Abstract
Background and Aim
Bowel preparation is a crucial factor affecting the diagnostic accuracy of colonoscopy, and few randomized control trials evaluated enhancement in bowel preparation. In this study, we aimed to evaluate the effectiveness of walking exercises on bowel preparation before a colonoscopy procedure.
Methods
The present study is a single-blind randomized controlled trial involving 262 patients scheduled for colonoscopy procedures. These patients were randomly assigned to two groups: an intervention group (n = 131) and a control group (n = 131). In the intervention group, participants followed a predetermined plan that included the consumption of specific liquids and foods, bisacodyl pills, polyethylene glycol powder, and a regimen of walking exercises in preparation for their colonoscopy. Conversely, individuals in the control group followed the same regimen but were not instructed to engage in walking exercises. On the day of the colonoscopy, both groups were assessed for their level of physical activity using a foot counter. Additionally, an experienced gastroenterologist evaluated and compared the bowel preparation between the two groups using the Boston Bowel Preparation Scale (BBPS).
Results
The number of footsteps recorded in the two groups exhibited a significant difference (P < 0.001). Although there was no statistically significant difference between the intervention and control groups in terms of mean BBPS scores (6.26 ± 1.9 vs. 6.29 ± 1.9, P = 0.416), individuals who took more than 6900 steps had significantly higher BBPS scores compared to those with fewer than 6900 footsteps (6.62 ± 1.8 vs. 5.92 ± 1.9, P = 0.003).In the univariate analysis, BBPS was found to be significantly associated with individuals under the age of 50 (OR: 2.45, 95% CI: 1.30–4.61, P = 0.006) and smoking status (OR: 0.41, 95% CI: 0.17–0.94, P = 0.043). In the multivariate analysis, the relationship between BBPS and age below 50 and smoking remained significant (OR: 2.50, 95% CI: 1.30–4.70, P = 0.005, and OR: 0.38, 95% CI: 0.16–0.93, P = 0.034, respectively).
Conclusion
A higher number of footsteps taken especially more than 6900 can significantly enhance bowel preparation; however, walking exercise as an intervention before colonoscopy is not significantly associated with BBPS. Also, older people and smokers seem to have fewer benefits from walking exercises for bowel preparation.
Trial registration
ISRCTN32724024 (Registration date:22/08/2018).
Keywords: Walking exercise, Colonoscopy, Bowel preparation, Randomized control trial, Gastrointestinal cancer, Bowel enhancement
Background
Colorectal cancers are widely recognized as among the most harmful forms of cancer worldwide [1–4]. International reports reveal that colorectal cancer ranks as the third most prevalent cancer and is also the third leading cause of cancer-related mortality across both genders [5]. Several risk factors have been associated with colorectal cancers, including low levels of physical activity, a high-fat diet, a high body mass index (BMI), limited consumption of vegetables and fruits, smoking, alcohol, consumption, and a family history of the disease [4, 6].
Screening for this cancer and early detection and removal of neoplastic colon polyps can reduce colorectal cancer mortality [7, 8]. Studies have shown that colonoscopy is the most effective screening and diagnostic method in colorectal cancer management, and its usage has been expanding dramatically in recent years [7, 9–12]. Colonoscopy provides the detection and removal of suspicious lesions, enhancement of adenoma detection rate, and decrease in colon cancer risk [13].
Intestinal cleansing is one of the crucial determinants of the operators’ performance during colonoscopy [14]. Better bowel preparation results in higher intestinal cleanliness providing the operators with a better view of possible lesions in the lumen [15]. In contrast, poor bowel preparation results in missing the detection of the lesions and a longer duration of the procedure [16, 17].
The current bowel preparation guidelines before colonoscopy recommend administering intestinal cleansers like polyethylene glycol (PEG), sodium phosphate, and simethicone 4–6 h before the procedures [18, 19].
Previous animal studies have shown that exercising can ease gastrointestinal (GI) system motility and better defecation [20, 21]; hence it can be hypothesized that mild walking exercise for outpatients can enhance their colonoscopy efficiency by increasing bowel cleanliness. However, limited clinical trial studies with controversial results have been performed on the effects of planned walking exercise on colon cleansing in colonoscopy [22, 23]; therefore, we performed this single-blind randomized study to identify exercise’s effect on bowel preparation before colonoscopy.
Materials and methods
Study design and setting
The present study is a single-blind randomized clinical trial (RCT) with an allocation ratio of 1:1. The study protocol was registered in International Standard Randomized Controlled Trial Number (ISRCTN) (Registration number: ISRCTN32724024, Registration date: 22/8/2018). The study population included the patients who were referred to Razi Hospital (Rasht, Guilan province, Iran) by a Gastroenterologist for colonoscopy procedures for diagnostic indications from June 2018 up to October 2018.
Patients with the following criteria were eligible for the study: (1) age was between 18 and 70 years old regardless of gender; (2) being aware of the study protocols or having an alert companion. Patients were excluded from the study if they had the following criteria: (1) showing allergic reactions to the drugs used in the study; (2) being a pregnant or lactating woman; (3) having underlying diseases that make the exercise uncomfortable for the patient, like heart, lung, malignant diseases, and diabetes mellitus; (4) having hip and knee joint replacement or any movement problems.
We used random sampling (Random Allocation) using the online software to allocate participants into two intervention and control groups. All patients were randomly divided into two groups of intervention (having walking exercise) and control (without walking exercise).
Bowel preparation protocol
The bowel preparation protocol was explained to the patients by a trained researcher. To ensure the correctness of the intervention, two phone calls were made to all participants on the day before the colonoscopy procedure. Written consent was obtained from patients to participate in the project. At each stage of the study, patients could leave the study at their discretion.
The protocol of bowel preparation before colonoscopy was as follows:
The day before the colonoscopy
The intervention group participants were supposed to eat a light breakfast at 8 a.m., without milk and other dairy products. From 8 a.m. to 12 p.m., they were allowed to drink only clear liquids in the amount of 8 to 10 glasses (such as water, tea, or lemon juice) and walk for 5 min. From 12 p.m. to 1 p.m., they were told to eat a light lunch (like a piece of cooked chicken with a slice of bread) and two bisacodyl pills and then walk for 5 min. Then the patients were not allowed to eat any solid food until the colonoscopy procedure. The patients were told to dissolve three packs of PEG in 3 L of water (approximately equivalent to 12 to 13 full glasses) drink about one glass of this solution (the first PEG solution) every 15 to 20 min from 3 p.m. and walk for 5 min.
The participants were supposed to eat two other bisacodyl pills at 6 p.m. and walk for 5 min. After finishing the first PEG solution, our patients were allowed to drink clear liquids such as water, tea, and completely strained fruit juice as much as they wanted and walk for 5 min. After finishing the first PEG solution, they were allowed to rest for about 2 h. They were supposed to dissolve two other packets of PEG powder in two liters of water (about eight glasses) and then drink them (the second PEG solution) from 9 p.m. every 15 to 20 min and walk for 5 min. After finishing the second PEG solution, the patients were forbidden to eat or drink anything until the colonoscopy.
The day before the colonoscopy for the control group was similar to the intervention group, but the participants were not told to walk.
Patients were given smart bands (Mi band 2, Xiaomi) and monitored to assess the number of footsteps taken. An alert patient companion observed all steps of the protocol for each patient.
The colonoscopy procedure
On the colonoscopy day, all patients had the procedure by an experienced gastroenterologist and two trained nurses. The gastroenterologist and the nurses were blind regarding the group of patients. Propofin and etomidate were used as narcotic drugs. A Fuji 600 series scope with a processor model 4050 was utilized. No distal attachments were used during the procedures. Our approach involved the application of Fice chromoendoscopy and the utilization of water/air insufflation. Two trained assistants recorded the total procedure and the intubation time.
Bowel preparation evaluation
Boston Bowel Preparation Scale (BBPS) was used to score bowel preparation [24]. In this criterion, the colon is divided into three parts, including 1-ascending colon, 2-transverse colon, 3-descending colon, and rectum [25]. Every part is scored from 0 to 3 based on the following:
Score 0: The colon was not visible without mucosal preparation due to the presence of solid stools.
Score 1: Some Parts of the mucous were seen in the colon, but others were not visible due to stains, stools, or opaque fluid.
Score 2: A small amount of residue, small pieces of stool, or opaque fluid existed, but the mucosal part of the colon was seen well.
Score 3: All mucosal layer of the colon was seen well without any stains, small pieces of stool, or opaque fluid.
The predictive scale used for bowel preparation was appropriate if BBPS score ≥ 2 was observed in each three parts of the colon.
Data collection
A trained assistant at the gastroenterology and liver research center initially registered individuals. Then the socioeconomic characteristics, social history (drug use, smoking, and alcohol consumption), chief complaint (reason for referral), defecation status, history of gastrointestinal or gynecological surgery, history of chronic diseases, history of previous colonoscopies and the number of footsteps (recorded by the smart band) were completed by a trained questioner. Also, during the colonoscopy, the rest of the questionnaire was completed by two trained questioners, including the time of the colonoscopy and the time of reaching the cecum. A gastroenterologist ultimately determined the final diagnosis, Boston score, and future colonoscopy status.
Data analysis
Data analysis was done using SPSS version 21 statistical software. In order to investigate the difference between the two intervention and control groups, the χ2 test and student t-test were used for categorical variables. One-way ANOVA test was used to evaluate the difference between bowel cleanliness scores in the two groups. In addition, univariate or multivariate regression analysis was used to find the influencing factors in bowel cleansing. A significance level was considered as P < 0.05. The sample size in each group was calculated at 131 people according to the prior pilot study and the mean ± SD for the intervention and control group (6.5 ± 1.8 and 5.6 ± 3.06, respectively).
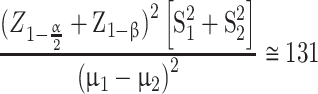 |
 |
 |
Results
Base line characteristics
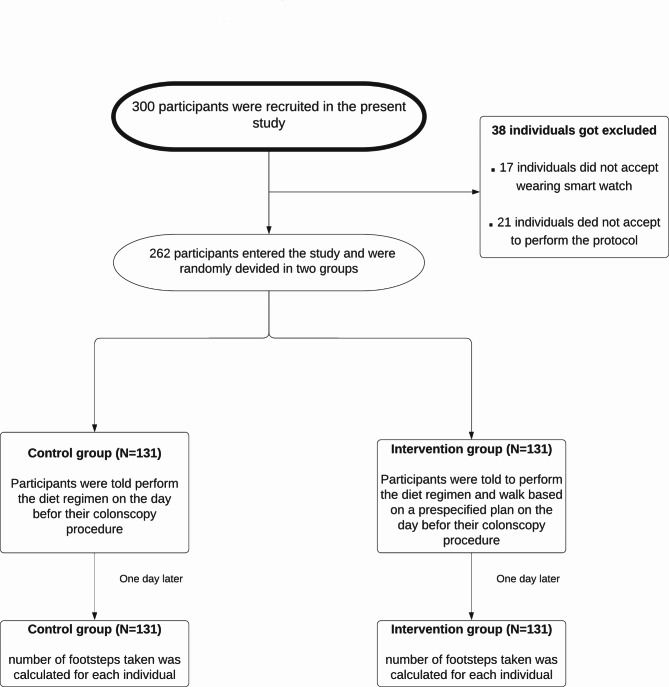
A total of 300 participants were recruited to contribute to the study. Of these, 38 individuals were excluded for various reasons, like failing to perform the trial protocol or not using the smart band. A total of 262 patients were finally divided into two groups of intervention (n = 131) and control (n = 131) (Fig. 1). The mean ages of the intervention and control groups were 50.90 ± 14.27 and 51.70 ± 14.10 years old, respectively. The basic information of the participants in the intervention and control groups is shown in Table 1. No significant difference was observed between the intervention and control groups except for the number of footsteps taken (8866.20 ± 4699.00 vs. 6120.60 ± 3975.00, P < 0.001). The trial was ended on 19 October 2018.
Fig. 1.
Consort flowchart of study
Table 1.
Baseline information of the intervention and the control groups
| Variable | Intervention group (n = 131) | Control group (n = 131) |
P-value |
|---|---|---|---|
| Age, mean ± SD* | 50.90 ± 14.27 | 51.70 ± 14.10 | 0.621 |
| Gender, % of men | 58 (9/42) | 67 (2/49) | 0.298 |
| Number of steps, mean ± SD | 8866.20 ± 4699.00 | 6120.60 ± 3975.00 | < 0.001 |
| BMI**, mean ± SD | 26.70 ± 4.50 | 26.80 ± 5.90 | 0.878 |
|
Smoking, % of positive responses |
11 (8.10) | 16 (11.70) | 0.320 |
| History of gastrointestinal surgery, % of positive responses | 22 (16.20) | 20 (14.70) | 0.718 |
| History of gynecological surgery, % of positive responses | 34 (25.10) | 36 (26.40) | 0.809 |
| History of chronic disease, % of positive responses | 59 (43.70) | 69 (50.70) | 0.223 |
| Colonoscopy time (min) | 20.20 (11.00) | 18.30 (9.00) | 0.123 |
| Time to receive cecum (min) | 9.80 (6.50) | 9.00 (6.80) | 0.329 |
*Standard Deviation, **Body Mass Index
Primary outcomes
Bowel cleanliness
Our results showed that although there was no significant difference between the intervention and control groups regarding mean BBPS (6.26 ± 1.9 vs. 6.29 ± 1.9, P = 0.885), there was a significant difference between the individuals with less than 6900 steps and more than 6900 steps. The mean BBPS score in the individuals with more than 6900 steps was significantly higher than individuals with less than 6900 steps (6.62 ± 1.8 vs. 5.92 ± 1.9, P = 0.003) (Table 2).
Table 2.
BBPS in different groups
| Groups | Mean ± SD | P value |
|---|---|---|
| Intervention group | 6.26 ± 1.9 | 0.885 |
| Control group | 6.29 ± 1.9 | |
| Individuals higher than 6900 footsteps | 6.62 ± 1.8 | 0.003 |
| Individuals less than 6900 footsteps | 5.92 ± 1.9 |
Secondary outcomes
Results of univariate analysis
The results of univariate regression analysis related to the factors affecting bowel cleansing (BBPS higher than 5 vs. less than 5) showed no significant correlation between gender (P = 0.125), walking exercise (P = 0.416), BMI (P = 0.904), diarrhea (P = 0.201), constipation (P = 0.399), history of chronic diseases (P = 0.051), history of gastrointestinal (P = 0.377) or gynecological surgeries (P = 0.881) with high BBPS. However, the association between BBPS with age less than 50 (P = 0.006) and smoking was significant (P = 0.043) (Table 3; Fig. 2).
Table 3.
Univariate analysis logistic regression analysis predicting factors affecting total BBPS higher than 5, right colon BBPS higher than 2, left colon BBPS higher than 2
| Variable | Univariate Analysis* of total BBPS higher than 5 | Univariate Analysis* of BBPS higher than 2 in right colon | Univariate Analysis* of BBPS higher than 2 in left colon | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR‡ | 95% CI | P value | OR‡ | 95% CI | P value | OR‡ | 95% CI | P value | |
|
Gender Man Woman |
0.63 | (0.35 − 0.113) | 0.125 | 0.68 | (0.33–1.43) | 0.318 | 0.38 | (0.17–0.82) | 0.014 |
|
Age, year < 50 ≥ 50 |
2.45 | (1.30–4.61) | 0.006 | 3.92 | (1.55–9.88) | 0.004 | 2.15 | (0.96–4.83) | 0.063 |
|
Walking Yes No |
1.27 | (0.71–2.26) | 0.416 | 1.21 | (0.58–2.52) | 0.609 | 1.21 | (0.58–2.51) | 0.609 |
|
BMI, kg/m2 < 25 ≥ 25 |
0.96 | (0.53–1.73) | 0.904 | 0.96 | (0.45–2.04) | 0.931 | 1.08 | (0.51–2.27) | 0.827 |
|
Diarrhea Yes No |
1.82 | (0.72–4.56) | 0.201 | 3.15 | (0.72–13.74) | 0.127 | 1.92 | (0.56–6.64) | 0.298 |
|
Constipation Yes No |
0.77 | (0.43–1.39) | 0.399 | 0.73 | (0.34–1.54) | 0.413 | 1.18 | (0.57–2.45) | 0.648 |
|
Smoking Yes No |
0.41 | (0.17–0.94) | 0.043 | 0.38 | (0.13–1.04) | 0.060 | 0.16 | (0.06–0.41) | < 0.001 |
|
History of gastrointestinal surgery Yes No |
1.47 | (0.62–3.53) | 0.377 | 1.06 | (0.38–2.94) | 0.903 | 1.01 | (0.36–2.79) | 0.980 |
|
History of gynecological surgery Yes No |
1.05 | (0.54–2.03) | 0.881 | 0.89 | (0.39–2.04) | 0.791 | 1.64 | (0.65–4.18) | 0.293 |
|
History of chronic disease Yes No |
0.56 | (0.31-1.00) | 0.051 | 0.54 | (0.15–1.14) | 0.107 | 0.94 | (0.45–1.95) | 0.874 |
*Pvalue based on the χ2 test, ‡Odds Ratio
Fig. 2.
Boston Bowel Preparation Score (BBPS) in two groups of intervention (Blue bar) and control (green bar)
The results of univariate regression analysis related to the factors affecting right colon cleansing (BBPS higher than 2 vs. less than 2) showed no significant correlation between gender (P = 0.318), walking exercise (P = 0.609), BMI (P = 0.931), diarrhea (P = 0.127), constipation (P = 0.413), history of chronic diseases (P = 0.107), history of gastrointestinal (P = 0.903) or gynecological surgeries (P = 0.791) and smoking (P = 0.060) with high BBPS. However, the association between BBPS with age less than 50 (P = 0.004) was significant (Table 3; Fig. 2).
The results of univariate regression analysis related to the factors affecting left bowel cleansing (BBPS higher than 2 vs. less than 2) showed no significant correlation between age (P = 0.063), walking exercise (P = 0.609), BMI (P = 0.827), diarrhea (P = 0.298), constipation (P = 0.648), history of chronic diseases (P = 0.874), history of gastrointestinal (P = 0.980) or gynecological surgeries (P = 0.293) with high BBPS. However, the association between BBPS with smoking (P < 0.001), and male gender (P = 0.014) was significant (Table 3; Fig. 2).
Results of multivariate analysis
The result of multivariate analysis showed a significant relationship between age less than 50 and total BBPS higher than 5, right colon BBPS higher than 2, and left colon BBPS higher than 2 (P = 0.005, P = 0.004 and P = 0.043 respectively). The relation between smoking and total BBPS and left colon BBPS was significant (P = 0.034 and P < 0.001, respectively); however, it showed no significant effect on right colon BBPS (P = 0.055) (Table 4).
Table 4.
Multivariate logistic regression analysis of predicting factors affecting total BBPS higher than 5, right colon BBPS higher than 2, left colon BBPS higher than 2
| Variable | Univariate Analysis* of total BBPS higher than 5 | Univariate Analysis* of BBPS higher than 2 in right colon | Univariate Analysis* of BBPS higher than 2 in left colon | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR‡ | 95% CI | P | OR‡ | 95% CI | P | OR‡ | 95% CI | P | |
|
Age, year < 50 ≥ 50 (Reference group) |
2.50 | (1.30–4.70) | 0.005 | 3.98 | (1.57–10.10) | 0.004 | 2.39 | (1.02–5.56) | 0.043 |
|
Smoking Yes No (Reference group) |
0.38 | (0.16–0.93) | 0.034 | 0.35 | (0.12–1.02) | 0.055 | 0.15 | (0.06–0.38) | < 0.001 |
*P value based on the χ2 test; **P value based on logistic regression, ‡ Odds Ratio
Discussion
Colonoscopy is a great tool for assessing large intestine and rectum abnormalities. It can be used for evaluating many GI diseases such as bleeding hemorrhoids [26, 27], Crohn’s disease [28], ulcerative colitis [29], chronic diarrhea [30, 31], rectum prolapse [32], colon polyps [33, 34], colon cancer [35] and occult blood in feces [36]. Using colonoscopy to screen colorectal cancer can lead to less incidence and mortality in the general population [37, 38]. Colonoscopy is the most common method used for colorectal cancer screening in the United States [39].
Bowel preparation before colonoscopy is essential and determines the imaging quality [40]. The terms “excellent”, “good”, “fair”, and “poor” are used to describe the quality of bowel preparation [41–44]. Poor bowel preparation can lead to missing the pathologic lesions in colonoscopy and wrong diagnoses [45–48].
There are some factors which can determine the quality of bowel preparation including age [49, 50], gender [50–52], underlying diseases such as diabetes [53, 54], cerebrovascular accidents [51], prior surgery [53] and socioeconomic status [49, 50, 55]. Although preparation is important, it is usually unpleasant for patients because of the bad taste of the used agents [41, 56, 57]. The two agents that are commonly administered for the preparation of colonoscopy are polyethylene glycol (PEG) and sodium phosphate (NaP) [58, 59]. PEG is not well tolerated in patients undergoing colonoscopy because of the high volume and bad taste, while the safety of NaP, especially in patients with renal failure, is challenging [60]. It has also been reported that 19.7% and 16.4% of patients undergoing colonoscopy suffer abdominal pain and vomiting during bowel preparation, respectively [61].
Several items can lead to better preparation, including following instructions precisely [49, 50] and the time of bowel preparation [62–65]. Recent studies suggested that walking exercises before colonoscopy might be effective in bowel preparation [66, 67].
In this study, although there was not a significant correlation between walking and bowel cleansing in the intervention and control groups, individuals with more than 6900 steps had significantly higher bowel cleansing scores (Fig. 3). Another study by Zhang et al. showed that patients who walk longer (regarding walking time) have better bowel preparation [68]. Another study by Gao et al. on three groups of patients (0 steps, 5000 steps, and 10,000 steps before the colonoscopy procedure) revealed that walking more than 10,000 steps can significantly increase BBPS compared to the other groups [23]. Another RCT study by Kim et al. on 383 patients revealed that walking exercises can significantly enhance bowel preparation, especially in non-obese patients under 65 years old and individuals without past abdominal surgery history [69]. A meta-analysis by Huang et al. found quantitative exercise before colonoscopy can increase bowel preparation in addition to reducing adverse effects of colonoscopy including vomiting, abdominal pain, and bloating [70].
Fig. 3.
Summary of study setting and results
In our study, younger individuals had better bowel preparation (Fig. 3). Some other studies also identified age as a risk factor for poor preparation of the bowel [53, 71, 72]. Older age is assumed to be related to higher comorbid diseases and lower colonic movements, which can lead to poorer cleansing of the colon [73–76].
In addition, in our study, smoking was another risk factor for poorer bowel preparation (Fig. 3). Other studies also confirm this finding [77, 78]. It is assumed that smokers are less likely to follow the instructions before colonoscopy precisely due to lower socioeconomic and health status [79].
Several mechanisms can justify the positive coloration between mild walking on colon cleansing before colonoscopy. One mechanism can be related to GI tract motility and secretion. GI tract movement is a complicated process resulting from interaction and coordination between multiple cell types like enteric neurons, interstitial cells of Cajal (ICC) and smooth muscle cells, which are located in the tunica vascularis layer. Throughout a process called excitation–contraction coupling, the coordinated contraction of smooth muscle cells leads to GI motility [80, 81]. It has been shown that exercise can enhance GI tract motility and glandular secretions [82–84], which leads to faster emptying of the GI tract there by a better intestinal clarity can be expected [85]. It has also been shown that intestinal clarity has a positive relationship with the time of exercising [86]. Hu et al. found that the elderly who can walk better can have better colonoscopy preparation than those who cannot walk properly [86]. Exercising can also reduce the adverse effects of solution in colonoscopy preparation, like abdominal pain and vomiting, which can be described by the effect of exercise on the enhancement of digestive blood circulation and glandular secretions. This enhancement can lead to better absorption and excretion of bowel-cleansing agents and a better tolerance of the patients [85].
Our study had some limitations. First, one expert gastroenterologist determined the BBPS. For more accurate data, we highly recommend future studies to assess BBPS by two gastroenterologists. Second, we assessed taken footsteps by a smart band which may not be accurate. We could not assess whether participants wore the band all the time before the colonoscopy. Also, we assessed the footsteps taken by individuals. We did not assess the quality of walking. We could not assess whether participants had mild exercise or moderate or severe.
Conclusion
In conclusion, a higher number of footsteps taken especially more than 6900 can significantly enhance bowel preparation; however, walking exercise as an intervention before colonoscopy is not significantly associated with BBPS. In addition, older age and smoking could inversely affect BBPS. This finding highlights more chance of misdiagnosis of pathologic conditions in colonoscopies of elderly and smoker patients.
Acknowledgements
We acknowledge BioRender since all the illustrations are created with BioRender.com.
Abbreviations
- BBPS
Boston Bowel Preparation Scale
- BMI
Body mass index
- PEG
polyethene glycol sodium phosphate
- OR
Odds Ratio
- SD
Standard deviation
- CI
Confidential interval
Authors’ contributions
G.R and F.MG designed the study. E.AS and S.H drafted the paper. S.M and H.D contributed to the study design. S.H and F.J are responsible for data analysis. S.M, G.R, and E.AS are responsible for data acquisition. E.AS prepared the graphical abstract. All authors are responsible for the interpretation of data, critical revision of intellectual content, and approval of this paper.
Funding
This study was supported by Guilan University of Medical Sciences) ISRCTN32724024, Registration date: 22/08/2018).
Data Availability
Data from the study can be provided from Corresponding Author on reasonable request.
Declarations
Ethics approval and consent to participate
The present study was approved by Guilan University of Medical Sciences ethical committee. The protocol registry number is IR.GUMS.REC.1397.113. Informed Consent was obtained from participants involved in the study. All methods were conducted in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132(5):1133–45. doi: 10.1002/ijc.27711. [DOI] [PubMed] [Google Scholar]
- 2.Van Leersum N, Janssen-Heijnen M, Wouters M, Rutten H, Coebergh JW, Tollenaar R, Lemmens V. Increasing prevalence of comorbidity in patients with colorectal cancer in the South of the Netherlands 1995–2010. Int J Cancer. 2013;132(9):2157–63. doi: 10.1002/ijc.27871. [DOI] [PubMed] [Google Scholar]
- 3.Maringe C, Mangtani P, Rachet B, Leon DA, Coleman MP, dos Santos Silva I. Cancer incidence in south asian migrants to England, 1986–2004: unraveling ethnic from socioeconomic differentials. Int J Cancer. 2013;132(8):1886–94. doi: 10.1002/ijc.27826. [DOI] [PubMed] [Google Scholar]
- 4.Keivanlou M-H, Amini-Salehi E, Hassanipour S, Mahapatro A, Raghuma N, Joukar F, Letafatkar N, Habibi A, Norouzi N, Aleali MS. Association between smoking and colorectal cancer in Eastern Mediterranean Regional Office (EMRO): a systematic review and meta-analysis. Saudi J Gastroenterology: Official J Saudi Gastroenterol Association. 2023;29(4):204. doi: 10.4103/sjg.sjg_163_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdifard E, Amini S, Bab S, Masroor N, Khachian A, Heidari M. Incidence trends of colorectal cancer in Iran during 2000–2009: a population-based study. Med J Islam Repub Iran. 2016;30:382–2. [PMC free article] [PubMed] [Google Scholar]
- 6.Rafiemanesh H, Pakzad R, Abedi M, Kor Y, Moludi J, Towhidi F, Reza Makhsosi B, Salehiniya H. Colorectal cancer in Iran: epidemiology and morphology trends. EXCLI J. 2016;15:738–44. doi: 10.17179/excli2016-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee RH. Quality colonoscopy: a matter of time, technique or technology? World J Gastroenterol. 2013;19(10):1517–22. doi: 10.3748/wjg.v19.i10.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonnenberg A, Delcò F, Inadomi JM. Cost-effectiveness of colonoscopy in screening for colorectal cancer. Ann Intern Med. 2000;133(8):573–84. doi: 10.7326/0003-4819-133-8-200010170-00007. [DOI] [PubMed] [Google Scholar]
- 9.Agah S, Faghihi A, Fereshtehnejad S, Shirali A, Hashemi S, Salami A, Khaleghi S, Nazifi A. Comparison of the Effects of Different Doses of Polyethylene Glycol 4000 (Pidrolax) Versus Castor Oil on Bowel Preparation for Colonoscopy: A Prospective Double Blind Randomized Clinical Trial. GOVARESH; Vol 11, No 4 (2006): Winter.
- 10.Filip D, Gao X, Angulo-Rodríguez L, Mintchev MP, Devlin SM, Rostom A, Rosen W, Andrews CN. Colometer: a real-time quality feedback system for screening colonoscopy. World J Gastroenterol. 2012;18(32):4270–7. doi: 10.3748/wjg.v18.i32.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson DA, Gurney MS, Volpe RJ, Jones DM, VanNess MM, Chobanian SJ, Avalos JC, Buck JL, Kooyman G, Cattau EL Jr. A prospective study of the prevalence of colonic neoplasms in asymptomatic patients with an age-related risk. Am J Gastroenterol 1990, 85(8). [PubMed]
- 12.Tan JJY, Tjandra J. Which is the optimal bowel preparation for colonoscopy–a meta-analysis. Colorectal Dis. 2006;8(4):247–58. doi: 10.1111/j.1463-1318.2006.00970.x. [DOI] [PubMed] [Google Scholar]
- 13.Guo F, Chen C, Holleczek B, Schöttker B, Hoffmeister M, Brenner H. Strong reduction of Colorectal Cancer incidence and mortality after screening colonoscopy: prospective cohort study from Germany. Am J Gastroenterol. 2021;116(5):967–75. doi: 10.14309/ajg.0000000000001146. [DOI] [PubMed] [Google Scholar]
- 14.Spadaccini M, Frazzoni L, Vanella G, East J, Radaelli F, Spada C, Fuccio L, Benamouzig R, Bisschops R, Bretthauer M, et al. Efficacy and tolerability of high- vs low-volume Split-Dose Bowel Cleansing Regimens for Colonoscopy: a systematic review and Meta-analysis. Clin Gastroenterol Hepatology: Official Clin Pract J Am Gastroenterological Association. 2020;18(7):1454–1465e1414. doi: 10.1016/j.cgh.2019.10.044. [DOI] [PubMed] [Google Scholar]
- 15.Guo X, Yang Z, Zhao L, Leung F, Luo H, Kang X, Li X, Jia H, Yang S, Tao Q, et al. Enhanced instructions improve the quality of bowel preparation for colonoscopy: a meta-analysis of randomized controlled trials. Gastrointest Endosc. 2017;85(1):90–97e96. doi: 10.1016/j.gie.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Kluge MA, Williams JL, Wu CK, Jacobson BC, Schroy PC, 3rd, Lieberman DA, Calderwood AH. Inadequate Boston Bowel Preparation scale scores predict the risk of missed neoplasia on the next colonoscopy. Gastrointest Endosc. 2018;87(3):744–51. doi: 10.1016/j.gie.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim WH, Cho YJ, Park JY, Min PK, Kang JK, Park IS. Factors affecting insertion time and patient discomfort during colonoscopy. Gastrointest Endosc. 2000;52(5):600–5. doi: 10.1067/mge.2000.109802. [DOI] [PubMed] [Google Scholar]
- 18.Wexner SD, Beck DE, Baron TH, Fanelli RD, Hyman N, Shen B, Wasco KE. A consensus document on bowel preparation before colonoscopy: prepared by a task force from the american society of Colon and rectal surgeons (ASCRS), the American Society for gastrointestinal endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) Gastrointest Endosc. 2006;63(7):894–909. doi: 10.1016/j.gie.2006.03.918. [DOI] [PubMed] [Google Scholar]
- 19.Connor A, Tolan D, Hughes S, Carr N, Tomson C. Consensus guidelines for the safe prescription and administration of oral bowel-cleansing agents. Gut. 2012;61(11):1525–32. doi: 10.1136/gutjnl-2011-300861. [DOI] [PubMed] [Google Scholar]
- 20.Dapoigny M, Sarna SK. Effects of physical exercise on colonic motor activity. Am J Physiology-Gastrointestinal Liver Physiol. 1991;260(4):G646–52. doi: 10.1152/ajpgi.1991.260.4.G646. [DOI] [PubMed] [Google Scholar]
- 21.Rao SS, Chamberlain M, Leistikow J, Gisolfi C. Effects of acute graded exercise on human colonic motility. GASTROENTEROLOGY-BALTIMORE THEN PHILADELPHIA. 1997;112:A801–1. [Google Scholar]
- 22.Kim HS, Park DH, Kim JW, Jee MG, Baik SK, Kwon SO, Lee DK. Effectiveness of walking exercise as a bowel preparation for colonoscopy: a randomized controlled trial. Am J Gastroenterol. 2005;100(9):1964. doi: 10.1111/j.1572-0241.2005.40373.x. [DOI] [PubMed] [Google Scholar]
- 23.Gao X, Bian Q, Ding W, Qian H, Li W, Zhang G, Li X. Effect of walking Exercise and Intestinal Cleansing interval on Bowel Preparation Quality, a Single-Blind, randomized controlled trial. Dig Dis Sci. 2023;68(1):193–201. doi: 10.1007/s10620-022-07526-4. [DOI] [PubMed] [Google Scholar]
- 24.Kluge MA, Williams JL, Wu CK, Jacobson BC, Schroy PC, III, Lieberman DA, Calderwood AH. Inadequate Boston Bowel Preparation scale scores predict the risk of missed neoplasia on the next colonoscopy. Gastrointest Endosc. 2018;87(3):744–51. doi: 10.1016/j.gie.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kluge MA, Williams JL, Wu CK, Jacobson BC, Schroy PC III, Lieberman DA, Calderwood AH. Inadequate Boston Bowel Preparation scale scores predict the risk of missed neoplasia on the next colonoscopy. Gastrointest Endosc 2017. [DOI] [PMC free article] [PubMed]
- 26.Lohsiriwat V. Approach to hemorrhoids. Curr Gastroenterol Rep. 2013;15(7):332. doi: 10.1007/s11894-013-0332-6. [DOI] [PubMed] [Google Scholar]
- 27.Lohsiriwat V. Hemorrhoids: from basic pathophysiology to clinical management. World J Gastroenterol. 2012;18(17):2009–17. doi: 10.3748/wjg.v18.i17.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn’s disease. Lancet (London England) 2017;389(10080):1741–55. doi: 10.1016/S0140-6736(16)31711-1. [DOI] [PubMed] [Google Scholar]
- 29.Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel J-F. Ulcerative colitis. Lancet (London England) 2017;389(10080):1756–70. doi: 10.1016/S0140-6736(16)32126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schiller LR, Pardi DS, Spiller R, Semrad CE, Surawicz CM, Giannella RA, Krejs GJ, Farthing MJ, Sellin JH. Gastro 2013 APDW/WCOG Shanghai working party report: chronic diarrhea: definition, classification, diagnosis. J Gastroenterol Hepatol. 2014;29(1):6–25. doi: 10.1111/jgh.12392. [DOI] [PubMed] [Google Scholar]
- 31.Schiller LR, Pardi DS, Sellin JH. Chronic diarrhea: diagnosis and management. Clin Gastroenterol Hepatology: Official Clin Pract J Am Gastroenterological Association. 2017;15(2):182–193e183. doi: 10.1016/j.cgh.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 32.Segal J, McKeown DG, Tavarez MM. Rectal prolapse. StatPearls edn. Treasure Island (FL): StatPearls Publishing Copyright © 2020, StatPearls Publishing LLC.; 2020.
- 33.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104(3):739–50. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 34.Angarita FA, Feinberg AE, Feinberg SM, Riddell RH, McCart JA. Management of complex polyps of the colon and rectum. Int J Colorectal Dis. 2018;33(2):115–29. doi: 10.1007/s00384-017-2950-1. [DOI] [PubMed] [Google Scholar]
- 35.Banerjee A, Pathak S, Subramanium VD, Murugesan GD, Verma R. Strategies for targeted drug delivery in treatment of colon cancer: current trends and future perspectives. Drug Discovery Today. 2017;22(8):1224–32. doi: 10.1016/j.drudis.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Li JN, Yuan SY. Fecal occult blood test in colorectal cancer screening. J Dig Dis. 2019;20(2):62–4. doi: 10.1111/1751-2980.12712. [DOI] [PubMed] [Google Scholar]
- 37.Doubeni CA, Corley DA, Quinn VP, Jensen CD, Zauber AG, Goodman M, Johnson JR, Mehta SJ, Becerra TA, Zhao WK, et al. Effectiveness of screening colonoscopy in reducing the risk of death from right and left colon cancer: a large community-based study. Gut. 2018;67(2):291–8. doi: 10.1136/gutjnl-2016-312712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan J, Xin L, Ma YF, Hu LH, Li ZS. Colonoscopy reduces Colorectal Cancer incidence and mortality in patients with non-malignant findings: a Meta-analysis. Am J Gastroenterol. 2016;111(3):355–65. doi: 10.1038/ajg.2015.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mankaney G, Sutton RA, Burke CA. Colorectal cancer screening: choosing the right test. Cleve Clin J Med. 2019;86(6):385–92. doi: 10.3949/ccjm.86a.17125. [DOI] [PubMed] [Google Scholar]
- 40.Rutherford CC, Calderwood AH. Update on Bowel Preparation for Colonoscopy. Curr Treat Options Gastroenterol. 2018;16(1):165–81. doi: 10.1007/s11938-018-0165-3. [DOI] [PubMed] [Google Scholar]
- 41.Burke CA, Church JM. Enhancing the quality of colonoscopy: the importance of bowel purgatives. Gastrointest Endosc. 2007;66(3):565–73. doi: 10.1016/j.gie.2007.03.1084. [DOI] [PubMed] [Google Scholar]
- 42.Aronchick CA, Lipshutz WH, Wright SH, Dufrayne F, Bergman G. A novel tableted purgative for colonoscopic preparation: efficacy and safety comparisons with colyte and Fleet Phospho-Soda. Gastrointest Endosc. 2000;52(3):346–52. doi: 10.1067/mge.2000.108480. [DOI] [PubMed] [Google Scholar]
- 43.Rostom A, Jolicoeur E, Dubé C, Grégoire S, Patel D, Saloojee N, Lowe C. A randomized prospective trial comparing different regimens of oral sodium phosphate and polyethylene glycol-based lavage solution in the preparation of patients for colonoscopy. Gastrointest Endosc. 2006;64(4):544–52. doi: 10.1016/j.gie.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 44.Rostom A, Jolicoeur E. Validation of a new scale for the assessment of bowel preparation quality. Gastrointest Endosc. 2004;59(4):482–6. doi: 10.1016/s0016-5107(03)02875-x. [DOI] [PubMed] [Google Scholar]
- 45.Hassan C, Bretthauer M, Kaminski MF, Polkowski M, Rembacken B, Saunders B, Benamouzig R, Holme O, Green S, Kuiper T, et al. Bowel preparation for colonoscopy: european society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy. 2013;45(2):142–50. doi: 10.1055/s-0032-1326186. [DOI] [PubMed] [Google Scholar]
- 46.Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc. 2003;58(1):76–9. doi: 10.1067/mge.2003.294. [DOI] [PubMed] [Google Scholar]
- 47.Chiu HM, Lin JT, Lee YC, Liang JT, Shun CT, Wang HP, Wu MS. Different bowel preparation schedule leads to different diagnostic yield of proximal and nonpolypoid colorectal neoplasm at screening colonoscopy in average-risk population. Dis Colon Rectum. 2011;54(12):1570–7. doi: 10.1097/DCR.0b013e318231d667. [DOI] [PubMed] [Google Scholar]
- 48.Chokshi RV, Hovis CE, Hollander T, Early DS, Wang JS. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest Endosc. 2012;75(6):1197–203. doi: 10.1016/j.gie.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Nguyen DL, Wieland M. Risk factors predictive of poor quality preparation during average risk colonoscopy screening: the importance of health literacy. J Gastrointest Liver Diseases: JGLD. 2010;19(4):369–72. [PubMed] [Google Scholar]
- 50.Chan WK, Saravanan A, Manikam J, Goh KL, Mahadeva S. Appointment waiting times and education level influence the quality of bowel preparation in adult patients undergoing colonoscopy. BMC Gastroenterol. 2011;11:86. doi: 10.1186/1471-230X-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ness RM, Manam R, Hoen H, Chalasani N. Predictors of inadequate bowel preparation for colonoscopy. Am J Gastroenterol. 2001;96(6):1797–802. doi: 10.1111/j.1572-0241.2001.03874.x. [DOI] [PubMed] [Google Scholar]
- 52.Lebwohl B, Wang TC, Neugut AI. Socioeconomic and other predictors of colonoscopy preparation quality. Dig Dis Sci. 2010;55(7):2014–20. doi: 10.1007/s10620-009-1079-7. [DOI] [PubMed] [Google Scholar]
- 53.Chung YW, Han DS, Park KH, Kim KO, Park CH, Hahn T, Yoo KS, Park SH, Kim JH, Park CK. Patient factors predictive of inadequate bowel preparation using polyethylene glycol: a prospective study in Korea. J Clin Gastroenterol. 2009;43(5):448–52. doi: 10.1097/MCG.0b013e3181662442. [DOI] [PubMed] [Google Scholar]
- 54.Taylor C, Schubert ML. Decreased efficacy of polyethylene glycol lavage solution (golytely) in the preparation of diabetic patients for outpatient colonoscopy: a prospective and blinded study. Am J Gastroenterol. 2001;96(3):710–4. doi: 10.1111/j.1572-0241.2001.03610.x. [DOI] [PubMed] [Google Scholar]
- 55.Rosenfeld G, Krygier D, Enns RA, Singham J, Wiesinger H, Bressler B. The impact of patient education on the quality of inpatient bowel preparation for colonoscopy. Can J Gastroenterology = Journal canadien de gastroenterologie. 2010;24(9):543–6. doi: 10.1155/2010/718628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones RM, Devers KJ, Kuzel AJ, Woolf SH. Patient-reported barriers to colorectal cancer screening: a mixed-methods analysis. Am J Prev Med. 2010;38(5):508–16. doi: 10.1016/j.amepre.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jover R, Zapater P, Polanía E, Bujanda L, Lanas A, Hermo JA, Cubiella J, Ono A, González-Méndez Y, Peris A, et al. Modifiable endoscopic factors that influence the adenoma detection rate in colorectal cancer screening colonoscopies. Gastrointest Endosc. 2013;77(3):381–389e381. doi: 10.1016/j.gie.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 58.Burke CA, Church JM. Enhancing the quality of colonoscopy: the importance of bowel purgatives. Gastrointest Endosc. 2007;66(3):565–573. doi: 10.1016/j.gie.2007.03.1084. [DOI] [PubMed] [Google Scholar]
- 59.Davis GR, Santa Ana CA, Morawski SG, Fordtran JS. Development of a lavage solution associated with minimal water and electrolyte absorption or secretion. Gastroenterology. 1980;78(5 Pt 1):991–5. [PubMed] [Google Scholar]
- 60.Vanner SJ, MacDonald PH, Paterson WG, Prentice RS, Da Costa LR, Beck IT. A randomized prospective trial comparing oral sodium phosphate with standard polyethylene glycol-based lavage solution (Golytely) in the preparation of patients for colonoscopy. Am J Gastroenterol. 1990;85(4):422–7. [PubMed] [Google Scholar]
- 61.Belsey J, Epstein O, Heresbach D. Systematic review: adverse event reports for oral sodium phosphate and polyethylene glycol. Aliment Pharmacol Ther. 2009;29(1):15–28. doi: 10.1111/j.1365-2036.2008.03837.x. [DOI] [PubMed] [Google Scholar]
- 62.Chan W-K, Azmi N, Mahadeva S, Goh K-L. Split-dose vs same-day reduced-volume polyethylene glycol electrolyte lavage solution for morning colonoscopy. World J Gastroenterology: WJG. 2014;20(39):14488. doi: 10.3748/wjg.v20.i39.14488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Church JM. Effectiveness of polyethylene glycol antegrade gut lavage bowel preparation for colonoscopy–timing is the key! Dis Colon Rectum. 1998;41(10):1223–5. doi: 10.1007/BF02258217. [DOI] [PubMed] [Google Scholar]
- 64.Gupta T, Mandot A, Desai D, Abraham P, Joshi A, Shah S. Comparison of two schedules (previous evening versus same morning) of bowel preparation for colonoscopy. Endoscopy. 2007;39(8):706–9. doi: 10.1055/s-2007-966375. [DOI] [PubMed] [Google Scholar]
- 65.Parra-Blanco A, Nicolas-Perez D, Gimeno-Garcia A, Grosso B, Jimenez A, Ortega J, Quintero E. The timing of bowel preparation before colonoscopy determines the quality of cleansing, and is a significant factor contributing to the detection of flat lesions: a randomized study. World J Gastroenterol. 2006;12(38):6161–6. doi: 10.3748/wjg.v12.i38.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Romero RV, Mahadeva S. Factors influencing quality of bowel preparation for colonoscopy. World J Gastrointest Endosc. 2013;5(2):39–46. doi: 10.4253/wjge.v5.i2.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noh CK, Kim IS, Lee GH, Park JW, Lee E, Park B, Hong HJ, Lim SG, Shin SJ, Kim JH, et al. Comparison of effectiveness between Abdominal Vibration Stimulation and walking Exercise for Bowel Cleansing before Therapeutic Colonoscopy. Gut Liver. 2020;14(4):468–76. doi: 10.5009/gnl19199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arya V, Gupta KA, Arya SV. Efficacy of bolus lukewarm saline and yoga postures as colonoscopy preparation: a pilot study. J Altern Complement Med. 2010;16(12):1269–77. doi: 10.1089/acm.2010.0166. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y, Wang Q, Zhenyun W, Jie G, Zhao Y, Yang X. Investigation on the effect of bowel preparation before colonoscopy and its influencing factors. Chin J Practical Nurs. 2017;33(14):1085–8. [Google Scholar]
- 70.Kim HS, Park DH, Kim JW, Jee MG, Baik SK, Kwon SO, Lee DK. Effectiveness of walking exercise as a bowel preparation for colonoscopy: a randomized controlled trial. Am J Gastroenterol. 2005;100(9):1964–9. doi: 10.1111/j.1572-0241.2005.40373.x. [DOI] [PubMed] [Google Scholar]
- 71.Huang L, Zhou W. A systematic review and meta-analysis examining the benefits of quantitative exercise intervention on effective bowel preparation prior to colonoscopy. Annals of Palliative Medicine. 2021;10(12):124782487–124712487. doi: 10.21037/apm-21-3378. [DOI] [PubMed] [Google Scholar]
- 72.Nguyen DL, Wieland M. Risk factors predictive of poor quality preparation during average risk colonoscopy screening: the importance of health literacy. J Gastrointest Liver Dis 2010, 19(4). [PubMed]
- 73.Chan W-K, Saravanan A, Manikam J, Goh K-L, Mahadeva S. Appointment waiting times and education level influence the quality of bowel preparation in adult patients undergoing colonoscopy. BMC Gastroenterol. 2011;11(1):1–9. doi: 10.1186/1471-230X-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chung YW, Han DS, Park KH, Kim KO, Park CH, Hahn T, Yoo K-S, Park SH, Kim JH, Park CK. Patient factors predictive of inadequate bowel preparation using polyethylene glycol: a prospective study in Korea. J Clin Gastroenterol. 2009;43(5):448–452. doi: 10.1097/MCG.0b013e3181662442. [DOI] [PubMed] [Google Scholar]
- 75.Gallagher P, O’Mahony D. Constipation in old age. Best Pract Res Clin Gastroenterol. 2009;23(6):875–87. doi: 10.1016/j.bpg.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 76.Heppner H, Christ M, Gosch M, Mühlberg W, Bahrmann P, Bertsch T, Sieber C, Singler K. Polypharmacy in the elderly from the clinical toxicologist perspective. Z für Gerontologie und Geriatrie. 2012;45(6):473–8. doi: 10.1007/s00391-012-0383-6. [DOI] [PubMed] [Google Scholar]
- 77.Cameron AJ, Shaw JE, Zimmet PZ. The metabolic syndrome: prevalence in worldwide populations. Endocrinol Metabolism Clin. 2004;33(2):351–75. doi: 10.1016/j.ecl.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 78.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Panitch J, Tin K, Gonuguntla V, Rahmani R. Sex, smoking and ethnicity: does it influence Bowel Preparation? 501. Official J Am Coll Gastroenterology| ACG. 2018;113:288. [Google Scholar]
- 80.Amitay EL, Niedermaier T, Gies A, Hoffmeister M, Brenner H. Risk factors of inadequate Bowel Preparation for Screening Colonoscopy. J Clin Med. 2021;10(12):2740. doi: 10.3390/jcm10122740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wong MCS, Ching JYL, Chan VCW, Lam TYT, Luk AKC, Tang RSY, Wong SH, Ng SC, Ng SSM, Wu JCY, et al. Determinants of Bowel Preparation Quality and its Association with Adenoma detection: a prospective Colonoscopy Study. Med (Baltim) 2016;95(2):e2251–1. doi: 10.1097/MD.0000000000002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sanders KM, Koh SD, Ro S, Ward SM. Regulation of gastrointestinal motility–insights from smooth muscle biology. Nat Reviews Gastroenterol Hepatol. 2012;9(11):633–45. doi: 10.1038/nrgastro.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Al-Shboul OA. The importance of interstitial cells of cajal in the gastrointestinal tract. Saudi J Gastroenterology: Official J Saudi Gastroenterol Association. 2013;19(1):3–15. doi: 10.4103/1319-3767.105909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim YS, Song BK, Oh JS, Woo SS. Aerobic exercise improves gastrointestinal motility in psychiatric inpatients. World J Gastroenterol. 2014;20(30):10577–84. doi: 10.3748/wjg.v20.i30.10577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Horner KM, Schubert MM, Desbrow B, Byrne NM, King NA. Acute exercise and gastric emptying: a meta-analysis and implications for appetite control. Sports Med. 2015;45(5):659–78. doi: 10.1007/s40279-014-0285-4. [DOI] [PubMed] [Google Scholar]
- 86.Peters HP, De Vries WR, Vanberge-Henegouwen GP, Akkermans LM. Potential benefits and hazards of physical activity and exercise on the gastrointestinal tract. Gut. 2001;48(3):435–9. doi: 10.1136/gut.48.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Y-Y, Yan M, Li J, Fu M-L, Xie L, Tang W, Wang Q-Y. Effects of walking exercise on bowel preparation in patients undergoing colonoscopy: evidence from systematic review and meta-analysis. Front Nurs. 2020;7(1):39–47. [Google Scholar]
- 88.Wenqing H, Wan Q. Correlation analysis between the walking function status and the quality of bowel preparation for colonoscopy in the elderly patients. Chin J Postgraduates Med. 2017;40(7):608–11. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from the study can be provided from Corresponding Author on reasonable request.