Abstract
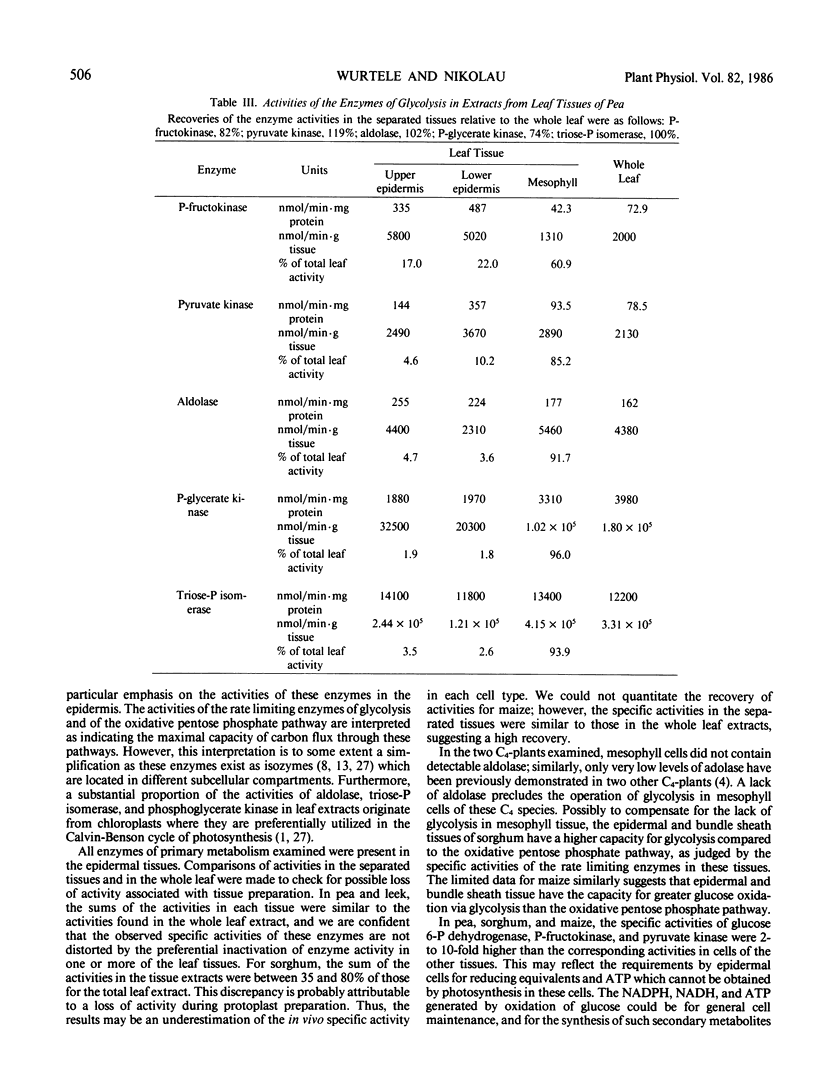
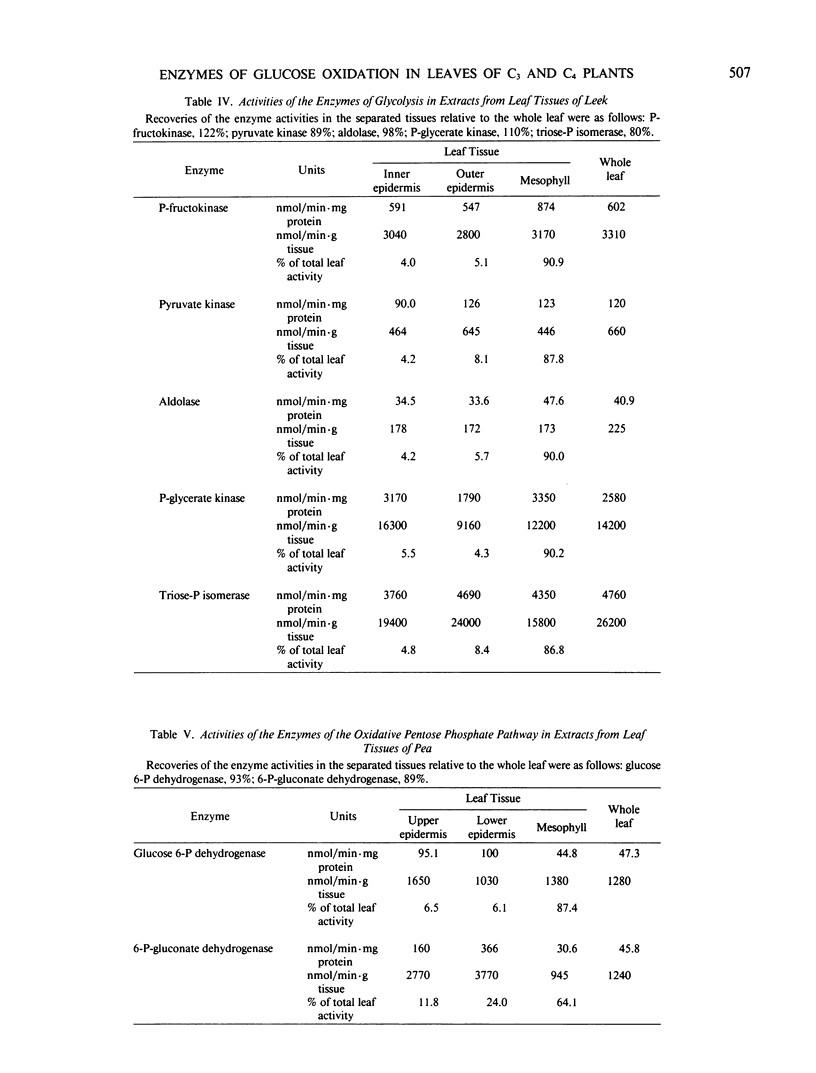
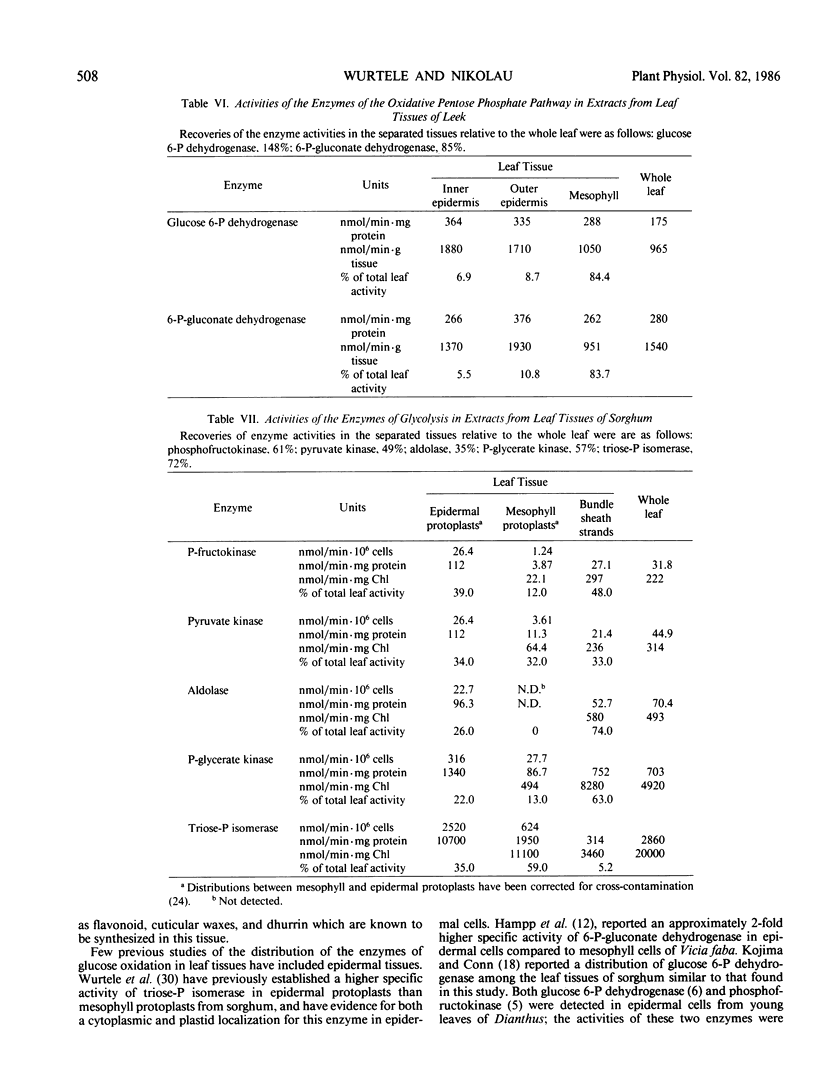
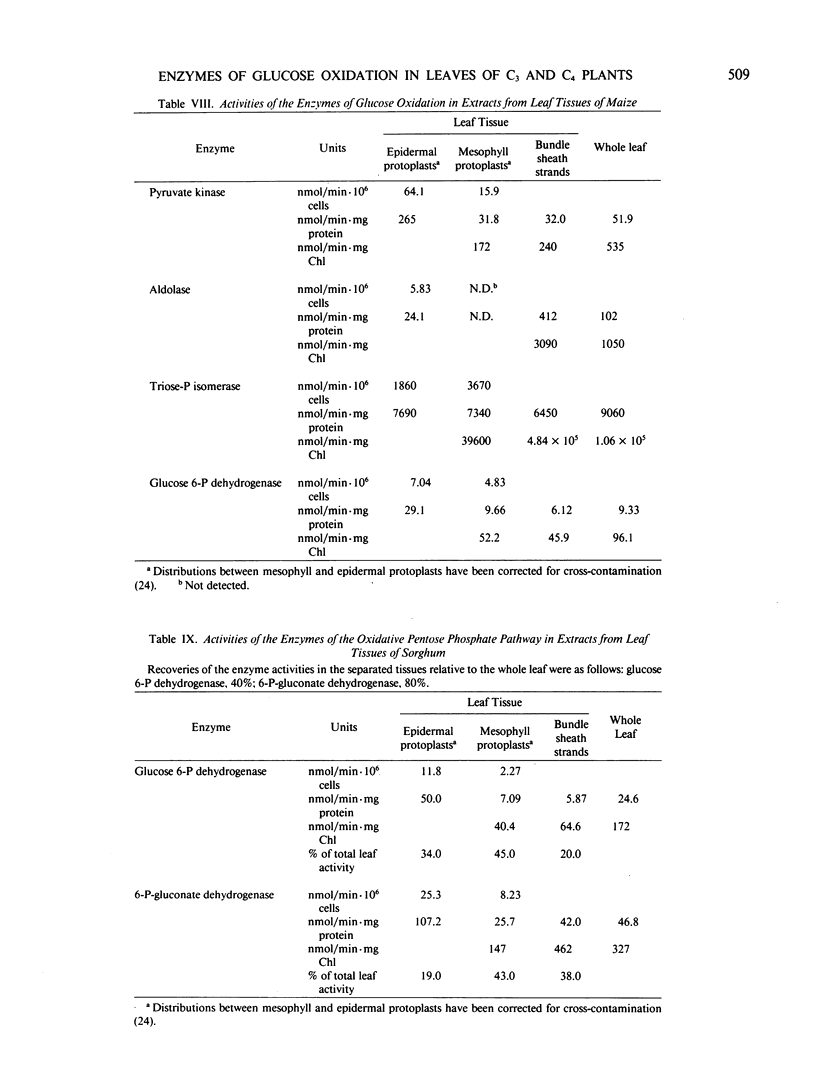
The distribution of the glycolytic enzymes, phosphofructokinase, aldolase, triosephosphate isomerase, phosphoglycerate kinase, pyruvate kinase, and the oxidative pentose phosphate pathway enzymes, glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase, was determined in the leaf tissues of two C3-plants, pea and leek, and two C4-plants, maize and sorghum. All enzymes examined were found in epidermal tissue. In pea, maize, and sorghum leaves, the specific activities of these enzymes were usually higher in the nonphotosynthetic epidermal tissue than in the photosynthetic tissues of the leaves. In leek leaves, which were etiolated, specific activities were similar in both epidermal and mesophyll tissue. The distribution of the rate limiting enzymes of glycolysis and the oxidative pentose phosphate pathways probably reflects the capacity of each tissue to generate NADH, NADPH, and ATP from the oxidation of glucose. This capacity appears to be greater in leaf tissues unable to generate reducing equivalents and ATP by photosynthesis, that is, in epidermal tissues and etiolated mesophyll tissue.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Croxdale J. G. Quantitative Measurements of Phosphofructokinase in the Shoot Apical Meristem, Leaf Primordia, and Leaf Tissues of Dianthus chinensis L. Plant Physiol. 1983 Sep;73(1):66–70. doi: 10.1104/pp.73.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland W. J., Dennis D. T. Plastid and cytosolic phosphofructokinase from the developing endosperm of Ricinus communis. I. Separation, purification, and initial characterization of the isozymes. Arch Biochem Biophys. 1980 Oct 1;204(1):302–309. doi: 10.1016/0003-9861(80)90037-5. [DOI] [PubMed] [Google Scholar]
- Hampp R., Outlaw W. H., Tarczynski M. C. Profile of Basic Carbon Pathways in Guard Cells and Other Leaf Cells of Vicia faba L. Plant Physiol. 1982 Dec;70(6):1582–1585. doi: 10.1104/pp.70.6.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrazdina G., Marx G. A., Hoch H. C. Distribution of Secondary Plant Metabolites and Their Biosynthetic Enzymes in Pea (Pisum sativum L.) Leaves : Anthocyanins and Flavonol Glycosides. Plant Physiol. 1982 Sep;70(3):745–748. doi: 10.1104/pp.70.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland R. J., Deluca V., Dennis D. T. Isoenzymes of pyruvate kinase in etioplasts and chloroplasts. Plant Physiol. 1979 May;63(5):903–907. doi: 10.1104/pp.63.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M., Conn E. E. Tissue Distributions of Chlorogenic Acid and of Enzymes Involved in Its Metabolism in Leaves of Sorghum bicolor. Plant Physiol. 1982 Sep;70(3):922–925. doi: 10.1104/pp.70.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M., Poulton J. E., Thayer S. S., Conn E. E. Tissue Distributions of Dhurrin and of Enzymes Involved in Its Metabolism in Leaves of Sorghum bicolor. Plant Physiol. 1979 Jun;63(6):1022–1028. doi: 10.1104/pp.63.6.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessire R., Stumpe P. K. Nature of the Fatty Acid Synthetase Systems in Parenchymal and Epidermal Cells of Allium porrum L. Leaves. Plant Physiol. 1983 Nov;73(3):614–618. doi: 10.1104/pp.73.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolau B. J., Wurtele E. S., Stumpf P. K. Tissue distribution of acetyl-coenzyme a carboxylase in leaves. Plant Physiol. 1984 Aug;75(4):895–901. doi: 10.1104/pp.75.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacold I., Anderson L. E. Chloroplast and Cytoplasmic Enzymes: VI. Pea Leaf 3-Phosphoglycerate Kinases. Plant Physiol. 1975 Feb;55(2):168–171. doi: 10.1104/pp.55.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippa M., Signorini M. 6-Phosphogluconate dehydrogenase from Candida utilis. Methods Enzymol. 1975;41:237–240. doi: 10.1016/s0076-6879(75)41054-0. [DOI] [PubMed] [Google Scholar]
- Wurtele E. S., Nikolau B. J., Conn E. E. Tissue Distribution of beta-Cyanoalanine Synthase in Leaves. Plant Physiol. 1984 Aug;75(4):979–982. doi: 10.1104/pp.75.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtele E. S., Thayer S. S., Conn E. E. Subcellular Localization of a UDP-Glucose:Aldehyde Cyanohydrin beta-Glucosyl Transferase in Epidermal Plastids of Sorghum Leaf Blades. Plant Physiol. 1982 Dec;70(6):1732–1737. doi: 10.1104/pp.70.6.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]


