Abstract
Background
Mammographic density (MD) is a strong risk factor for breast cancer. We aimed to evaluate the association between MD and breast cancer related risk factors among average-risk women in rural China.
Methods
This is a population-based screening study. 12518 women aged 45–64 years with complete MD data from three maternal and childcare hospitals in China were included in the final analysis. ORs and 95%CIs were estimated using generalized logit model by comparing each higher MD (BI-RADS b, c, d) to the lowest group (BI-RADS a). The cumulative logistic regression model was used to estimate the ORtrend (95%CI) and Ptrend by treating MD as an ordinal variable.
Results
Older age (ORtrend = 0.81, 95%CI: 0.79–0.81, per 2-year increase), higher BMI (ORtrend = 0.73, 95%CI: 0.71–0.75, per 2 kg/m2), more births (ORtrend = 0.47, 95%CI: 0.41–0.54, 3 + vs. 0–1), postmenopausal status (ORtrend = 0.42, 95%CI: 0.38–0.46) were associated with lower MD. For parous women, longer duration of breastfeeding was found to be associated with higher MD when adjusting for study site, age, BMI, and age of first full-term birth (ORtrend = 1.53, 95%CI: 1.27–1.85, 25 + months vs. no breastfeeding; ORtrend = 1.45, 95%CI: 1.20–1.75, 19–24 months vs. no breastfeeding), however, the association became non-significant when adjusting all covariates. Associations between examined risk factors and MD were similar in premenopausal and postmenopausal women except for level of education and oral hormone drug usage. Higher education was only found to be associated with an increased proportion of dense breasts in postmenopausal women (ORtrend = 1.08, 95%CI: 1.02–1.15). Premenopausal women who ever used oral hormone drug were less likely to have dense breasts, though the difference was marginally significant (OR = 0.54, P = 0.045). In postmenopausal women, we also found the proportion of dense breasts increased with age at menopause (ORtrend = 1.31, 95%CI: 1.21–1.43).
Conclusions
In Chinese women with average risk for breast cancer, we found MD was associated with age, BMI, menopausal status, lactation, and age at menopausal. This finding may help to understand the etiology of breast cancer and have implications for breast cancer prevention in China.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-023-11444-7.
Keywords: Mammographic density, Risk factor, Breast cancer
Background
Breast cancer is an important public health problem, and has become the most frequently diagnosed malignancy worldwide. The incidence rate in Asia is relatively lower compared with that in Western countries, but the gap is narrower and 45.4% of new breast cancer cases are diagnosed in Asia countries since it is the most populous continent [1]. Breast cancer is a complex disease with various etiological causes. Nonmodifiable factors known to increase the risk of breast cancer include age, family history of breast cancer [2], reproductive factors [3] and genetic mutation [4]. Modifiable risk factors include postmenopausal obesity [5], alcohol consumption [6] and physical inactivity [7].
Apart from those mentioned above, mammographic density (MD) is a strong risk factor for breast cancer. MD represents the percentage of radiologically dense area of breast, which, comprised of more stromal and epithelial tissues, appears white on mammography images [8]. Previous studies have constantly found that women with 75% or greater percent of MD had four to six times higher risk of breast cancer compared with women with density in less than 10% [9, 10]. MD is modifiable and decreased with age [11]. Other modifiable and nonmodifiable factors were found to be associated with MD, such as alcohol consumption [12], body mass index (BMI) [13], dietary factors [14], reproductive factors [15]. This suggested many of risk factors that increased breast cancer risk might work through their effects on MD. Breast dense tissues also diminished the capacity of detecting breast cancers by mammogram and thus increase the risk of interval breast cancer between screening tests [16].
Asian women had smaller breast size, smaller absolute breast dense volume, higher percent breast density compared to Caucasian women [17–19]. The Breast Imaging Reporting and Data System (BI-RADS) divided MD into four categories based on quantitative assessment, which provided the risk for developing breast cancer to clinicians [20]. The BI-RADS density classification was frequently used for MD assessment in breast cancer screening programs in China and the worldwide. Asian women were more frequently reported to have heterogeneously or extremely dense breasts than Caucasian women. Prior studies have evaluated associations between MD and breast cancer related risk factors in Chinese women. However, these studies were mainly focused on women with high risk of breast cancer who were lived in urban areas [21] or only examined a limited number of risk factors [22].
Henan, Shanxi, and Sichuan locate at Eastern China, North China, and Southwest China, with GDP per capita lower than the national average level. Apart from Henan, the rest two provinces are mountainous areas, which would impede the dissemination of health service. The breast cancer incidence in Henan, Shanxi, and Sichuan province ranked in the 8th, 15th, and 32nd out of 34 provinces [23], which represent the higher, middle, and lower level of breast cancer incidence in China.
Therefore, this study aimed to describe the distribution of MD and estimates the association of MD with selected known breast cancer risk factors according to menopausal status among general-risk Chinese women living in rural areas.
Methods
Study population
Study subjects were a subset of participants aged 45–64 years old who participated in a breast cancer screening trial that was conducted in rural areas of China. This study was led by Cancer Hospital, Chinese Academy of Medical Science and was conducted between Feb, 2018 and Feb, 2022. In this trial, women aged 35–64 years old without history of breast cancer, had lived for more than six months in their local communities, and were able to understand and provide written informed consent form (ICF) were recruited. Those who were pregnant, lactating, or plan to become pregnant at the time; had been screened for breast cancer within prior three years; had been diagnosed or treated for malignancies in the prior 12 months; showed suspicious signs of breast cancer even without indication of breast imaging exams were excluded before registration. All eligible women underwent breast ultrasound, those who aged 45–64 years old also underwent mammogram. In this study, participants aged 45–64 years old from Zezhou (Shanxi province), Xinmi (Henan Province), and Mianyang (Sichuan Province) with completed mammogram data were included.
Mammographic density
For each participant, craniocaudal (CC) and mediolateral (MLO) views were obtained using full-field digital mammograms (SN-DR3, Shenzhen Shengnuo and Selenia Dimensions, Hologic) in each hospital and were interpreted by experienced radiologists. MD was recorded using the American College of Radiology’s Breast Imaging Reporting and Data System (BI-RADS), which categorizes the mammographic density into four categories: BI-RADS a, indicating that the breasts are almost entirely fatty; BI-RADS b, indicating that there are scattered areas of fibroglandular density; BI-RADS c, indicating that the breast are heterogeneously dense; and BI-RADS d, indicating that the breasts are extremely dense.
Covariates
All eligible women completed the questionnaire before undertaking mammogram examination, which included demographic information (age, weight, height, education, family history of breast cancer, smoking status, and alcohol drinking status), reproductive factors (age at menarche, menopausal status, parity). Oral hormone drug use included estrogen and progesterone, and other hormone drugs such as glucocorticoids, and thyroid hormones. BMI was calculated as weight (kg) divided by height squared (m2) then was divided into four categories (< 18.5, 18.5–23.9, 24–27.9, 28 +) based on the criteria of weight for adults released by the National Health Commission of China in 2013. Since the number of nulliparous women was small, nulliparous women and those who have ever had one childbirth were collapsed into one group. For parous women, the cumulative time of breastfeeding was also recorded. Women who reported had no menstruation in the past 12 months were recorded as postmenopausal and age of last menstruation was recorded for them. Family history of breast cancer was defined as breast cancer occurred in first-degree, second-degree, or third-degree relatives and were grouped as binary variables because of the small number of breast cancer in each relative degree. The smoking status was defined as have never smoked (never), smoked regularly in the past six months (currently smoking), and only smoked six months before (was smoking). The alcohol drinking status was defined as have never drank (never), drank regularly in the last six months (currently drinking), only drank six months before (was drinking). The smoking and alcohol drinking status was grouped as binary variables since most of the women never smoked nor drank.
Statistical analysis
The difference of demographic and reproductive factors among four MD categories were assessed using one-way analysis of variance (ANOVA) for continuous variable or Chi square test for categorical variables. Polytomous logistic regression was used to compare each higher MD (BI-RADS b, c, and d) to BI-RADS a, Odds Ratios (ORs) and 95% confidence intervals (95%CIs) were estimated using generalized logit model. The ORtrend (95%CI) and Ptrend were estimated using cumulative logistic regression model with defining MD as an ordinal variable.
The final multivariable cumulative model included study site, age (per two years), education (none-elementary. Middle school, high school, college), BMI (per two units), age at menarche (≤ 13, 14–15, 16 +), parity (≤ 1, 2, 3 +), menopause status (pre- vs. postmenopausal), family history of breast cancer (yes vs. no). For parous women, we also adjusted age at first full term birth (≤ 20, 21–24, 25–29, 30 +) and breastfeeding (no breastfeeding, 1–6 months, 7–12 months, 13–18 months, 19–24 months, 25 +). For postmenopausal women, age at menopause (≤ 45, 45–50, 51 +) was additionally adjusted. Smoking and oral hormone drug use were not associated with MD in the univariate analysis and therefore was not included in the final multivariable analysis. We also excluded the alcohol drinking from final analysis because only 347 (2.77%) women drank alcohol currently or six months before. We also did stratified analysis with menopausal status as the stratified factors. The MD was grouped as dense group (BI-RADS c-d) and non-dense group (BI-RADS a-b) in stratified analysis due to the small number of participants in some extreme groups. The significance level was 0.05 for two-sided tests. All statistical analysis was performed using SAS 9.4 software (SAS Institute Inc., Cary, NC).
Results
Baseline characteristics of the study participants
A total of 12,518 women aged 45–64 years were included in this analysis. The demographic characteristics were described in Table 1. Overall, the mean age was 51.53 (SD, 4.45) years and the mean BMI was 23.9 kg/m2 (SD: 2.26). 50.16% of the participants were postmenopausal. 39.66% of the women had more than nine years of education (high school and above). Most women were parous (99.24%), and reported that they gave first full term birth at age 21–29 (92.20%), had breastfed (95.88%). Almost all participants reported that they had never smoked (99.59%) nor drank alcohol (97.23). 1.66% of the women reported a family history of breast cancer within three blood degrees.
Table 1.
Demographic characteristics and breast cancer risk factors of participants
| All | BI-RADS a | BI-RADS b | BI-RADS c | BI-RADS d | Pa | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 12,518 | n = 693 | n = 3042 | n = 6717 | n = 2066 | ||||||||
| n | % | n | % | n | % | n | % | n | % | |||
| age | ||||||||||||
| Mean (SD) | 51.53 | 4.45 | 55.28 | 4.53 | 53.46 | 4.58 | 51.04 | 4.11 | 49.00 | 3.15 | < 0.01 | |
| 45–49 | 5394 | 43.09 | 102 | 14.72 | 769 | 25.28 | 3107 | 46.26 | 1416 | 68.54 | < 0.01 | |
| 50–54 | 4237 | 33.85 | 204 | 29.44 | 1110 | 36.49 | 2385 | 35.51 | 538 | 26.04 | ||
| 55–59 | 2303 | 18.40 | 275 | 39.68 | 888 | 29.19 | 1037 | 15.44 | 103 | 4.99 | ||
| 60–64 | 584 | 4.67 | 112 | 16.16 | 275 | 9.04 | 188 | 2.80 | 9 | 0.44 | ||
| education | ||||||||||||
| None-elementary | 1987 | 15.88 | 144 | 20.81 | 532 | 17.49 | 1103 | 16.43 | 208 | 10.07 | < 0.01 | |
| Middle school | 5563 | 44.46 | 354 | 51.16 | 1436 | 47.22 | 2951 | 43.95 | 822 | 39.79 | ||
| High school | 2547 | 20.35 | 124 | 17.92 | 680 | 22.36 | 1328 | 19.78 | 415 | 20.09 | ||
| College and above | 2416 | 19.31 | 70 | 10.12 | 393 | 12.92 | 1332 | 19.84 | 621 | 30.06 | ||
| missing | 5 | 1 | 1 | 3 | 0 | |||||||
| BMI (kg/m2) | ||||||||||||
| Mean (SD) | 23.9 | 2.66 | 25.36 | 2.91 | 24.38 | 2.72 | 23.85 | 2.58 | 22.85 | 2.30 | < 0.01 | |
| < 18.5 | 120 | 0.96 | 2 | 0.29 | 23 | 0.76 | 55 | 0.82 | 40 | 1.94 | < 0.01 | |
| 18.5–23.9 | 6644 | 53.12 | 235 | 33.96 | 1393 | 45.85 | 3594 | 53.55 | 1422 | 68.86 | ||
| 24–27.9 | 4837 | 38.67 | 332 | 47.98 | 1323 | 43.55 | 2622 | 39.06 | 560 | 27.12 | ||
| 28 + | 906 | 7.24 | 123 | 17.77 | 299 | 9.84 | 441 | 6.57 | 43 | 2.08 | ||
| missing | 11 | 1 | 4 | 5 | 1 | |||||||
| age at menarche | ||||||||||||
| ≤ 13 | 3837 | 30.66 | 189 | 27.27 | 859 | 28.26 | 2141 | 31.87 | 648 | 31.40 | < 0.01 | |
| 14–15 | 4824 | 38.55 | 227 | 32.76 | 1125 | 37.01 | 2597 | 38.66 | 875 | 42.39 | ||
| 16 + | 3853 | 30.79 | 277 | 39.97 | 1056 | 34.74 | 1979 | 29.46 | 541 | 26.21 | ||
| missing | 4 | 0 | 2 | 0 | 2 | |||||||
| parity | ||||||||||||
| 0 | 95 | 0.76 | 3 | 0.43 | 17 | 0.56 | 51 | 0.76 | 24 | 1.16 | < 0.01 | |
| 1 | 4501 | 36.00 | 119 | 17.17 | 929 | 30.60 | 2504 | 37.30 | 949 | 46.05 | ||
| 2 | 6383 | 51.05 | 394 | 56.85 | 1594 | 52.50 | 3428 | 51.07 | 967 | 46.92 | ||
| 3 + | 1524 | 12.19 | 177 | 25.54 | 496 | 16.34 | 730 | 10.87 | 121 | 5.87 | ||
| missing | 15 | 0 | 6 | 4 | 5 | |||||||
| age at first full term birthb | ||||||||||||
| ≤ 20 | 537 | 4.33 | 22 | 3.19 | 138 | 4.57 | 307 | 4.61 | 70 | 3.44 | < 0.01 | |
| 21–24 | 7133 | 57.52 | 414 | 60.00 | 1840 | 60.97 | 3777 | 56.75 | 1102 | 54.13 | ||
| 25–29 | 4300 | 34.68 | 231 | 33.48 | 964 | 31.94 | 2324 | 34.92 | 781 | 38.36 | ||
| 30 + | 430 | 3.47 | 23 | 3.33 | 76 | 2.52 | 248 | 3.73 | 83 | 4.08 | ||
| missing | 8 | 0 | 1 | 6 | 1 | |||||||
| breastfeedingb | ||||||||||||
| No breastfeeding | 510 | 4.12 | 18 | 2.61 | 99 | 3.29 | 298 | 4.49 | 95 | 4.67 | < 0.01 | |
| 1–6 months | 661 | 5.34 | 12 | 1.74 | 163 | 5.41 | 373 | 5.61 | 113 | 5.56 | ||
| 7–12 months | 3169 | 25.60 | 107 | 15.51 | 686 | 22.78 | 1694 | 25.50 | 682 | 33.55 | ||
| 13–18 months | 1420 | 11.47 | 64 | 9.28 | 320 | 10.63 | 766 | 11.53 | 270 | 13.28 | ||
| 19–24 months | 2993 | 24.18 | 195 | 28.26 | 769 | 25.54 | 1541 | 23.19 | 488 | 24.00 | ||
| 25 months + | 3625 | 29.29 | 294 | 42.61 | 974 | 32.35 | 1972 | 29.68 | 385 | 18.94 | ||
| missing | 30 | 0 | 8 | 18 | 4 | |||||||
| menopausal status | ||||||||||||
| premenopausal | 6228 | 49.84 | 113 | 16.31 | 879 | 28.95 | 3626 | 54.06 | 1610 | 78.12 | < 0.01 | |
| postmenopausal | 6269 | 50.16 | 580 | 83.69 | 2157 | 71.05 | 3081 | 45.94 | 451 | 21.88 | ||
| missing | 21 | 0 | 6 | 10 | 5 | |||||||
| age at menopausec | ||||||||||||
| ≤ 45 | 1009 | 16.13 | 116 | 20.03 | 329 | 15.30 | 474 | 15.40 | 90 | 20.04 | < 0.01 | |
| 46–50 | 3208 | 51.28 | 271 | 46.80 | 1111 | 51.65 | 1593 | 51.77 | 233 | 51.89 | ||
| 51 + | 2039 | 32.59 | 192 | 33.16 | 711 | 33.05 | 1010 | 32.82 | 126 | 28.06 | ||
| missing | 13 | 1 | 6 | 4 | 2 | |||||||
| oral hormone drug use | ||||||||||||
| never | 12,229 | 98.61 | 675 | 98.54 | 2947 | 98.10 | 6582 | 98.75 | 2025 | 98.93 | 0.12 | |
| 1–5 years | 134 | 1.08 | 9 | 1.31 | 44 | 1.46 | 66 | 0.99 | 15 | 0.73 | ||
| 5 years + | 38 | 0.31 | 1 | 0.15 | 13 | 0.43 | 17 | 0.26 | 7 | 0.34 | ||
| missing | 117 | 8 | 38 | 52 | 19 | |||||||
| family history of breast cancer | ||||||||||||
| no | 12,289 | 98.31 | 688 | 99.28 | 2985 | 98.42 | 6602 | 98.39 | 2014 | 97.58 | < 0.05 | |
| yes | 211 | 1.69 | 5 | 0.72 | 48 | 1.58 | 108 | 1.61 | 50 | 2.42 | ||
| missing | 18 | 0 | 9 | 7 | 2 | |||||||
| smoking | ||||||||||||
| never | 12,452 | 99.50 | 693 | 1.00 | 3022 | 99.44 | 6680 | 99.46 | 2057 | 99.56 | 0.25 | |
| yes | 62 | 0.50 | 0 | 0 | 0.00 | 17 | 0.56 | 36 | 0.54 | 9 | 0.44 | |
| missing | 4 | 0 | 3 | 1 | 0 | |||||||
| alcohol drinking | ||||||||||||
| never | 12,167 | 97.23 | 688 | 99.28 | 2933 | 96.51 | 6540 | 97.38 | 2006 | 97.10 | < 0.01 | |
| yes | 347 | 2.77 | 0 | 5 | 0.72 | 106 | 3.49 | 176 | 2.62 | 60 | 2.90 | |
| missing | 4 | 0 | 3 | 1 | 0 | |||||||
aresults are from one-way analysis of variance (ANOVA) for continuous variables and Chi square test for categorical variables
bparous women only
cpostmenopausal women only
The distribution of mammographic density
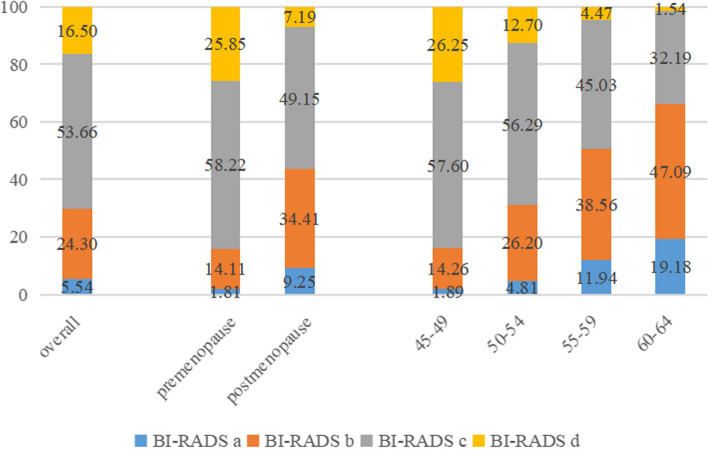
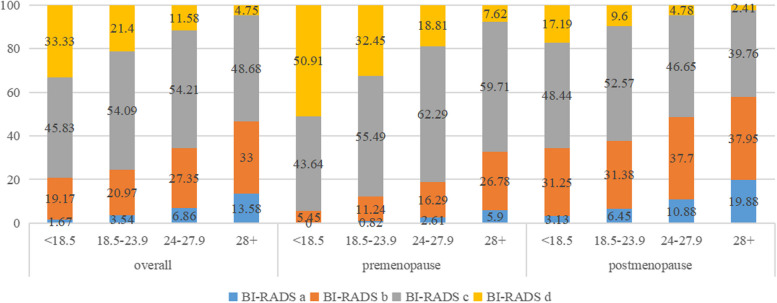
The distribution of mammographic density by age groups and menopausal status were shown in Fig. 1. Overall, the proportion of mammographic density rated as almost entirely fatty (BI-RADS a), scattered fibrograndular densities (BI-RADS b), heterogeneously dense (BI-RADS c), and extremely dense (BI-RADS d) were 5.54%, 24.30%, 53.66%, and 16.50%, respectively. The proportion of dense breasts (BI-RADS c-d) decreased from 83.85% for women aged 45–49 to 33.73% for women aged 60–64 (Ptrend < 0.01). MD also decreased with BMI both in premenopausal and postmenopausal women (Ptrend < 0.01) (Fig. 2). MD also varied by level of education, age at menarche, parity, age at first full term birth, breastfeeding, menopause status, family history of breast cancer, alcohol drinking (P Chi-square or ANOVA < 0.05, Table 1).
Fig. 1.
Distribution of MD by age groups and menopausal status
Fig. 2.
Distribution of MD by BMI stratified by menopausal status
Factors associated with MD
The association between selected demographic and reproductive factors with MD was examined using multivariable polytomous regression model and cumulative logistic regression model. We found than older age (ORtrend = 0.81, 95%CI: 0.79–0.81, per 2-year increase), higher BMI (ORtrend = 0.73, 95%CI: 0.71–0.75, per 2 kg/m2), more births (ORtrend = 0.47, 95%CI: 0.41–0.54, 3 + vs. 0–1), postmenopausal status (ORtrend = 0.42, 95%CI: 0.0.38–0.46) were associated with lower MD (Table 2). For parous women, longer duration of breastfeeding was found to be associated with higher MD when adjusting for study site age, BMI, and age of first full-term birth (ORtrend = 1.53, 95%CI: 1.27–1.85, 25 + months vs. no breastfeeding; ORtrend = 1.45, 95%CI: 1.20–1.75, 19–24 months vs. no breastfeeding), however, the association became non-significant when adjusting all covariates (Table 2). Associations between examined risk factors and MD were similar in premenopausal and postmenopausal women except for level of education and oral hormone drug usage. Higher education was only found to be associated with an increased proportion of dense breasts in postmenopausal women (ORtrend = 1.08, 95%CI: 1.02–1.15). Premenopausal women who ever used oral hormone drug were less likely to have dense breasts, though the difference was marginally significant (OR = 0.54, P = 0.045). We observed that BMI had stronger effect on reducing MD among premenopausal women than postmenopausal women (ORpre = 0.70, 95%CI: 0.66–0.74; ORpost = 0.78, 95%CI: 0.75–0.81). In postmenopausal women, we also found the proportion of dense breasts increased with age at menopause (ORtrend = 1.31, 95%CI: 1.21–1.43) (Table 3).
Table 2.
Associations between selected characteristics and mammographic density
| BI-RADS b vs. a | BI-RADS c vs. a | BI-RADS d vs. a | ORtrend (95%CI)b | Ptrendb | |
|---|---|---|---|---|---|
| OR (95%CI)a | OR (95%CI)a | OR (95%CI)a | |||
| age | |||||
| per 2 years | 0.88 (0.84–0.92) | 0.75 (0.71–0.78) | 0.62 (0.59–0.66) | 0.81 (0.79–0.82) | < 0.01 |
| education | |||||
| None-elementary | 1 | 1 | 1 | 1 | |
| Middle school | 1.07 (0.85–1.34) | 0.96 (0.77–1.20) | 0.99 (0.75–1.29) | 0.91 (0.83–1.01) | 0.08 |
| High school | 1.42 (1.07–1.89) | 1.27 (0.97–1.68) | 1.46 (1.05–2.03) | 1.00 (0.88–1.12) | 0.93 |
| College | 0.87 (0.61–1.24) | 1.00 (0.70–1.41) | 1.22 (0.83–1.80) | 1.14 (1.00–1.30) | < 0.05 |
| BMI | |||||
| per 2 kg/m2 | 0.78 (0.74–0.83) | 0.64 (0.60–0.68) | 0.46 (0.43–0.50) | 0.73 (0.71–0.75) | < 0.01 |
| age at menarche | |||||
| ≤ 13 | 1 | 1 | 1 | 1 | |
| 14–15 | 1.25 (1.00–1.56) | 1.21 (0.98–1.51) | 1.22 (0.96–1.55) | 1.04 (0.95–1.13) | 0.41 |
| 16 + | 1.16 (0.93–1.44) | 1.08 (0.87–1.34) | 1.07 (0.84–1.37) | 0.98 (0.90–1.08) | 0.70 |
| parity | |||||
| ≤ 1 | 1 | 1 | 1 | 1 | |
| 2 | 0.89 (0.69–1.16) | 0.61 (0.48–0.79) | 0.47 (0.36–0.61) | 0.65 (0.59–0.71) | < 0.01 |
| 3 + | 0.72 (0.53–0.98) | 0.37 (0.27–0.50) | 0.24 (0.17–0.35) | 0.47 (0.41–0.54) | < 0.01 |
| age at first full term birthc | |||||
| ≤ 20 | 1 | 1 | 1 | 1 | |
| 21–24 | 0.83 (0.51–1.34) | 0.81 (0.50–1.30) | 0.83 (0.49–1.42) | 0.96 (0.81–1.14) | 0.64 |
| 25–29 | 0.73 (0.44–1.19) | 0.76 (0.47–1.25) | 0.74 (0.43–1.29) | 0.94 (0.78–1.13) | 0.49 |
| 30 + | 0.38 (0.19–0.75) | 0.45 (0.23–0.88) | 0.45 (0.21–0.94) | 0.86 (0.66–1.11) | 0.25 |
| breastfeedingc | |||||
| No breastfeeding | 1 | 1 | 1 | ||
| 1–6 months | 1.16 (0.52–2.60) | 0.89 (0.40–1.97) | 1.10 (0.47–2.56) | 0.94 (0.74–1.18) | 0.59 |
| 7–12 months | 1.00 (0.56–1.77) | 0.82 (0.47–1.43) | 0.89 (0.49–1.61) | 0.95 (0.79–1.14) | 0.58 |
| 13–18 months | 0.99 (0.55–1.80) | 0.76 (0.42–1.35) | 0.76 (0.41–1.43) | 0.87 (0.71–1.06) | 0.16 |
| 19–24 months | 0.99 (0.56–1.75) | 0.78 (0.45–1.36) | 0.72 (0.39–1.31) | 0.83 (0.68–1.01) | 0.06 |
| 25 months + | 1.02 (0.58–1.80) | 0.91 (0.52–1.58) | 0.75 (0.41–1.37) | 0.89 (0.73–1.09) | 0.25 |
| menopausal status | |||||
| premenopausal | 1 | 1 | 1 | 1 | |
| postmenopausal | 0.60 (0.46–0.77) | 0.29 (0.23–0.38) | 0.16 (0.12–0.21) | 0.42 (0.38–0.46) | < 0.01 |
| age at menopaused | |||||
| ≤ 45 | 1 | 1 | 1 | 1 | |
| 46–50 | 1.52 (1.17–1.97) | 1.75 (1.35–2.27) | 1.70 (1.18–2.44) | 1.25 (1.09–1.44) | < 0.01 |
| 51 + | 1.78 (1.34–2.36) | 2.66 (2.00–3.53) | 3.46 (2.26–5.28) | 1.70 (1.45–1.98) | < 0.01 |
| family history of breast cancer | |||||
| no | 1 | 1 | 1 | 1 | |
| yes | 1.92 (0.75–4.93) | 1.77 (0.69–4.51) | 2.34 (0.88–6.22) | 1.18 (0.90–1.54) | 0.23 |
aPolytomous logistic regression was used to estimate ORs and 95%CIs comparing each BI-RADS categories of (b, c, d) to BI-RADS a. adjusted factors: study site, age, education, BMI, age at menarche, parity, menopausal status, family history of breast cancer
bCumulative logistic regression and Wald Chi square test was used to estimate ORtrend and Ptrend with MD modeled as an ordinal variable comparing higher to lower BI-RADS categories. Model was adjusted for the same factors as above
cParous women only. Model was additionally adjusted for age at first full term birth and breastfeeding
dpostmenopausal women only
Table 3.
Associations between selected characteristics and mammographic density by menopausal status
| Premenopausal (n = 6228) | Postmenopausal (n = 6269) | |||
|---|---|---|---|---|
| OR (95%CI)a, b | P | OR (95%CI)a, c | P | |
| age | ||||
| per 2 years | 0.83 (0.78–0.87) | < 0.01 | 0.81 (0.79–0.83) | < 0.01 |
| education | ||||
| None-elementary | 1 | 1 | ||
| Middle school | 0.99 (0.79–1.24) | 0.94 | 0.86 (0.74–1.00) | 0.05 |
| High school | 0.92 (0.71–1.20) | 0.54 | 0.98 (0.82–1.16) | 0.79 |
| College | 1.10 (0.83–1.44) | 0.52 | 1.25 (1.01–1.55) | 0.04 |
| ORtrend (95%CI); Ptrendd | 1.03 (0.95–1.12) | 0.47 | 1.08 (1.02–1.15) | < 0.05 |
| BMI | ||||
| per 2 kg/m2 | 0.70 (0.66–0.74) | < 0.01 | 0.78 (0.75–0.81) | < 0.01 |
| age at menarche | ||||
| ≤ 13 | 1 | 1 | ||
| 14–15 | 0.94 (0.79–1.11) | 0.48 | 1.06 (0.92–1.21) | 0.43 |
| 16 + | 0.91 (0.75–1.10) | 0.33 | 0.99 (0.86–1.14) | 0.90 |
| ORtrend (95%CI); Ptrendd | 0.99 (0.90–1.08) | 0.76 | 1.02 (0.96–1.10) | 0.51 |
| parity | ||||
| 0–1 | 1 | 1 | ||
| 2 | 0.62 (0.51–0.75) | < 0.01 | 0.64 (0.56–0.75) | < 0.01 |
| 3 + | 0.49 (0.36–0.65) | < 0.01 | 0.44 (0.36–0.54) | < 0.01 |
| ORtrend (95%CI); Ptrendd | 0.82 (0.72–0.93) | < 0.01 | 0.80 (0.73–0.87) | < 0.01 |
| age at first full term birthe | ||||
| ≤ 20 | 1 | 1 | ||
| 21–24 | 0.96 (0.69–1.32) | 0.79 | 0.97 (0.72–1.30) | 0.83 |
| 25–29 | 0.89 (0.63–1.25) | 0.49 | 1.08 (0.80–1.47) | 0.61 |
| 30 + | 1.25 (0.74–2.09) | 0.41 | 0.89 (0.57–1.38) | 0.60 |
| ORtrend (95%CI); Ptrendd | 1.01 (0.89–1.14) | 0.91 | 1.12 (1.02–1.23) | < 0.05 |
| breastfeedinge | ||||
| No breastfeeding | 1 | 1 | ||
| 1–6 months | 1.05 (0.63–1.75) | 0.85 | 0.69 (0.47–1.01) | 0.05 |
| 7–12 months | 0.94 (0.63–1.40) | 0.76 | 0.80 (0.58–1.08) | 0.15 |
| 13–18 months | 0.82 (0.54–1.27) | 0.38 | 0.73 (0.53–1.02) | 0.07 |
| 19–24 months | 0.88 (0.58–1.34) | 0.55 | 0.72 (0.52–1.00) | 0.05 |
| 25 months + | 0.94 (0.61–1.44) | 0.78 | 0.83 (0.60–1.15) | 0.26 |
| ORtrend (95%CI); Ptrendd | 1.03 (0.97–1.10) | 0.33 | 1.06 (1.01–1.11) | < 0.05 |
| age at menopause | ||||
| ≤ 45 | - | - | 1 | |
| 46–50 | - | - | 1.23 (1.06–1.44) | < 0.01 |
| 51 + | - | - | 1.70 (1.43–2.02) | < 0.01 |
| ORtrend (95%CI); Ptrendd | - | - | 1.31 (1.21–1.43) | < 0.01 |
| oral hormone drug use | ||||
| never | 1 | 1 | ||
| yes | 0.54 (0.30–0.99) | 0.045 | 0.85 (0.58–1.27) | 0.43 |
| family history of breast cancer | ||||
| no | 1 | 1 | ||
| yes | 1.05 (0.61–1.81) | 0.87 | 1.07 (0.68–1.68) | 0.78 |
aBinary logistic regression was used to estimate the ORs and 95%CIs comparing the dense (BI-RADS a-b) to non-dense breast group (BI-RADS c-d)
bAdjusted for study site, age, education, BMI, age at menarche, parity, family history of breast cancer
cAdjusted for study site, age, education, BMI, age at menarche, parity, family history of breast cancer, age at menopause
dORtrend and Ptrend were estimated and obtained by treating categorical variables as continuous variables
eParous women only
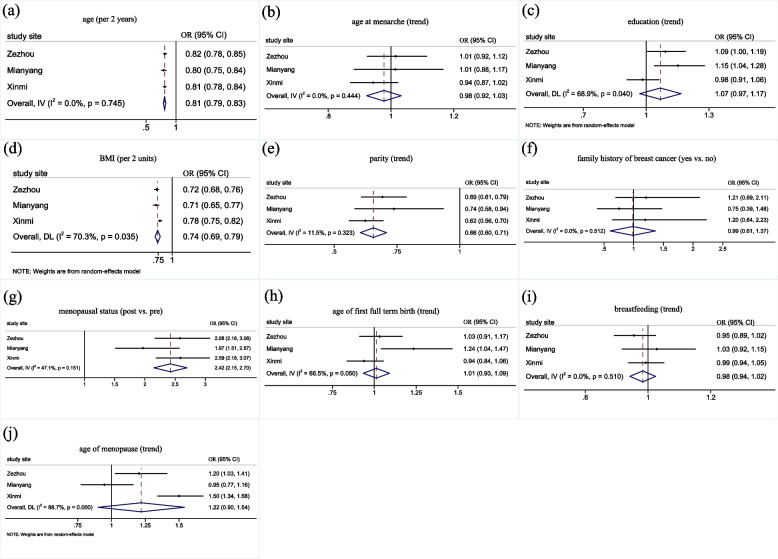
Associations of MD with examined risk factors were assessed by study sites using meta-analysis (Fig. 3). Among all risk factors, the significant heterogeneity for education, BMI, age at menopause (Pheterogeneity < 0.05) were found among three study sites. The demographic characteristics of participants from different study sites were available in Additional file Table S1. In sensitivity analysis, we analyzed the association of the same factors when combining MD into two categories (dense breasts vs. non-dense breasts) and observed the same associations (data are available in Additional file Table S2).
Fig. 3.
Associations between risk factors with MD by study site: a age, b age at menarche, c education, d BMI, e parity, f family history of breast cancer, g menopausal status, h age of first full term birth, i breastfeeding, j age of menopause. The study site specific ORtrend for having dense breasts (BI-RADS c-d) compared with non-dense breasts (BI-RADS a-b) were estimated by treating categorical variables as continuous variables. All models were adjusted for age (per 2 years), BMI (per 2 units), education (none-elementary, middle school, high school, college), age at menarche (≤ 13, 14–15, 16 +), parity (≤ 1, 2, 3 +), menopause status (pre vs. post), family history of breast cancer (yes vs. no). Age of first full term birth (≤ 20, 21–24, 25–29, 30 +) and breastfeeding (no breastfeeding, 1–6 months, 7–12 months, 13–18 months, 19–24 months, 25 months +) was estimated only for parous women and adjusted by each other. The age of menopause (≤ 45, 46–50, 51 +) was estimated only for postmenopausal women
Discussion
In this study, we described the distribution of MD among Chinese women with average risk for breast cancer and assessed the associations between known breast cancer risk factors with MD in this population. To the best of our knowledge, this is the first large study conducted among women with general risk of breast cancer in rural China. The associations we found in this study were consistent with that reported in previous studies, and were similar in pre-menopausal and postmenopausal women.
Previous studies comparing the MD between Caucasian and Asian women found that Asian women had higher percent density and dense volume, especially in premenopausal woman [17, 19]. In our study, approximately 60% of the women aged 45–64 years were classified as heterogeneously dense or extremely dense breasts, this number was even higher in women aged 45–49 years. Our result was higher than Sung et al. [21] (55%, age: 45–69 years) and Dai et al. [22] (49%, age: 45–64 years) had reported which conducted among urban women. This could be explained by differences in life and diet styles [14] between those living in urban and rural areas. Higher MD does not necessarily lead to higher incidence of breast cancer due to the complexity etiology of breast cancer including age, family history of breast cancer [2], age of menarche, age of first full-term pregnancy, HRT use [3], genetic mutation [4], BMI [5], alcohol consumption [6], and physical inactivity [7]. The older demographics, fewer childbirth, and older age at first full-term pregnancy of the urban women may contribute to the higher incidence of breast cancer in this population.
The high proportion of dense breasts in rural women highlighted that accessible and accurate breast cancer screening modalities other than mammography are needed for those women living in rural areas since they are more likely to have dense breasts. Previous studies found an increased sensitivity of adding digital breast tomosynthesis (DBT), hand-held ultrasound, automated breast ultrasound, and magnetic resonance imaging (MRI) to MG in women with either higher risk of breast cancer or dense breasts [24]. To maximize benefits using limited resources, which is the problems of rural areas, adopting ultrasound-based breast cancer screening in rural areas might be a feasible approach. On the other hand, developing a risk-stratified screening program incorporating MD with other common risk factors could improve resource allocation [25].
Age and BMI, as expected, were inversely associated with MD. Previous studies found that MD decreased with age in healthy Taiwanese women and Western women [26]. Breast lobules involution that characterized by the reduction in number and size of acini in lobules is positively associated with increasing aging. Furthermore, breast glandular elements and collagen are progressively replaced by fatty tissue as women aging [27]. These two mechanisms might explain the inverse association between age and MD. However, lobular involution and fatty areas were found to be association with reduced risk of breast cancer [27–29]. Cumulative exposure to MD, which reflects the cumulative exposure to factors that promote the carcinogenesis, may explain the paradox observation that with increasing age, MD decreases and breast lobular regresses, whereas breast cancer risk increases [8, 10, 30, 31]. BMI is positively associated with non-dense area of the mammogram, thus, is negatively associated with MD [32]. BMI and MD are positively associated with increased breast cancer risk in postmenopausal women, but are inversely associated with each other. Norman F. Body et al. noted that BMI and MD could independently perform through different pathways in breast cancer development [33]. In our study, we found BMI had stronger effect on reducing MD in premenopausal women. Norman F. Boyd et.al. also found a stronger inverse association between BMI and MD in both premenopausal breast cancer patients and non-patients than postmenopausal women.
The association between parity and MD was well-established through previous studies. Parous status and higher number of births were associated with decreased MD in Chinese and other populations [21, 22, 34]. Menopausal status was also proven to be independently associated with MD from prior studies [21, 22, 34, 35]. Our findings showed an inverse association between number of births, menopausal status and MD after adjustment for age and other cofounders, which were in consistent with previous evidences. In our study, longer than 19 months of breastfeeding was found to be significantly associated with decreased MD when adjusting for study site, age, BMI, and age of first full-term birth though the association became nonsignificant when additionally adjusted for education, age at menarche, menopausal status and family history of breast cancer. Advanced age at first full term birth and limited breastfeeding were associated with increased risk of breast cancer both in China and other countries [3, 36]. However, their associations with MD are less consistent and more investigations are warranted [34, 37–39].
Previous studies evaluating relationships between age at menarche and MD produced inconsistent results. Most reported finding no evidence of an association between age at menarche and MD, some reported a positive association between them, while very few reported an inverse association [34]. Sarah V. Ward et al. first demonstrated associations between age at menarche and MD across 22 different countries. They found a small positive association between later age at menarche and both increasing per cent and absolute dense area [40]. Both Dai, Hongji et al. [22] and Hyuna Sung et al. [21] found no evidence of an association between age at menarche and MD in Chinese populations, which is consistent with our result. Amita G. Ghadge et al. [41] reported that pubertal mammary gland development may affect adult MD and cancer risk through complexed mechanisms involving endocrine regulators, paracrine regulator, genetic and epigenetic determinants. Therefore, the paradox epidemiological findings may be attributed to the complex mechanisms of mammary gland development.
Estrogen and progesterone, which are the main components of hormone replacement therapy, are important in the development of breast cancer, especially hormone receptor-related tumors [42]. Valerie A.M. al. reported that MD increased by 2.4% with the use of hormone therapy [43]. Celia Byrne et al. investigated the effect of estrogen and progestin therapy on MD by randomly assigning participants to estrogen plus progestin or placebo therapy, they found that mean MD increased by 9.73% after at least one year of using estrogen and progestin therapy and this change was associated with increased breast cancer risk [44], Norman F. Boyd et al. reported that the association between MD change and hormone therapy in postmenopausal women were greater in women who later developed breast cancer than those who didn’t [45]. However, another study assessed the association between blood levels of estradiol, progesterone, prolactin, sex hormone binding globulin and MD change found that total estradiol and progesterone levels were unrelated to MD in both pre- and post- menopausal women [46]. In our study, a small number of women who ever used hormone drug. We found only marginal evidence that oral hormone drug use in premenopausal women were less likely to have dense breasts. In our study, in addition to progesterone and estrogen, glucocorticoids and thyroid hormones were also recorded as hormone drug use, which accounted for 9.5% (8/84) of premenopausal women whoever took hormone drugs. This might confound the association between sex hormone and MD. Apart from epidemiological evidences, there is a need for more researches on the underlying mechanisms between hormone factors and MD, and the genetic and environmental factors that influence the levels of these factors.
The main strength of this study was the large number of understudied Chinese women that came from areas with limited medical resources. We also evaluated associations for pre- and pos- tmenopausal women separately. Limitations of this study include the visual and qualitative assessment of MD, which is user dependent. Quantitative assessments convey additional information on breast density. However, Inger T Gram et al. reported that both the qualitative and quantitative methods capture the same overall associations with risk factors for breast cancer in postmenopausal women [47]. Computer-assisted methods are highly consistent and producible, but have limited application because they require either digital mammography or trained observer, whereas BI-RADS score is widely used in routine breast cancer screening activities. Future studies using computer-assisted methods could provide more accurate estimation of MD. All information about potential breast cancer risk factors were self-reported, thus the accuracy may be suboptimal. Questionnaires were checked for any logical error or missing value by project team members before each participant leaving the study sites, minimized the error rates.
In conclusion, most of the Chinese women with general risk for breast cancer have dense breasts. We found MD was associated with some established breast cancer risk factors. This finding may help to understand the etiology of breast cancer and have implications for breast cancer prevention in low resource areas where mammographic screening is not practicable to general population.
Supplementary Information
Additional file 1: Table S1 Demographic characteristics and breast cancer risk factors of participants and Table S2 Associations between selected characteristics and mammographic density are included in the additional file. Table S1. Described the demographic characteristics and breast cancer risk factors of participants from each study sites. Table S2. Showed results of association of the same factors when combining MD into two categories (dense breasts vs. non-dense breasts).
Acknowledgements
We thank staffs from the following hospitals for their valuable contributions: Zezhou maternal & child health care hospital, Xinmi maternal & child health care hospital, and Mianyang maternal & child health care hospital.
Abbreviations
- MD
Mammographic density
- BI-RADS
Breast imaging reporting and data system
- OR
Odds ratio
- CI
Confidence interval
- BMI
Body mass index
- ICF
Informed consent form
- CC
Craniocaudal
- MLO
Mediolateral
- ANOVA
One-way analysis of variance
Authors’ contributions
All co-authors contributed significantly to this study: HY contributed to the data analysis and drafted the manuscript. WR, PX, MJ contributed to the data analysis. ZL, SZ, and HC contributed to data collection. YQ contributed to the study design. HY, WR, PX, MJ and YQ interpreted the results. All authors read and approved the final manuscript submitted for publication.
Funding
This design of the study and data collection were founded by Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CIFMS 2017-I2M-B&R-03). Data analysis and interpretation were founded by Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CIFMS 2017-I2M-B&R-03) and China Postdoctoral Science Foundation (2021T140068).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the institutional review board of the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical Sciences (IRB approval number 19/109–1893). And the ethics committees of each participating hospitals were approved this study. All methods were performed in accordance with the ethical standards of the Institutional Review of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical Sciences, all participant hospitals, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all participants.
Consent for publication
Consent for publication was obtained from all participants.
Competing interests
Author MJ is the 2018 GE Healthcare Call for proposal Awardee. Other authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Eccles DM, Pichert G. Familial non-BRCA1/BRCA2-associated breast cancer. Lancet Oncol. 2005;6(9):705–711. doi: 10.1016/S1470-2045(05)70318-1. [DOI] [PubMed] [Google Scholar]
- 3.Lambertini M, Santoro L, Del Mastro L, Nguyen B, Livraghi L, Ugolini D, Peccatori FA, Azim HA., Jr Reproductive behaviors and risk of developing breast cancer according to tumor subtype: a systematic review and meta-analysis of epidemiological studies. Cancer Treat Rev. 2016;49:65–76. doi: 10.1016/j.ctrv.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Easton DF, Pharoah PD, Antoniou AC, Tischkowitz M, Tavtigian SV, Nathanson KL, Devilee P, Meindl A, Couch FJ, Southey M, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372(23):2243–2257. doi: 10.1056/NEJMsr1501341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J, Yang DL, Chen ZZ, Gou BF. Associations of body mass index with cancer incidence among populations, genders, and menopausal status: a systematic review and meta-analysis. Cancer Epidemiol. 2016;42:1–8. doi: 10.1016/j.canep.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Seitz HK, Pelucchi C, Bagnardi V, La Vecchia C. Epidemiology and pathophysiology of alcohol and breast cancer: Update 2012. Alcohol Alcohol. 2012;47(3):204–212. doi: 10.1093/alcalc/ags011. [DOI] [PubMed] [Google Scholar]
- 7.Bellocco R, Marrone G, Ye W, Nyrén O, Adami HO, Mariosa D, Lagerros YT. A prospective cohort study of the combined effects of physical activity and anthropometric measures on the risk of post-menopausal breast cancer. Eur J Epidemiol. 2016;31(4):395–404. doi: 10.1007/s10654-015-0064-z. [DOI] [PubMed] [Google Scholar]
- 8.Boyd NF, Martin LJ, Bronskill M, Yaffe MJ, Duric N, Minkin S. Breast tissue composition and susceptibility to breast cancer. J Natl Cancer Inst. 2010;102(16):1224–1237. doi: 10.1093/jnci/djq239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCormack VA, dos Santos SI. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1159–1169. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 10.Boyd NF, Rommens JM, Vogt K, Lee V, Hopper JL, Yaffe MJ, Paterson AD. Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol. 2005;6(10):798–808. doi: 10.1016/S1470-2045(05)70390-9. [DOI] [PubMed] [Google Scholar]
- 11.Krishnan K, Baglietto L, Stone J, Simpson JA, Severi G, Evans CF, MacInnis RJ, Giles GG, Apicella C, Hopper JL. Longitudinal study of mammographic density measures that predict breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2017;26(4):651–660. doi: 10.1158/1055-9965.EPI-16-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziembicki S, Zhu J, Tse E, Martin LJ, Minkin S, Boyd NF. The association between alcohol consumption and breast density: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2017;26(2):170–178. doi: 10.1158/1055-9965.EPI-16-0522. [DOI] [PubMed] [Google Scholar]
- 13.Yochum L, Tamimi RM, Hankinson SE. Birthweight, early life body size and adult mammographic density: a review of epidemiologic studies. Cancer Causes Control. 2014;25(10):1247–1259. doi: 10.1007/s10552-014-0432-0. [DOI] [PubMed] [Google Scholar]
- 14.Masala G, Ambrogetti D, Assedi M, Giorgi D, Del Turco MR, Palli D. Dietary and lifestyle determinants of mammographic breast density. A longitudinal study in a Mediterranean population. Int J Cancer. 2006;118(7):1782–1789. doi: 10.1002/ijc.21558. [DOI] [PubMed] [Google Scholar]
- 15.Vachon CM, Kuni CC, Anderson K, Anderson VE, Sellers TA. Association of mammographically defined percent breast density with epidemiologic risk factors for breast cancer (United States) Cancer Causes Control. 2000;11(7):653–662. doi: 10.1023/A:1008926607428. [DOI] [PubMed] [Google Scholar]
- 16.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, Jong RA, Hislop G, Chiarelli A, Minkin S, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 17.Rajaram N, Mariapun S, Eriksson M, Tapia J, Kwan PY, Ho WK, Harun F, Rahmat K, Czene K, Taib NA, et al. Differences in mammographic density between Asian and Caucasian populations: a comparative analysis. Breast Cancer Res Treat. 2017;161(2):353–362. doi: 10.1007/s10549-016-4054-y. [DOI] [PubMed] [Google Scholar]
- 18.Chen Z, Wu AH, Gauderman WJ, Bernstein L, Ma H, Pike MC, Ursin G. Does mammographic density reflect ethnic differences in breast cancer incidence rates? Am J Epidemiol. 2004;159(2):140–147. doi: 10.1093/aje/kwh028. [DOI] [PubMed] [Google Scholar]
- 19.Maskarinec G, Meng L, Ursin G. Ethnic differences in mammographic densities. Int J Epidemiol. 2001;30(5):959–965. doi: 10.1093/ije/30.5.959. [DOI] [PubMed] [Google Scholar]
- 20.Harvey JA, Bovbjerg VE. Quantitative assessment of mammographic breast density: relationship with breast cancer risk. Radiology. 2004;230(1):29–41. doi: 10.1148/radiol.2301020870. [DOI] [PubMed] [Google Scholar]
- 21.Sung H, Ren J, Li J, Pfeiffer RM, Wang Y, Guida JL, Fang Y, Shi J, Zhang K, Li N, et al. Breast cancer risk factors and mammographic density among high-risk women in urban China. NPJ Breast Cancer. 2018;4:3. doi: 10.1038/s41523-018-0055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai H, Yan Y, Wang P, Liu P, Cao Y, Xiong L, Luo Y, Pan T, Ma X, Wang J, et al. Distribution of mammographic density and its influential factors among Chinese women. Int J Epidemiol. 2014;43(4):1240–1251. doi: 10.1093/ije/dyu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cancer Atlas in China. National cancer center. Beijing: SinoMaps Press; 2018.
- 24.Vourtsis A, Berg WA. Breast density implications and supplemental screening. Eur Radiol. 2019;29(4):1762–1777. doi: 10.1007/s00330-018-5668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gail MH, Pfeiffer RM. Breast cancer risk model requirements for counseling, prevention, and screening. J Natl Cancer Inst. 2018;110(9):994–1002. doi: 10.1093/jnci/djy013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shia WC, Wu HK, Huang YL, Lin LS, Chen DR. Mammographic density distribution of healthy Taiwanese women and its naturally decreasing trend with age. Sci Rep. 2018;8(1):14937. doi: 10.1038/s41598-018-32923-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milanese TR, Hartmann LC, Sellers TA, Frost MH, Vierkant RA, Maloney SD, Pankratz VS, Degnim AC, Vachon CM, Reynolds CA, et al. Age-related lobular involution and risk of breast cancer. J Natl Cancer Inst. 2006;98(22):1600–1607. doi: 10.1093/jnci/djj439. [DOI] [PubMed] [Google Scholar]
- 28.Pettersson A, Hankinson SE, Willett WC, Lagiou P, Trichopoulos D, Tamimi RM. Nondense mammographic area and risk of breast cancer. Breast Cancer Res. 2011;13(5):R100. doi: 10.1186/bcr3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baglietto L, Krishnan K, Stone J, Apicella C, Southey MC, English DR, Hopper JL, Giles GG. Associations of mammographic dense and nondense areas and body mass index with risk of breast cancer. Am J Epidemiol. 2014;179(4):475–483. doi: 10.1093/aje/kwt260. [DOI] [PubMed] [Google Scholar]
- 30.Boyd N, Berman H, Zhu J, Martin LJ, Yaffe MJ, Chavez S, Stanisz G, Hislop G, Chiarelli AM, Minkin S, et al. The origins of breast cancer associated with mammographic density: a testable biological hypothesis. Breast Cancer Res. 2018;20(1):17. doi: 10.1186/s13058-018-0941-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flugelman AA, Burton A, Keinan-Boker L, Stein N, Kutner D, Shemesh L, Boyd N. Correlation between cumulative mammographic density and age-specific incidence of breast cancer: a biethnic study in Israel. Int J Cancer. 2022;150(12):1968–1977. doi: 10.1002/ijc.33957. [DOI] [PubMed] [Google Scholar]
- 32.Woolcott CG, Cook LS, Courneya KS, Boyd NF, Yaffe MJ, Terry T, Brant R, McTiernan A, Bryant HE, Magliocco AM, et al. Associations of overall and abdominal adiposity with area and volumetric mammographic measures among postmenopausal women. Int J Cancer. 2011;129(2):440–448. doi: 10.1002/ijc.25676. [DOI] [PubMed] [Google Scholar]
- 33.Boyd NF, Martin LJ, Sun L, Guo H, Chiarelli A, Hislop G, Yaffe M, Minkin S. Body size, mammographic density, and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2086–2092. doi: 10.1158/1055-9965.EPI-06-0345. [DOI] [PubMed] [Google Scholar]
- 34.Alexeeff SE, Odo NU, McBride R, McGuire V, Achacoso N, Rothstein JH, Lipson JA, Liang RY, Acton L, Yaffe MJ, et al. Reproductive factors and mammographic density: associations among 24,840 women and comparison of studies using digitized film-screen mammography and full-field digital mammography. Am J Epidemiol. 2019;188(6):1144–1154. doi: 10.1093/aje/kwz033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyd N, Martin L, Stone J, Little L, Minkin S, Yaffe M. A longitudinal study of the effects of menopause on mammographic features. Cancer Epidemiol Biomarkers Prev. 2002;11(10 Pt 1):1048–1053. [PubMed] [Google Scholar]
- 36.Gao YT, Shu XO, Dai Q, Potter JD, Brinton LA, Wen W, Sellers TA, Kushi LH, Ruan Z, Bostick RM, et al. Association of menstrual and reproductive factors with breast cancer risk: results from the Shanghai Breast Cancer Study. Int J Cancer. 2000;87(2):295–300. doi: 10.1002/1097-0215(20000715)87:2<295::AID-IJC23>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 37.Yang L, Jacobsen KH. A systematic review of the association between breastfeeding and breast cancer. J Womens Health (Larchmt) 2008;17(10):1635–1645. doi: 10.1089/jwh.2008.0917. [DOI] [PubMed] [Google Scholar]
- 38.Shang MY, Guo S, Cui MK, Zheng YF, Liao ZX, Zhang Q, Piao HZ. Influential factors and prediction model of mammographic density among Chinese women. Medicine (Baltimore) 2021;100(28):e26586. doi: 10.1097/MD.0000000000026586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butler LM, Gold EB, Greendale GA, Crandall CJ, Modugno F, Oestreicher N, Quesenberry CP, Jr, Habel LA. Menstrual and reproductive factors in relation to mammographic density: the Study of Women's Health Across the Nation (SWAN) Breast Cancer Res Treat. 2008;112(1):165–174. doi: 10.1007/s10549-007-9840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ward SV, Burton A, Tamimi RM, Pereira A, Garmendia ML, Pollan M, Boyd N, Dos-Santos-Silva I, Maskarinec G, Perez-Gomez B, et al. The association of age at menarche and adult height with mammographic density in the International Consortium of Mammographic Density. Breast Cancer Res. 2022;24(1):49. doi: 10.1186/s13058-022-01545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghadge AG, Dasari P, Stone J, Thompson EW, Robker RL, Ingman WV. Pubertal mammary gland development is a key determinant of adult mammographic density. Semin Cell Dev Biol. 2021;114:143–158. doi: 10.1016/j.semcdb.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 42.Bao PP, Shu XO, Gao YT, Zheng Y, Cai H, Deming SL, Ruan ZX, Su Y, Gu K, Lu W, et al. Association of hormone-related characteristics and breast cancer risk by estrogen receptor/progesterone receptor status in the shanghai breast cancer study. Am J Epidemiol. 2011;174(6):661–671. doi: 10.1093/aje/kwr145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCormack VA, Perry NM, Vinnicombe SJ, Dos Santos SI. Changes and tracking of mammographic density in relation to Pike's model of breast tissue aging: a UK longitudinal study. Int J Cancer. 2010;127(2):452–461. doi: 10.1002/ijc.25053. [DOI] [PubMed] [Google Scholar]
- 44.Byrne C, Ursin G, Martin CF, Peck JD, Cole EB, Zeng D, Kim E, Yaffe MD, Boyd NF, Heiss G, et al. Mammographic density change with estrogen and progestin therapy and breast cancer risk. J Natl Cancer Inst. 2017;109(9):djx001. doi: 10.1093/jnci/djx001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boyd NF, Melnichouk O, Martin LJ, Hislop G, Chiarelli AM, Yaffe MJ, Minkin S. Mammographic density, response to hormones, and breast cancer risk. J Clin Oncol. 2011;29(22):2985–2992. doi: 10.1200/JCO.2010.33.7964. [DOI] [PubMed] [Google Scholar]
- 46.Boyd NF, Stone J, Martin LJ, Jong R, Fishell E, Yaffe M, Hammond G, Minkin S. The association of breast mitogens with mammographic densities. Br J Cancer. 2002;87(8):876–882. doi: 10.1038/sj.bjc.6600537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gram IT, Bremnes Y, Ursin G, Maskarinec G, Bjurstam N, Lund E. Percentage density, Wolfe's and Tabár's mammographic patterns: agreement and association with risk factors for breast cancer. Breast Cancer Res. 2005;7(5):R854–861. doi: 10.1186/bcr1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1 Demographic characteristics and breast cancer risk factors of participants and Table S2 Associations between selected characteristics and mammographic density are included in the additional file. Table S1. Described the demographic characteristics and breast cancer risk factors of participants from each study sites. Table S2. Showed results of association of the same factors when combining MD into two categories (dense breasts vs. non-dense breasts).
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.