A divalent copper one-dimensional ribbon copper(II) coordination polymer, with 1,3-bis(pyridin-3-yl)urea and succinate ligands, was structurally characterized by single-crystal X-ray diffraction.
Keywords: crystal structure, coordination polymer, ribbon topology, copper, succinate
Abstract
In the title compound, [Cu2(C4H4O4)2(C11H10N4O)]
n
, mono-periodic coordination polymer ribbons are held into the crystal structure by means of N—H⋯O hydrogen bonding and crystal packing forces.
Structure description
The title compound was isolated during an exploratory synthetic effort aiming to produce a copper coordination polymer containing both succinate (succ) and 1,3-bis(pyridin-3-yl)urea (or 3,3′-dipyridylurea, 3-dpu) ligands. Previously, our group had isolated a series of cadmium succinate coordination polymers featuring isomeric dipyridylamide coligands. Structural topologies were highly dependent on the specific dipyridylamide ligand used (Uebler et al., 2013 ▸).
The asymmetric unit of the title compound contains two divalent Cu atoms, two crystallographically distinct fully deprotonated succ ligands, and a full 3-dpu ligand. The Cu1 and Cu2 atoms display [NO4] square-pyramidal coordination environments, with elongated apical positions occupied by pyridyl N-atom donors from 3-dpu ligands. Their basal planes comprise four carboxylate O-atom donors from four different succ ligands (Table 1 ▸). The Cu1 and Cu2 atoms possess trigonality factors τ of 0.044 and 0.035 (Addison et al., 1984 ▸), indicating only a slight variance from idealized square-pyramidal geometry. Complete coordination environments and ligand sets are shown in Fig. 1 ▸.
Table 1. Selected geometric parameters (Å, °).
| Cu1—O2 | 1.960 (4) | Cu2—O1 | 1.979 (4) |
| Cu1—O4i | 2.023 (4) | Cu2—O3i | 1.990 (4) |
| Cu1—O6 | 1.963 (4) | Cu2—O5 | 1.980 (4) |
| Cu1—O8i | 1.961 (4) | Cu2—O7i | 1.973 (4) |
| Cu1—N4ii | 2.197 (5) | Cu2—N1 | 2.167 (5) |
| O2—Cu1—O4i | 90.48 (18) | O1—Cu2—O3i | 89.16 (18) |
| O2—Cu1—O6 | 87.41 (18) | O1—Cu2—O5 | 90.34 (18) |
| O2—Cu1—O8i | 166.45 (17) | O1—Cu2—N1 | 95.10 (17) |
| O2—Cu1—N4ii | 91.11 (18) | O3i—Cu2—N1 | 99.11 (18) |
| O4i—Cu1—N4ii | 89.25 (17) | O5—Cu2—O3i | 166.52 (17) |
| O6—Cu1—O4i | 169.11 (17) | O5—Cu2—N1 | 94.36 (18) |
| O6—Cu1—N4ii | 101.47 (18) | O7i—Cu2—O1 | 168.62 (18) |
| O8i—Cu1—O4i | 88.52 (17) | O7i—Cu2—O3i | 88.50 (18) |
| O8i—Cu1—O6 | 91.03 (18) | O7i—Cu2—O5 | 89.35 (19) |
| O8i—Cu1—N4ii | 102.38 (18) | O7i—Cu2—N1 | 96.27 (18) |
Symmetry codes: (i)
 ; (ii)
; (ii)
 .
.
Figure 1.
The copper coordination environments in the title compound with the full ligand set and the complete {Cu2(OCO)4} paddlewheel cluster. Displacement ellipsoids are drawn at the 50% probability level. Color code: Cu dark blue, O red, N light blue, and C black. H-atom positions are represented as sticks. The symmetry codes are as listed in Table 1 ▸.
The carboxylate groups of the succ ligands bridge Cu1 and Cu2 atoms in a syn–syn fashion, giving rise to {Cu2(OCO)4} paddlewheel dimers with a Cu⋯Cu separation of 2.657 (1) Å. The full span of the gauche-conformation succ ligands connect the dimeric clusters into [Cu2(succ)2] n coordination polymer chains oriented parallel to the b crystal direction (Fig. 2 ▸). The 3-dpu ligands, which adopt a syn conformation, conjoin Cu1 and Cu2 along the top and bottom of the [Cu2(succ)2] n chain motifs, affording [Cu2(succ)2(3-dpu)] n coordination polymer ribbons oriented parallel to the b crystal direction (Fig. 3 ▸).
Figure 2.
The [Cu2(succ)2] n coordination polymer chain in the title compound, featuring {Cu2(OCO)4} paddlewheel clusters.
Figure 3.
A [Cu2(succ)2(3-dpu)] n coordination polymer ribbon in the title compound, with a [Cu2(succ)2] n chain motif drawn in red.
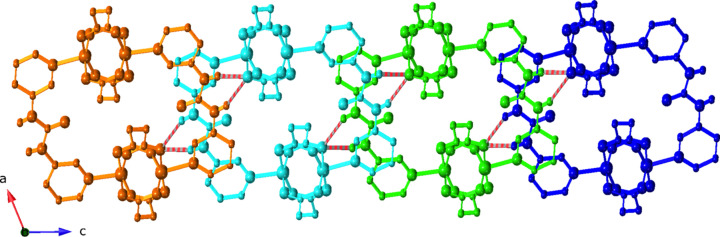
Regarding supramolecular interactions, adjacent [Cu2(succ)2(3-dpu)] n motifs aggregate into supramolecular layers parallel to the bc crystal planes by means of N—H⋯O hydrogen bonding between the urea groups of 3-dpu ligands in one ribbon, and succ carboxylate O atoms in the next ribbon (Fig. 4 ▸). In turn, the supramolecular layers aggregate into the three-dimensional crystal structure of the title compound by crystal packing forces (Fig. 5 ▸). Details regarding the hydrogen bonding patterns in the title compound are listed in Table 2 ▸.
Figure 4.
Supramolecular layer formed by N—H⋯O hydrogen bonding (hatched bonds) between [Cu2(succ)2(3-dpu)] n ribbon motifs.
Figure 5.
Aggregation of supramolecular layer motifs in the title compound.
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H2⋯O4iii | 0.88 | 2.21 | 3.042 (6) | 157 |
| N3—H3⋯O4iii | 0.88 | 2.36 | 3.174 (6) | 154 |
Symmetry code: (iii)
 .
.
Synthesis and crystallization
Cu(NO3)2·2.5H2O (86 mg, 0.37 mmol), succinic acid (succH2; 44 mg, 0.37 mmol), 3,3′-dipyridylurea (3-dpu; 79 mg, 0.37 mmol), and 0.75 ml of a 1.0 M NaOH solution were placed into 10 ml distilled water in a Teflon-lined acid digestion bomb. The bomb was sealed and heated in an oven at 393 K for 48 h, and then cooled slowly to 273 K. Green crystals of the title complex were obtained in 43% yield.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 3 ▸. All H atoms were placed in calculated positions and refined with a riding model.
Table 3. Experimental details.
| Crystal data | |
| Chemical formula | [Cu2(C4H4O4)2(C11H10N4O)] |
| M r | 573.45 |
| Crystal system, space group | Monoclinic, P21/c |
| Temperature (K) | 173 |
| a, b, c (Å) | 15.587 (2), 6.7579 (10), 20.942 (3) |
| β (°) | 111.614 (2) |
| V (Å3) | 2050.9 (5) |
| Z | 4 |
| Radiation type | Mo Kα |
| μ (mm−1) | 2.14 |
| Crystal size (mm) | 0.24 × 0.12 × 0.05 |
| Data collection | |
| Diffractometer | Bruker APEXII CCD |
| Absorption correction | Multi-scan (SADABS; Bruker, 2013 ▸) |
| T min, T max | 0.568, 0.745 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 15941, 3758, 2378 |
| R int | 0.110 |
| (sin θ/λ)max (Å−1) | 0.603 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.056, 0.151, 0.99 |
| No. of reflections | 3758 |
| No. of parameters | 307 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 1.12, −0.60 |
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S2414314623007472/bh4078sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314623007472/bh4078Isup2.hkl
CCDC reference: 2290886
Additional supporting information: crystallographic information; 3D view; checkCIF report
full crystallographic data
Crystal data
| [Cu2(C4H4O4)2(C11H10N4O)] | F(000) = 1160 |
| Mr = 573.45 | Dx = 1.857 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 15.587 (2) Å | Cell parameters from 2446 reflections |
| b = 6.7579 (10) Å | θ = 2.8–25.1° |
| c = 20.942 (3) Å | µ = 2.14 mm−1 |
| β = 111.614 (2)° | T = 173 K |
| V = 2050.9 (5) Å3 | Plate, green |
| Z = 4 | 0.24 × 0.12 × 0.05 mm |
Data collection
| Bruker APEXII CCD diffractometer | 3758 independent reflections |
| Radiation source: sealed tube | 2378 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.110 |
| Detector resolution: 8.36 pixels mm-1 | θmax = 25.4°, θmin = 1.4° |
| ω scans | h = −18→18 |
| Absorption correction: multi-scan (SADABS; Bruker, 2013) | k = −8→8 |
| Tmin = 0.568, Tmax = 0.745 | l = −25→25 |
| 15941 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: dual |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.056 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.151 | H-atom parameters constrained |
| S = 0.99 | w = 1/[σ2(Fo2) + (0.0685P)2] where P = (Fo2 + 2Fc2)/3 |
| 3758 reflections | (Δ/σ)max = 0.001 |
| 307 parameters | Δρmax = 1.12 e Å−3 |
| 0 restraints | Δρmin = −0.60 e Å−3 |
Special details
| Experimental. Data was collected using a BRUKER CCD (charge coupled device) based diffractometer equipped with an Oxford low-temperature apparatus operating at 173 K. A suitable crystal was chosen and mounted on a nylon loop using Paratone oil. Data were measured using omega scans of 0.5° per frame for 30 s. The total number of images were based on results from the program COSMO where redundancy was expected to be 4 and completeness to 0.83Å to 100%. Cell parameters were retrieved using APEX II software and refined using SAINT on all observed reflections.Data reduction was performed using the SAINT software which corrects for Lp. Scaling and absorption corrections were applied using SADABS6 multi-scan technique, supplied by George Sheldrick. The structure was solved by the direct method using the SHELXT program and refined by least squares method on F2, SHELXL, incorporated in OLEX2. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cu1 | 0.76095 (5) | 0.56430 (10) | 0.20555 (4) | 0.0199 (2) | |
| Cu2 | 0.77124 (5) | 0.49295 (10) | 0.33295 (4) | 0.0198 (2) | |
| O1 | 0.6695 (3) | 0.6855 (6) | 0.3154 (2) | 0.0241 (10) | |
| O2 | 0.6730 (3) | 0.7626 (6) | 0.2124 (2) | 0.0257 (11) | |
| O3 | 0.6784 (3) | 1.2834 (6) | 0.2892 (2) | 0.0250 (10) | |
| O4 | 0.6616 (3) | 1.3542 (6) | 0.1809 (2) | 0.0228 (10) | |
| O5 | 0.8617 (3) | 0.7122 (6) | 0.3540 (2) | 0.0272 (11) | |
| O6 | 0.8587 (3) | 0.7601 (6) | 0.2474 (2) | 0.0269 (11) | |
| O7 | 0.8683 (3) | 1.3085 (6) | 0.3309 (2) | 0.0284 (11) | |
| O8 | 0.8527 (3) | 1.3529 (6) | 0.2213 (2) | 0.0232 (10) | |
| O9 | 0.5334 (3) | 0.3287 (7) | 0.4400 (2) | 0.0355 (12) | |
| N1 | 0.7897 (3) | 0.4419 (7) | 0.4394 (3) | 0.0190 (12) | |
| N2 | 0.6521 (4) | 0.3177 (7) | 0.5442 (3) | 0.0226 (12) | |
| H2 | 0.665061 | 0.298117 | 0.588321 | 0.027* | |
| N3 | 0.5065 (4) | 0.2208 (7) | 0.5341 (3) | 0.0225 (12) | |
| H3 | 0.533315 | 0.195629 | 0.578293 | 0.027* | |
| N4 | 0.2765 (4) | 0.1280 (7) | 0.4043 (2) | 0.0207 (12) | |
| C1 | 0.6429 (4) | 0.7848 (8) | 0.2603 (3) | 0.0196 (14) | |
| C2 | 0.5697 (4) | 0.9394 (8) | 0.2500 (3) | 0.0194 (14) | |
| H2A | 0.579259 | 1.002925 | 0.294759 | 0.023* | |
| H2B | 0.508546 | 0.874023 | 0.234080 | 0.023* | |
| C3 | 0.5692 (4) | 1.0992 (9) | 0.1984 (3) | 0.0190 (14) | |
| H3A | 0.576689 | 1.034695 | 0.158311 | 0.023* | |
| H3B | 0.508195 | 1.165147 | 0.181940 | 0.023* | |
| C4 | 0.6433 (4) | 1.2555 (8) | 0.2259 (3) | 0.0181 (14) | |
| C5 | 0.8876 (4) | 0.7960 (9) | 0.3111 (3) | 0.0197 (14) | |
| C6 | 0.9644 (4) | 0.9500 (8) | 0.3372 (3) | 0.0225 (15) | |
| H6A | 0.961873 | 1.010462 | 0.379568 | 0.027* | |
| H6B | 1.024551 | 0.881653 | 0.349782 | 0.027* | |
| C7 | 0.9604 (4) | 1.1147 (9) | 0.2866 (3) | 0.0240 (15) | |
| H7A | 1.021401 | 1.180573 | 0.301650 | 0.029* | |
| H7B | 0.948973 | 1.054800 | 0.241052 | 0.029* | |
| C8 | 0.8870 (4) | 1.2704 (9) | 0.2789 (3) | 0.0213 (15) | |
| C9 | 0.8712 (5) | 0.4774 (9) | 0.4890 (3) | 0.0280 (16) | |
| H9 | 0.922054 | 0.513711 | 0.476843 | 0.034* | |
| C10 | 0.8834 (5) | 0.4625 (9) | 0.5578 (3) | 0.0279 (16) | |
| H10 | 0.942091 | 0.487920 | 0.592295 | 0.034* | |
| C11 | 0.8108 (4) | 0.4111 (9) | 0.5757 (3) | 0.0250 (15) | |
| H11 | 0.818417 | 0.401257 | 0.622687 | 0.030* | |
| C12 | 0.7253 (4) | 0.3733 (8) | 0.5245 (3) | 0.0213 (14) | |
| C13 | 0.7181 (5) | 0.3899 (9) | 0.4566 (3) | 0.0234 (15) | |
| H13 | 0.660291 | 0.363379 | 0.421060 | 0.028* | |
| C14 | 0.5621 (5) | 0.2911 (9) | 0.5012 (3) | 0.0235 (15) | |
| C15 | 0.2276 (5) | 0.0979 (9) | 0.4441 (4) | 0.0260 (16) | |
| H15 | 0.163956 | 0.066116 | 0.423285 | 0.031* | |
| C16 | 0.2677 (5) | 0.1121 (8) | 0.5150 (3) | 0.0253 (15) | |
| H16 | 0.231447 | 0.093317 | 0.542417 | 0.030* | |
| C17 | 0.3608 (4) | 0.1539 (9) | 0.5456 (3) | 0.0249 (15) | |
| H17 | 0.389672 | 0.160856 | 0.594176 | 0.030* | |
| C18 | 0.4114 (4) | 0.1852 (8) | 0.5044 (3) | 0.0188 (14) | |
| C19 | 0.3656 (5) | 0.1700 (9) | 0.4340 (3) | 0.0251 (15) | |
| H19 | 0.399984 | 0.190872 | 0.405368 | 0.030* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cu1 | 0.0226 (5) | 0.0173 (4) | 0.0204 (4) | 0.0010 (3) | 0.0088 (4) | 0.0007 (3) |
| Cu2 | 0.0227 (5) | 0.0170 (4) | 0.0207 (4) | 0.0002 (3) | 0.0092 (4) | 0.0002 (3) |
| O1 | 0.030 (3) | 0.021 (2) | 0.025 (3) | 0.0066 (19) | 0.015 (2) | 0.0068 (19) |
| O2 | 0.034 (3) | 0.021 (2) | 0.025 (3) | 0.009 (2) | 0.015 (2) | 0.0029 (19) |
| O3 | 0.031 (3) | 0.023 (2) | 0.022 (3) | −0.007 (2) | 0.010 (2) | −0.0049 (19) |
| O4 | 0.026 (3) | 0.024 (2) | 0.018 (2) | −0.0066 (19) | 0.008 (2) | 0.0026 (19) |
| O5 | 0.032 (3) | 0.022 (2) | 0.025 (3) | −0.012 (2) | 0.007 (2) | 0.001 (2) |
| O6 | 0.032 (3) | 0.024 (3) | 0.028 (3) | −0.006 (2) | 0.015 (2) | −0.006 (2) |
| O7 | 0.029 (3) | 0.026 (3) | 0.032 (3) | 0.012 (2) | 0.012 (2) | 0.003 (2) |
| O8 | 0.025 (3) | 0.022 (2) | 0.021 (2) | 0.0053 (19) | 0.007 (2) | 0.0023 (19) |
| O9 | 0.028 (3) | 0.049 (3) | 0.027 (3) | −0.003 (2) | 0.006 (2) | 0.013 (2) |
| N1 | 0.019 (3) | 0.015 (3) | 0.019 (3) | 0.002 (2) | 0.004 (2) | 0.002 (2) |
| N2 | 0.029 (3) | 0.019 (3) | 0.024 (3) | 0.001 (2) | 0.015 (3) | 0.004 (2) |
| N3 | 0.029 (3) | 0.017 (3) | 0.021 (3) | 0.001 (2) | 0.009 (3) | 0.005 (2) |
| N4 | 0.028 (3) | 0.015 (3) | 0.019 (3) | −0.002 (2) | 0.008 (3) | 0.001 (2) |
| C1 | 0.024 (4) | 0.012 (3) | 0.025 (4) | −0.009 (3) | 0.012 (3) | −0.006 (3) |
| C2 | 0.018 (3) | 0.018 (3) | 0.024 (3) | −0.002 (3) | 0.010 (3) | 0.004 (3) |
| C3 | 0.018 (3) | 0.020 (3) | 0.021 (3) | −0.001 (3) | 0.009 (3) | −0.002 (3) |
| C4 | 0.016 (3) | 0.010 (3) | 0.026 (4) | 0.005 (2) | 0.005 (3) | −0.002 (3) |
| C5 | 0.013 (3) | 0.020 (3) | 0.024 (4) | 0.005 (3) | 0.003 (3) | 0.005 (3) |
| C6 | 0.017 (4) | 0.020 (4) | 0.028 (4) | −0.004 (3) | 0.005 (3) | −0.003 (3) |
| C7 | 0.021 (4) | 0.023 (4) | 0.028 (4) | 0.001 (3) | 0.009 (3) | −0.002 (3) |
| C8 | 0.021 (4) | 0.016 (3) | 0.032 (4) | −0.007 (3) | 0.014 (3) | −0.004 (3) |
| C9 | 0.023 (4) | 0.036 (4) | 0.028 (4) | −0.001 (3) | 0.013 (3) | 0.001 (3) |
| C10 | 0.024 (4) | 0.029 (4) | 0.021 (4) | −0.001 (3) | −0.002 (3) | 0.008 (3) |
| C11 | 0.032 (4) | 0.026 (4) | 0.017 (3) | 0.006 (3) | 0.009 (3) | 0.000 (3) |
| C12 | 0.028 (4) | 0.012 (3) | 0.027 (4) | −0.001 (3) | 0.014 (3) | 0.001 (3) |
| C13 | 0.031 (4) | 0.021 (4) | 0.019 (3) | 0.002 (3) | 0.011 (3) | −0.001 (3) |
| C14 | 0.027 (4) | 0.017 (3) | 0.029 (4) | 0.003 (3) | 0.012 (3) | 0.002 (3) |
| C15 | 0.019 (4) | 0.019 (4) | 0.040 (4) | 0.002 (3) | 0.011 (3) | −0.003 (3) |
| C16 | 0.038 (4) | 0.014 (3) | 0.033 (4) | −0.001 (3) | 0.024 (4) | 0.005 (3) |
| C17 | 0.026 (4) | 0.026 (4) | 0.024 (4) | 0.001 (3) | 0.011 (3) | −0.002 (3) |
| C18 | 0.024 (4) | 0.015 (3) | 0.018 (3) | 0.000 (3) | 0.008 (3) | 0.001 (3) |
| C19 | 0.029 (4) | 0.021 (4) | 0.027 (4) | 0.001 (3) | 0.013 (3) | −0.001 (3) |
Geometric parameters (Å, º)
| Cu1—O2 | 1.960 (4) | C2—H2A | 0.9900 |
| Cu1—O4i | 2.023 (4) | C2—H2B | 0.9900 |
| Cu1—O6 | 1.963 (4) | C2—C3 | 1.526 (8) |
| Cu1—O8i | 1.961 (4) | C3—H3A | 0.9900 |
| Cu1—N4ii | 2.197 (5) | C3—H3B | 0.9900 |
| Cu2—O1 | 1.979 (4) | C3—C4 | 1.515 (8) |
| Cu2—O3i | 1.990 (4) | C5—C6 | 1.528 (8) |
| Cu2—O5 | 1.980 (4) | C6—H6A | 0.9900 |
| Cu2—O7i | 1.973 (4) | C6—H6B | 0.9900 |
| Cu2—N1 | 2.167 (5) | C6—C7 | 1.522 (8) |
| O1—C1 | 1.265 (7) | C7—H7A | 0.9900 |
| O2—C1 | 1.264 (7) | C7—H7B | 0.9900 |
| O3—C4 | 1.248 (7) | C7—C8 | 1.519 (8) |
| O4—C4 | 1.271 (7) | C9—H9 | 0.9500 |
| O5—C5 | 1.249 (7) | C9—C10 | 1.386 (9) |
| O6—C5 | 1.263 (7) | C10—H10 | 0.9500 |
| O7—C8 | 1.254 (7) | C10—C11 | 1.361 (9) |
| O8—C8 | 1.256 (7) | C11—H11 | 0.9500 |
| O9—C14 | 1.218 (7) | C11—C12 | 1.392 (8) |
| N1—C9 | 1.333 (8) | C12—C13 | 1.389 (8) |
| N1—C13 | 1.340 (7) | C13—H13 | 0.9500 |
| N2—H2 | 0.8800 | C15—H15 | 0.9500 |
| N2—C12 | 1.401 (8) | C15—C16 | 1.387 (9) |
| N2—C14 | 1.371 (8) | C16—H16 | 0.9500 |
| N3—H3 | 0.8800 | C16—C17 | 1.383 (9) |
| N3—C14 | 1.376 (8) | C17—H17 | 0.9500 |
| N3—C18 | 1.400 (8) | C17—C18 | 1.383 (8) |
| N4—C15 | 1.335 (8) | C18—C19 | 1.385 (8) |
| N4—C19 | 1.327 (8) | C19—H19 | 0.9500 |
| C1—C2 | 1.504 (8) | ||
| O2—Cu1—O4i | 90.48 (18) | O3—C4—O4 | 125.4 (5) |
| O2—Cu1—O6 | 87.41 (18) | O3—C4—C3 | 119.0 (5) |
| O2—Cu1—O8i | 166.45 (17) | O4—C4—C3 | 115.5 (5) |
| O2—Cu1—N4ii | 91.11 (18) | O5—C5—O6 | 126.1 (6) |
| O4i—Cu1—N4ii | 89.25 (17) | O5—C5—C6 | 117.9 (6) |
| O6—Cu1—O4i | 169.11 (17) | O6—C5—C6 | 115.9 (5) |
| O6—Cu1—N4ii | 101.47 (18) | C5—C6—H6A | 108.5 |
| O8i—Cu1—O4i | 88.52 (17) | C5—C6—H6B | 108.5 |
| O8i—Cu1—O6 | 91.03 (18) | H6A—C6—H6B | 107.5 |
| O8i—Cu1—N4ii | 102.38 (18) | C7—C6—C5 | 115.0 (5) |
| O1—Cu2—O3i | 89.16 (18) | C7—C6—H6A | 108.5 |
| O1—Cu2—O5 | 90.34 (18) | C7—C6—H6B | 108.5 |
| O1—Cu2—N1 | 95.10 (17) | C6—C7—H7A | 108.6 |
| O3i—Cu2—N1 | 99.11 (18) | C6—C7—H7B | 108.6 |
| O5—Cu2—O3i | 166.52 (17) | H7A—C7—H7B | 107.6 |
| O5—Cu2—N1 | 94.36 (18) | C8—C7—C6 | 114.6 (5) |
| O7i—Cu2—O1 | 168.62 (18) | C8—C7—H7A | 108.6 |
| O7i—Cu2—O3i | 88.50 (18) | C8—C7—H7B | 108.6 |
| O7i—Cu2—O5 | 89.35 (19) | O7—C8—O8 | 126.2 (6) |
| O7i—Cu2—N1 | 96.27 (18) | O7—C8—C7 | 117.2 (6) |
| C1—O1—Cu2 | 119.2 (4) | O8—C8—C7 | 116.6 (5) |
| C1—O2—Cu1 | 127.8 (4) | N1—C9—H9 | 119.2 |
| C4—O3—Cu2iii | 123.8 (4) | N1—C9—C10 | 121.6 (6) |
| C4—O4—Cu1iii | 122.6 (4) | C10—C9—H9 | 119.2 |
| C5—O5—Cu2 | 124.8 (4) | C9—C10—H10 | 120.2 |
| C5—O6—Cu1 | 121.1 (4) | C11—C10—C9 | 119.7 (6) |
| C8—O7—Cu2iii | 125.0 (4) | C11—C10—H10 | 120.2 |
| C8—O8—Cu1iii | 120.7 (4) | C10—C11—H11 | 120.3 |
| C9—N1—Cu2 | 120.0 (4) | C10—C11—C12 | 119.5 (6) |
| C9—N1—C13 | 119.1 (5) | C12—C11—H11 | 120.3 |
| C13—N1—Cu2 | 120.7 (4) | C11—C12—N2 | 118.4 (6) |
| C12—N2—H2 | 116.9 | C13—C12—N2 | 123.7 (6) |
| C14—N2—H2 | 116.9 | C13—C12—C11 | 117.9 (6) |
| C14—N2—C12 | 126.1 (5) | N1—C13—C12 | 122.3 (6) |
| C14—N3—H3 | 116.7 | N1—C13—H13 | 118.8 |
| C14—N3—C18 | 126.5 (5) | C12—C13—H13 | 118.8 |
| C18—N3—H3 | 116.7 | O9—C14—N2 | 123.6 (6) |
| C15—N4—Cu1iv | 129.1 (4) | O9—C14—N3 | 122.9 (6) |
| C19—N4—Cu1iv | 111.2 (4) | N2—C14—N3 | 113.4 (6) |
| C19—N4—C15 | 118.6 (6) | N4—C15—H15 | 119.2 |
| O1—C1—C2 | 118.4 (5) | N4—C15—C16 | 121.5 (6) |
| O2—C1—O1 | 124.7 (6) | C16—C15—H15 | 119.2 |
| O2—C1—C2 | 116.9 (5) | C15—C16—H16 | 120.3 |
| C1—C2—H2A | 108.9 | C17—C16—C15 | 119.4 (6) |
| C1—C2—H2B | 108.9 | C17—C16—H16 | 120.3 |
| C1—C2—C3 | 113.5 (5) | C16—C17—H17 | 120.5 |
| H2A—C2—H2B | 107.7 | C18—C17—C16 | 119.0 (6) |
| C3—C2—H2A | 108.9 | C18—C17—H17 | 120.5 |
| C3—C2—H2B | 108.9 | C17—C18—N3 | 120.1 (6) |
| C2—C3—H3A | 108.6 | C17—C18—C19 | 117.7 (6) |
| C2—C3—H3B | 108.6 | C19—C18—N3 | 122.1 (6) |
| H3A—C3—H3B | 107.5 | N4—C19—C18 | 123.7 (6) |
| C4—C3—C2 | 114.8 (5) | N4—C19—H19 | 118.2 |
| C4—C3—H3A | 108.6 | C18—C19—H19 | 118.2 |
| C4—C3—H3B | 108.6 | ||
| Cu1—O2—C1—O1 | 4.2 (9) | N4—C15—C16—C17 | −1.5 (9) |
| Cu1—O2—C1—C2 | −175.2 (4) | C1—C2—C3—C4 | −78.3 (7) |
| Cu1iii—O4—C4—O3 | 5.2 (8) | C2—C3—C4—O3 | −22.2 (8) |
| Cu1iii—O4—C4—C3 | −177.9 (4) | C2—C3—C4—O4 | 160.7 (5) |
| Cu1—O6—C5—O5 | 2.4 (9) | C5—C6—C7—C8 | 75.8 (7) |
| Cu1—O6—C5—C6 | 179.4 (4) | C6—C7—C8—O7 | 32.4 (8) |
| Cu1iii—O8—C8—O7 | 0.4 (9) | C6—C7—C8—O8 | −149.6 (5) |
| Cu1iii—O8—C8—C7 | −177.5 (4) | C9—N1—C13—C12 | 0.7 (9) |
| Cu1iv—N4—C15—C16 | 167.8 (4) | C9—C10—C11—C12 | 0.3 (9) |
| Cu1iv—N4—C19—C18 | −169.4 (5) | C10—C11—C12—N2 | 179.0 (6) |
| Cu2—O1—C1—O2 | 4.5 (8) | C10—C11—C12—C13 | 0.0 (9) |
| Cu2—O1—C1—C2 | −176.2 (4) | C11—C12—C13—N1 | −0.5 (9) |
| Cu2iii—O3—C4—O4 | 0.4 (8) | C12—N2—C14—O9 | −6.2 (9) |
| Cu2iii—O3—C4—C3 | −176.4 (4) | C12—N2—C14—N3 | 175.0 (5) |
| Cu2—O5—C5—O6 | 2.4 (9) | C13—N1—C9—C10 | −0.3 (9) |
| Cu2—O5—C5—C6 | −174.6 (4) | C14—N2—C12—C11 | 174.2 (5) |
| Cu2iii—O7—C8—O8 | 5.8 (9) | C14—N2—C12—C13 | −6.8 (9) |
| Cu2iii—O7—C8—C7 | −176.4 (4) | C14—N3—C18—C17 | −166.6 (6) |
| Cu2—N1—C9—C10 | 174.5 (5) | C14—N3—C18—C19 | 16.7 (9) |
| Cu2—N1—C13—C12 | −174.1 (4) | C15—N4—C19—C18 | −0.2 (9) |
| O1—C1—C2—C3 | 157.7 (5) | C15—C16—C17—C18 | 1.6 (9) |
| O2—C1—C2—C3 | −22.9 (8) | C16—C17—C18—N3 | −177.7 (5) |
| O5—C5—C6—C7 | −150.1 (6) | C16—C17—C18—C19 | −0.9 (9) |
| O6—C5—C6—C7 | 32.6 (8) | C17—C18—C19—N4 | 0.3 (9) |
| N1—C9—C10—C11 | −0.2 (10) | C18—N3—C14—O9 | −1.0 (10) |
| N2—C12—C13—N1 | −179.5 (5) | C18—N3—C14—N2 | 177.8 (5) |
| N3—C18—C19—N4 | 177.0 (5) | C19—N4—C15—C16 | 0.8 (9) |
Symmetry codes: (i) x, y−1, z; (ii) −x+1, y+1/2, −z+1/2; (iii) x, y+1, z; (iv) −x+1, y−1/2, −z+1/2.
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H2···O4v | 0.88 | 2.21 | 3.042 (6) | 157 |
| N3—H3···O4v | 0.88 | 2.36 | 3.174 (6) | 154 |
| C2—H2A···O3 | 0.99 | 2.48 | 2.815 (7) | 100 |
| C3—H3A···O2 | 0.99 | 2.38 | 2.744 (7) | 101 |
| C6—H6A···O7 | 0.99 | 2.47 | 2.825 (7) | 100 |
| C7—H7B···O6 | 0.99 | 2.47 | 2.824 (8) | 101 |
| C9—H9···O5 | 0.95 | 2.74 | 3.196 (8) | 110 |
| C13—H13···O9 | 0.95 | 2.17 | 2.800 (8) | 123 |
| C19—H19···O2iv | 0.95 | 2.35 | 2.965 (8) | 122 |
| C19—H19···O4vi | 0.95 | 2.84 | 3.126 (7) | 98 |
| C19—H19···O9 | 0.95 | 2.15 | 2.786 (8) | 124 |
Symmetry codes: (iv) −x+1, y−1/2, −z+1/2; (v) x, −y+3/2, z+1/2; (vi) −x+1, y−3/2, −z+1/2.
Funding Statement
Funding for this research was provided by: Lyman Briggs College, Michigan State University.
References
- Addison, A. W., Rao, T. N., Reedijk, J., van Rijn, J. & Verschoor, G. C. (1984). J. Chem. Soc. Dalton Trans. pp. 1349–1356.
- Bruker (2009). COSMO. Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2013). SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Palmer, D. (2020). CrystalMakerX. Crystal Maker Software, Begbroke, England.
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Uebler, J. W., Pochodylo, A. L., Staples, R. J. & LaDuca, R. L. (2013). Cryst. Growth Des. 13, 2220–2232.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S2414314623007472/bh4078sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314623007472/bh4078Isup2.hkl
CCDC reference: 2290886
Additional supporting information: crystallographic information; 3D view; checkCIF report