The title compound crystallizes with Z = 6 in space group P21/m.
Keywords: crystal structure, triazolium salt, heterocyclic ionic compound
Abstract
An ionic compound consisting of a triazolium cation and bromide anion, C7H14N3
+·Br−, has been synthesized and structurally characterized using single-crystal X-ray diffraction and NMR. The compound crystallizes in the monoclinic space group P21/m with the non-hydrogen atoms of one cation lying on general positions and the others lying on a mirror plane. One bromide ion also lies on the mirror. The extended structure exhibits only weak intermolecular interactions between heterocyclic C—H groups and Br− ions.
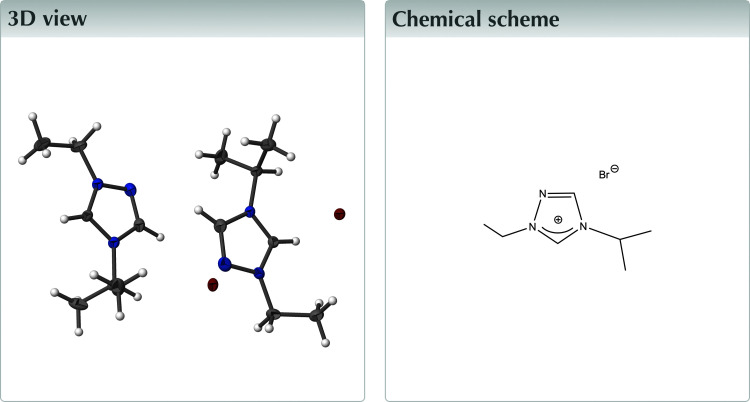
Structure description
Asymmetric 1,2,4-triazolium cations are of interest due to their utility as cations in ionic liquids (ILs) and as precursors to N-heterocyclic carbenes (NHCs) (Dwivedi et al., 2014 ▸; Nelson, 2015 ▸; Strassner et al., 2013 ▸; Riederer et al., 2011 ▸; Chianese et al., 2004 ▸). The crystal structures of several triazolium salts have been reported (Peña Hueso et al., 2022 ▸; Kumasaki et al., 2021 ▸; Ponjan et al., 2020 ▸; Guino-o et al., 2015 ▸). We have synthesized many imidazolium and triazolium salts as precursors in the synthesis of NHC complexes of rhodium and iridium (Castaldi et al., 2021 ▸; Gnanamgari et al., 2007 ▸; Idrees et al., 2017 ▸; Nichol et al., 2011 ▸; Newman et al., 2021 ▸; Rushlow et al., 2022 ▸).
The molecular structure of the title compound is shown in Fig. 1 ▸. There are one and a half molecules in the asymmetric unit with the non-hydrogen atoms of the N1 cation (except C4) and Br1 lying on the (x, 3/4, z) mirror plane. All the atoms of the N4 cation and Br2 occupy general positions. The bond lengths in the triazolium rings indicate aromaticity with C—N bonds exhibiting distances in the range of 1.305 (2)–1.366 (2) Å and N—N bond distances near 1.365 Å; the N—C—N bond angles in the triazolium ring range from 106.93 (18) to 111.35 (18)°. The C1—N2—C5—C6 torsion angle of the ethyl side chain in the N1 cation is constrained to be 0° by symmetry and the corresponding C8—N5—C12—C13 torsion angle in the N4 cation is 24.4 (2)°.
Figure 1.
The molecular structure of the title compound with displacement ellipsoids drawn at the 50% probability level. The N1 molecule (except C4) and Br1 lie on the (x, 3/4, z) mirror plane. Atom C4i is generated by the symmetry operation x,
 − y, z.
− y, z.
The crystal packing of the title compound is displayed in Fig. 2 ▸. There are weak non-classical hydrogen-bonding interactions between the heterocyclic C—H groupings and bromide ions. These weak interactions are shown as dotted red lines in Fig. 2 ▸ and summarized in Table 1 ▸.
Figure 2.

Crystal packing of the title compound shown along the a axis. Non-classical C—H⋯Br hydrogen-bonding interactions are shown as dotted red lines.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C1—H1⋯Br1i | 0.95 | 2.67 | 3.610 (2) | 170 |
| C2—H2⋯Br2i | 0.95 | 2.70 | 3.6344 (18) | 166 |
| C7—H7⋯Br2i | 0.95 | 2.69 | 3.6316 (15) | 170 |
| C8—H8⋯Br2i | 0.95 | 2.68 | 3.5635 (15) | 156 |
Symmetry code: (i)
 .
.
Synthesis and crystallization
1-Ethyl triazole was purchased from AmBeed. All other compounds used in the syntheses of the title compound were obtained from Sigma-Aldrich. All materials in the synthesis were used as received. The synthesis was performed under nitrogen using reagent grade solvents, which were used as received without further purification. NMR spectra were recorded at room temperature in CDCl3 on a 400 MHz Varian spectrometer and referenced to the residual solvent peak (δ in p.p.m.).
1-Ethyl-1,2,4-triazole (2.01 g, 20.61 mmol) and isopropyl bromide (10.14 g, 82.4 mmol) were added to toluene (20 ml) and the mixture was refluxed for 48 h. Once cooled, the liquid was decanted, the white solid product that formed was washed with ether, filtered, and dried. The title compound crystallized as clear needles by slow diffusion of pentane into a CH2Cl2 solution. Yield: 1.04 g (23%). 1H NMR: CDCl3, δ (p.p.m.) 11.99 (s, 1 H, N—C5H—N), 8.85 (s, 1 H, N—C3H—N), 5.13 (m, 1 H, CH(CH3)2), 4.63 (q, 2 H, N—CH2), 1.74 (d, 6 H, CH(CH3)2), 1.65 (t, 3 H, CH2CH3). 13C NMR: δ (p.p.m.) 142.27 (N—CH—N), 141.84 (N—CH—N), 53.15 [CH(CH3)2], 48.36 (N—CH2), 23.14 [CH(CH3)2], 14.22 (N—CH2CH3).
Refinement
Crystal data, data collection, and structure refinement details are summarized in Table 2 ▸.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | C7H14N3 +·Br− |
| M r | 220.12 |
| Crystal system, space group | Monoclinic, P21/m |
| Temperature (K) | 100 |
| a, b, c (Å) | 8.1283 (2), 21.3822 (7), 8.6376 (2) |
| β (°) | 101.713 (3) |
| V (Å3) | 1469.96 (7) |
| Z | 6 |
| Radiation type | Mo Kα |
| μ (mm−1) | 4.14 |
| Crystal size (mm) | 0.38 × 0.25 × 0.04 |
| Data collection | |
| Diffractometer | Rigaku XtaLAB Synergy-S |
| Absorption correction | Multi-scan (CrysAlis PRO; Rigaku OD; 2022 ▸) |
| T min, T max | 0.483, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 23168, 3747, 3142 |
| R int | 0.036 |
| (sin θ/λ)max (Å−1) | 0.667 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.022, 0.052, 1.04 |
| No. of reflections | 3747 |
| No. of parameters | 168 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.38, −0.29 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2414314623007848/hb4448sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314623007848/hb4448Isup2.hkl
Supporting information file. DOI: 10.1107/S2414314623007848/hb4448Isup3.cml
CCDC reference: 2293675
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
AM was supported in this work by the Millersville University Murley Summer Undergraduate Research Fellowship.
full crystallographic data
Crystal data
| C7H14N3+·Br− | F(000) = 672 |
| Mr = 220.12 | Dx = 1.492 Mg m−3 |
| Monoclinic, P21/m | Mo Kα radiation, λ = 0.71073 Å |
| a = 8.1283 (2) Å | Cell parameters from 9609 reflections |
| b = 21.3822 (7) Å | θ = 3.1–28.2° |
| c = 8.6376 (2) Å | µ = 4.14 mm−1 |
| β = 101.713 (3)° | T = 100 K |
| V = 1469.96 (7) Å3 | Plate, colourless |
| Z = 6 | 0.38 × 0.25 × 0.04 mm |
Data collection
| Rigaku XtaLAB Synergy-S diffractometer | 3747 independent reflections |
| Radiation source: micro-focus sealed X-ray tube, PhotonJet (Mo) X-ray Source | 3142 reflections with I > 2σ(I) |
| Mirror monochromator | Rint = 0.036 |
| Detector resolution: 10.0000 pixels mm-1 | θmax = 28.3°, θmin = 2.6° |
| ω scans | h = −10→10 |
| Absorption correction: multi-scan (CrysAlis PRO; Rigaku OD; 2022) | k = −27→28 |
| Tmin = 0.483, Tmax = 1.000 | l = −11→11 |
| 23168 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: dual |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.022 | H-atom parameters constrained |
| wR(F2) = 0.052 | w = 1/[σ2(Fo2) + (0.0235P)2 + 0.254P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.04 | (Δ/σ)max = 0.001 |
| 3747 reflections | Δρmax = 0.38 e Å−3 |
| 168 parameters | Δρmin = −0.29 e Å−3 |
| 0 restraints |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Br1 | 0.65535 (3) | 0.750000 | 0.86475 (2) | 0.01997 (6) | |
| Br2 | 0.36575 (2) | 0.58378 (2) | 1.16793 (2) | 0.01912 (5) | |
| N1 | 0.5536 (2) | 0.750000 | 0.34816 (19) | 0.0180 (4) | |
| N2 | 0.3012 (2) | 0.750000 | 0.22004 (19) | 0.0181 (4) | |
| N3 | 0.2904 (2) | 0.750000 | 0.3756 (2) | 0.0225 (4) | |
| C1 | 0.4576 (3) | 0.750000 | 0.2031 (2) | 0.0175 (4) | |
| H1 | 0.495511 | 0.750000 | 0.106069 | 0.021* | |
| C2 | 0.4471 (3) | 0.750000 | 0.4505 (2) | 0.0209 (4) | |
| H2 | 0.482097 | 0.750000 | 0.562368 | 0.025* | |
| C3 | 0.7407 (3) | 0.750000 | 0.3888 (3) | 0.0277 (5) | |
| H3 | 0.777584 | 0.750000 | 0.506529 | 0.033* | |
| C4 | 0.8056 (2) | 0.80896 (8) | 0.3239 (2) | 0.0324 (4) | |
| H4A | 0.772489 | 0.809040 | 0.208337 | 0.049* | |
| H4B | 0.928441 | 0.810248 | 0.355041 | 0.049* | |
| H4C | 0.757925 | 0.845690 | 0.366538 | 0.049* | |
| C5 | 0.1468 (3) | 0.750000 | 0.0987 (3) | 0.0315 (6) | |
| H5A | 0.079611 | 0.787411 | 0.112921 | 0.038* | 0.5 |
| H5B | 0.079611 | 0.712589 | 0.112921 | 0.038* | 0.5 |
| C6 | 0.1791 (3) | 0.750000 | −0.0638 (3) | 0.0353 (6) | |
| H6A | 0.225946 | 0.790528 | −0.085598 | 0.053* | 0.5 |
| H6B | 0.073605 | 0.742719 | −0.139517 | 0.053* | 0.5 |
| H6C | 0.259308 | 0.716754 | −0.073790 | 0.053* | 0.5 |
| N4 | 0.45707 (16) | 0.58697 (5) | 0.70657 (14) | 0.0163 (3) | |
| N5 | 0.71989 (15) | 0.59178 (5) | 0.80363 (14) | 0.0170 (3) | |
| N6 | 0.70540 (17) | 0.59818 (6) | 0.64405 (15) | 0.0234 (3) | |
| C7 | 0.5439 (2) | 0.59462 (7) | 0.58810 (18) | 0.0223 (3) | |
| H7 | 0.493358 | 0.597046 | 0.478994 | 0.027* | |
| C8 | 0.57173 (19) | 0.58536 (6) | 0.84100 (17) | 0.0167 (3) | |
| H8 | 0.550409 | 0.580476 | 0.944460 | 0.020* | |
| C9 | 0.27440 (18) | 0.57739 (7) | 0.69275 (18) | 0.0198 (3) | |
| H9 | 0.249096 | 0.576878 | 0.801314 | 0.024* | |
| C10 | 0.1780 (2) | 0.63095 (8) | 0.6001 (2) | 0.0296 (4) | |
| H10A | 0.218468 | 0.670794 | 0.649669 | 0.044* | |
| H10B | 0.057966 | 0.626396 | 0.599636 | 0.044* | |
| H10C | 0.195525 | 0.630178 | 0.491108 | 0.044* | |
| C11 | 0.2264 (2) | 0.51438 (8) | 0.61529 (19) | 0.0287 (4) | |
| H11A | 0.240093 | 0.515644 | 0.505186 | 0.043* | |
| H11B | 0.109054 | 0.505046 | 0.618208 | 0.043* | |
| H11C | 0.299190 | 0.481809 | 0.672508 | 0.043* | |
| C12 | 0.88457 (19) | 0.59542 (8) | 0.9095 (2) | 0.0242 (3) | |
| H12A | 0.917169 | 0.639848 | 0.927513 | 0.029* | |
| H12B | 0.969276 | 0.574799 | 0.859048 | 0.029* | |
| C13 | 0.8832 (2) | 0.56445 (8) | 1.06588 (18) | 0.0246 (3) | |
| H13A | 0.847326 | 0.520823 | 1.048013 | 0.037* | |
| H13B | 0.804907 | 0.586585 | 1.119266 | 0.037* | |
| H13C | 0.996325 | 0.565779 | 1.132088 | 0.037* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Br1 | 0.02633 (12) | 0.01928 (12) | 0.01483 (11) | 0.000 | 0.00543 (8) | 0.000 |
| Br2 | 0.01946 (8) | 0.02267 (9) | 0.01542 (8) | −0.00045 (5) | 0.00400 (6) | −0.00014 (5) |
| N1 | 0.0180 (9) | 0.0217 (9) | 0.0145 (8) | 0.000 | 0.0036 (7) | 0.000 |
| N2 | 0.0193 (9) | 0.0205 (9) | 0.0149 (8) | 0.000 | 0.0043 (7) | 0.000 |
| N3 | 0.0242 (10) | 0.0261 (10) | 0.0194 (9) | 0.000 | 0.0094 (7) | 0.000 |
| C1 | 0.0181 (10) | 0.0194 (11) | 0.0142 (10) | 0.000 | 0.0015 (8) | 0.000 |
| C2 | 0.0265 (11) | 0.0219 (11) | 0.0154 (10) | 0.000 | 0.0066 (9) | 0.000 |
| C3 | 0.0196 (11) | 0.0469 (15) | 0.0148 (10) | 0.000 | −0.0009 (9) | 0.000 |
| C4 | 0.0238 (8) | 0.0396 (10) | 0.0359 (9) | −0.0128 (7) | 0.0108 (7) | −0.0165 (8) |
| C5 | 0.0159 (11) | 0.0541 (16) | 0.0222 (12) | 0.000 | −0.0021 (9) | 0.000 |
| C6 | 0.0224 (12) | 0.0617 (18) | 0.0196 (11) | 0.000 | −0.0007 (9) | 0.000 |
| N4 | 0.0165 (6) | 0.0197 (7) | 0.0132 (6) | 0.0001 (5) | 0.0040 (5) | 0.0014 (4) |
| N5 | 0.0169 (6) | 0.0181 (7) | 0.0171 (6) | −0.0005 (5) | 0.0059 (5) | 0.0009 (4) |
| N6 | 0.0244 (7) | 0.0275 (7) | 0.0204 (7) | −0.0008 (5) | 0.0093 (5) | −0.0004 (5) |
| C7 | 0.0236 (8) | 0.0278 (9) | 0.0167 (7) | −0.0001 (6) | 0.0068 (6) | −0.0005 (6) |
| C8 | 0.0170 (7) | 0.0164 (7) | 0.0170 (7) | 0.0000 (5) | 0.0041 (6) | 0.0013 (5) |
| C9 | 0.0138 (7) | 0.0284 (9) | 0.0169 (7) | −0.0003 (6) | 0.0023 (6) | 0.0025 (6) |
| C10 | 0.0242 (8) | 0.0386 (10) | 0.0273 (8) | 0.0118 (7) | 0.0077 (7) | 0.0102 (7) |
| C11 | 0.0231 (8) | 0.0329 (9) | 0.0280 (8) | −0.0061 (7) | 0.0003 (7) | −0.0008 (7) |
| C12 | 0.0134 (7) | 0.0286 (9) | 0.0302 (9) | −0.0012 (6) | 0.0035 (6) | 0.0032 (7) |
| C13 | 0.0187 (8) | 0.0305 (9) | 0.0232 (8) | 0.0006 (6) | 0.0013 (6) | −0.0004 (6) |
Geometric parameters (Å, º)
| N1—C1 | 1.335 (2) | N4—C7 | 1.366 (2) |
| N1—C2 | 1.357 (3) | N4—C8 | 1.3337 (19) |
| N1—C3 | 1.490 (3) | N4—C9 | 1.4795 (19) |
| N2—N3 | 1.364 (2) | N5—N6 | 1.3662 (18) |
| N2—C1 | 1.308 (3) | N5—C8 | 1.316 (2) |
| N2—C5 | 1.463 (3) | N5—C12 | 1.4618 (19) |
| N3—C2 | 1.307 (3) | N6—C7 | 1.305 (2) |
| C3—C4 | 1.517 (2) | C9—C10 | 1.520 (2) |
| C3—C4i | 1.517 (2) | C9—C11 | 1.520 (2) |
| C5—C6 | 1.480 (3) | C12—C13 | 1.507 (2) |
| C1—N1—C2 | 106.43 (18) | C7—N4—C9 | 128.25 (12) |
| C1—N1—C3 | 126.56 (18) | C8—N4—C7 | 106.21 (13) |
| C2—N1—C3 | 127.01 (17) | C8—N4—C9 | 125.39 (13) |
| N3—N2—C5 | 119.20 (18) | N6—N5—C12 | 120.41 (13) |
| C1—N2—N3 | 111.63 (16) | C8—N5—N6 | 111.24 (12) |
| C1—N2—C5 | 129.18 (18) | C8—N5—C12 | 128.29 (13) |
| C2—N3—N2 | 103.67 (18) | C7—N6—N5 | 104.00 (13) |
| N2—C1—N1 | 106.93 (18) | N6—C7—N4 | 111.31 (13) |
| N3—C2—N1 | 111.35 (18) | N5—C8—N4 | 107.24 (13) |
| N1—C3—C4i | 109.14 (11) | N4—C9—C10 | 109.85 (12) |
| N1—C3—C4 | 109.14 (11) | N4—C9—C11 | 108.78 (12) |
| C4—C3—C4i | 112.4 (2) | C11—C9—C10 | 112.16 (13) |
| N2—C5—C6 | 112.79 (19) | N5—C12—C13 | 111.39 (13) |
| N2—N3—C2—N1 | 0.000 (1) | N5—N6—C7—N4 | 0.69 (16) |
| N3—N2—C1—N1 | 0.000 (1) | N6—N5—C8—N4 | 0.42 (15) |
| N3—N2—C5—C6 | 180.000 (1) | N6—N5—C12—C13 | −158.90 (13) |
| C1—N1—C2—N3 | 0.000 (1) | C7—N4—C8—N5 | 0.01 (15) |
| C1—N1—C3—C4i | −61.59 (13) | C7—N4—C9—C10 | −56.70 (19) |
| C1—N1—C3—C4 | 61.59 (13) | C7—N4—C9—C11 | 66.41 (18) |
| C1—N2—N3—C2 | 0.000 (1) | C8—N4—C7—N6 | −0.46 (16) |
| C1—N2—C5—C6 | 0.000 (1) | C8—N4—C9—C10 | 128.34 (14) |
| C2—N1—C1—N2 | 0.000 (1) | C8—N4—C9—C11 | −108.55 (15) |
| C2—N1—C3—C4i | 118.41 (13) | C8—N5—N6—C7 | −0.69 (16) |
| C2—N1—C3—C4 | −118.41 (13) | C8—N5—C12—C13 | 24.4 (2) |
| C3—N1—C1—N2 | 180.000 (1) | C9—N4—C7—N6 | −176.18 (13) |
| C3—N1—C2—N3 | 180.000 (1) | C9—N4—C8—N5 | 175.89 (12) |
| C5—N2—N3—C2 | 180.000 (1) | C12—N5—N6—C7 | −177.94 (13) |
| C5—N2—C1—N1 | 180.000 (1) | C12—N5—C8—N4 | 177.41 (13) |
Symmetry code: (i) x, −y+3/2, z.
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C1—H1···Br1ii | 0.95 | 2.67 | 3.610 (2) | 170 |
| C2—H2···Br2ii | 0.95 | 2.70 | 3.6344 (18) | 166 |
| C7—H7···Br2ii | 0.95 | 2.69 | 3.6316 (15) | 170 |
| C8—H8···Br2ii | 0.95 | 2.68 | 3.5635 (15) | 156 |
Symmetry code: (ii) x, y, z−1.
References
- Castaldi, K. T., Astashkin, A. V., Albert, D. R. & Rajaseelan, E. (2021). IUCrData, 6, x211142. [DOI] [PMC free article] [PubMed]
- Chianese, A. R., Kovacevic, A., Zeglis, B. M., Faller, J. W. & Crabtree, R. H. (2004). Organometallics, 23, 2461–2468.
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Dwivedi, S., Gupta, S. & Das, S. (2014). Curr. Organocatalysis, 1, 13–39.
- Gnanamgari, D., Moores, A., Rajaseelan, E. & Crabtree, R. H. (2007). Organometallics, 26, 1226–1230.
- Guino-o, M. A., Talbot, M. O., Slitts, M. M., Pham, T. N., Audi, M. C. & Janzen, D. E. (2015). Acta Cryst. E71, 628–635. [DOI] [PMC free article] [PubMed]
- Idrees, K. B., Astashkin, A. V. & Rajaseelan, E. (2017). IUCrData, 2, x171081.
- Kumasaki, M., Gontani, S., Mori, K., Matsumoto, S. & Inoue, K. (2021). Acta Cryst. C77, 197–201. [DOI] [PubMed]
- Nelson, D. J. (2015). Eur. J. Inorg. Chem. pp. 2012–2027.
- Newman, E. B., Astashkin, A. V., Albert, D. R. & Rajaseelan, E. (2021). IUCrData, 6, x210836. [DOI] [PMC free article] [PubMed]
- Nichol, G. S., Rajaseelan, J., Walton, D. P. & Rajaseelan, E. (2011). Acta Cryst. E67, m1860–m1861. [DOI] [PMC free article] [PubMed]
- Peña Hueso, A., Esparza Ruiz, A. & Flores Parra, A. (2022). IUCrData, 7, x220172. [DOI] [PMC free article] [PubMed]
- Ponjan, N., Aroonchat, P. & Chainok, K. (2020). Acta Cryst. E76, 137–140. [DOI] [PMC free article] [PubMed]
- Riederer, S. K., Bechlars, B., Herrmann, W. A. & Kühn, F. E. (2011). Dalton Trans. 40, 41–43. [DOI] [PubMed]
- Rigaku OD (2022). CrysAlis PRO. Rigaku Oxford Diffraction, Yarnton, England.
- Rushlow, J., Astashkin, A. V., Albert, D. R. & Rajaseelan, E. (2022). IUCrData, 7, x220685. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Strassner, T., Unger, Y., Meyer, D., Molt, O., Münster, I. & Wagenblast, G. (2013). Inorg. Chem. Commun. 30, 39–41.
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2414314623007848/hb4448sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314623007848/hb4448Isup2.hkl
Supporting information file. DOI: 10.1107/S2414314623007848/hb4448Isup3.cml
CCDC reference: 2293675
Additional supporting information: crystallographic information; 3D view; checkCIF report



