Abstract
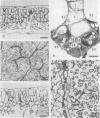
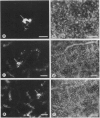
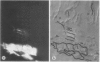
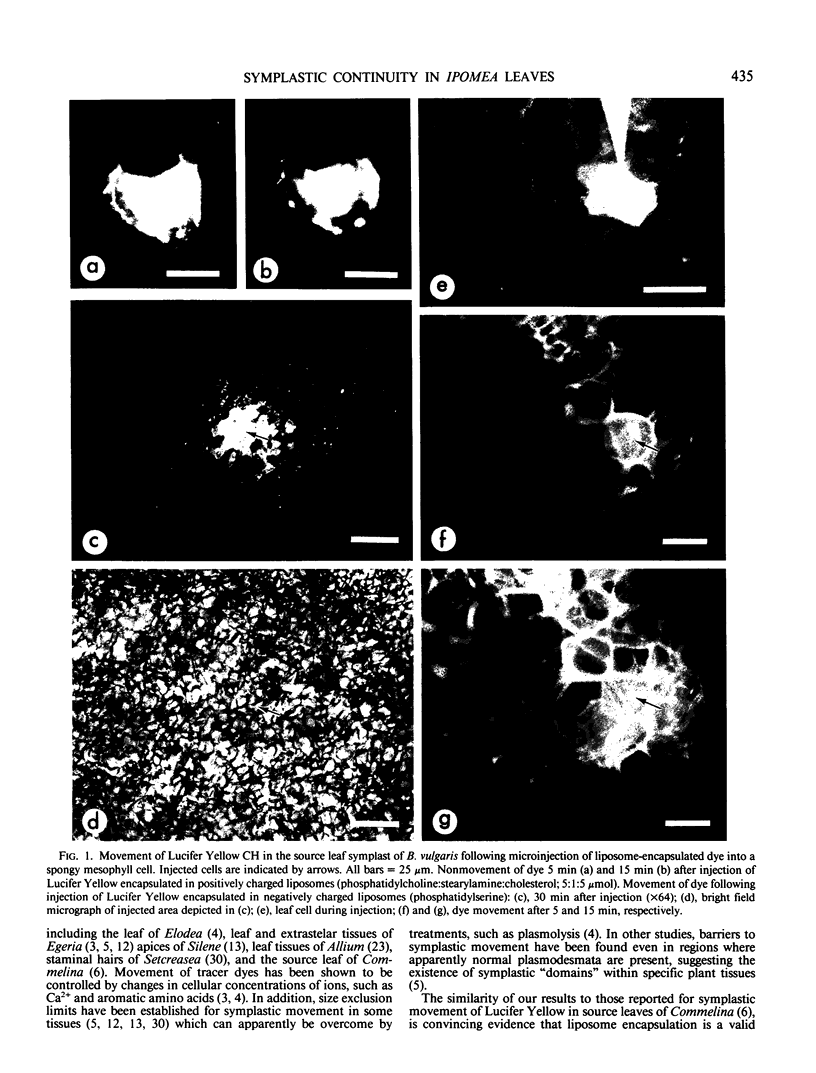
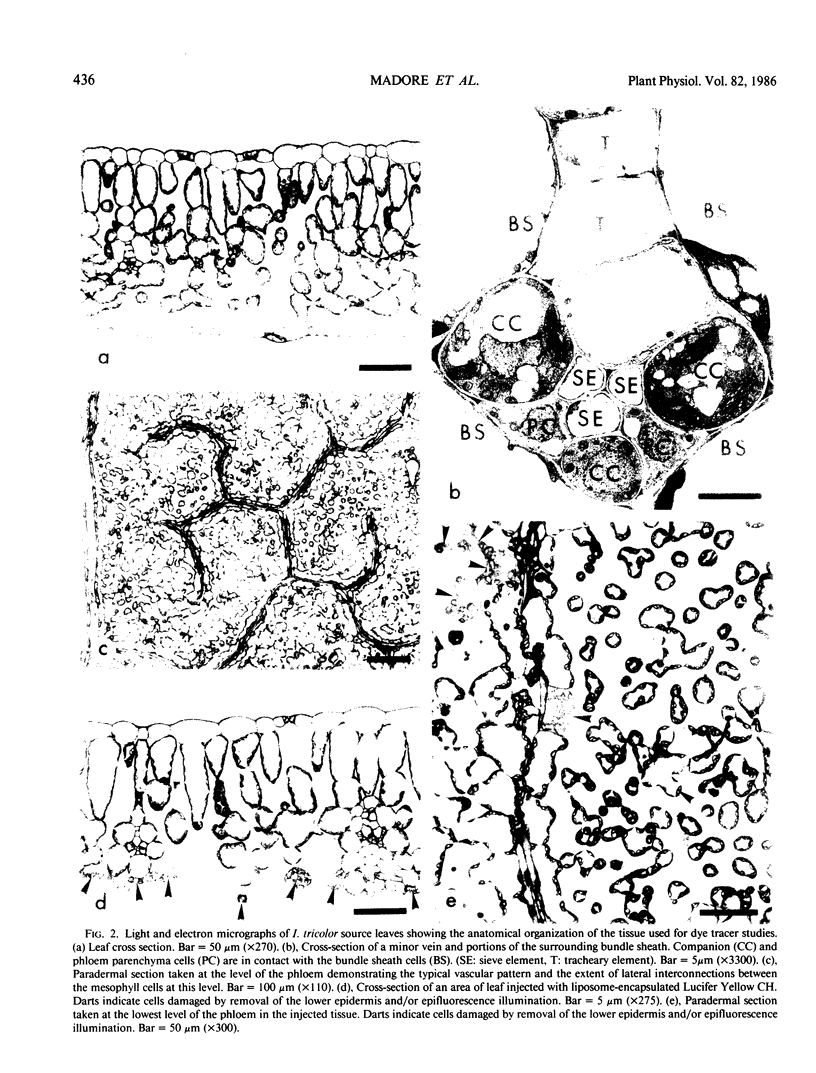
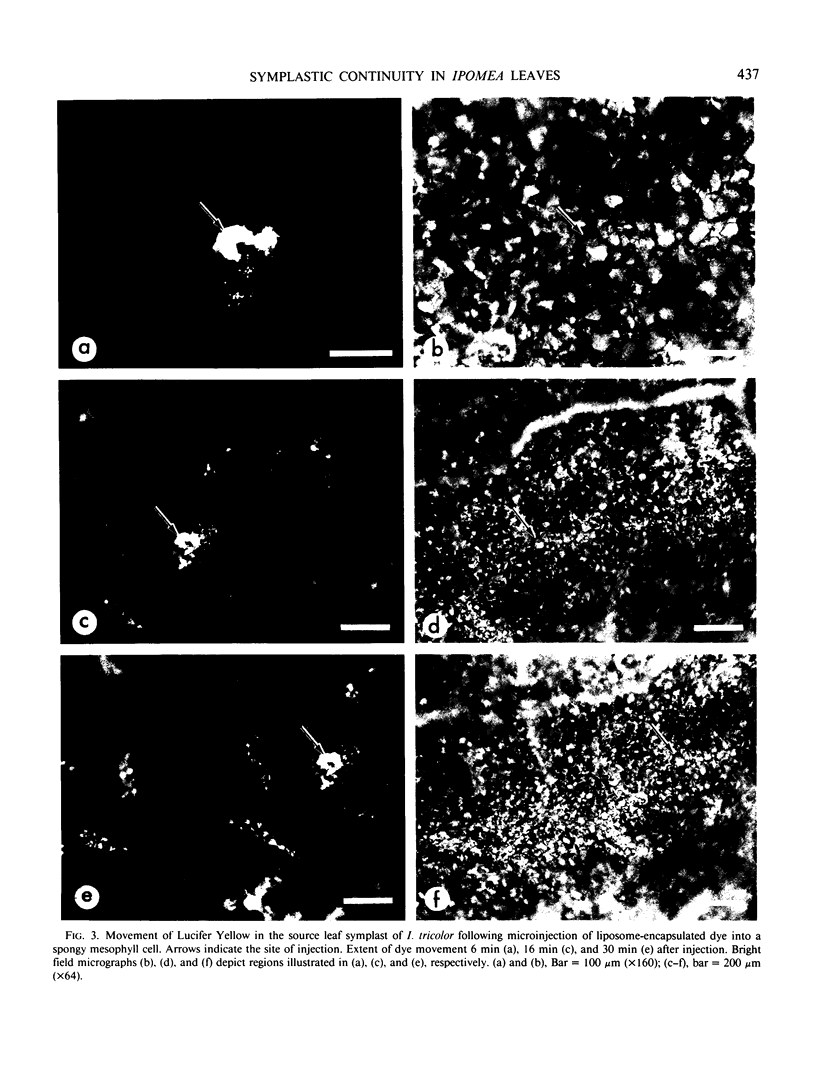
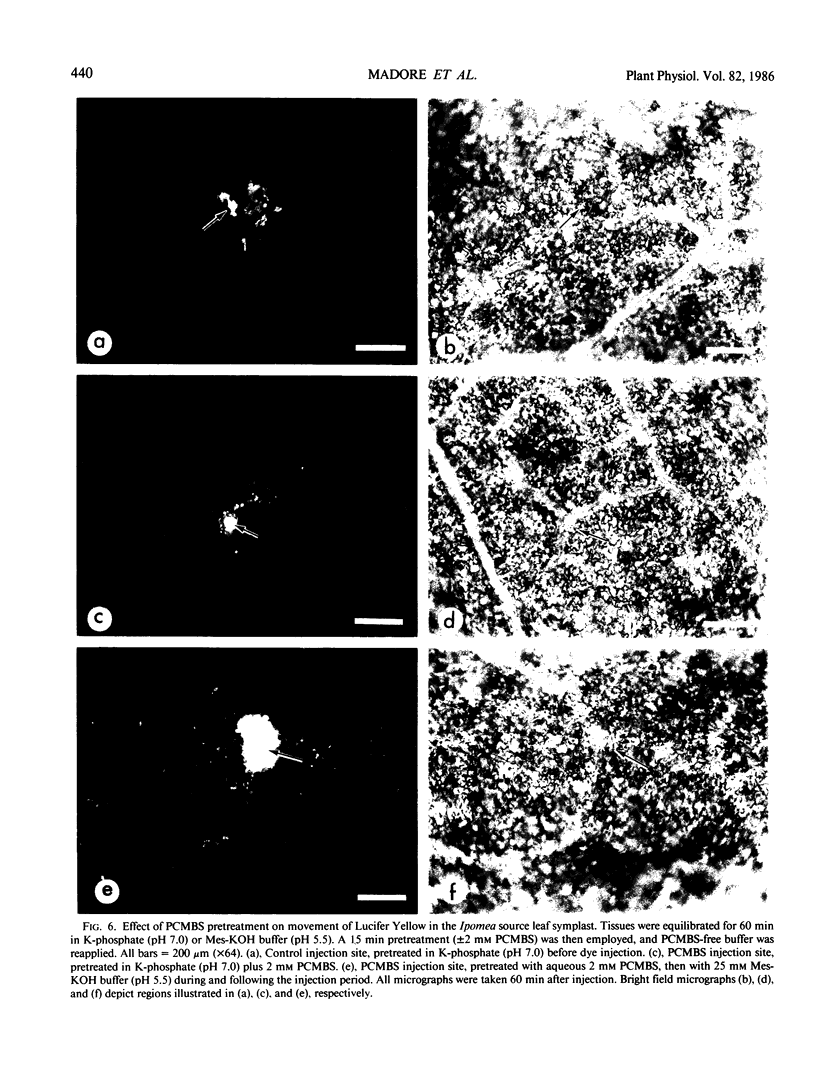
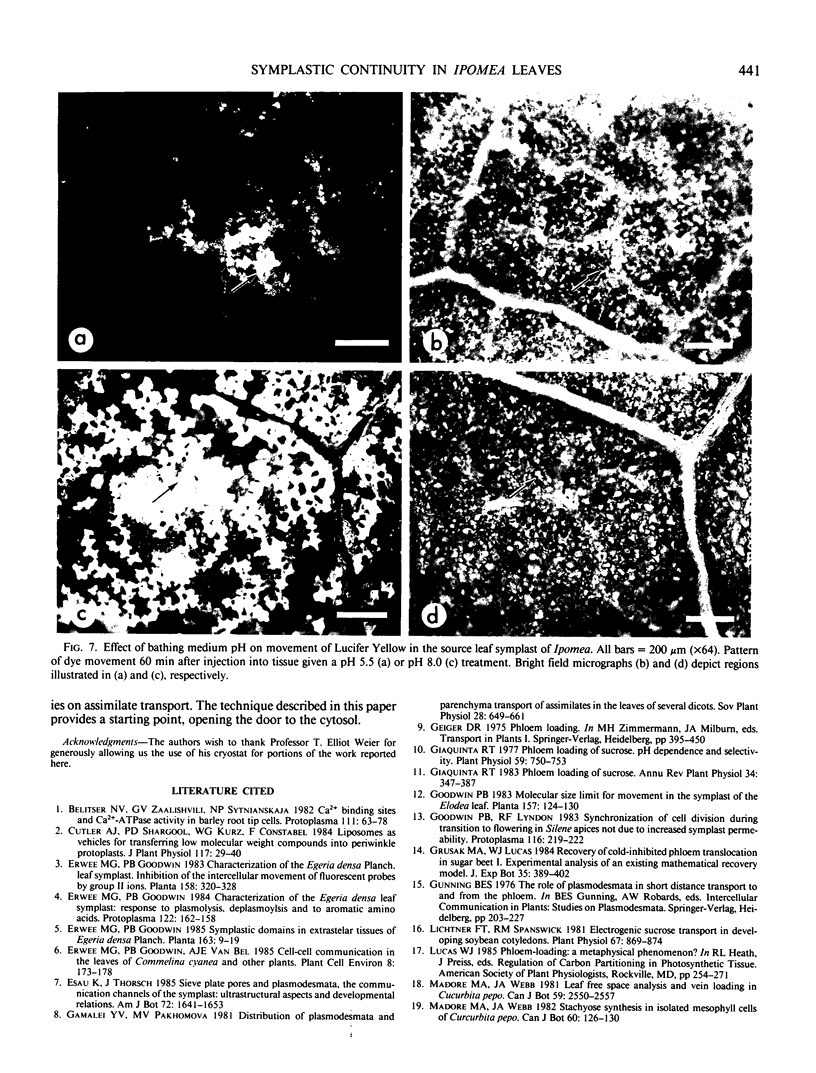
A novel method for the delivery of the fluorescent dye Lucifer Yellow CH to the cytosol of a source leaf mesophyll cell was devised which utilized a preencapsulation of the dye in phospholipid vesicles (liposomes). The liposomes were easily injected into the vacuoles of leaf cells of Beta vulgaris or Ipomea tricolor, where fusion with the tonoplast resulted in the release of the dye into the cytosol. Subsequent cell-to-cell movement of the dye was readily followed by fluorescence microscopy. Using this liposome technique symplastic continuity from the the mesophyll to the minor veins of the source leaf of Ipomea tricolor was demonstrated. This agreed with ultrastructural studies which demonstrated the presence of plasmodesmata between all cells from the mesophyll to the minor veins. The symplastic movement of dye from the injected mesophyll cell to the minor veins was unaffected by pretreatment of the leaf tissues with 2 millimolar p-chloromercuribenzenesulfonic acid. Pretreatment of the leaf tissues at alkaline pH (3-[N-morpholino] propanesulfonic acid-KOH, pH 8.0) had no apparent effect on dye movement between adjacent mesophyll cells but inhibited the movement of dye into and along the minor veins. Thus, although there were no apparent barriers to symplastic solute movement in this leaf, symplastic barriers could be imposed by the experimental conditions used.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman J. D., Keenan C. M., Lamb S. R., Hanson W. L., Waits V. B. Leishmania donovani: oral efficacy and toxicity of formycin B in the infected hamster. Exp Parasitol. 1983 Oct;56(2):215–221. doi: 10.1016/0014-4894(83)90065-6. [DOI] [PubMed] [Google Scholar]
- Giaquinta R. Phloem Loading of Sucrose: pH Dependence and Selectivity. Plant Physiol. 1977 Apr;59(4):750–755. doi: 10.1104/pp.59.4.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtner F. T., Spanswick R. M. Electrogenic sucrose transport in developing soybean cotyledons. Plant Physiol. 1981 Apr;67(4):869–874. doi: 10.1104/pp.67.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M'batchi B., Pichelin D., Delrot S. The Effect of Sugars on the Binding of [Hg]-p-Chloromercuribenzenesulfonic Acid to Leaf Tissues. Plant Physiol. 1985 Oct;79(2):537–542. doi: 10.1104/pp.79.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard J. W., Lucas W. J. Sucrose and Glucose Uptake into Beta vulgaris Leaf Tissues : A Case for General (Apoplastic) Retrieval Systems. Plant Physiol. 1982 Nov;70(5):1436–1443. doi: 10.1104/pp.70.5.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papahadjopoulos D., Vail W. J. Incorporation of macromolecules within large unilamellar vesicles (LUV). Ann N Y Acad Sci. 1978;308:259–267. doi: 10.1111/j.1749-6632.1978.tb22028.x. [DOI] [PubMed] [Google Scholar]
- Pick U. Liposomes with a large trapping capacity prepared by freezing and thawing of sonicated phospholipid mixtures. Arch Biochem Biophys. 1981 Nov;212(1):186–194. doi: 10.1016/0003-9861(81)90358-1. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]