Abstract
Background
To determine the safety and efficacy of biological agents used in the treatment of systemic lupus erythematosus (SLE) in adults.
Methods
Systematic review and meta-analysis following PRISMA guidelines.
Data sources
MEDLINE (through Pubmed), EMBASE, Cochrane library, Clinicaltrials.gov, Australianclinicaltrials.gov.au, ANZCTR.org.au and WHO International Clinical Trials Registry Platform for studies published from 20 May 2021 and 15 years prior. A grey literature search was performed and completed on 31 May 2021.
Study criteria
Phase II, III or quasi randomised controlled trials, studies with only cerebral or cutaneous lupus were excluded. Data extraction: Two authors independently screened studies for eligibility, extracted, reviewed data for accuracy, and used the Cochrane tool to assess risk of bias.
Results
Forty-four studies were identified, consisting of 15 groups of drugs and 25 different biological agents, totalling 16,889 patients. The main outcomes assessed included Systemic Lupus Erythematosus Responder Index (SRI), BILAG-Based Composite Lupus Assessment (BICLA) and combined combined/partial renal remission (CRR/PRR).
Four groups of biologics were found to improve outcomes. Anti-interferons: Anifrolumab increased BICLA response and SRI 5 to 8, decreased prednisone dosages, with increased herpes zoster infections, but fewer serious adverse events. Sifalimumab improved SRI but also increased herpes zoster infections. Anti BAFF/BLyS and/or APRIL: Belimumab consistently improved SRI 4, decreased prednisone dosages, increased combined CRR/PRR, and had no adverse safety outcomes. Tabalumab increased SRI 5 at 52 weeks with no steroid sparing effect but was associated with increased infusion related adverse events. Telitacicept improved SRI 4 at 52 weeks, with no increased adverse events, though data was rather sparse. Anti CD-20 monoclonal antibody, Obinutuzumab increased combined CRR/PRR at 1 and 2 years. Anti IL12/23 monoclonal antibody, Ustekinumab, increased SRI 4 to 6, but not BICLA at 24 weeks, with no concerning safety outcomes.
Conclusion
Multiple biologic agents are shown in high quality studies to have a significant therapeutic impact on outcomes in SLE.
Supplementary Information
The online version contains supplementary material available at 10.1186/s41927-023-00358-3.
Keywords: Systemic lupus erythematosus, Renal lupus, Biologics
Background
Systemic Lupus Erythematosus (SLE) is an autoimmune disease of unknown aetiology with multiple manifestations including musculoskeletal, renal, haematological, serosal, and neuropsychiatric involvement. Treatment for SLE to date is centred on immunosuppression and anti-inflammatory therapy, depending on the degree of end organ involvement. Pregnant and non-pregnant lupus patients benefit from the use of hydroxychloroquine (HCQ), with reductions in lupus flares, end organ damage, loss of bone mass, thrombosis, cumulative steroid usage and increased long term survival [1]. Non-steroidal anti-inflammatory drugs (NSAIDs) may be used to manage milder manifestations such as musculoskeletal or mucocutaneous manifestations. Chronic glucocorticoid (GC) therapy is associated with cumulative dose toxicity. However, given its efficacy it is often used in lower doses as a component of maintenance therapy, or in higher doses for the treatment of disease flares depending on the severity of end organ involvement. Other immunosuppressants used include mycophenolate mofetil (MMF), cyclophosphamide (CYC) and calcineurin inhibitors such as tacrolimus (TAC) and azathioprine (AZA). “Standard of care” therapy is typically defined in clinical trials to include these agents.
Multiple biological agents have recently emerged as potential novel treatments for SLE. In this review we aim to summarise the available data from randomised controlled trials for the efficacy of biologics in SLE, and to highlight potential therapies which require further data.
Methods
All phase II, and III clinical trials or randomised control trials or quasi randomised controlled trial enrolling adult patients with SLE according to standard criteria, examining biologic agent/s compared to placebo, other immunosuppressive drug/s or standard of care were examined.
Outcome measures included change in validated disease activity indices such as SLEDAI, SELENA-SLEDAI, SLEDAI-2 K, BILAG, BILAG-2004, SLICC/ACR score. Adverse events and death were also recorded.
Search methods are documented in the online supplement. Two authors independently examined all studies and extracted data. Dichotomous outcome results were expressed as risk ratios (RR) with 95% confidence intervals (CI), with data pooled using random effects models. Data with continuous outcomes were not measured in this review. The Cochrane risk of bias tool was used by both authors to independently assess the quality of included studies.
Results
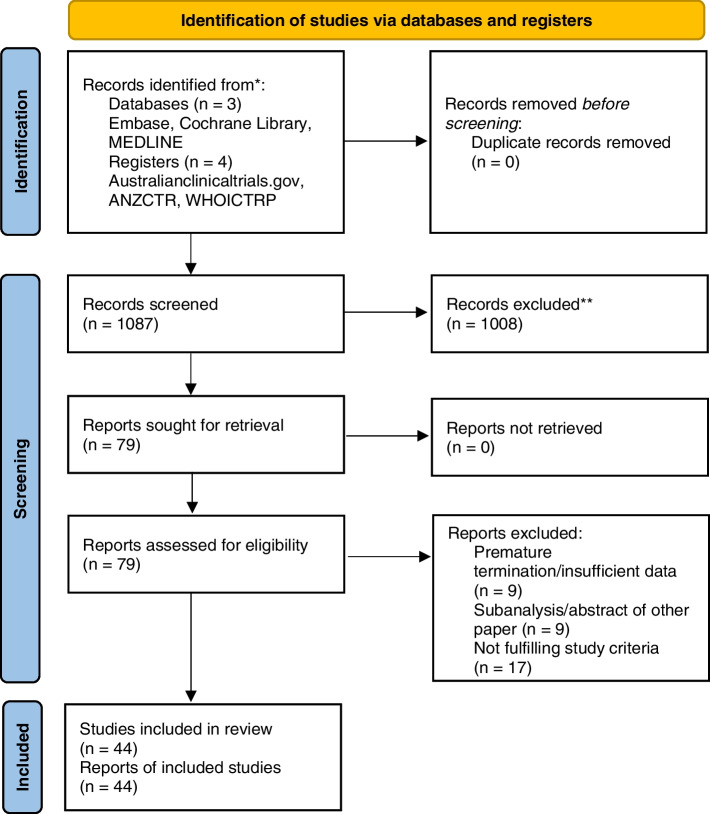
One thousand eighty-seven studies were identified. Seventy-nine studies were further assessed. Forty-four studies were included with 16,889 patients, 15 distinct drug groups and 25 biological agents. Characteristics of the studies including patient characteristics and study protocols are summarised in the supplementary information. PRISMA flow diagram is shown below (Fig. 1), and the PRISMA checklist is included in the supplementary information.
Fig. 1.
PRISMA flow
CD80/86 inhibition
CD80/86 is expressed by antigen presenting cells such as plasmacytoid dendritic cells and B cells. CD80/86 ligate CD28, a co-stimulatory receptor expressed on T cells. CD28 stimulation in conjunction with T cell receptor engagement prolongs and increases T cell differentiation and production of IL2, with subsequent B cell proliferation and differentiation into antibody producing plasma cells.
Abatacept
Abatacept is a fusion protein composed of a CTLA-4 molecule linked to the Fc portion of IgG1. This selectively and competitively antagonises CD80 and CD86 receptors on an antigen presenting cell, limiting CD28 mediated T cell activation.
Four studies [2–5] included 1017 patients. Three of the studies recruited patients with lupus nephritis whereas Merrill 2010 [2] excluded patients with renal involvement.
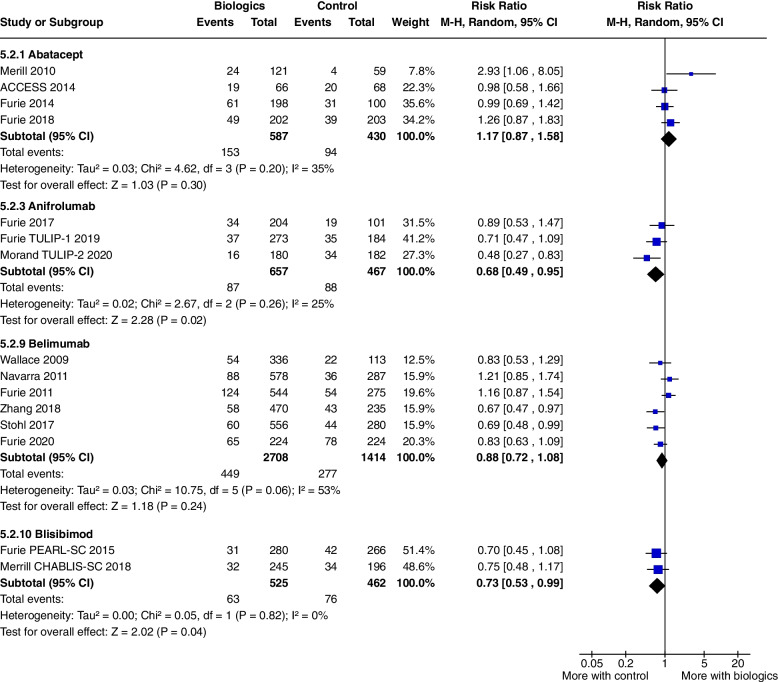
No outcomes achieved significance. Serious adverse events were significantly raised only in Merrill 2010 (RR 2.93, CI 1.06 to 8.05, P = 0.04) but not in the pooled data of all the Abatacept studies (RR 1.17, CI 0.87 to 1.58, P = 0.30) (Fig. 2).
Fig. 2.
Serious adverse events
Anti-interferon monoclonal antibody
T1 IFN is considered the canonical SLE cytokine impairing immune tolerance through multiple mechanisms. Three anti-interferon monoclonal antibodies have been assessed in this review. Anifrolumab which binds to both IFN-α/β receptors, Rontalizumab and Sifalimumab which selectively bind to IFN-α receptors.
Anifrolumab
Anifrolumab is a fully human, IgG1k monoclonal antibody that binds to IFN-α/β receptor and prevents signalling by all types of I IFNs.
Three studies addressed the use of Anifrolumab in SLE: Furie 2017 [6], Furie 2019 [7] and Morand 2020 [8] and included 1124 patients. The main outcomes studied were SRI and BICLA response.
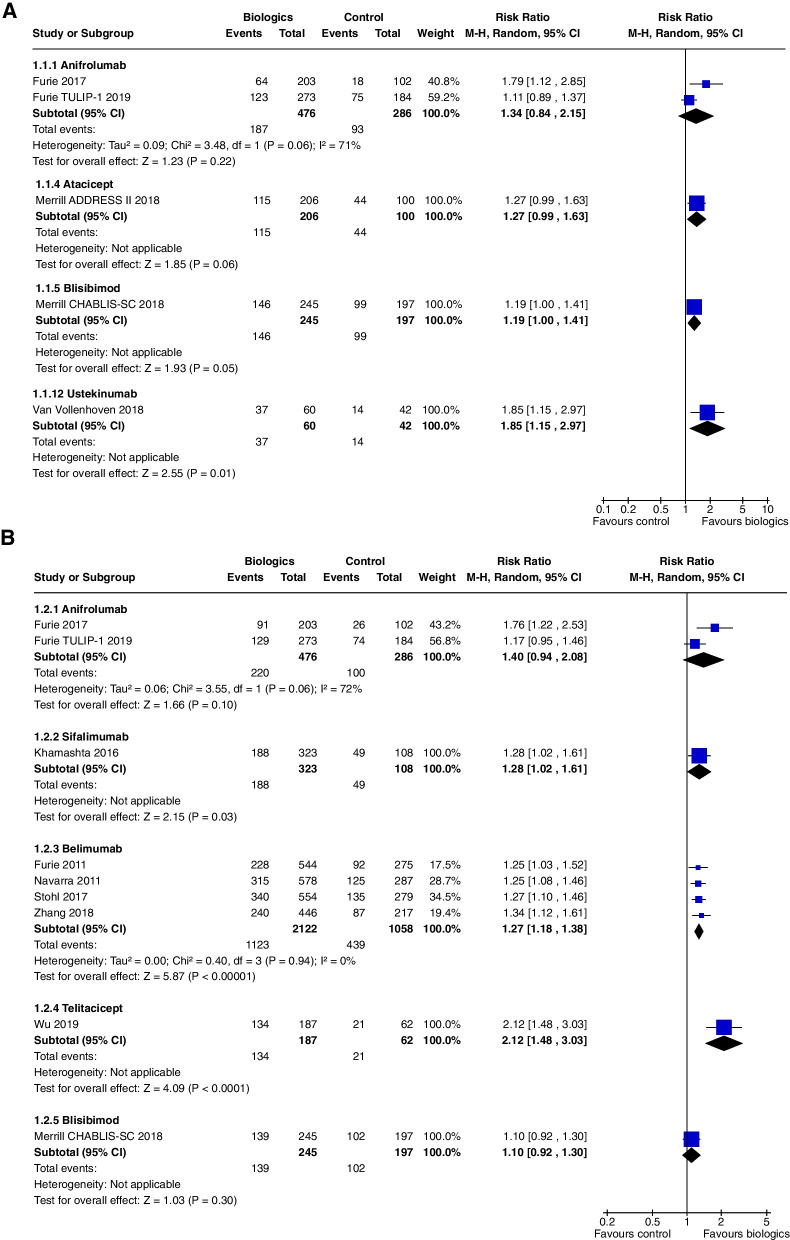
SRI 4 at 24 weeks did not achieve statistical significance (RR 1.34, CI 0.84 to 2.15, P = 0.22, 2 studies [6, 7]), though results from Furie 2017 alone were significant (RR 1.79, CI 1.12 to 2.85, P = 0.01) (Fig. 3a).
Fig. 3.
A SRI 4 at 24 weeks, B SRI 4 at 52 weeks
SRI 4 at 52 weeks did not achieve statistical significance (RR 1.40, CI 0.94 to 2.08, P = 0.10, 2 studies [6, 7]), though results from Furie 2017 alone were significant (RR 1.76, CI 1.22 to 2.53, P = 0.002) (Fig. 3b).
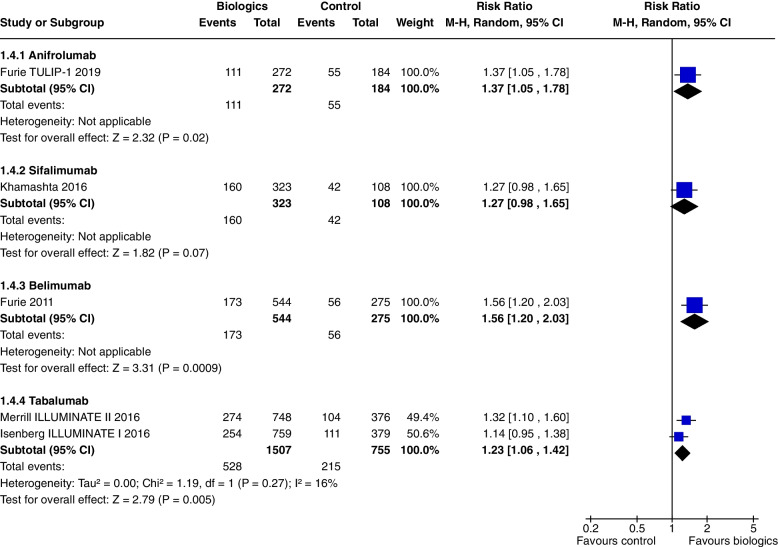
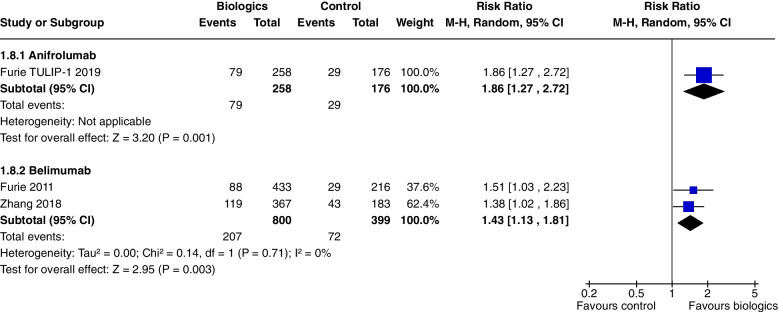
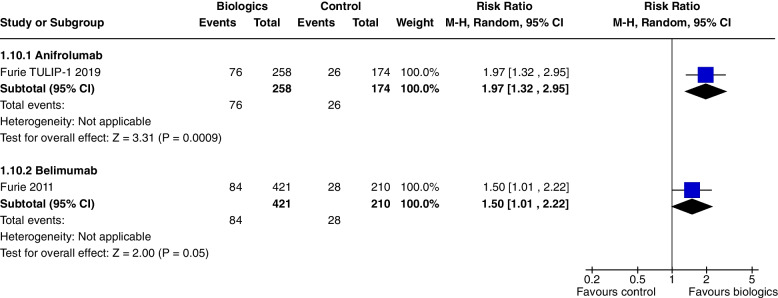
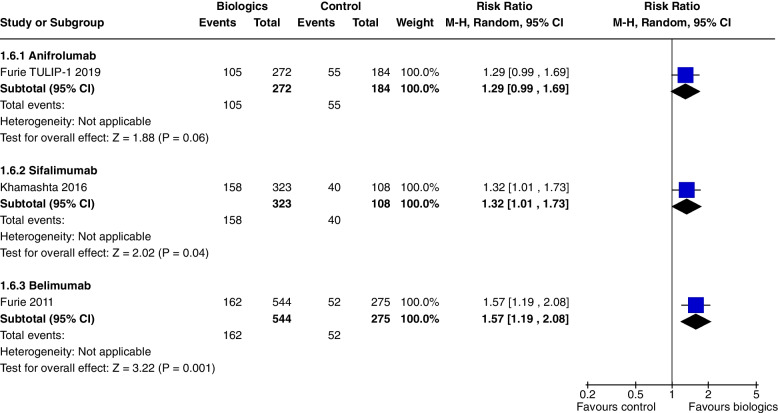
In a single study [7] at 52 weeks, Anifrolumab significantly increased SRI 5 (RR 1.37, CI 1.05 to 1.78, P = 0.02) (Fig. 4), SRI 7 (RR 1.86, CI 1.27 to 2.72, P = 0.001) (Fig. 5), and SRI 8 (RR 1.97, CI 1.32 to 2.95, P = 0.0009) (Fig. 6), but not SRI 6 (RR 1.29, CI 0.99 to 1.69, P = 0.06), though the results trended towards significance (Fig. 7).
Fig. 4.
SRI 5 at 52 weeks
Fig. 5.
SRI 7 at 52 weeks
Fig. 6.
SRI 8 at 52 weeks
Fig. 7.
SRI 6 at 52 weeks
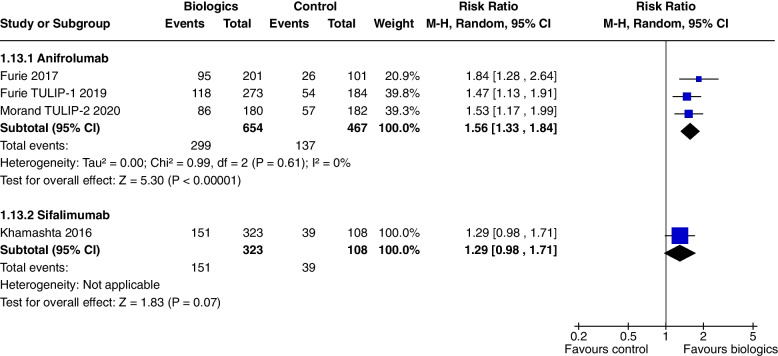
Anifrolumab significantly increased BICLA response at 52 weeks in all 3 studies (RR 1.56, CI 1.33 to 1.84, P < 0000.1) (Fig. 8).
Fig. 8.
BICLA at 52 weeks
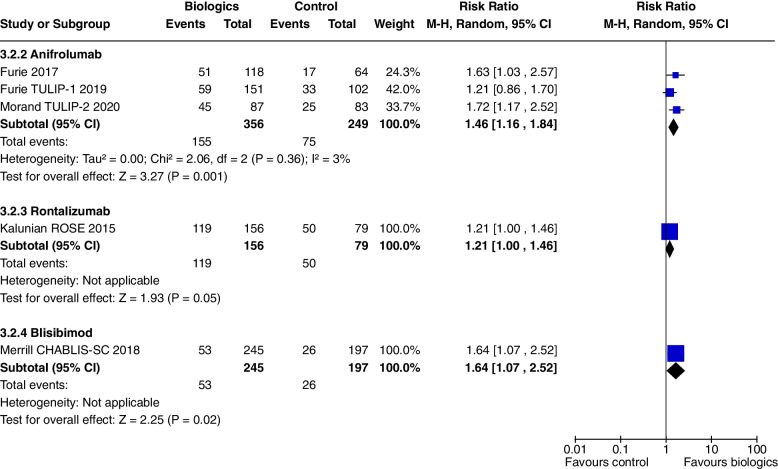
Prednisone dose reduction to < 10 mg/day was increased with Anifrolumab treatment (RR 1.46, CI 1.16 to 1.84, P = 0.001, 3 studies) (Fig. 9).
Fig. 9.
Change in prednisone dosages to ≤ 10 mg/day
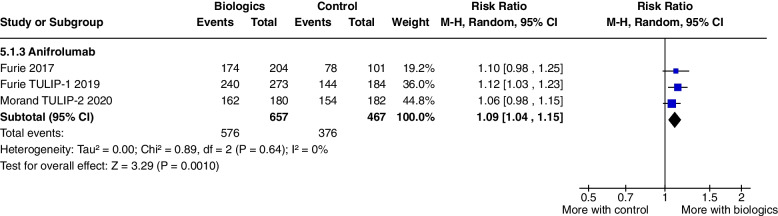
Adverse events were increased with Anifrolumab treatment, (RR 1.09, CI 1.04 to 1.15, P = 0.001, 3 studies) (Fig. 10), with a higher incidence of herpes zoster infections, but there were significantly fewer serious adverse events, (RR 0.68, CI 0.49 to 0.95, P = 0.02, 3 studies) compared to controls (Fig. 2). The other safety outcomes did not reach statistical significance.
Fig. 10.
Adverse events
Rontalizumab
Rontalizumab is a human anti-IFN-α monoclonal antibody that binds to all 12 IFN-α subtypes preventing signalling through the type I IFN receptor.
One study [9] including 238 patients addressed the use of Rontalizumab in SLE: Kalunian 2016 Patients with lupus nephritis were excluded. At 24 weeks, Rontalizumab did not improve SRI 4 (RR 1.11, CI 0.83 to 1.48, P = 0.47) (Fig. 3a), though there were steroid sparing benefits with an increased number of patients tapering their steroids to a prednisone equivalent of ≤ 10 mg/day (RR 1.21, CI 1.0 to 1.46, P = 0.05) (Fig. 9). There were no significant differences in safety outcomes.
Sifalimumab
Sifalimumab is a fully human, immunoglobulin G1 κ monoclonal antibody that binds to and neutralises the majority of IFN-α subtypes.
In a single study [10], at 52 weeks, Sifalimumab improved SRI 4, (RR 1.28, CI 1.02 to 1.61, P = 0.03) (Fig. 3b) and SRI 6 (RR 1.32, CI 1.01 to 1.73, P = 0.04) (Fig. 7). SRI 5 at 52 weeks (RR1.27, CI 0.98 to 1.65, P = 0.07) (Fig. 4) and BICLA at 52 weeks (RR 1.29, CI 0.98 to 1.71, P = 0.07) (Fig. 8) trended towards but did not achieve significance. There were no steroid sparing benefits, with no difference in the reduction in prednisone < 7.5 mg/day with 25% reduction from baseline dosage (RR 1.21, CI 0.41 to 3.54, P = 0.73) (Fig. 11). Adverse events were not increased with the use of Sifalimumab, though there were higher rates of herpes zoster compared to placebo (5.9% vs 0.9%).
Fig. 11.
Change in prednisone dosages to ≤ 7.5 mg and > 25% reduction from baseline dosage
Anti BAFF/BLyS and APRIL monoclonal antibody
BAFF and APRIL are cytokines from the TNF family, secreted by most myeloid and lymphoid cells, and bind to TACI, BCMA and BAFF receptors. Ligation of BAFF receptors promote B cell survival, immunoglobulin class switching and secretion. BAFF binds to all 3 receptors, whereas APRIL only binds to TACI and BCMA. Blisibimod and Tabalumab inhibit soluble and membrane bound BAFF and Belimumab binds to soluble human BAFF. Atacicept and Telitacicept block both BlyS and APRIL.
Belimumab
Belimumab is a human IgG1 monoclonal antibody that binds soluble human BlyS. It is currently only indicated for use in SLE not responding to standard of care therapy.
Seven studies [11–17] including 4022 patients. Furie 2020 [16] and Atisha Fregoso 2021 [17] included patients with lupus nephritis.
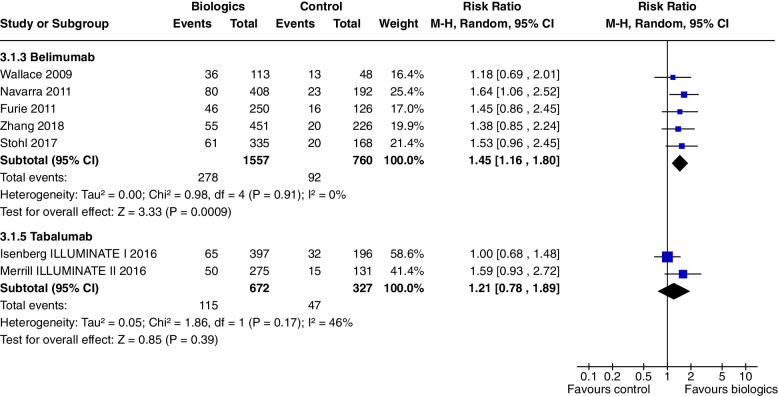
At 52 weeks, Belimumab use significantly increased SRI 4 (RR 1.27, CI 1.18 to 1.38, P < 0.0001, 4 studies) (Fig. 3b). In a single study at 52 weeks, improvements were demonstrated in SRI 5 (RR 1.56, CI 1.20 to 2.03, P = 0.0009) (Fig. 4), SRI 6 (RR 1.57, CI 1.19 to 2.08, P = 0.001) (Fig. 7) and SRI 8 (RR 1.50, CI 1.01 to 2.22, P = 0.05) (Fig. 6). In two studies, SRI 7 at 52 weeks significantly increased (RR 1.43, CI 1.13 to 1.81, P = 0.003) (Fig. 5).
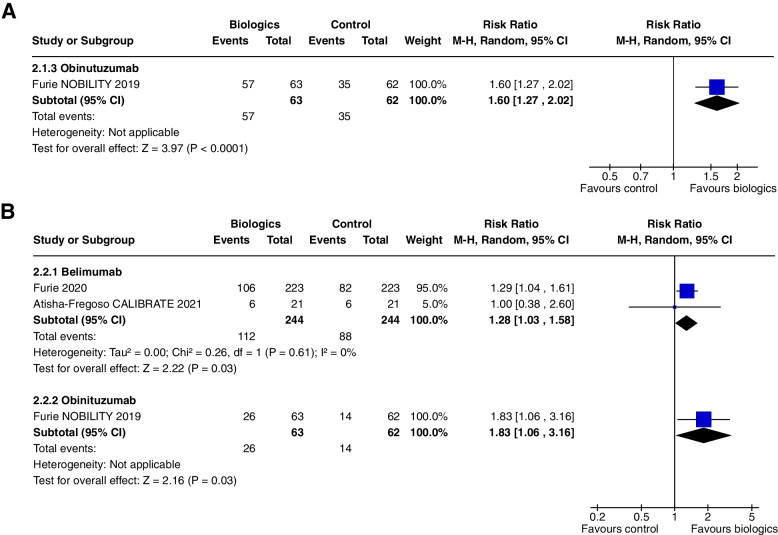
Belimumab did not alter CRR/PRR at 1 year (RR 1.28, CI 0.67 to 2.45, P = 0.45, 1 study) but showed a significant effect at 2 years (RR 1.29, CI 1.04 to 1.61, P = 0.03, 2 studies) (Fig. 12b).
Fig. 12.
A Combined complete and partial renal remission at 1 year, B combined complete and partial renal remission at 2 years
Belimumab significantly increased the number of patients able reduce prednisone dosages to ≤ 7.5 mg/day (RR 1.45, CI 1.16 to 1.80, P = 0.0009, 5 studies (Fig. 11).
There were no significant difference in serious adverse events (RR 0.88, 0.72 to 1.08, P = 0.24, 6 studies) (Fig. 2) or in other reported safety outcomes.
Blisibimod
Blisibimod is a selective inhibitor of soluble BAFF and membrane-bound BAFF, composed of a tetrameric BAFF binding domain fused to a human IgG1. Two studies [18, 19] included 988 patients. Patients with severe lupus nephritis were excluded.
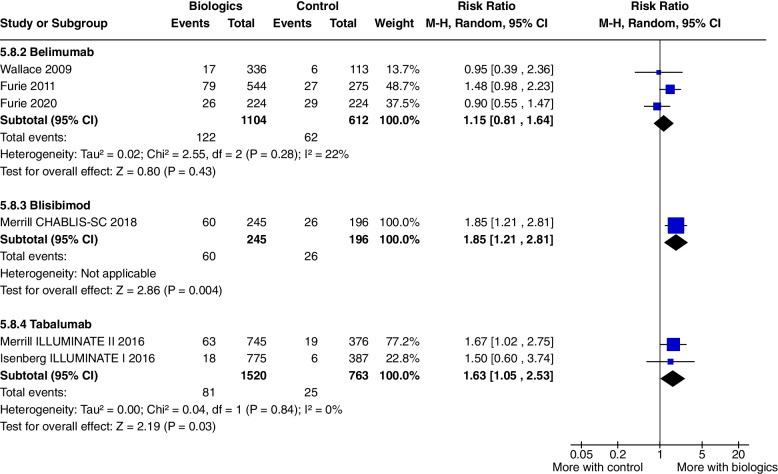
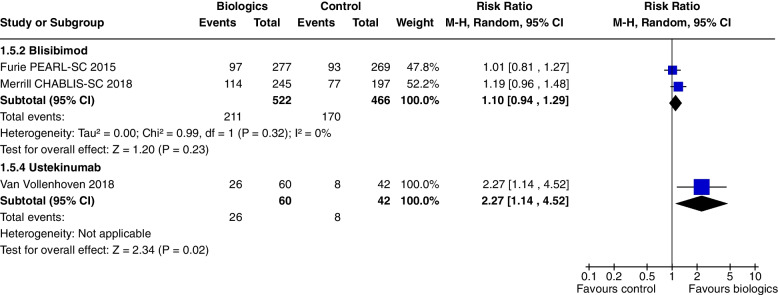
Blisibimod increased SRI 4 at 24 weeks only in Merrill 2018 [19], (RR 1.19, CI 1.00 to 1.41, P = 0.05, 2 studies) (Fig. 3a), but not SRI 4 and 6 at 52 weeks or SRI 5 to 8 at 24 weeks. Blisibimod reduced prednisone dosage below 10 mg/day (RR 1.64, CI 1.07 to 2.52, P = 0.02, 1 study [19] (Fig. 9).
Infusion related adverse events were increased (RR 1.85, CI 1.21 to 2.81, P = 0.004, 1 study [19] (Fig. 13). There were no significant increase in other adverse events.
Fig. 13.
Infusion related adverse events
Tabalumab
Tabalumab is a fully human IgG4 monoclonal antibody, that binds and neutralises both membrane and soluble BAFF. Two studies [20, 21] included 2262 patients. Patients with severe lupus nephritis were excluded.
Tabalumab significantly increased SRI 5 at 52 weeks (RR 1.23, CI 1.06 to 1.42, P = 0.005, 2 studies) (Fig. 4). Tabalumab did not significantly decrease prednisone doses (RR 1.21, CI 0.78 to 1.89, P = 0.39, 2 studies).
Infusion related adverse events were significantly higher with Tabalumab, (RR 1.63, CI 1.05 to 2.53, P = 0.03, 2 studies) (Fig. 13). Tabalumab did not increase withdrawals from the study, serious infections or death.
Atacicept
Atacicept is a recombinant fusion protein comprising the extracellular domain of the TACI receptor joined to a human IgG1 Fc domain that blocks B-cell activating factor BlyS and APRIL.
Two studies [22, 23] included 767 patients. Patients with lupus nephritis were excluded.
In one study [23], Atacicept did not increase SRI 4 (RR 1.27, CI 0.99 to 1.63, P = 0.06) (Fig. 3a), SRI 6 (RR 1.13, CI 0.79 to 1.62, P = 0.49) and BICLA (RR 1.13, CI 0.87 to 1.47, P = 0.36) at 24 weeks.
There were no steroid sparing benefits or significant differences in the safety outcomes.
Telitacicept
Telitacicept is a fusion protein comprising a recombinant TACI receptor fused to the Fc domain of human IgG, which binds to and neutralises the BLyS and APRIL, suppressing development and maturation of plasma cells and mature B cells.
One study [24] included 202 patients. Patients with severe lupus nephritis were excluded.
SRI 4 at 52 weeks was significantly increased with Telitacicept (RR 2.12, CI 1.48 to 3.03, P < 0.00001) (Fig. 3b).
There were no significant differences in reported safety outcomes of adverse events, serious adverse events and death.
Anti-CD20 monoclonal antibody
Three anti CD20 monoclonal antibodies are examined in this review, Rituximab (murine-human chimeric), Ocrelizumab and Obinutuzumab (humanised).
Obinutuzumab
Obinutuzumab is a recombinant type II anti-CD20 and IgG1 Fc-optimised humanised monoclonal antibody, which has improved mAb-FcγRIIIA interaction and direct and immune effector cell-mediated cytotoxicity compared to Rituximab.
One study [25] included 125 patients. Patients with lupus nephritis ISPN/RPS 2003 class III/IV were included in the study.
Combined CRR/PRR at 1 year was increased (RR 1.60, CI 1.27 to 2.02, P < 0.0001) (Fig. 12a) and 2 years (RR 1.83, CI 1.06 to 3.16, P = 0.03) (Fig. 12b).
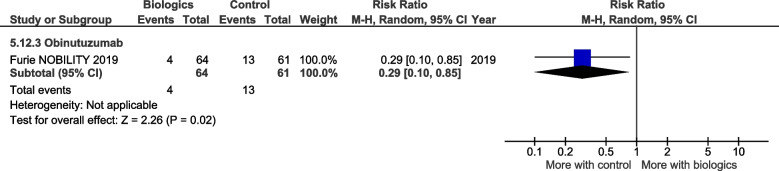
There were fewer grade 3 or higher related infectious events with Obinutuzumab (RR 0.29, CI 0.10 to 0.85, P = 0.02) (Fig. 14), but no significant differences in the other safety outcomes.
Fig. 14.
Grade 3 or higher related infectious events
Ocrelizumab
Ocrelizumab is a humanised monoclonal antibody against CD20 and may have greater antibody dependent cellular toxicity and less complement dependent cytotoxicty compared to Rituximab which is a chimeric monoclonal antibody. One study [26] included 378 patients with lupus nephritis.
Combined CRR/PRR at 1 year was not increased (RR 1.22, CI 0.97 to 1.55, P = 0.09).
There were no significant differences in the pooled safety outcomes, but a higher rate of serious infections were seen in patients receiving MMF compared to ELNT induction.
Rituximab
Rituximab is a type 1 chimeric anti-CD20 monoclonal antibody directed to the CD20 antigen on the surface of B lymphocytes, causing apoptosis, complement activation and cell mediated cytotoxicity.
Two studies [27, 28] included 401 patients. Rovin 2012 [28] only included patients with lupus nephritis class III/IV ± V. Rituximab did not increase CRR/PRR at 1 year, (RR 1.24, CI 0.90 to 1.71, P = 0.19, 1 study [28]). There was no reduction in the number of patients achieving prednisone < 10 mg/day (RR 0.81, CI 0.37 to 1.80, P = 0.60), 1 study [27].
Ustekinumab
Ustekinumab is a fully humanised monoclonal antibody against the p40 subunit found on both IL-12 and IL-23. IL-12 has a key role in inducing Th cell differentiation to Th1 cells, and IL-23 in Th17 cell activation and subsequent IL-17 secretion.
One study [29] included 102 patients. Patients with lupus nephritis class III/IV were excluded.
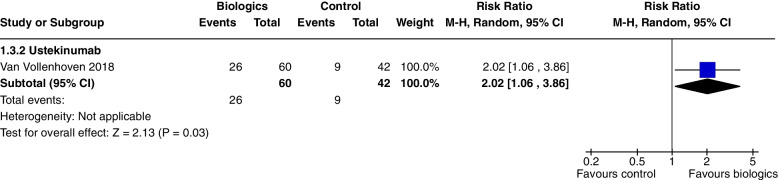
Ustekinumab increased SRI 4 at 24 weeks (RR 1.85, CI 1.15 to 2.97, P = 0.01) (Fig. 3a), SRI 5 at 24 weeks (RR 2.02, CI 1.06 to 3.86, P = 0.03) (Fig. 15) and SRI 6 at 24 weeks (RR 2.27, CI 1.14 to 4.52, P = 0.02) (Fig. 16), but not BICLA at 24 weeks (P = 0.86). Ustekinumab use did not increase any adverse events.
Fig. 15.
SRI 5 at 24 weeks
Fig. 16.
SRI 6 at 24 weeks
Group of drugs without significant results
There were no significant outcomes in the disease activity indices, composite responder rates or adverse events in the following group of drugs; anti-dsDNA complexing Abetimus [30] selective JAK 1 and 2 inhibitors Baricitinib [31], BTK inhibitors Evobrutinib [32], Fenebrutinib [33], high affinity cereblon ligand CC-220/Iberdomide [34, 35], tolerogenic peptides Edratide [36], anti CD22 monoclonal antibody Epratuzumab [37–39], anti IL-6 antibody PF-04326921 [40] Vobarilizumab [41] anti IL-10 monoclonal antibody BT063 [42], P140 peptide Lupuzor [43] and recombinant soluble human FcyRIIb SM101 [44].
Summary of findings
The main results of this review are presented in the summary of findings tables. Outcomes with significant results presented include composite outcomes, renal outcomes, glucocorticoid dose reduction, and adverse events. The complete GRADE tables are shown below (Refer Table 1: Composite outcomes, Table 2: Renal outcomes, Table 3: Glucocorticoid dose reduction, Table 4: Adverse events).
Table 1.
Composite outcomes
| Biologics compared to placebo for the treatment of Systemic Lupus Erythematosus measured by composite responder rates | |||||
|---|---|---|---|---|---|
| Patient or population: Systemic Lupus Erythematosus Setting: Inpatients then outpatients Intervention: Biologics Comparison: Standard of care, placebo | |||||
| Outcomes | № of participants (studies) Follow-up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
| Risk with Standard of care | Risk difference with Biologics | ||||
| (SRI) 4 at 52 weeks—Anifrolumab | 762 (2 RCTs) | ⨁⨁⨁◯ Moderatea,b,c,e | RR 1.40 (0.94 to 2.08) | 350 per 1,000 | 140 more per 1,000 (21 fewer to 378 more) |
| (SRI) 4 at 52 weeks—Sifalimumab | 431 (1 RCT) |
⨁⨁⨁◯ Moderatef |
RR 1.28 (1.02 to 1.61) | 454 per 1,000 | 127 more per 1,000 (9 more to 277 more) |
| (SRI) 4 at 52 weeks—Belimumab | 3180 (4 RCTs) |
⨁⨁⨁⨁ Highh |
RR 1.27 (1.18 to 1.38) | 415 per 1,000 | 112 more per 1,000 (75 more to 158 more) |
| (SRI) 4 at 52 weeks—Telitacicept | 249 (1 RCT) |
⨁⨁◯◯ Lowd,e,f,g |
RR 2.12 (1.48 to 3.03) | 339 per 1,000 | 379 more per 1,000 (163 more to 688 more) |
| BICLA response at 52 weeks—Anifrolumab | 1121 (3 RCTs) |
⨁⨁⨁◯ Moderatea,b,c,i |
RR 1.56 (1.33 to 1.84) | 293 per 1,000 | 164 more per 1,000 (97 more to 246 more) |
| BICLA response at 52 weeks—Sifalimumab | 431 (1 RCT) |
⨁⨁⨁◯ Moderatee,f |
RR 1.29 (0.98 to 1.71) | 361 per 1,000 | 105 more per 1,000 (7 fewer to 256 more) |
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect
Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect
CI Confidence interval, RR Risk ratio
The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI)
Explanations
aallocation concealment method not stated
brandomisation method not stated
cselective reporting, multiple analyses of data
dWide CI
esingle study
fDid not meet OIS criterion
gstudy protocols unavailable
hI2 = 0, significant P and consistently overlapping CI
iselective reporting, change in primary outcome resulting in significant outcome (Morand 2020)
Table 2.
Renal outcomes
| Biologics compared to placebo for the treatment of Systemic Lupus Erythematosus measured by renal outcomes | |||||
|---|---|---|---|---|---|
| Patient or population: Systemic Lupus Erythematosus Setting: Inpatients then outpatients Intervention: Biologics Comparison: Standard of care, placebo | |||||
| Outcomes | № of participants (studies) Follow-up |
Certainty of the evidence (GRADE) |
Relative effect (95% CI) |
Anticipated absolute effects | |
| Risk with placebo | Risk difference with Renal outcomes | ||||
| Partial and/or complete renal response by 1 year—Abatacept | 377 (2 RCTs) |
⨁⨁◯◯ Lowa,b,c,d |
RR 0.98 (0.78 to 1.23) | 436 per 1,000 | 9 fewer per 1,000 (96 fewer to 100 more) |
| Partial and/or complete renal response by 1 year—Belimumab | 43 (1 RCT) |
⨁◯◯◯ Very lowc,d,e |
RR 1.28 (0.67 to 2.45) | 409 per 1,000 | 115 more per 1,000 (135 fewer to 593 more) |
| Partial and/or complete renal response by 1 year—Obinutuzumab | 125 (1 RCT) |
⨁⨁⨁◯ Moderated |
RR 1.60 (1.27 to 2.02) | 565 per 1,000 | 339 more per 1,000 (152 more to 576 more) |
| Partial and/or complete renal response by 1 year—Ocrelizumab | 223 (1 RCT) |
⨁⨁◯◯ Lowa,b,d,f |
RR 1.22 (0.97 to 1.55) | 547 per 1,000 | 120 more per 1,000 (16 fewer to 301 more) |
| Partial and/or complete renal response by 1 year—Rituximab | 144 (1 RCT) |
⨁⨁⨁◯ Moderatea,b,d |
RR 1.24 (0.90 to 1.71) | 458 per 1,000 | 110 more per 1,000 (46 fewer to 325 more) |
| Partial and/or complete renal response by 2 years—Belimumab | 488 (2 RCTs) |
⨁◯◯◯ Very lowd,g,h |
RR 1.28 (1.03 to 1.58) | 361 per 1,000 | 101 more per 1,000 (11 more to 209 more) |
| Partial and/or complete renal response by 2 years—Obinituzumab | 125 (1 RCT) |
⨁⨁⨁◯ Moderatec,d |
RR 1.83 (1.06 to 3.16) | 226 per 1,000 | 187 more per 1,000 (14 more to 488 more) |
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect
Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect
CI Confidence interval, RR Risk ratio
The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI)
Explanations
arandomisation method not specified
ballocation concealment method not specified
cWide CI
dNot meeting OIS criteria
enot blinded, open label
fattrition bias, premature termination of study with incomplete reporting of primary endpoints
gnot blinded, open label (Atisha Fregoso 2021)
hsignificant P value and 0% heterogeneity but CI from Atisha Fregoso wide and overlaps significant/non significance
Table 3.
Glucocorticoid dose reduction
| Biologics compared to placebo for the treatment of Systemic Lupus Erythematosus measured by glucocorticoid dose reduction | |||||
|---|---|---|---|---|---|
| Patient or population: Systemic Lupus Erythematosus 1. Setting: Inpatients then outpatients Intervention: Biologics Comparison: Standard of care, placebo | |||||
| Outcomes | № of participants (studies) Follow-up |
Certainty of the evidence (GRADE) |
Relative effect (95% CI) | Anticipated absolute effects | |
| Risk with placebo | Risk difference with Glucocorticoid dose | ||||
| number of patients with prednisone equivalent ≤ 7.5 mg/day, with reduction ≥ 25% from baseline—Belimumab | 2317 (5 RCTs) |
⨁⨁⨁◯ Moderatec,d |
RR 1.45 (1.16 to 1.80) | 121 per 1,000 | 54 more per 1,000 (19 more to 97 more) |
| number of patients with prednisone equivalent ≤ 7.5 mg/day, with reduction ≥ 25% from baseline—Tabalumab | 999 (2 RCTs) |
⨁◯◯◯ Very lowa,b,d,e |
RR 1.21 (0.78 to 1.89) | 144 per 1,000 | 30 more per 1,000 (32 fewer to 128 more) |
| number of patients with prednisone equivalent ≤ 10 mg/day—Anifrolumab | 605 (3 RCTs) |
⨁⨁◯◯ Lowa,d,e,f |
RR 1.46 (1.16 to 1.84) | 301 per 1,000 | 139 more per 1,000 (48 more to 253 more) |
| number of patients with prednisone equivalent ≤ 10 mg/day—Rontalizumab | 235 (1 RCT) |
⨁⨁⨁◯ Moderatea,c,d |
RR 1.21 (1.00 to 1.46) | 633 per 1,000 | 133 more per 1,000 (0 fewer to 291 more) |
| number of patients with prednisone equivalent ≤ 10 mg/day—Blisibimod | 442 (1 RCT) |
⨁⨁⨁◯ Moderatec,d |
RR 1.64 (1.07 to 2.52) | 132 per 1,000 | 84 more per 1,000 (9 more to 201 more) |
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect
Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect
CI Confidence interval, RR Risk ratio
The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI)
Explanations
aallocation concealment method not stated
bWide CI
cSingle study
dNot meeting OIS criteria
erandomisation method not stated
fselective reporting bias, multiple analyses of data
Table 4.
Adverse events
| Biologics compared to placebo for the treatment of Systemic Lupus Erythematosus measured by adverse events | |||||
|---|---|---|---|---|---|
| Patient or population: Systemic Lupus Erythematosus Setting: Inpatients then outpatients Intervention: Biologics Comparison: Standard of care, placebo | |||||
| Outcomes | № of participants (studies) Follow-up |
Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
| Risk with placebo | Risk difference with Adverse events | ||||
| AEs—Anifrolumab | 1124 (3 RCTs) |
⨁⨁⨁◯ Moderatea |
RR 1.09 (1.04 to 1.15) | 805 per 1,000 | 72 more per 1,000 (32 more to 121 more) |
| AEs—CC-220 | 330 (2 RCTs) |
⨁⨁◯◯ Lowa,b |
RR 1.23 (0.84 to 1.80) | 363 per 1,000 | 83 more per 1,000 (58 fewer to 290 more) |
| Serious AEs—Abatacept | 1017 (4 RCTs) |
⨁⨁⨁◯ Moderatea |
RR 1.17 (0.87 to 1.58) | 219 per 1,000 | 37 more per 1,000 (28 fewer to 127 more) |
| Serious AEs—Anifrolumab | 1124 (3 RCTs) |
⨁⨁⨁◯ Moderatea |
RR 0.68 (0.49 to 0.95) | 188 per 1,000 | 60 fewer per 1,000 (96 fewer to 9 fewer) |
| Serious AEs—Belimumab | 4122 (6 RCTs) |
⨁⨁⨁◯ Moderatee |
RR 0.88 (0.72 to 1.08) | 196 per 1,000 | 24 fewer per 1,000 (55 fewer to 16 more) |
| Serious AEs—Blisibimod | 987 (2 RCTs) |
⨁⨁⨁◯ Moderatea |
RR 0.73 (0.53 to 0.99) | 165 per 1,000 | 44 fewer per 1,000 (77 fewer to 2 fewer) |
| Treatment related AEs—Belimumab | 1989 (3 RCTs) |
⨁⨁⨁◯ Moderatea |
RR 1.12 (0.99 to 1.26) | 334 per 1,000 | 40 more per 1,000 (3 fewer to 87 more) |
| Treatment related AEs—Blisibimod | 987 (2 RCTs) |
⨁⨁⨁⨁ High |
RR 1.26 (0.89 to 1.78) | 314 per 1,000 | 82 more per 1,000 (35 fewer to 245 more) |
| Treatment related AEs—CC-220 | 330 (2 RCTs) | ⨁⨁⨁◯ Moderatea | RR 1.39 (1.02 to 1.90) | 319 per 1,000 | 124 more per 1,000 (6 more to 287 more) |
| Infusion related AE—Belimumab | 1716 (3 RCTs) |
⨁⨁◯◯ Lowa,c,d |
RR 1.15 (0.81 to 1.64) | 101 per 1,000 | 15 more per 1,000 (19 fewer to 65 more) |
| Infusion related AE—Blisibimod | 441 (1 RCT) |
⨁⨁⨁◯ Moderatea |
RR 1.85 (1.21 to 2.81) | 133 per 1,000 | 113 more per 1,000 (28 more to 240 more) |
| Infusion related AE—Tabalumab | 2283 (2 RCTs) |
⨁⨁⨁◯ Moderatea |
RR 1.63 (1.05 to 2.53) | 33 per 1,000 | 21 more per 1,000 (2 more to 50 more) |
| Infection related grade 3 or higher AE—Obinutuzumab | 125 (1 RCT) |
⨁⨁⨁◯ Moderatea |
RR 0.29 (0.10 to 0.85) | 213 per 1,000 | 151 fewer per 1,000 (192 fewer to 32 fewer) |
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect
Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect
CI Confidence interval, RR Risk ratio
The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI)
Explanations
adidn't meet OIS criteria
bhigh heterogeneity
callocation concealment method not stated
dwide CI
ehigh heterogeneity and 2 studies suggesting reduction in events, 4 don't
Risk of bias
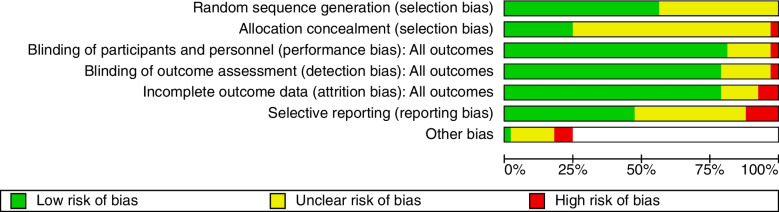
Risk of bias graph (Fig. 17) and risk of bias summary (Fig. 18) is shown below.
Fig. 17.
Risk of bias graph
Fig. 18.
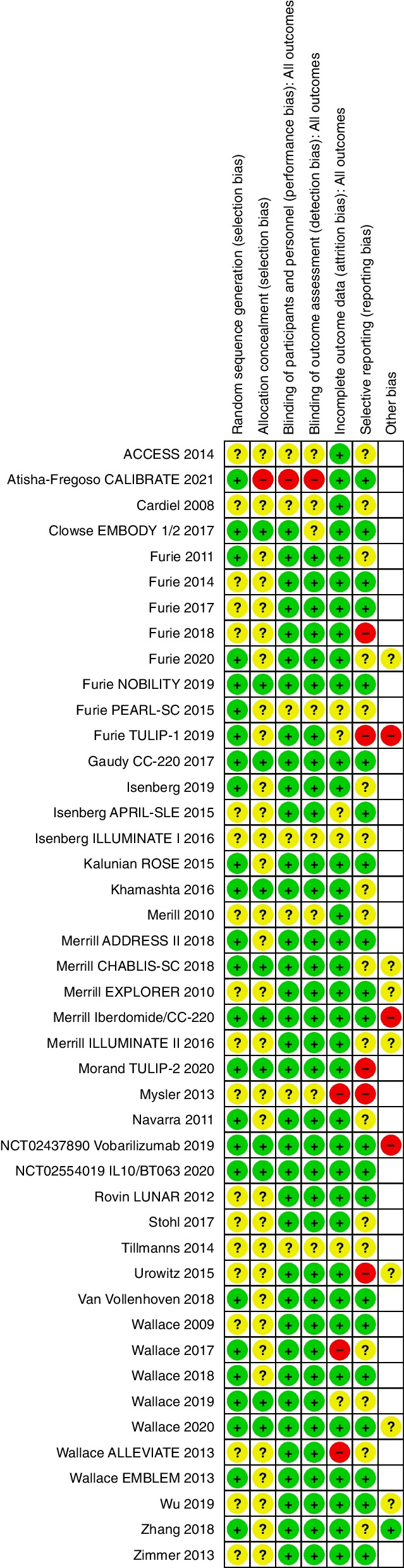
Risk of bias summary
Discussion
Summary of main positive and negative outcomes of our study.
We have summarised the RCT data available on 25 biological agents from 15 different drug groups in the treatment of SLE. The majority of these drugs have limited data available and will require further trials to determine their efficacy in various patient groups.
Currently Belimumab is shown to have the most significant data suggesting that it is effective in SLE without a major adverse effect profile. There is high quality evidence showing Belimumab improves composite outcomes measured by SRI. The level of evidence for other biologics with significant outcomes range from low to moderate (Summary of findings: Composite outcomes). Other newer treatments have shown significant efficacy but in more specific outcomes and will need further trials to clearly delineate their strengths and weaknesses.
The main outcomes assessed in these studies were SRI, BICLA, and combined CRR/PRR. Of the 25 biologic agents, only anti-interferon, anti BAFF/BLyS and/or APRIL, anti IL12/23 and anti CD20 monoclonal antibodies were found to improve outcomes.
Anifrolumab increased BICLA response at 52 weeks, SRI 5 to 8 in a single study (Furie 2019), decreased prednisone dosages, with increased adverse events with herpes zoster infections, but with lesser serious adverse events. Sifalimumab also improved SRI but also increased herpes zoster infections. Among the anti BAFF/Blys and/or APRIL monoclonal antibodies, Belimumab consistently improved SRI 4, decreased prednisone dosages, increased combined CRR/PRR in a single study, and had no adverse safety outcomes. Tabalumab increased SRI 5 at 52 weeks with no steroid sparing effect but was associated with increased infusion related adverse events. Telitacicept also improved SRI 4 at 52 weeks, without data on its effect on steroid dosages. Of the three anti CD-20 monoclonal antibodies, only Obinutuzumab increased combined CRR/PRR at 1 and 2 years, with lower grade 3 or higher infectious events. The single anti IL12/23 monoclonal antibody, Ustekinumab, increased SRI 4 to 6, but not BICLA at 24 weeks, with no concerning safety outcomes.
Despite positive results in some of these biologics, several of their developments have since been terminated. There are no further trials planned for Tabalumab ( two phase III trials) by the parent pharmaceutical company as it was not felt to have reached significant efficacy compared to existing treatments, and Sifalimumab's development (one phase IIb trial) has been ceased in favour of Anifrolumab. Following the completion of this review, a phase III trial of Ustekinumab involving 516 patients showed no superiority compared to placebo when measuring SRI 4 as a primary endpoint [45].
Our review did not include non-biologics such as the calcineurin inhibitor Voclosporin which has shown benefit in proteinuria reduction in patients with lupus nephritis [46].
The other remaining drug classes and biological agents did not improve any of the outcomes assessed in the study and had no other notable safety outcomes.
Difficulties with outcome measures
Prior to the introduction of SRI and BICLA, trials reported outcomes using individual BILAG, SLICC, SLEDAI based scores such as SLEDAI-2 K and SELENA-SLEDAI as their outcome measures. There were inconsistencies with how these scores were reported to denote significant results. Examples included BILAG as a numerical score determined by the study authors (and outcomes reporting changes in percentages, mean BILAG score differences compared to baseline) and differing organ domain severity scores (eg 1A and 2B, 1A and 1B, B only, C in all domains) and SLEDAI based metrics using varying decrease in points, expressed in means, medians or percentage of changes in baseline values.
SRI4 response is defined as SLEDAI improvement of 4 points or more, PGA not worsening by 0.3 points or more (10% or more), and BILAG having no new As and not having two or more new Bs. SRI 5, 6 and 7 correspond to an increase in improvement in SLEDAI points, without changes to the other criteria. BICLA response is defined as a reduction of all baseline BILAG-2004 A and B domain scores to B/C/D and C/D, no worsening in any organ system; no worsening of SLEDAI-2 K score from baseline, and no worsening ≥ 0.3 points (< 10% worsening) in Physician's Global Assessment, and no non-protocol treatment (new or increased immunosuppressives, antimalarials, corticosteroids or premature discontinuation of study treatment).
Comparing SRI and BICLA, SRI places more emphasis on SLEDAI improvement which does not evaluate the degree of individual component improvement, compared to the more comprehensive BILAG based BICLA, which does not evaluate for serological improvements. Ohmura 2021 [47] summarises the differences between the existing SLE activity indexes in clinical trials. Quality of life outcome measures also suffered from the aforementioned issues.
As SRI and BICLA incorporates a standardised change in BILAG, SELENA-SLEDAI/SLEDAI-2 K and PGA in their scoring, the authors of this study decided to omit data reporting other disease and quality of life outcomes outside of SRI and BICLA. This is mainly to maximise data that can be appropriately compared across studies, the main utility of a systematic review such as this.
Studies of lupus nephritis also did not use standardised definitions of complete or partial renal remissions (Table 5: Renal outcomes). Neither did they provide adequate reporting on other renal outcomes time to ESRD, or changes in serum creatinine/eGFR.
Table 5.
Renal outcomes
| Study | Complete renal remission | Partial renal remission |
|---|---|---|
| Abatacept | ||
| ACCESS 2014 [4] |
UPCR < 0.5 based on a 24-h urine collection, Serum creatinine ≤ 1.2 mg/dl or ≤ 125% of baseline Adherence to the prednisone taper to 10 mg/day by week12 |
UPCR 50% improvement from baseline Serum creatinine ≤ 1.2 mg/dl or ≤ 125% of baseline Adherence to the prednisone taper to 10 mg/day by week12 |
| Furie 2014 [3] |
EGFR 90% of screening level if normal at screening visit or eGFR 90% of 6-month, pre-flare value if abnormal at screening, UPCR 0.26 gm/gm (30 mg/mmole) Inactive urinary sediment (RBCs and WBCs per hpf within normal limits, no RBC or WBC casts All complete response criteria had to be met once again, 4 weeks after they were initially achieved |
Inactive urinary sediment regardless of the screening value UPCR 50% improvement from screening value eGFR ≥ 90% of screening value if eGFR 60–89 ≥ 50% improvement in eGFR if screening eGFR was between 15–59, or eGFR ≥ 90% of the screening or 6 month pre-flare value |
| Furie 2018 [5] |
Maintenance of GFR UPCR ≤ 0.5 Absence of urinary cellular casts Pednisone ≤ 10 mg/day |
None |
| Belimumab | ||
| Furie 2020 [16] |
UPCR of < 0.5 eGFR that was no worse than 10% below the preflare value or ≥ 90 No rescue therapy |
≥ 50% decrease in the uPCR and either uPCR < 1.0, if the baseline ratio was ≤ 3.0 or < 3.0, if the baseline ratio was > 3.0 |
| Atisha-Fregoso 2021 [17] |
UPCR of < 0.5 based on a 24-h urine sample collection eGFR of ≥ 120 or if the value was < 120, then > 80% of the eGFR recorded at the time of study entry Adherence to the prednisone dosing provisions. (prednisone 40 mg/day with taper to 10 mg/day by week 12, and ≤ 10 mg/day through week 96.) |
eGFR no more than 10% below the baseline value or within normal range |
| Ocrelizumab | ||
| Mysler 2013 [26] |
Serum creatinine ≤ 25% increase from baseline UPCR < 0.5 |
Serum creatinine ≤ 25% above baseline 50% improvement in UPCR, if baseline ratio > 3.0, then UPCR < 3.0 |
| Obinutuzumab | ||
| Furie 2019 [7] |
Maintenance of eGFR, UPCR) ≤ 0.5 Absence of urinary cellular casts Prednisone ≤ 10 mg/day |
Serum creatinine ≤ 15% above baseline value No urinary red cell casts and either RBCs/HPF ≤ 50% above baseline or < 10 RBCs/HPF 50% improvement in UPCR, with one of following conditions met: If baseline UPCR is ≤ 3.0, then a UPCR of < 1.0 If baseline UPCR > 3.0, then a UPCR of < 3.0 |
| Rituximab | ||
| Rovin 2012 [28] |
Normal creatinine level if it was abnormal at baseline or a creatinine level of ≤ 115% of baseline if it was normal at baseline Inactive urinary sediment (< 5 RBCs/hpf and absence of RBC casts); and UPCR ratio < 0.5 |
Creatinine level ≤ 115% of baseline RBCs/hpf ≤ 50% above baseline and no RBC casts At least a 50% decrease in the UPC ratio to < 1.0 (if the baseline UPC ratio was ≤ 3.0) or to ≤ 3.0 (if the baseline UPC ratio was > 3.0) |
Comparison with other systematic reviews
Four other reviews examined the use of biologic agents in the treatment of SLE. A meta-analysis by Oon 2018 [48] that Belimumab, Tabalumab and Epratuzumab had steroid sparing effects, which differed from our finding of only Belimumab had a significant steroid sparing effect. We did not include the data of steroid doses in the Epratuzumab studies of Wallace (EMBLEM) 2013 and Clowse 2017 as they were reported as mean ± SD and Wallace 2013 (ALLEVIATE) which reported them as medians without sufficient IQR data. Borba 2014 [49] which assessed 7 biologic agents similarly concluded that Belimumab improved disease response in the outcomes assessed compared to placebo. Singh 2021 [50] assessed 6 RCTs of Belimumab and concluded that Belimumab was effective in increasing SELENA-SLEDAI (≥ 4 point improvement) and reduction in glucocorticoid dosages. Sciascia 2017 [51] assessed the efficacy of Belimumab in renal outcomes and reported a decrease in proteinuria in patients treated with Belimumab but were unable to arrive to any conclusions for other parameters of renal response due to differing criteria across the studies. We did not include data describing renal outcomes such as number of and time to renal flares, and proteinuria due to the heterogeneous methods of reporting them across the studies, limiting their applicability in a systematic review.
Conclusions
Recommendations for patient treatments
Based on current data, Anifrolumab, Sifalimumab, Belimumab, Tabalumab, Telitacicept, are effective treatments in the treatment of SLE without lupus nephritis. Anifrolumab and Belimumab are useful in decreasing the steroid burden in these patients when compared to other biologics. In patients with lupus nephritis, Belimumab and Obinutuzumab are effective treatments. There is insufficient data to recommend for or against the use of biologics in CNS lupus due to their exclusion from trials. Patients treated with Anifrolumab or Sifalimumab should consider herpes zoster vaccination prior to commencing treatment.
Recommendations for further research
Our review has revealed and summarised a wealth of studies in the treatment of SLE with biological agents and demonstrated the limited availability of data in many of these agents and the need for further studies to elucidate the efficacy of each agent in SLE treatment.
Comparison between agents will need to emerge as a research question in the near future. Other potential areas to consider will be the combination of treatments from different drug groups to improve the overall efficacy of disease control over time.
Studies involving biologics in SLE have heterogeneous endpoints and duration. The majority of the studies selectively excluded renal lupus involvement, though the criteria for exclusion varied widely, from active urinary sediment and mildly decreased eGFR to rapidly progressing glomerulonephritis. As lupus nephritis remains a leading cause of morbidity and mortality in SLE, a larger number of trials with a standardised definition of renal composite end points is required.
Trials in the treatment of SLE need to standardise outcomes and reporting in order that results can contribute to a coherent picture of treatment efficacy and safety.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- AM
Antimalarials
- ACR
American College of Rheumatology
- APRIL
A proliferation-inducing ligand
- AZA
Azathioprine
- BAFF
B-cell activating factor
- BCMA
B cell maturation antigen
- BICLA
BILAG-Based Composite Lupus Assessment
- BID
Twice daily
- BILAG
British Isles Lupus Assessment Group
- BlyS
B-lymphocyte stimulator
- CYC
Cyclophosphamide
- eGFR
Estimated glomerular filtration rate (measured in ml per minute per 1.73 m2)
- EULAR
European League Against Rheumatism
- HPF
High powered field
- IFN
Interferon
- IV
Intravenous
- JAK
Janus kinase
- MMF
Mycophenolate mofetil
- MFS
Mycophenolate sodium
- MTX
Methotrexate
- NSAIDS
Nonsteroidal anti inflammatory drugs
- QD
Once daily
- RPGN
Rapidly progressing glomerulonephritis
- RBC
Red blood cell
- SC
Subcutaneous
- SLE
Systemic lupus erythromatosus
- SLEDAI
Systemic Lupus Erythematosus Disease Activity Index
- SLICC
Systemic Lupus International Collaborating Clinics
- SRI
Systemic Lupus Erythematosus Responder Index
- TACI
Transmembrane activator and calcium modulator and cySM101clophylin ligand interactor
- UPCR
Urine protein to creatinine ratio
- WBC
White blood cell
Authors’ contributions
PP and JC reviewed existing literature and designed the review with input from GW and SJ. JC undertook the role of lead author for this review. GW and SJ reviewed and verified statistical data and the writing of this review. The manuscript was approved by all authors.
Funding
The authors did not receive any funding for this review.
Availability of data and materials
The published article contains summarised versions of significant results generated and analysed during this study. A full set of the unedited data presented via forest plots is provided in the supplementary files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, et al. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis. 2010;69:20–28. doi: 10.1136/ard.2008.101766. [DOI] [PubMed] [Google Scholar]
- 2.Merrill JT, Burgos-Vargas R, Westhovens R, et al. The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62:3077–3087. doi: 10.1002/art.27601. [DOI] [PubMed] [Google Scholar]
- 3.Furie R, Nicholls K, Cheng T-T, et al. Efficacy and safety of Abatacept in lupus nephritis: a twelve-month, randomized, double-blind study: Abatacept in lupus nephritis. Arthritis Rheumatol. 2014;66:379–389. doi: 10.1002/art.38260. [DOI] [PubMed] [Google Scholar]
- 4.The ACCESS Trial Group Treatment of lupus nephritis with Abatacept: the Abatacept and cyclophosphamide combination efficacy and safety study: Abatacept in lupus nephritis. Arthritis Rheumatol. 2014;66:3096–3104. doi: 10.1002/art.38790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furie R, Dooley MA, Wofsy D, et al. OP0253 A phase iii randomised, double-blind, placebo-controlled study to evaluate the efficacy and safety of abatacept or placebo on standard of care in patients with active class iii or iv lupus nephritis. Ann Rheum Dis. 2018;77:176–177. [Google Scholar]
- 6.Furie R, Khamashta M, Merrill JT, et al. Anifrolumab, an Anti-Interferon-α Receptor Monoclonal Antibody, in moderate-to-severe systemic lupus erythematosus: Anifrolumab in moderate-to-severe SLE. Arthritis Rheumatol. 2017;69:376–386. doi: 10.1002/art.39962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furie RA, Morand EF, Bruce IN, et al. Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1): a randomised, controlled, phase 3 trial. Lancet Rheumatol. 2019;1:e208–e219. doi: 10.1016/S2665-9913(19)30076-1. [DOI] [PubMed] [Google Scholar]
- 8.Morand EF, Furie R, Tanaka Y, et al. Trial of Anifrolumab in active systemic lupus erythematosus. N Engl J Med. 2020;382:211–221. doi: 10.1056/NEJMoa1912196. [DOI] [PubMed] [Google Scholar]
- 9.Kalunian KC, Merrill JT, Maciuca R, et al. A phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon-α) in patients with systemic lupus erythematosus (ROSE) Ann Rheum Dis. 2016;75:196–202. doi: 10.1136/annrheumdis-2014-206090. [DOI] [PubMed] [Google Scholar]
- 10.Khamashta M, Merrill JT, Werth VP, et al. Sifalimumab, an anti-interferon-α monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2016;75:1909–1916. doi: 10.1136/annrheumdis-2015-208562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallace DJ, Stohl W, Furie RA, et al. A phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus. Arthritis Care Res. 2009;61:1168–1178. doi: 10.1002/art.24699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furie R, Petri M, Zamani O, et al. A phase III, randomized, placebo-controlled study of Belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63:3918–3930. doi: 10.1002/art.30613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navarra SV, Guzmán RM, Gallacher AE, et al. Efficacy and safety of Belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:721–731. doi: 10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- 14.Stohl W, Schwarting A, Okada M, et al. Efficacy and safety of subcutaneous Belimumab in systemic lupus erythematosus: a fifty-two–week randomized, double-blind placebo-controlled study. Arthritis Rheumatol. 2017;69:1016–1027. doi: 10.1002/art.40049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang F, Bae S-C, Bass D, et al. A pivotal phase III, randomised, placebo-controlled study of belimumab in patients with systemic lupus erythematosus located in China, Japan and South Korea. Ann Rheum Dis. 2018;77:355–363. doi: 10.1136/annrheumdis-2017-211631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furie R, Rovin BH, Houssiau F, et al. Two-year, randomized, controlled trial of Belimumab in lupus nephritis. N Engl J Med. 2020;383:1117–1128. doi: 10.1056/NEJMoa2001180. [DOI] [PubMed] [Google Scholar]
- 17.Atisha-Fregoso Y, Malkiel S, Harris KM, et al. Phase II Randomized Trial of Rituximab Plus Cyclophosphamide Followed by Belimumab for the Treatment of Lupus Nephritis. Arthritis Rheumatol Hoboken NJ. 10.1002/art.41466. Epub ahead of print 4 August 2020. [DOI] [PMC free article] [PubMed]
- 18.Furie RA, Leon G, Thomas M, et al. A phase 2, randomised, placebo-controlled clinical trial of blisibimod, an inhibitor of B cell activating factor, in patients with moderate-to-severe systemic lupus erythematosus, the PEARL-SC study. Ann Rheum Dis. 2015;74:1667–1675. doi: 10.1136/annrheumdis-2013-205144. [DOI] [PubMed] [Google Scholar]
- 19.Merrill JT, Shanahan WR, Scheinberg M, et al. Phase III trial results with blisibimod, a selective inhibitor of B-cell activating factor, in subjects with systemic lupus erythematosus (SLE): results from a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2018;77:883–889. doi: 10.1136/annrheumdis-2018-213032. [DOI] [PubMed] [Google Scholar]
- 20.Isenberg DA, Petri M, Kalunian K, et al. Efficacy and safety of subcutaneous tabalumab in patients with systemic lupus erythematosus: results from ILLUMINATE-1, a 52-week, phase III, multicentre, randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2016;75:323–331. doi: 10.1136/annrheumdis-2015-207653. [DOI] [PubMed] [Google Scholar]
- 21.Merrill JT, van Vollenhoven RF, Buyon JP, et al. Efficacy and safety of subcutaneous tabalumab, a monoclonal antibody to B-cell activating factor, in patients with systemic lupus erythematosus: results from ILLUMINATE-2, a 52-week, phase III, multicentre, randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2016;75:332–340. doi: 10.1136/annrheumdis-2015-207654. [DOI] [PubMed] [Google Scholar]
- 22.Isenberg D, Gordon C, Licu D, et al. Efficacy and safety of atacicept for prevention of flares in patients with moderate-to-severe systemic lupus erythematosus (SLE): 52-week data (APRIL-SLE randomised trial) Ann Rheum Dis. 2015;74:2006–2015. doi: 10.1136/annrheumdis-2013-205067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merrill JT, Wallace DJ, Wax S, et al. Efficacy and safety of Atacicept in patients with systemic lupus erythematosus: results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled, parallel-arm Phase IIb Study. Arthritis Rheumatol. 2018;70:266–276. doi: 10.1002/art.40360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.RemeGen Co., Ltd. A Phase 2, Randomized, Double-Blind, Multicenter Study of Telitacicept for Injection (RC18) in Subjects With IgA Nephropathy. 2021. Clinical Trial Registration NCT04905212, clinicaltrials.gov, https://clinicaltrials.gov/ct2/show/NCT04905212. Accessed 1 Nov 2021.
- 25.A Phase II Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Obinutuzumab or Placebo in Combination with Mycophenolate Mofetil in Patients with Active Class III or IV Lupus Nephritis. ACR Meeting Abstracts, https://acrabstracts.org/abstract/a-phase-ii-randomized-double-blind-placebo-controlled-study-to-evaluate-the-efficacy-and-safety-of-obinutuzumab-or-placebo-in-combination-with-mycophenolate-mofetil-in-patients-with-active-class-iii/. Accessed 27 Aug 2021.
- 26.Mysler EF, Spindler AJ, Guzman R, et al. Efficacy and safety of Ocrelizumab in active proliferative lupus nephritis: results from a randomized, double-blind, phase III study: Ocrelizumab in lupus nephritis. Arthritis Rheum. 2013;65:2368–2379. doi: 10.1002/art.38037. [DOI] [PubMed] [Google Scholar]
- 27.Merrill JT, Neuwelt CM, Wallace DJ, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: The randomized, double-blind, phase ii/iii systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010;62:222–233. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rovin BH, Furie R, Latinis K, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: The lupus nephritis assessment with rituximab study. Arthritis Rheum. 2012;64:1215–1226. doi: 10.1002/art.34359. [DOI] [PubMed] [Google Scholar]
- 29.van Vollenhoven RF, Hahn BH, Tsokos GC, et al. Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study. Lancet Lond Engl. 2018;392:1330–1339. doi: 10.1016/S0140-6736(18)32167-6. [DOI] [PubMed] [Google Scholar]
- 30.Cardiel MH, Tumlin JA, Furie RA, et al. Abetimus sodium for renal flare in systemic lupus erythematosus: results of a randomized, controlled phase III trial. Arthritis Rheum. 2008;58:2470–2480. doi: 10.1002/art.23673. [DOI] [PubMed] [Google Scholar]
- 31.Wallace DJ, Furie RA, Tanaka Y, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 2 trial. The Lancet. 2018;392:222–231. doi: 10.1016/S0140-6736(18)31363-1. [DOI] [PubMed] [Google Scholar]
- 32.Wallace DJ, Martin EC, Ona V, et al. 212 Phase 2, randomized, double-blind, placebo-controlled, dose-finding study, evaluating the Bruton’s tyrosine kinase inhibitor evobrutinib in patients with systemic lupus erythematosus: study design. Lupus Sci Med. 6. 10.1136/lupus-2019-lsm.212. Epub ahead of print 1 April 2019.
- 33.Isenberg D, Furie R, Jones NS, et al. Efficacy, Safety, and Pharmacodynamic Effects of the Bruton’s Tyrosine Kinase Inhibitor, Fenebrutinib (GDC-0853), in Systemic Lupus Erythematosus. Arthritis Rheumatol. 10.1002/art.41811. [DOI] [PubMed]
- 34.Gaudy A, Ye Y, Korish S, et al. SAT0225 Cereblon modulator CC-220 decreases naÏve and memory B cells and plasmacytoid dendritic cells in systemic lupus erythematosus (SLE) patients: exposure-response results from a phase 2A proof of concept study. In: Poster Presentations. BMJ Publishing Group Ltd and European League Against Rheumatism, p. 858.2–859. 10.1136/annrheumdis-2017-eular.3036.
- 35.Efficacy and Safety of Iberdomide in Patients with Active Systemic Lupus Erythematosus: 24-Week Results of a Phase 2, Randomized, Placebo-Controlled Study - ACR Meeting Abstracts, https://acrabstracts.org/abstract/efficacy-and-safety-of-iberdomide-in-patients-with-active-systemic-lupus-erythematosus-24-week-results-of-a-phase-2-randomized-placebo-controlled-study/. Accessed 27 Aug 2021.
- 36.Urowitz MB, Isenberg DA, Wallace DJ. Safety and efficacy of hCDR1 (Edratide) in patients with active systemic lupus erythematosus: results of phase II study. Lupus Sci Med. 2015;2:e000104. doi: 10.1136/lupus-2015-000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallace DJ, Gordon C, Strand V, et al. Efficacy and safety of epratuzumab in patients with moderate/severe flaring systemic lupus erythematosus: results from two randomized, double-blind, placebo-controlled, multicentre studies (ALLEVIATE) and follow-up. Rheumatology. 2013;52:1313–1322. doi: 10.1093/rheumatology/ket129. [DOI] [PubMed] [Google Scholar]
- 38.Wallace DJ, Kalunian K, Petri MA, et al. Efficacy and safety of epratuzumab in patients with moderate/severe active systemic lupus erythematosus: results from EMBLEM, a phase IIb, randomised, double-blind, placebo-controlled, multicentre study. Ann Rheum Dis. 2014;73:183–190. doi: 10.1136/annrheumdis-2012-202760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clowse MEB, Wallace DJ, Furie RA, et al. Efficacy and safety of epratuzumab in moderately to severely active systemic lupus erythematosus: results from two phase III randomized, double-blind, placebo-controlled trials: efficacy and safety of Epratuzumab in SLE. Arthritis Rheumatol. 2017;69:362–375. doi: 10.1002/art.39856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wallace DJ, Strand V, Merrill JT, et al. Efficacy and safety of an interleukin 6 monoclonal antibody for the treatment of systemic lupus erythematosus: a phase II dose-ranging randomised controlled trial. Ann Rheum Dis. 2017;76:534–542. doi: 10.1136/annrheumdis-2016-209668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ablynx. A Phase II Multicenter, Randomized, Double-blind, Placebo Controlled, Dose-range Finding Study to Evaluate the Safety and Efficacy of ALX-0061 Administered Subcutaneously in Subjects With Moderate to Severe Active Systemic Lupus Erythematosus. 2019. Clinical Trial Registration NCT02437890, clinicaltrials.gov, https://clinicaltrials.gov/ct2/show/NCT02437890. Accessed 15 June 2021.
- 42.Biotest. A Prospective, Double-blind, Randomized, Placebo-controlled, Repeated Dose, Multicentre Phase IIa Proof-of-Concept Study With BT063 in Subjects With Systemic Lupus Erythematosus. 2020. Clinical Trial Registration study/NCT02554019, clinicaltrials.gov, https://clinicaltrials.gov/ct2/show/study/NCT02554019. Accessed 15 June 2021.
- 43.Zimmer R, Scherbarth HR, Rillo OL, et al. Lupuzor/P140 peptide in patients with systemic lupus erythematosus: a randomised, double-blind, placebo-controlled phase IIb clinical trial. Ann Rheum Dis. 2013;72:1830–1835. doi: 10.1136/annrheumdis-2012-202460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tillmanns S. SM101, a Novel Recombinant, Soluble, Human FcγIIB Receptor, in the Treatment of Systemic Lupus Erythematosus: Results of a Double-Blind, Placebo-Controlled Multicenter Study. ACR Meeting Abstracts, https://acrabstracts.org/abstract/sm101-a-novel-recombinant-soluble-human-fcγiib-receptor-in-the-treatment-of-systemic-lupus-erythematosus-results-of-a-double-blind-placebo-controlled-multicenter-study/. Accessed 28 Aug 2021.
- 45.van Vollenhoven RF, Kalunian KC, Dörner T, et al. Phase 3, multicentre, randomised, placebo-controlled study evaluating the efficacy and safety of ustekinumab in patients with systemic lupus erythematosus. Ann Rheum Dis. 2022;81:1556–1563. doi: 10.1136/ard-2022-222858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rovin BH, Teng YKO, Ginzler EM, et al. Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2021;397:2070–2080. doi: 10.1016/S0140-6736(21)00578-X. [DOI] [PubMed] [Google Scholar]
- 47.Ohmura K. Which is the best SLE activity index for clinical trials? Mod Rheumatol. 2021;31:20–28. doi: 10.1080/14397595.2020.1775928. [DOI] [PubMed] [Google Scholar]
- 48.Oon S, Huq M, Godfrey T, et al. Systematic review, and meta-analysis of steroid-sparing effect, of biologic agents in randomized, placebo-controlled phase 3 trials for systemic lupus erythematosus. Semin Arthritis Rheum. 2018;48:221–239. doi: 10.1016/j.semarthrit.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Borba HHL, Wiens A, de Souza TT, et al. Efficacy and safety of biologic therapies for systemic lupus erythematosus treatment: systematic review and meta-analysis. BioDrugs Clin Immunother Biopharm Gene Ther. 2014;28:211–228. doi: 10.1007/s40259-013-0074-x. [DOI] [PubMed] [Google Scholar]
- 50.Singh JA, Shah NP, Mudano AS. Belimumab for systemic lupus erythematosus. Cochrane Database Syst Rev. 10.1002/14651858.CD010668.pub2. Epub ahead of print 2021. [DOI] [PMC free article] [PubMed]
- 51.Sciascia S, Radin M, Yazdany J, et al. Efficacy of belimumab on renal outcomes in patients with systemic lupus erythematosus: A systematic review. Autoimmun Rev. 2017;16:287–293. doi: 10.1016/j.autrev.2017.01.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The published article contains summarised versions of significant results generated and analysed during this study. A full set of the unedited data presented via forest plots is provided in the supplementary files.