The cation of the title compound exhibits point group symmetry
 , with the central NiII atom in a trans [N4O2] coordination environment.
, with the central NiII atom in a trans [N4O2] coordination environment.
Keywords: synthesis, X-ray crystal structure, NiII complex, ethylenediamine metal complex, vicinal diamine derivative, DFT, Hirshfeld surface analysis, distorted octahedral complex, trans-Ni complex.
Abstract
In the hydrated complex salt, [Ni(C9H14N2)2(H2O)2]Cl2·2H2O, the asymmetric unit comprises of half of the complex cation along with one chloride anion and one non-coordinating water molecule. The central nickel(II) atom is located on an inversion center and is coordinated in a trans octahedral fashion by four N atoms from two bidentate 1,2-diamino-1-phenylpropane ligands in the equatorial plane, and by two oxygen atoms from two water molecules occupying the axial sites. The five-membered chelate ring is in a slightly twisted envelope conformation. The crystal packing features O—H⋯Cl, N—H⋯O and N—H⋯Cl hydrogen bonds. Hirshfeld surface analysis revealed that the most important contributions to the crystal packing are from H⋯H (56.4%), O⋯H/H⋯O (16.4%) and H⋯Cl (13.3%) interactions. The crystal void volume was calculated to be 15.17%.
1. Chemical context
Unsymmetrically substituted vicinal diamines are an important class of organic compounds widely used as chelating agents. They are important structural units that have been used for decades, including in asymmetric synthesis. Besides possessing anticancer activities (Gayathri et al., 2017 ▸), their metal complexes, being analogues of cis-platin, play crucial roles in biological processes including metal-ion-involved metabolism. In addition, such metal complexes find extensive applications in the materials field (Hussain et al., 2019 ▸; Rajeshwari et al., 2021 ▸). Such complexes with nickel(II) and bio-active unsymmetrically substituted vicinal diamines are interesting since nickel is found to be a major trace element, playing a crucial role as a catalytic center in many important metabolic enzymes. Exploring the molecular structure of such NiII complexes with bio-active diamine ligands becomes inevitable in order to understand their properties and find possible applications in materials and medicinal chemistry.
In this context, we report here on the synthesis, crystal structure and Hirshfeld surface analysis of the complex salt trans-diaquabis(1-phenylpropane-1,2-diamine-κ2
N,N′)nickel(II) dichloride dihydrate, [Ni(C9H14N2)2(H2O)2]Cl2·2H2O, (I).
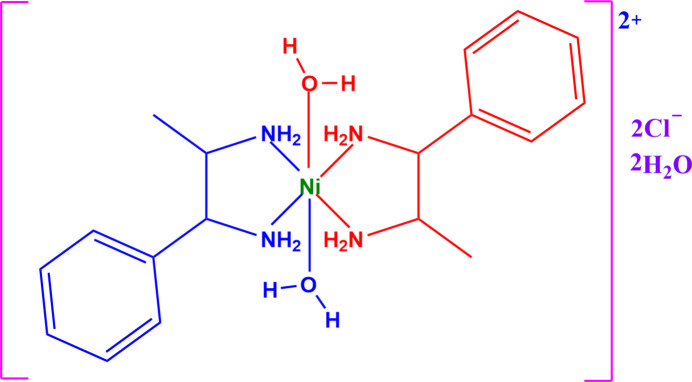
2. Structural commentary
The asymmetric unit of (I) consists of half of the cationic complex, one chloride anion and one non-coordinating water molecule. The central NiII atom is located on an inversion center, and the other half of the cationic complex is generated by symmetry operation −x, −y + 1, −z + 1. The nickel(II) atom shows a distorted trans-octahedral coordination by four N atoms from two bidentate 1,2-diamino-1-phenylpropane ligands in the equatorial plane, and by two oxygen atoms from two water molecules in the axial sites (Fig. 1 ▸). The five-membered (Ni1/N1/C3/C1/N2) chelate ring is in a slightly twisted envelope conformation with puckering parameters (Cremer & Pople, 1975 ▸) of Q(2) = 0.346 (4) Å, φ(2) = 86.6 (4)°, closest pucker descriptor: twisted on C3—C1. The Ni1—O2 bond length is 2.158 (2) Å whereas the Ni1—N bonds are shorter with 2.092 (2) Å to N1 and 2.070 (3) Å to N2. The differences in Ni—N bond lengths may be due to the influence of unsymmetrical substitutions at C3 and C1. The cis-N1—Ni1—N2 bond angle is found to be 82.45 (9)° and that of cis-N1—Ni1—N2i is 97.55 (9)°. The N1—Ni1—O2 bond angle is 91.79 (9)° and that of N1i—Ni1—O2 is 88.21 (9)°. The bond lengths and angles in the complex cation of (I) are comparable with those in similar structures (Sbai et al., 2002 ▸; Li et al., 2005 ▸; Chen et al., 2006 ▸; Kim & Lee, 2002 ▸).
Figure 1.
View of the molecular structure of (I), showing displacement ellipsoids at the 30% probability level and spheres of arbitrary radius for the H atoms. [Symmetry code: (i) −x, −y + 1, −z + 1.]
3. Supramolecular features
The crystal packing of (I) involves hydrogen bonding of the coordinating water molecule (O2) to the chloride anion, and of the amino groups to the non-coordinating water molecule (O1) and the chloride anion (Table 1 ▸, Fig. 2 ▸), establishing a layered arrangement parallel to (100).
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O2—H2A⋯Cl2i | 0.85 | 2.29 | 3.134 (2) | 173 |
| O2—H2B⋯Cl2 | 0.88 | 2.26 | 3.132 (2) | 170 |
| N1—H1A⋯O1 | 0.89 | 2.48 | 3.313 (5) | 157 |
| N1—H1B⋯Cl2 | 0.89 | 2.69 | 3.464 (3) | 146 |
| N2—H2C⋯Cl2i | 0.84 (5) | 2.68 (5) | 3.460 (3) | 155 (4) |
| N2—H2D⋯Cl2ii | 0.81 (5) | 2.86 (5) | 3.378 (3) | 124 (4) |
Symmetry codes: (i)
 ; (ii)
; (ii)
 .
.
Figure 2.
A partial packing diagram of (I) viewed along the b axis showing the O—H⋯Cl, N—H⋯O and N—H⋯Cl hydrogen-bonding interactions as dashed lines.
4. Hirshfeld surface analysis
The Hirshfeld surface analysis of (I) was carried out with CrystalExplorer (Spackman et al., 2021 ▸). Fig. 3 ▸ shows the Hirshfeld surface plotted over d norm in the range −0.495 to 1.462 a.u. where the intense red spots represent the shortest intermolecular contacts between nearest molecules, the blue spots the longest contacts and the white ones medium contacts. In this respect, interactions shorter than the van der Waals radii between O—H⋯Cl, N—H⋯Cl or N—H⋯O are shown as bright-red spots. The two-dimensional fingerprint plots are plotted in Fig. 4 ▸. The most important contributions to the crystal packing are from H⋯H (56.4%), O⋯H/H⋯O (16.4%) and H⋯Cl (13.3%) interactions.
Figure 3.
Hirshfeld surface plotted over d norm for (I).
Figure 4.
Two-dimensional-fingerprint plots of (I).
5. HOMO-LUMO
Fig. 5 ▸ represents the HOMO and LUMO of the cationic complex of (I), visualized using TONTO calculations in CrystalExplorer at the B3LYP/6-31 G(d,p) level. From the HOMO representation, it can be seen that the electrons reside mostly over the metal and water molecules, whereas in the LUMO representation, the electrons are delocalized and largely reside over the metal and amino groups.
Figure 5.
HOMO (a) and LUMO (b) of (I).
6. Crystal void
In order to assess the mechanical stability of the crystal of (I), void analysis (Turner et al., 2011 ▸) was performed with CrystalExplorer. The void volume of the crystal of (I) (Fig. 6 ▸), was calculated to be 188.48 Å3, i.e., 15.17% of the crystal volume, which shows that the crystal is tightly packed.
Figure 6.
Representation of the crystal voids in the crystal structure of (I).
7. Database survey
A search of the Cambridge Structural Database (CSD, Version 5.44, updated June 2023; Groom et al., 2016 ▸) using the molecular moiety (II) depicted in Fig. 7 ▸ for the basic skeleton of (I), with a transition-metal atom of period 4 at the center, omitting aromatic H, methyl and methine H atoms, water molecules, O and Cl atoms, gave 46 hits. There are no close matches in the CSD since the title compound possesses an unsymmetrically substituted vicinal diamine ligand.
Figure 7.
The molecular moiety (II) used for the CSD database search.
8. Synthesis and crystallization
The salt (I) was synthesized by addition of 1,2-diamino-1-phenylpropane (0.02 mol), prepared by the procedure reported by Noller & Baliah (1948 ▸) and Thennarasu & Perumal (2002 ▸), to a nickel dichloride hexahydrate (0.01 mol) solution in methanol (20 ml) with stirring under ice-cold conditions. The mixture was stirred in an ice bath for nearly 1 h and the pinkish-red solid formed was filtered and washed with chloroform. The schematic synthesis is shown in Fig. 8 ▸. Purification and growth of single crystals suitable for X-ray analysis was accomplished by recrystallization from methanol and slow evaporation (m.p. 497 K).
Figure 8.
Synthesis scheme of (I).
9. Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. The H atoms attached to C atoms were placed in calculated positions (with aromatic C—H = 0.93, methyl group C—H = 0.96 and methine C—H = 0.98 Å). The H atoms attached to N1 were placed at N1—H1A = 0.89 and N1—H1B = 0.89 Å. The H atoms attached to N2 were freely refined with N2—H2C = 0.84 (5) and N2—H2D = 0.81 (5) Å. O1 is the O atom of the non-coordinating water molecule. The two H-atom positions around this O atom were not discernible from difference-Fourier maps and are not included in the model. The H atoms attached to O2 were placed at O2—H2A = 0.85 and O2—H2B = 0.88 Å. All H atoms (except the freely refined H2C and H2D atoms) were included as riding contributions with isotropic displacement parameters U iso(H) = 1.2 and 1.5U eq(C), 1.2U eq(N) and 1.5U eq(O).
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | [Ni(C9H14N2)2(H2O)2]Cl2·2H2O |
| M r | 502.12 |
| Crystal system, space group | Monoclinic, P21/c |
| Temperature (K) | 298 |
| a, b, c (Å) | 12.1130 (7), 7.3062 (4), 14.0441 (8) |
| β (°) | 91.589 (2) |
| V (Å3) | 1242.42 (12) |
| Z | 2 |
| Radiation type | Mo Kα |
| μ (mm−1) | 1.02 |
| Crystal size (mm) | 0.31 × 0.27 × 0.21 |
| Data collection | |
| Diffractometer | Bruker D8 Quest XRD |
| Absorption correction | Multi-scan (SADABS; Krause et al., 2015 ▸) |
| T min, T max | 0.634, 0.746 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 15989, 3619, 2841 |
| R int | 0.020 |
| (sin θ/λ)max (Å−1) | 0.704 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.061, 0.140, 1.08 |
| No. of reflections | 3619 |
| No. of parameters | 144 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 1.52, −0.91 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989023008538/wm5696sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989023008538/wm5696Isup3.hkl
Supporting information file. DOI: 10.1107/S2056989023008538/wm5696Isup4.cdx
CCDC reference: 2210342
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
Authors thank DST PURSE Phase II, Department of Chemistry, Annamalai University for support of the single-crystal XRD data collection.
supplementary crystallographic information
Crystal data
| [Ni(C9H14N2)2(H2O)2]Cl2·2H2O | Dx = 1.331 Mg m−3 |
| Mr = 502.12 | Melting point: 497 K |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 12.1130 (7) Å | Cell parameters from 7663 reflections |
| b = 7.3062 (4) Å | θ = 3.1–30.0° |
| c = 14.0441 (8) Å | µ = 1.02 mm−1 |
| β = 91.589 (2)° | T = 298 K |
| V = 1242.42 (12) Å3 | Block, pink |
| Z = 2 | 0.31 × 0.27 × 0.21 mm |
| F(000) = 524 |
Data collection
| Bruker D8 Quest XRD diffractometer | 2841 reflections with I > 2σ(I) |
| Detector resolution: 7.3910 pixels mm-1 | Rint = 0.020 |
| ω and φ scans | θmax = 30.0°, θmin = 3.1° |
| Absorption correction: multi-scan (SADABS; Krause et al., 2015) | h = −17→16 |
| Tmin = 0.634, Tmax = 0.746 | k = −10→10 |
| 15989 measured reflections | l = −19→17 |
| 3619 independent reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.061 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.140 | w = 1/[σ2(Fo2) + (0.0335P)2 + 2.3825P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.08 | (Δ/σ)max < 0.001 |
| 3619 reflections | Δρmax = 1.52 e Å−3 |
| 144 parameters | Δρmin = −0.91 e Å−3 |
| 0 restraints | Extinction correction: SHELXL-2019/3 (Sheldrick, 2015b), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.011 (2) |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Ni1 | 0.000000 | 0.500000 | 0.500000 | 0.03444 (17) | |
| O1 | 0.2039 (4) | 0.2818 (7) | 0.2674 (3) | 0.1230 (15) | |
| O2 | −0.08403 (19) | 0.7604 (3) | 0.49802 (16) | 0.0480 (5) | |
| H2A | −0.067927 | 0.830472 | 0.544916 | 0.072* | |
| H2B | −0.067224 | 0.824263 | 0.447313 | 0.072* | |
| Cl2 | 0.00298 (9) | 0.99682 (12) | 0.32983 (6) | 0.0590 (3) | |
| N1 | 0.1278 (2) | 0.6032 (3) | 0.41843 (16) | 0.0393 (5) | |
| H1A | 0.163790 | 0.511552 | 0.391321 | 0.047* | |
| H1B | 0.100305 | 0.675472 | 0.372616 | 0.047* | |
| N2 | 0.1041 (2) | 0.5800 (4) | 0.61171 (18) | 0.0402 (5) | |
| C1 | 0.2120 (3) | 0.6298 (7) | 0.5760 (2) | 0.0656 (11) | |
| H1 | 0.247413 | 0.511203 | 0.565627 | 0.079* | |
| C2 | 0.2870 (3) | 0.7202 (6) | 0.6481 (3) | 0.0608 (10) | |
| H2E | 0.356214 | 0.748091 | 0.619709 | 0.091* | |
| H2F | 0.299490 | 0.639331 | 0.701219 | 0.091* | |
| H2G | 0.253580 | 0.831281 | 0.669762 | 0.091* | |
| C3 | 0.2038 (3) | 0.7078 (7) | 0.4816 (3) | 0.0670 (11) | |
| H3 | 0.167920 | 0.826718 | 0.489949 | 0.080* | |
| C4 | 0.3132 (3) | 0.7513 (6) | 0.4357 (2) | 0.0568 (9) | |
| C5 | 0.3882 (4) | 0.6251 (7) | 0.4081 (3) | 0.0793 (13) | |
| H5 | 0.374550 | 0.501135 | 0.416994 | 0.095* | |
| C6 | 0.4867 (4) | 0.6819 (9) | 0.3659 (4) | 0.0890 (16) | |
| H6 | 0.538556 | 0.595730 | 0.347740 | 0.107* | |
| C7 | 0.5056 (3) | 0.8635 (9) | 0.3517 (3) | 0.0806 (15) | |
| H7 | 0.570103 | 0.901948 | 0.323347 | 0.097* | |
| C8 | 0.4309 (4) | 0.9858 (7) | 0.3788 (4) | 0.0824 (14) | |
| H8 | 0.443916 | 1.109789 | 0.368966 | 0.099* | |
| C9 | 0.3354 (3) | 0.9320 (7) | 0.4208 (3) | 0.0669 (10) | |
| H9 | 0.284926 | 1.020016 | 0.439403 | 0.080* | |
| H2C | 0.079 (3) | 0.667 (6) | 0.644 (3) | 0.068 (13)* | |
| H2D | 0.114 (4) | 0.503 (6) | 0.653 (3) | 0.076 (14)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Ni1 | 0.0403 (3) | 0.0322 (3) | 0.0311 (3) | −0.0052 (2) | 0.00578 (18) | 0.00010 (19) |
| O1 | 0.126 (3) | 0.115 (3) | 0.128 (4) | 0.001 (3) | 0.006 (3) | 0.009 (3) |
| O2 | 0.0588 (13) | 0.0360 (11) | 0.0495 (12) | 0.0009 (10) | 0.0076 (10) | 0.0012 (9) |
| Cl2 | 0.0953 (7) | 0.0415 (4) | 0.0409 (4) | 0.0030 (4) | 0.0133 (4) | −0.0025 (3) |
| N1 | 0.0426 (12) | 0.0424 (13) | 0.0331 (11) | −0.0084 (10) | 0.0038 (9) | 0.0001 (10) |
| N2 | 0.0450 (13) | 0.0439 (14) | 0.0318 (12) | −0.0060 (11) | 0.0047 (10) | 0.0005 (11) |
| C1 | 0.0497 (19) | 0.107 (3) | 0.0399 (17) | −0.026 (2) | 0.0018 (14) | 0.0001 (19) |
| C2 | 0.0518 (19) | 0.082 (3) | 0.0479 (19) | −0.0132 (19) | −0.0065 (15) | −0.0045 (18) |
| C3 | 0.058 (2) | 0.095 (3) | 0.0470 (19) | −0.031 (2) | 0.0043 (16) | −0.0007 (19) |
| C4 | 0.0435 (17) | 0.086 (3) | 0.0412 (16) | −0.0226 (18) | 0.0046 (13) | −0.0004 (17) |
| C5 | 0.102 (4) | 0.063 (3) | 0.074 (3) | −0.008 (3) | 0.015 (3) | 0.002 (2) |
| C6 | 0.072 (3) | 0.120 (5) | 0.076 (3) | 0.029 (3) | 0.012 (2) | −0.008 (3) |
| C7 | 0.049 (2) | 0.126 (5) | 0.068 (3) | −0.027 (3) | 0.0143 (18) | 0.000 (3) |
| C8 | 0.084 (3) | 0.079 (3) | 0.085 (3) | −0.027 (3) | 0.019 (3) | 0.007 (3) |
| C9 | 0.057 (2) | 0.080 (3) | 0.065 (2) | −0.001 (2) | 0.0106 (18) | 0.005 (2) |
Geometric parameters (Å, º)
| Ni1—N2i | 2.070 (3) | C2—H2E | 0.9600 |
| Ni1—N2 | 2.070 (3) | C2—H2F | 0.9600 |
| Ni1—N1i | 2.092 (2) | C2—H2G | 0.9600 |
| Ni1—N1 | 2.092 (2) | C3—C4 | 1.523 (5) |
| Ni1—O2i | 2.158 (2) | C3—H3 | 0.9800 |
| Ni1—O2 | 2.158 (2) | C4—C5 | 1.359 (6) |
| O2—H2A | 0.8524 | C4—C9 | 1.364 (6) |
| O2—H2B | 0.8799 | C5—C6 | 1.409 (7) |
| N1—C3 | 1.475 (4) | C5—H5 | 0.9300 |
| N1—H1A | 0.8900 | C6—C7 | 1.362 (8) |
| N1—H1B | 0.8900 | C6—H6 | 0.9300 |
| N2—C1 | 1.458 (4) | C7—C8 | 1.335 (7) |
| N2—H2C | 0.84 (5) | C7—H7 | 0.9300 |
| N2—H2D | 0.81 (5) | C8—C9 | 1.371 (6) |
| C1—C3 | 1.444 (5) | C8—H8 | 0.9300 |
| C1—C2 | 1.496 (5) | C9—H9 | 0.9300 |
| C1—H1 | 0.9800 | ||
| N2i—Ni1—N2 | 180.0 | C3—C1—H1 | 103.4 |
| N2i—Ni1—N1i | 82.45 (9) | N2—C1—H1 | 103.4 |
| N2—Ni1—N1i | 97.55 (9) | C2—C1—H1 | 103.4 |
| N2i—Ni1—N1 | 97.55 (9) | C1—C2—H2E | 109.5 |
| N2—Ni1—N1 | 82.45 (9) | C1—C2—H2F | 109.5 |
| N1i—Ni1—N1 | 180.0 | H2E—C2—H2F | 109.5 |
| N2i—Ni1—O2i | 92.17 (11) | C1—C2—H2G | 109.5 |
| N2—Ni1—O2i | 87.83 (11) | H2E—C2—H2G | 109.5 |
| N1i—Ni1—O2i | 91.79 (9) | H2F—C2—H2G | 109.5 |
| N1—Ni1—O2i | 88.21 (9) | C1—C3—N1 | 111.9 (3) |
| N2i—Ni1—O2 | 87.83 (11) | C1—C3—C4 | 115.7 (3) |
| N2—Ni1—O2 | 92.17 (11) | N1—C3—C4 | 112.9 (3) |
| N1i—Ni1—O2 | 88.21 (9) | C1—C3—H3 | 105.0 |
| N1—Ni1—O2 | 91.79 (9) | N1—C3—H3 | 105.0 |
| O2i—Ni1—O2 | 180.00 (11) | C4—C3—H3 | 105.0 |
| Ni1—O2—H2A | 114.9 | C5—C4—C9 | 118.5 (4) |
| Ni1—O2—H2B | 111.0 | C5—C4—C3 | 125.1 (4) |
| H2A—O2—H2B | 104.7 | C9—C4—C3 | 116.4 (4) |
| C3—N1—Ni1 | 108.45 (19) | C4—C5—C6 | 120.1 (5) |
| C3—N1—H1A | 110.0 | C4—C5—H5 | 120.0 |
| Ni1—N1—H1A | 110.0 | C6—C5—H5 | 120.0 |
| C3—N1—H1B | 110.0 | C7—C6—C5 | 119.8 (5) |
| Ni1—N1—H1B | 110.0 | C7—C6—H6 | 120.1 |
| H1A—N1—H1B | 108.4 | C5—C6—H6 | 120.1 |
| C1—N2—Ni1 | 110.06 (19) | C8—C7—C6 | 119.5 (4) |
| C1—N2—H2C | 110 (3) | C8—C7—H7 | 120.2 |
| Ni1—N2—H2C | 114 (3) | C6—C7—H7 | 120.2 |
| C1—N2—H2D | 107 (3) | C7—C8—C9 | 121.1 (5) |
| Ni1—N2—H2D | 115 (3) | C7—C8—H8 | 119.4 |
| H2C—N2—H2D | 101 (4) | C9—C8—H8 | 119.4 |
| C3—C1—N2 | 112.0 (3) | C4—C9—C8 | 121.1 (4) |
| C3—C1—C2 | 118.2 (4) | C4—C9—H9 | 119.5 |
| N2—C1—C2 | 114.3 (3) | C8—C9—H9 | 119.5 |
| Ni1—N2—C1—C3 | 32.1 (5) | C1—C3—C4—C9 | −113.5 (5) |
| Ni1—N2—C1—C2 | 169.9 (3) | N1—C3—C4—C9 | 115.7 (4) |
| N2—C1—C3—N1 | −44.5 (5) | C9—C4—C5—C6 | 0.6 (7) |
| C2—C1—C3—N1 | 179.4 (4) | C3—C4—C5—C6 | 179.8 (4) |
| N2—C1—C3—C4 | −175.8 (4) | C4—C5—C6—C7 | −0.9 (8) |
| C2—C1—C3—C4 | 48.2 (6) | C5—C6—C7—C8 | 0.6 (8) |
| Ni1—N1—C3—C1 | 33.9 (4) | C6—C7—C8—C9 | 0.1 (8) |
| Ni1—N1—C3—C4 | 166.5 (3) | C5—C4—C9—C8 | 0.1 (7) |
| C1—C3—C4—C5 | 67.3 (6) | C3—C4—C9—C8 | −179.2 (4) |
| N1—C3—C4—C5 | −63.4 (5) | C7—C8—C9—C4 | −0.4 (7) |
Symmetry code: (i) −x, −y+1, −z+1.
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O2—H2A···Cl2ii | 0.85 | 2.29 | 3.134 (2) | 173 |
| O2—H2B···Cl2 | 0.88 | 2.26 | 3.132 (2) | 170 |
| N1—H1A···O1 | 0.89 | 2.48 | 3.313 (5) | 157 |
| N1—H1B···Cl2 | 0.89 | 2.69 | 3.464 (3) | 146 |
| N2—H2C···Cl2ii | 0.84 (5) | 2.68 (5) | 3.460 (3) | 155 (4) |
| N2—H2D···Cl2iii | 0.81 (5) | 2.86 (5) | 3.378 (3) | 124 (4) |
Symmetry codes: (ii) −x, −y+2, −z+1; (iii) x, −y+3/2, z+1/2.
References
- Bruker (2017). APEX3 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Chen, Z.-L., Zhang, Y.-Z. & Liang, F.-P. (2006). Acta Cryst. E62, m2287–m2289.
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc. 97, 1354–1358.
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Gayathri, A., Rajeswari, K., Vidhyasagar, T. & Selvanayagam, S. (2017). Acta Cryst. E73, 1878–1881. [DOI] [PMC free article] [PubMed]
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Hussain, A., AlAjmi, M. F., Rehman, Md. T., Amir, S., Husain, F. M., Alsalme, A., Siddiqui, M. A., Alkhedairy, A. A. & Khan, R. A. (2019). Sci. Rep. 9, 5237, 1–17. [DOI] [PMC free article] [PubMed]
- Kim, C.-H. & Lee, S.-G. (2002). Acta Cryst. C58, m421–m423. [DOI] [PubMed]
- Krause, L., Herbst-Irmer, R., Sheldrick, G. M. & Stalke, D. (2015). J. Appl. Cryst. 48, 3–10. [DOI] [PMC free article] [PubMed]
- Li, M. T., Wang, C.-G., Wu, Y. & Fu, X.-C. (2005). Acta Cryst. E61, m1613–m1615.
- Noller, C. R. & Baliah, V. (1948). J. Am. Chem. Soc. 70, 3853–3855. [DOI] [PubMed]
- Rajeshwari, K., Anantha Lakshmi, P. V., Archana, J. & Sumakanth, M. (2021). Appl. Organom Chem. 35, e6100.
- Sbai, F., Chkirate, K., Regragui, R., Essassi, E. M. & Pierrot, M. (2002). Acta Cryst. E58, m337–m339. [DOI] [PubMed]
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Spackman, P. R., Turner, M. J., McKinnon, J. J., Wolff, S. K., Grimwood, D. J., Jayatilaka, D. & Spackman, M. A. (2021). J. Appl. Cryst. 54, 1006–1011. [DOI] [PMC free article] [PubMed]
- Spek, A. L. (2020). Acta Cryst. E76, 1–11. [DOI] [PMC free article] [PubMed]
- Thennarasu, S. & Perumal, P. T. (2002). Molecules, 7, 487–493.
- Turner, M. J., McKinnon, J. J., Jayatilaka, D. & Spackman, M. A. (2011). CrystEngComm, 13, 1804–1813.
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989023008538/wm5696sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989023008538/wm5696Isup3.hkl
Supporting information file. DOI: 10.1107/S2056989023008538/wm5696Isup4.cdx
CCDC reference: 2210342
Additional supporting information: crystallographic information; 3D view; checkCIF report










