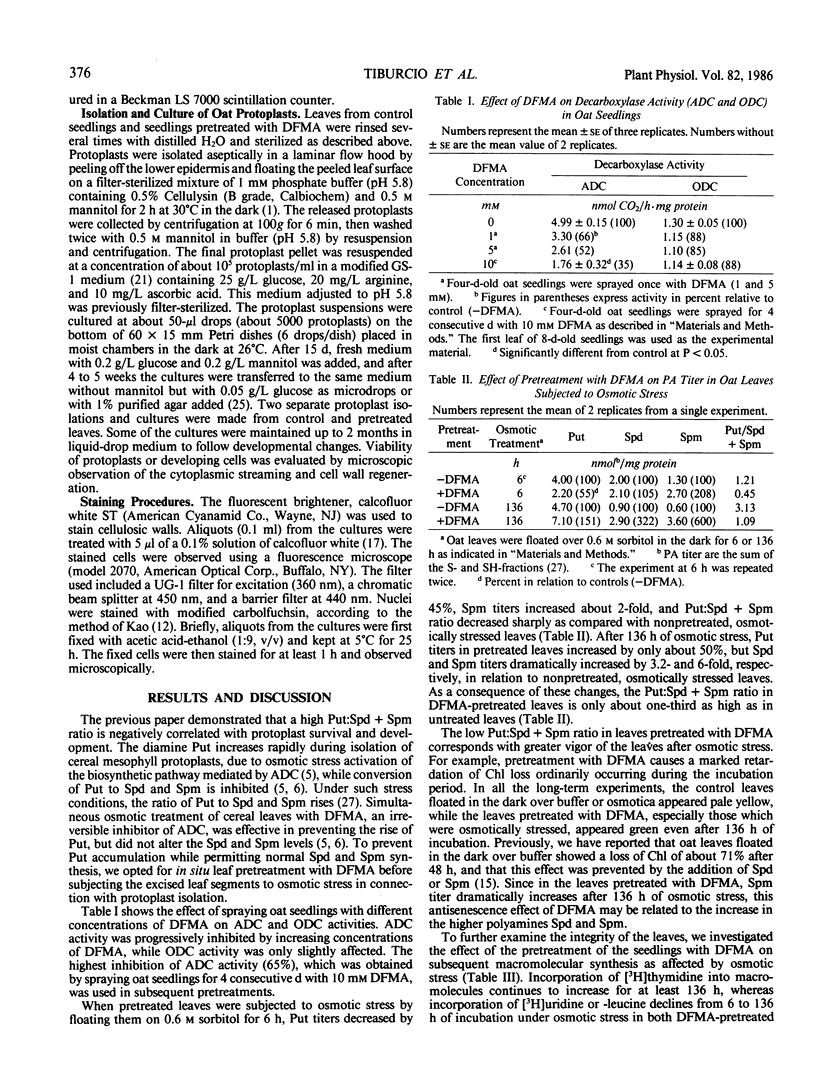
Abstract
We have attempted to improve the viability of cereal mesophyll protoplasts by pretreatment of leaves with dl-α-difluoromethylarginine (DFMA), a specific `suicide' inhibitor of the enzyme (arginine decarboxylase) responsible for their osmotically induced putrescine accumulation. Leaf pretreatment with DFMA before a 6 hour osmotic shock caused a 45% decrease of putrescine and a 2-fold increase of spermine titer. After 136 hours of osmotic stress, putrescine titer in DFMA-pretreated leaves increased by only 50%, but spermidine and spermine titers increased dramatically by 3.2- and 6-fold, respectively. These increases in higher polyamines could account for the reduced chlorophyll loss and enhanced ability of pretreated leaves to incorporate tritiated thymidine, uridine, and leucine into macromolecules. Pretreatment with DFMA significantly improved the overall viability of the protoplasts isolated from these leaves. The results support the view that the osmotically induced rise in putrescine and blockage of its conversion to higher polyamines may contribute to the lack of sustained cell division in cereal mesophyll protoplasts, although other undefined factors must also play a major role.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman A., Kaur-Sawhney R., Galston A. W. Stabilization of Oat Leaf Protoplasts through Polyamine-mediated Inhibition of Senescence. Plant Physiol. 1977 Oct;60(4):570–574. doi: 10.1104/pp.60.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Flores H. E., Galston A. W. Osmotic stress-induced polyamine accumulation in cereal leaves : I. Physiological parameters of the response. Plant Physiol. 1984 May;75(1):102–109. doi: 10.1104/pp.75.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores H. E., Galston A. W. Polyamines and plant stress: activation of putrescine biosynthesis by osmotic shock. Science. 1982 Sep 24;217(4566):1259–1261. doi: 10.1126/science.217.4566.1259. [DOI] [PubMed] [Google Scholar]
- Guarino L. A., Cohen S. S. Mechanism of toxicity of putrescine in Anacystis nidulans. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3660–3664. doi: 10.1073/pnas.76.8.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heby O. Role of polyamines in the control of cell proliferation and differentiation. Differentiation. 1981;19(1):1–20. doi: 10.1111/j.1432-0436.1981.tb01123.x. [DOI] [PubMed] [Google Scholar]
- Kaur-Sawhney R., Flores H. E., Galston A. W. Polyamine-induced DNA Synthesis and Mitosis in Oat Leaf Protoplasts. Plant Physiol. 1980 Feb;65(2):368–371. doi: 10.1104/pp.65.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slocum R. D., Kaur-Sawhney R., Galston A. W. The physiology and biochemistry of polyamines in plants. Arch Biochem Biophys. 1984 Dec;235(2):283–303. doi: 10.1016/0003-9861(84)90201-7. [DOI] [PubMed] [Google Scholar]
- Tiburcio A. F., Ingersoll R., Galston A. W. Modified alkaloid pattern in developing tobacco callus. Plant Sci. 1985;38:207–212. doi: 10.1016/0168-9452(85)90040-8. [DOI] [PubMed] [Google Scholar]
- Tiburcio A. F., Kaur-Sawhney R., Ingersoll R. B., Galston A. W. Correlation between polyamines and pyrrolidine alkaloids in developing tobacco callus. Plant Physiol. 1985;78:323–326. doi: 10.1104/pp.78.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiburcio A. F., Masdeu M. A., Dumortier F. M., Galston A. W. Polyamine metabolism and osmotic stress. I. Relation to protoplast viability. Plant Physiol. 1986;82:369–374. doi: 10.1104/pp.82.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasilewska L. D., Kleczkowsi K. Phytohormone induced changes in the nuclear RNA population of plant protoplasts. FEBS Lett. 1974 Aug 25;44(2):164–168. doi: 10.1016/0014-5793(74)80717-9. [DOI] [PubMed] [Google Scholar]


