technologies, Abingdon, England.
- Ben Haj Hassen, L., Ezzayani, K., Rousselin, Y., Stern, C., Nasri, H. & Schulz, C. E. (2016). J. Mol. Struct. 1110, 138–142.
- Bruker (2007). SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2012). APEX2. Bruker AXS, Inc., Madison, WI, USA.
- Burla, M. C., Caliandro, R., Camalli, M., Carrozzini, B., Cascarano, G. L., De Caro, L., Giacovazzo, C., Polidori, G. & Spagna, R. (2005). J. Appl. Cryst. 38, 381–388.
- Burnett, M. N. & Johnson, C. K. (1996). Report ORNL-6895, Oak Ridge National Laboratory, Tennessee, USA.
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Gismelseed, A., Bominaar, E. L., Bill, E., Trautwein, A. X., Winkler, H., Nasri, H., Doppelt, P., Mandon, D., Fischer, J. & Weiss, R. (1990). Inorg. Chem. 29, 2741–2749.
- Gouterman, M., Wagnière, G. H. & Snyder, L. C. (1963). J. Mol. Spectrosc. 11, 108–127.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Hachem, I., Belkhiria, M. S., Giorgi, M., Schulz, C. E. & Nasri, H. (2009). Polyhedron, 28, 954–958.
- Jameson, G. B. & Ibers, J. A. (1979). Inorg. Chem. 18, 1200–1208.
- Krause, L., Herbst-Irmer, R., Sheldrick, G. M. & Stalke, D. (2015). J. Appl. Cryst. 48, 3–10. [DOI] [PMC free article] [PubMed]
- Lee, D.-S., Park, S.-Y., Yamane, K., Obayashi, E., Hori, H. & Shiro, Y. (2001). Biochemistry, 40, 2669–2677. [DOI] [PubMed]
- Le Plouzennec, M., Bondon, A. & Simonneaux, G. (1984). Inorg. Chem. 23, 4398–4399.
- Nasri, S., Brahmi, J., Turowska-Tyrk, I., Schulz, C. E. & Nasri, H. (2017). J. Organomet. Chem. 846, 176–184.
- Rath, S. P., Olmstead, M. M. & Balch, A. L. (2004). Inorg. Chem. 43, 7648–7655. [DOI] [PubMed]
- Salzmann, R., McMahon, M. T., Godbout, N., Sanders, L. K., Wojdelski, M. & Oldfield, E. (1999). J. Am. Chem. Soc. 121, 3818–3828.
- Scheidt, W. R. & Reed, C. A. (1981). Chem. Rev. 81, 543–555.
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Simonneaux, G., Hindre, F. & Le Plouzennec, M. (1989). Inorg. Chem. 28, 823–825.
- Spek, A. L. (2020). Acta Cryst. E76, 1–11. [DOI] [PMC free article] [PubMed]
- St. George, R. C. C. & Pauling, L. (1951). Science, 114, 629–634. [DOI] [PubMed]
- Turner, M. J., McKinnon, J. J., Wolff, S. K., Grimwood, D. J., Spackman, P. R., Jayatilaka, D. & Spackman, M. A. (2017). Crystal Explorer 17. The University of Western Australia.
- Walker, F. A., Nasri, H., Turowska-Tyrk, I., Mohanrao, K., Watson, C. T., Shokhirev, N. V., Debrunner, P. G. & Scheidt, W. R. (1996). J. Am. Chem. Soc. 118, 12109–12118.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989023008083/hb8075sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989023008083/hb8075Isup2.hkl
CCDC reference: 975663
Additional supporting information: crystallographic information; 3D view; checkCIF report









