Abstract
Management of stable coronary artery disease (CAD) has been based on the assumption that flow-limiting atherosclerotic obstructions are the proximate cause of angina and myocardial ischemia in most patients and represent an important target for revascularization. However, the role of revascularization in reducing long-term cardiac events in these patients has been limited mainly to those with left main disease, 3-vessel disease with diabetes, or decreased ejection fraction. Mounting evidence indicates that nonepicardial coronary causes of angina and ischemia, including coronary microvascular dysfunction, vasospastic disorders, and derangements of myocardial metabolism, are more prevalent than flow-limiting stenoses, raising concerns that many important causes other than epicardial CAD are neither considered nor probed diagnostically. There is a need for a more inclusive management paradigm that uncouples the singular association between epicardial CAD and revascularization and better aligns diagnostic approaches that tailor treatment to the underlying mechanisms and precipitants of angina and ischemia in contemporary clinical practice.
Keywords: coronary microvascular dysfunction, epicardial coronary artery disease, myocardial ischemia, percutaneous coronary intervention, revascularization, stable angina
Since the advent of coronary angiography more than 60 years ago, stable coronary artery disease (CAD) management has been based on the plausible assumption that “significant” flow-limiting atherosclerotic obstructions of epicardial coronary arteries are the proximate cause of angina and myocardial ischemia in most cases. This belief, supported by anatomic and physiologic evidence that obstructive coronary stenoses can result in regional ischemia and may, in the acute setting, cause acute myocardial infarction (MI), has profoundly influenced our approach to CAD management. In acute MI patients, either percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) can restore coronary flow and improve event-free survival.1,2 There is also a prevalent belief that epicardial coronary stenosis remains the dominant cause or sine qua non of stable angina and ischemia. While, indeed, revascularization may reduce incident cardiac events in high-risk subsets with stable CAD (eg, left main disease, 3-vessel CAD with diabetes, and decreased ejection fraction), evidence from multiple randomized controlled trials (RCTs) has shown that revascularization of epicardial coronary obstructions, particularly with the use of PCI, does not reduce mortality or morbidity compared with guideline-directed medical therapy (GDMT) in the great majority of stable CAD patients.3,4
While revascularization of epicardial stenoses provides better symptom relief and improved quality of life compared with GDMT, recurrence of angina ranges between 20% and 30% within a year after successful PCI5 and up to 40% within 3 years,6 frequently leading to subsequent coronary angiography and repeated PCI. However, because repeat angiography often reveals no evidence of in-stent restenosis or residual coronary obstruction, it is essential to consider nonobstructive causes of angina. Thus, an often-unforeseen consequence of focusing disproportionately on epicardial coronary obstruction is that other pathogenetically important causes of angina and ischemia may not be considered. These causes include epicardial or microvascular coronary vasospasm, coronary microvascular dysfunction (CMD), and derangements of myocardial energy or metabolism.7
Accordingly, there is a need for a new, more broadly inclusive, management paradigm for patients with stable angina that uncouples the often-singular association between obstructive CAD and revascularization. Because there are many other potential pathogenetic mechanisms responsible for angina and ischemia, it is essential to identify diagnostic and therapeutic approaches to better tailor appropriate treatment of both obstructive and nonobstructive causes of myocardial ischemia. In so doing, a more pathogenetically directed approach to diagnosing and treating angina and ischemia would more likely align pharmacologic and procedural interventions as complementary and synergistic for a broader population of stable CAD patients.
LESSONS LEARNED FROM RECENT COMPARATIVE EFFECTIVENESS TRIALS
Earlier RCTs3,4 showed no incremental benefit of revascularization in reducing mortality, MI, and repeated revascularization when added to GDMT, which included multifaceted pharmacologic secondary prevention and lifestyle intervention. Those studies, however, had limitations, eg, inclusion of low-risk subjects, those with mild to moderate baseline ischemia, use of bare-metal or first-generation drug-eluting stents, and lack of blinding before diagnostic coronary angiography, that may have resulted in exclusion of subjects with severe angiographic obstructive disease. The ISCHEMIA (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches) randomized patients with moderate-to-severe inducible ischemia to an initial invasive strategy with revascularization (third-generation drug-eluting stents or CABG) plus GDMT vs an initial conservative strategy of GDMT alone.8 It found no benefit of an invasive approach on the primary endpoint (cardiovascular death, MI, resuscitated sudden cardiac death, or hospitalization for unstable angina or heart failure) or secondary endpoint (cardiovascular death or MI). The invasive strategy did result in a statistically significant quality of life improvement, although the overall effect was modest and concentrated mainly in the ~20% of patients with weekly to daily angina.9
In addition, a meta-analysis of GDMT with or without PCI in patients with stable CAD (10 RCTs comprising 12,125 patients, including ISCHEMIA)10 confirmed that PCI did not reduce mortality or MI vs GDMT alone, though the invasive strategy was associated with fewer follow-up revascularizations and improved anginal symptoms.
To evaluate potential bias in unblinded trials, the efficacy of PCI for the treatment of angina was studied in a placebo-controlled trial, which showed no incremental improvement in treadmill walking time, angina relief, or quality of life with PCI + GDMT vs a placebo procedure + GDMT.11 While limited by the small sample size and short follow-up, this study raises the issue of whether the observed salutary effect on angina relief attributed to PCI in earlier unblinded trials was due, at least in part, to a placebo effect.12
Finally, we should recognize that managing patients with stable angina must include informed and well considered decision making involving the patient, family, and physician. Both invasive and conservative approaches may be appropriate and should not be viewed as competing treatment approaches but rather as complementary and potentially additive strategies to enhance optimal patient-centered outcomes.13,14
WHY REVASCULARIZATION MAY NOT BE A THERAPEUTIC SOLUTION IN MANY STABLE ANGINA PATIENTS
In contrast to type 1 MI, for which prompt revascularization is indicated,1,2 revascularization has not been shown to reduce cardiac events in most stable CAD patients.3,4,8,15 Because atherosclerosis is fundamentally a systemic vascular and inflammatory condition affecting epicardial arteries and coronary microcirculation as well as other vascular beds, appropriate GDMT management of ischemia and atherosclerosis must include lifestyle modification (diet, exercise, tobacco cessation), intensive risk factor control, and multifaceted pharmacologic secondary prevention (targeting hypertension, dyslipidemia, diabetes, and perhaps inflammation), and, when angina is present, effective symptom control.16,17
Important observational data from recent large registries indicate that self-reported angina may improve or resolve over time with medical therapy in most stable CAD patients,18 and subsequent revascularization may be needed in only a minority of patients (~5%) during 5-year follow-up.19 Because angina may relapse or remit over time and coronary plaques may become quiescent, an appropriate assessment of angina requires careful follow-up and systematic ascertainment of patient-reported symptoms and quality of life. Thus, a sufficient time horizon (3–6 months) is often required for an empiric course of GDMT to be adequately evaluated and efficacy assessed.20,21 Finally, difficulty in achieving optimal GDMT should not necessarily represent justification to refer patients for revascularization, particularly if a sufficient empiric trial has not been implemented19–22 or if symptoms are infrequent and mild. Instead, effective GDMT can be achieved by an iterative process that entails collaboration with patients, along with education and counseling, toward a goal of largely patient-directed self-care.23–25
Nevertheless, ensuring that patients are treated optimally with both lifestyle intervention and multifaceted pharmacologic secondary prevention is time and labor intensive, and many cardiologists to whom patients are referred for specific diagnostic testing, including invasive angiography and revascularization, may lack resources to oversee the intensification of medical therapy personally. Therefore, more inclusive and coordinated team-management strategies incorporating physician extenders (nurse practitioners, physician assistants, pharmacists) are needed to facilitate optimization of GDMT and improve patient care.23 Using standardized care pathways and management algorithms may further enhance the use of these proven approaches.24,25 Finally, implementation of GDMT likewise remains suboptimal in patients undergoing revascularization,26–28 and such therapies must be similarly prioritized to reduce incident events following revascularization.
IMPORTANCE OF DIAGNOSING ANGINA AND ISCHEMIA ACCORDING TO THE UNDERLYING PATHOGENETIC CAUSE(S)
Essential insights on the need for a more encompassing view of the many causes and precipitants of angina and ischemia derive from the SCOT-HEART (Scottish Computed Tomography of the Heart) trial.29 There, most patients with known or suspected stable CAD did not have flow-limiting stenoses, indicating that the vast majority (approximately 4 in 5 individuals) had underlying causes of angina and ischemia not attributed to epicardial stenoses (Figure 1). For this reason, a purely anatomic diagnostic approach using invasive coronary angiography or coronary computed tomography angiography (CCTA) may fail to diagnose microvascular and/or vasospastic angina as treatable causes of angina, leaving many patients in whom no obstructive coronary lesions are identified and thence falsely reassured that ischemia is not present. Often such patients are discharged from cardiology, at which point myriad potential (and costly) noncardiac causes are probed rather than pursuing a more diligent evaluation of nonepicardial coronary causes of angina.
FIGURE 1. Clinical Assessment of Myocardial Ischemia and CAD.
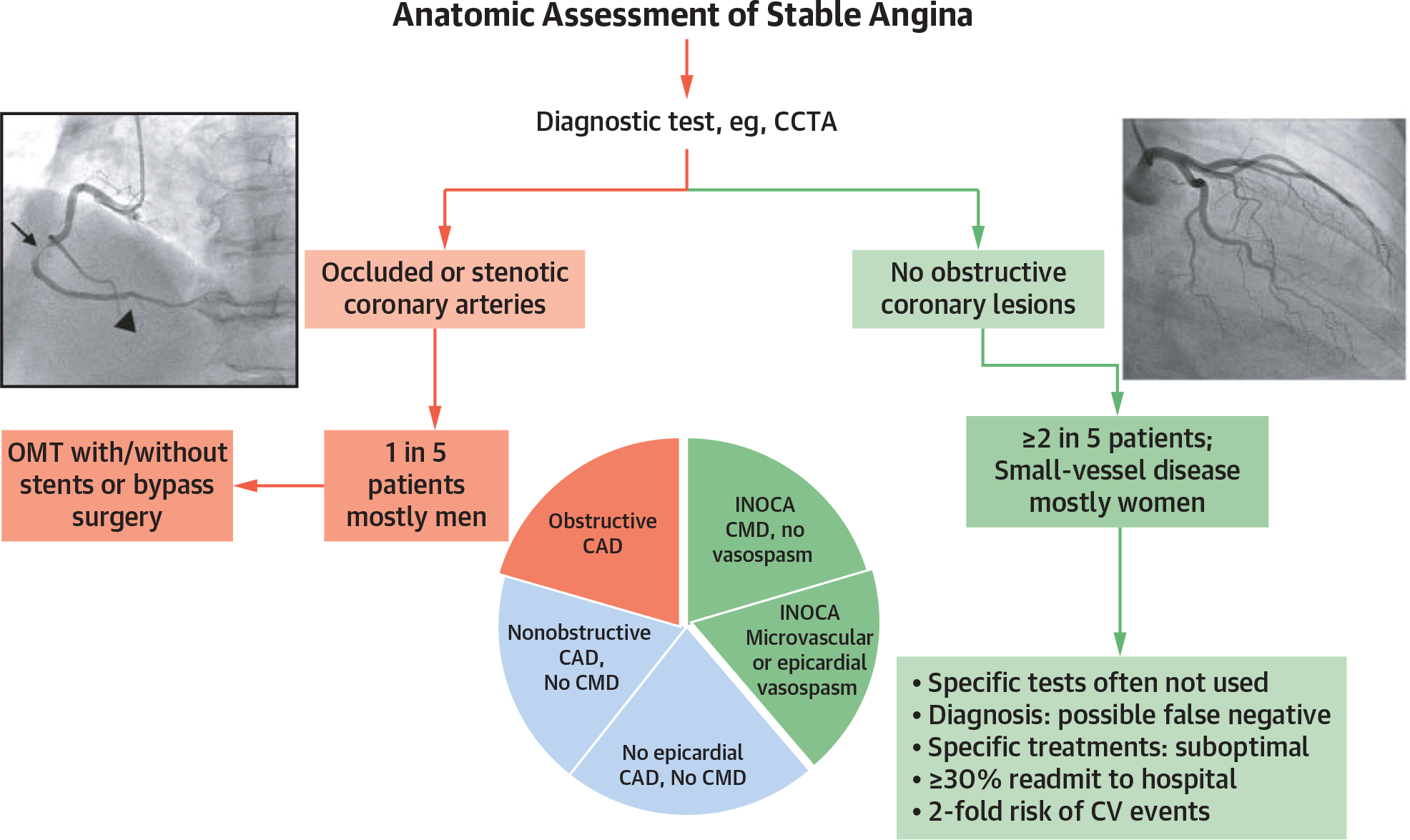
In SCOT-HEART,33 most patients with suspected stable coronary artery disease (CAD) did not have epicardial coronary obstructions, with the vast majority (~4 in 5) having angina and/or ischemia not due to epicardial stenoses. CCTA = coronary computed tomography angiography; CMD = coronary microvascular dysfunction; CV = cardiovascular; INOCA = ischemia with no obstructive coronary arteries; MI = myocardial infarction; OMT = optimal medical therapy.
This is particularly important for women, because most patients with ischemia and no obstructive coronary arteries (INOCA) are female.30 Heart disease in women is underrecognized and undertreated, particularly INOCA, where failure to account for microvascular and vasospastic angina within the primarily noninvasive anatomic imaging strategy may result in misdiagnosis.31 Certain stakeholder organizations have recognized that using CCTA as the primary diagnostic testing strategy in angina patients may help only in diagnosing obstructive epicardial CAD, which is not the most common cause of angina and is even less common in women than men.32
Indeed, a large observational study of almost 400,000 angina patients undergoing elective coronary angiography found that, among those with a positive noninvasive stress test, only 41% had obstructive CAD,33 indicating a need to embrace a more inclusive management approach that includes many other pathophysiologic mechanisms, including CMD and coronary vasospasm (epicardial and/or microvascular).34–36 Similarly, the 2019 European Society of Cardiology guidelines on chronic coronary syndromes showed that, among patients with typical angina in the most common age range for detecting stable CAD (50–59 years), 68% of men and 87% of women did not have obstructive coronary stenoses,37 and the CorMicA (Coronary Microvascular Angina) trial36 and others34 revealed that approximately 45% of patients presenting with angina or ischemia did not have CAD at angiography. Yet nearly 90% of these patients demonstrated objective evidence of coronary vasomotor dysfunction,38 including 81% with CMD. Thus, in a sizable proportion of suspected stable CAD patients, CMD or epicardial vasoconstriction can contribute to angina, and because functional mechanisms may coexist with obstructive CAD, these ischemia precipitants are not necessarily mutually exclusive and may often occur in the same patient.39Accordingly, a complete medical evaluation of stable angina patients should characterize the natural history, cardiovascular risk factors, physical examination, and pharmacotherapy (including treatment response, medication intolerance, and adherence). Treadmill exercise testing remains useful to assess functional capacity, response to the physiologic stress of exercise, and limiting symptoms and features of inducible ischemia (notably symptoms and electrocardiographic changes). The response to treatment can be diagnostically informative, and the initial management plan should include antianginal drug therapy, such as short-acting nitrates and either a beta-blocker or a calcium-channel blocker. This initial approach complements referral for CCTA because heart rate control (target 60 beats/min) is required for optimal imaging.
HOW TO DIAGNOSE AND MANAGE VASOSPASM, MICROVASCULAR DYSFUNCTION, AND OTHER CAUSES OF MYOCARDIAL ISCHEMIA
Both the 2021 AHA/ACC chest pain guideline40 and the 2019 European Society of Cardiology chronic coronary syndromes guideline37 delineate the 3 different mechanisms of stable angina (obstructive CAD, coronary vasospasm, and CMD). However, a fundamental limitation is the lack of a standard diagnostic evaluation for all patients with suspected angina. Although anginal chest discomfort is “the alarm system of the heart” and often the cardinal symptom of myocardial ischemia, it does not provide specificity on its cause. Therefore, it is critical not only to rule in or rule out obstructive CAD but also to establish the cause of myocardial ischemia and to prove or disprove the ischemic origin of symptoms. Such a diagnostic evaluation that comprehensively assesses anatomic and functional coronary alterations would help to confirm or exclude the diagnosis of myocardial ischemia and determine the precipitating cause whenever possible.
Myocardial perfusion imaging using positron emission tomography or cardiovascular magnetic resonance imaging is useful for diagnosing CMD.41 These noninvasive imaging techniques provide quantitative and qualitative information on inducible myocardial ischemia. Dynamic first-pass vasodilator stress/rest positron emission tomography uses radiotracers (eg, 82Rb, 13N-ammonia, 15O-H2O) and quantifies absolute myocardial blood flow.42 Advances with stress cardiovascular magnetic imaging include fully automatic pixelwise quantitative mapping of myocardial perfusion.43,44 This method generates pixel-encoded maps of myocardial blood flow (mL/min per g tissue) during vasodilator stress and at rest. Postprocessing software gives accurate measurements for both regional and global stress and resting myocardial blood flow and myocardial perfusion reserve (the ratio of stress to rest myocardial blood flow). A myocardial perfusion reserve <2.0, in the absence of obstructive CAD, is widely accepted as the CMD threshold associated with adverse outcomes.41
An algorithm for practical assessment of the multiple causes of angina and ischemia is proposed in the Central Illustration. It outlines a pragmatic approach stemming from current international guideline recommendations and results of landmark studies.3,8,29 It supports an initial evidence-based approach, including lifestyle interventions and pharmacologic secondary prevention with GDMT, to achieve and maintain multiple cardiovascular treatment targets for blood pressure, lipids, and glycemic levels as per the current U.S.40 and European guidelines.37 This algorithm endorses selective functional or anatomic imaging to identify high-risk subsets of stable CAD patients for whom revascularization is more appropriate than medical therapy alone.
CENTRAL ILLUSTRATION. Management Algorithm for Obstructive and Nonobstructive Coronary Causes of Angina.
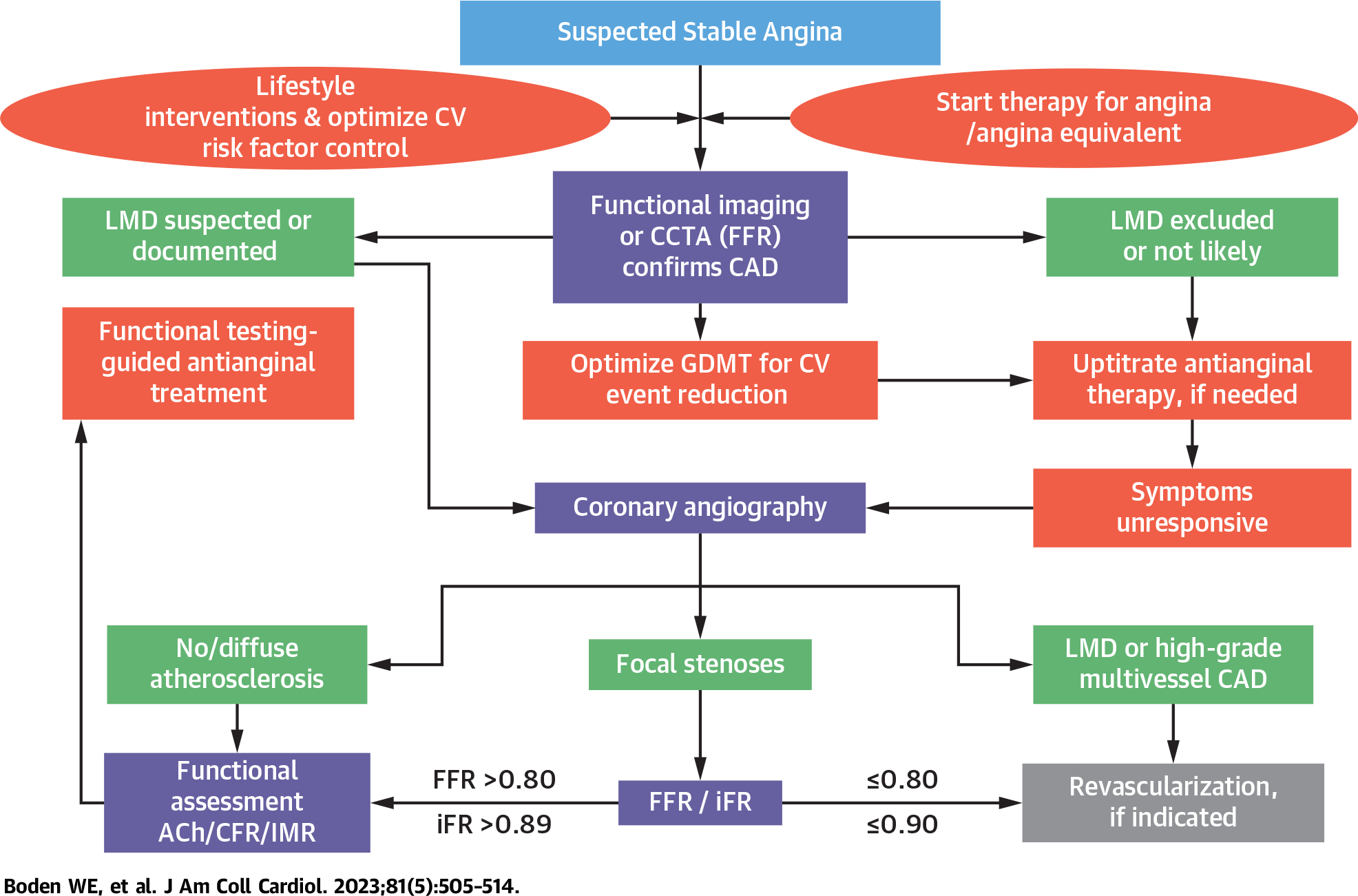
A more inclusive management paradigm for stable coronary artery disease (CAD) patients that addresses the many pathogenetic mechanisms responsible for angina and ischemia is necessary to identify diagnostic and therapeutic approaches that would better tailor the appropriate treatment of obstructive and nonobstructive causes of myocardial ischemia to the underlying ischemia precipitants. Such an approach seeks to promote both evidence-based pharmacologic secondary prevention and procedural interventions as complementary and potentially additive treatments to optimize the management of stable angina patients. ACh = acetylcholine; CCTA = coronary computed tomography angiography; CFR = coronary flow reserve; CV = cardiovascular; FFR = fractional flow reserve (a hyperemic pressure ratio);GDMT = guideline-directed medical therapy; iFR = instantaneous free wave ratio; IMR = index of microvascular resistance; LMD = left main disease.
If noninvasive studies identify a very low angina threshold and/or a large area of ischemic myocardium at risk during noninvasive stress testing, CCTA or invasive coronary angiography is appropriate to exclude left main and/or high-grade multivessel CAD. In all other chronic stable angina patients, an initial trial of empiric antianginal treatment is an important initial step and up-titrating dosages or adding agents for symptom control, as needed, is advocated.21,22 Stable CAD patients with angina should receive at least 2 antianginal drug classes and adjusted over 3 to 6 months before referral for revascularization, particularly if anginal symptoms are mild or infrequent (Figure 2).
FIGURE 2. Antianginal Treatment Directed to the Mechanism Responsible for Ischemia.
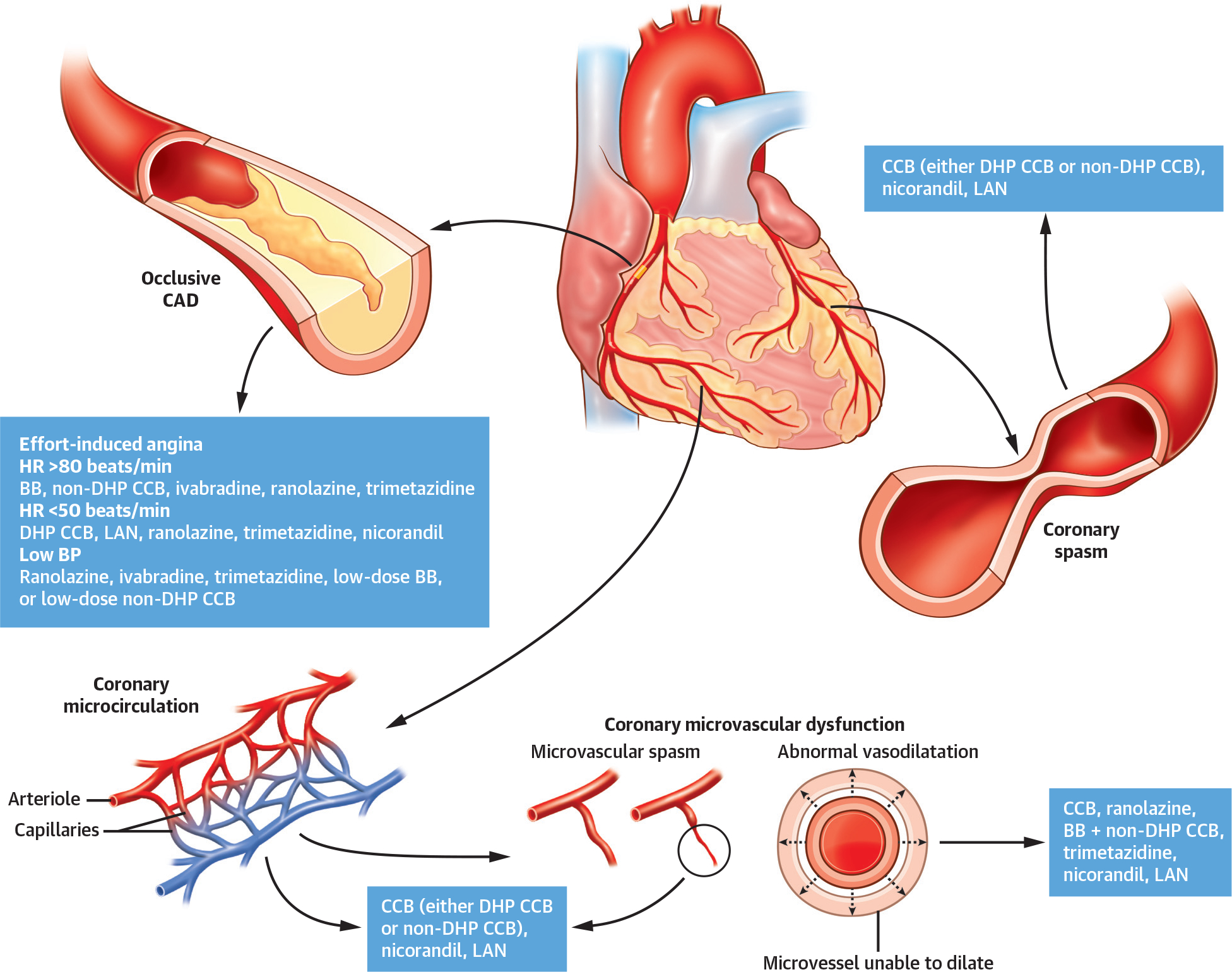
For exertional angina, antianginal drugs that reduce myocardial oxygen consumption (ie, beta-blockers [BBs], nondihydropyridine calcium-channel blockers [CCBs], or ivabradine) are most efficacious, whereas for variable threshold angina or coronary microvascular dysfunction, agents that improve myocardial oxygen utilization (ie, ranolazine or trimetazidine) are suitable treatment options. CCBs are the preferred option for epicardial or microvascular spasm, but nitrates and nicorandil may also be appropriate. BP = blood pressure; CAD = coronary artery disease; DHP = dihydropyridine; HR = heart rate; LAN = long-acting nitrate.
In those with persistent or recurrent ischemic symptoms despite intensive symptomatic treatment, coronary angiography is indicated to identify patients with flow-limiting stenoses who might benefit from myocardial revascularization. In patients without obstructive stenosis, the functional assessment of coronary circulation, including acetylcholine testing for spasm, coronary flow reserve, and microvascular resistance, should be considered to guide subsequent pharmacologic treatment. This algorithm allows tailoring of the diagnostic workup to the clinical situation (Central Illustration) and places less emphasis on CCTA, which, as currently used, is unable to detect functional coronary alterations (endothelial dysfunction or vasospasm) responsible for ischemia. In SCOT-HEART,29 nonfatal MI at 5 years was lower in the CCTA-guided group than in the standard care group, but there was no effect on mortality. Secondary prevention therapy, including aspirin and statins, was higher in the CCTA-guided group, further implying that disclosure of atherosclerosis resulted in linked therapy.
Ideally, the above-proposed diagnostic evaluation should be performed in all stable angina patients in whom obstructive CAD has been excluded, but, from a practical standpoint, many centers will not have access to such sophisticated testing modalities or may lack the skill or expertise to undertake such evaluations, and there are potential cost-effectiveness concerns that need to be considered as well. Therefore, we advocate additional diagnostic testing, described above, only after obstructive CAD has been excluded and only if symptoms do not improve (or if they worsen) despite appropriate antianginal therapy of at least 2 drug classes.21
WHY WE NEED A PARADIGM SHIFT IN OUR APPROACH TO ANGINA AND ISCHEMIA
Cardiologists should reappraise their thinking of angina and prioritize the following: 1) angina may be due to obstructive CAD and/or INOCA; 2) most patients presenting with chronic angina do not have epicardial coronary obstructions; 3) if CCTA is the initial diagnostic test and obstructive coronary stenoses are excluded, subsequent testing should include stress perfusion imaging, positron emission tomography, and/or invasive functional coronary angiography with pharmacologic testing to detect coronary microvascular or vasospastic mechanisms that may require more targeted therapy; and 4) most INOCA patients are women, and a diagnostic strategy with a singular focus on defining epicardial coronary obstructions may be inadequate.
Of interest, a comprehensive noninvasive diagnostic approach that contemplates both anatomic and functional issues may be provided by multimodality imaging such as PET/CCTA or “dynamic” CCTA and could be viewed as a noninvasive “one-stop shop” model to diagnose angina and suspected CAD, both obstructive and nonobstructive.45 Ongoing RCTs will determine whether dynamic CCTA fulfills this promise.
PHARMACOLOGIC MANAGEMENT TARGETING THE PRECIPITANTS OF ANGINA AND ISCHEMIA
A reduction of coronary/myocardial flow reserve may reflect ischemia due to epicardial stenoses, impaired microvascular function, or both, even in the same patient, as noted above. In this setting, drugs that reduce myocardial oxygen consumption (beta-blockers, nondihydropyridine calcium-channel blockers, or ivabradine) or optimize myocardial oxygen utilization (ranolazine or trimetazidine) are likely the best option. Their combination can also be considered (Figure 2). Alternatively, ischemia can also be caused by epicardial or microvascular spasm. In this setting, vasodilators (calcium-channel blockers, nitrates, or nicorandil) are most appropriate, and their combination can also be considered. Thus, to the extent possible, it is highly desirable to tailor pharmacologic therapies to the underlying causes and precipitants of ischemia.
CONCLUSIONS: WHERE DO WE GO FROM HERE?
The time has come for a paradigm shift in managing stable CAD patients. First, we need to expand our current scientific thinking about the many causes and mechanisms of both angina and myocardial ischemia and uncouple the narrow association of ischemia with obstructive epicardial disease46 as the guiding approach to management. Both angina and ischemia have many causes, but obstructive epicardial disease may or may not be the underlying pathogenetic mechanism (Central Illustration, Figure 2). Therefore, our nomenclature should reflect the actual causes of ischemia and angina beyond the currently used terms “coronary” and “disease,” both of which connote epicardial coronary obstruction and are perhaps too narrowly restrictive. A more inclusive and descriptive nomenclature might be considered, such as “acute and chronic myocardial ischemic syndromes.”47
Second, we must embrace a more enlightened management approach. Assessments of ischemia that do not delineate abnormal coronary angiographic findings should not necessarily shift diagnostic and therapeutic considerations to noncardiac causes of angina but rather to exploring nonepicardial coronary causes (eg, CMD and vasospastic disorders). We must remain mindful that the evaluation and treatment of angina and ischemia need to be tailored to the individual patient and that adoption of available diagnostic tools required for personalized approaches in clinical practice remains challenging.
Third, we must invest in developing newer management strategies and health care delivery models that may better align with treatments proven to benefit patients and society.48–52 Proven secondary prevention strategies and lifestyle interventions in contemporary GDMT continue to be underutilized, particularly in the United States, where as few as 40% to 50% of eligible CAD subjects are treated according to established clinical practice guidelines, including those who have been revascularized.26,49,51 A recent Viewpoint52 addressing the new coronary artery revascularization recommendations53 underscoresthe critical importance of concomitant preventive therapies in enhancing event-free survival and improving outcomes in stable CAD patients who had undergone CABG or PCI. In this way, perhaps we can rebalance patient management in a way that does not view procedural and pharmacologic interventions as competing treatments but rather as complementary and additive therapeutic approaches best suited to achieving optimal clinical outcomes and symptom relief for our patients.
HIGHLIGHTS.
Several mechanisms other than obstructive coronary artery disease may cause myocardial ischemia.
A conservative approach to management, including noninvasive testing, lifestyle interventions, and goal-directed multifaceted medical therapy, is evidence based and often effective in patients with stable angina.
Pharmacologic and procedural approaches to stable ischemic heart disease are complementary, and integrating these can optimize outcomes.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
This publication was supported by an educational grant from Servier, Suresnes Cedex, France. Support in the development of the manuscript was also provided by Liberum IME, London, United Kingdom. Dr Boden receives research grant support from AbbVie, Amarin Pharmaceuticals, Amgen, AstraZeneca, Sanofi, Massachusetts Veterans Epidemiology Research and Information Center, VA New England Healthcare System’s Clinical Trials Network, and the National Heart, Lung, and Blood Institute (NHLBI), where he served as national coprincipal investigator for the ISCHEMIA trial; is on the Board of Directors for the Boston VA Research Institute; receives consulting fees from Amarin Pharmaceuticals, Amgen, Janssen Pharmaceuticals, Metuchen Pharmaceuticals, and Servier; is on the VA Cooperative Studies Program data monitoring committee; and has received speaking honoraria from Amarin, Amgen, Janssen Pharmaceuticals, Pfizer, and Servier. Dr Marzilli lectures for Servier, Menarini, Degussa Pharma Group, Baldacci, Abbott, and AstraZeneca. Dr Crea has received personal speaker fees from Amgen, AstraZeneca, Servier, and BMS; and is a member of the advisory board for GlyCardial Diagnostics. Dr Mancini has received grants, advisory board appointments, and honoraria for educational lectures from Amgen, Sanofi, NovoNordisk, Lilly/Boehringer Ingelheim, and HLI Therapeutics; is on the advisory board for Esperion; and receives reports on grants from the National Institutes of Health (NIH) for the COURAGE and ISCHEMIA trials. Dr Weintraub has received research support from Amarin Corporation, NIH, and Centers for Disease Control and Prevention; and has served as a consultant for Amarin Corporation, AstraZeneca, Pfizer, Janssen, SC Pharma, Lexicon, Faraday, and The Medicines Company. Dr Taqueti is supported by NIH grant K23HL135438. Dr Pepine receives research grant support from NIH/NHLBI (R21AG063143, K08 HL130945, K01 HL138172, R01 HL146158, AG065141, R01 HL152162, R21 HL152264, R01 HL132448, UM1 HL087366, UM1 HL087318), NIH/National Center for Advancing Translational Sciences (UL1 TR001427), U.S. Department of Defense (W81XWH-17-2-0030, WARRIOR), Biocardia, Brigham and Women’s Hospital, CSL Behring, Cytori Therapeutics, DCRI, GE Healthcare, Mesoblast, and Pfizer; receives consultant fees/honoraria from Caladrius Biosciences, Slack, and Xylocor; and receives grant support from the Gatorade Foundation through the University of Florida Department of Medicine and from the McJunkin Family Foundation, Plantation, Florida. Dr Escaned has received speaker and advisory board honoraria from Abbott Laboratories and Philips. Dr Al-Lamee has received speaker honoraria from Philips Volcano, Abbott Vascular, and Menarini Pharmaceuticals. Dr Gowdak has received congress-related travel expenses from Servier; has participated in clinical trials sponsored by Servier and Angion Biomedica; has been a speaker for Servier, Boehringer-Lilly, and Abbott; is an advisory board member for Servier; and has prepared written scientific material for Servier and Abbott. Dr Berry is employed by the University of Glasgow, which holds consultancy and research agreements for his work with Abbott Vascular, AstraZeneca, Auxilius Pharma, Boehringer Ingelheim, Causeway Therapeutics, Coroventis, Genentech, GSK, HeartFlow, Menarini, Neovasc, Siemens Healthcare, and Valo Health; and receives research funding from the British Heart Foundation (grant RE/18/6134217), Chief Scientist Office, Engineering and Physical Sciences Research Council (EP/R511705/1, EP/S030875/1), European Union (754946–2), Medical Research Council (MR/S018905/1), and UKRI (MC/PC/20014). Dr Kaski has received speaker honoraria from A. Menarini Farmaceutica lnternazionale, Servier, and Bayer UK. Dr Escaned has reported that he has no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- CABG
coronary artery bypass grafting
- CAD
coronary artery disease
- CCTA
coronary computed tomography angiography
- CMD
coronary microvascular dysfunction
- GDMT
guideline-directed medical therapy
- INOCA
ischemia and no obstructive coronary arteries
- MI
myocardial infarction
- PCI
percutaneous coronary Intervention
- RCT
randomized controlled trial
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
REFERENCES
- 1.Grines C, Patel A, Zijlstra F, et al. Primary coronary angioplasty compared with intravenous thrombolytic therapy for acute myocardial infarction: six-month follow up and analysis of individual patient data from randomized trials. Am Heart J. 2003;145:47–57. [DOI] [PubMed] [Google Scholar]
- 2.Kirov H, Caldonazo T, Rahouma M, et al. A systematic review and meta-analysis of percutaneous coronary intervention compared to coronary artery bypass grafting in non–ST-elevation acute coronary syndrome. Sci Rep. 2022;12:5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. [DOI] [PubMed] [Google Scholar]
- 4.BARI 2D Study Group, Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben-Yehuda O, Kazi DS, Bonafede M, et al. Angina and associated healthcare costs following percutaneous coronary intervention: a real-world analysis from a multi-payer database. Catheter Cardiovasc Interv. 2016;88:1017–1024. [DOI] [PubMed] [Google Scholar]
- 6.Crea F, Bairey Merz CN, Beltrame JF, et al. Mechanisms and diagnostic evaluation of persistent or recurrent angina following percutaneous coronary revascularization. Eur Heart J. 2019;40:2455–2462. [DOI] [PubMed] [Google Scholar]
- 7.Kaski J-C, Crea F, Gersh BJ, Camici PG. Reappraisal of ischemic heart disease. Circulation. 2018;138:1463–1480. [DOI] [PubMed] [Google Scholar]
- 8.Maron DJ, Hochman JS, Reynolds HR, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382:1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spertus JA, Jones PG, Maron DJ, et al. Health-status outcomes with invasive or conservative care in coronary disease. N Engl J Med. 2020;382:1408–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah R, Nayyar M, Le FK, et al. A meta-analysis of optimal medical therapy with or without percutaneous coronary intervention in patients with stable coronary artery disease. Coron Artery Dis. 2022;33:91–97. [DOI] [PubMed] [Google Scholar]
- 11.Al-Lamee R, Thompson D, Dehbi H-M, et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet. 2018;391:31–40. [DOI] [PubMed] [Google Scholar]
- 12.Rajkumar CA, Nijjer SS, Cole GD, Al-Lamee R, Francis DP. Faith healing” and “subtraction anxiety” in unblinded trials of procedures: lessons from DEFER and FAME-2 for end points in the ISCHEMIA Trial. Circ Cardiovasc Qual Outcomes. 2018;11:e004665. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari R, Camici PG, Crea F, et al. Expert consensus document: a “diamond” approach to personalized treatment of angina. Nat Rev Cardiol. 2018;15:120–132. [DOI] [PubMed] [Google Scholar]
- 14.Yanagawa B, Puskas JD, Bhatt DL, Verma S. The coronary heart team. Curr Opin Cardiol. 2017;32:627–632. [DOI] [PubMed] [Google Scholar]
- 15.de Bruyne B, Fearon WF, Pijls NHJ, et al. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med. 2014;371:1208–1217. [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM. From CANTOS to CIRT to COLCOT to clinic: will all atherosclerosis patients soon be treated with combination lipid-lowering and inflammation-inhibiting agents? Circulation. 2020;141:787–789. [DOI] [PubMed] [Google Scholar]
- 17.Weintraub WS, Daniels SR, Burke LE, et al. Value of primordial and primary prevention for cardiovascular disease: a policy statement from the American Heart Association. Circulation. 2011;124:967–990. [DOI] [PubMed] [Google Scholar]
- 18.Orsini E, Marzilli M, Zito GB, et al. Clinical outcomes of newly diagnosed, stable angina patients managed according to current guidelines. The ARCA (Arca Registry for Chronic Angina) registry: a prospective, observational, nationwide study. Int J Cardiol. 2022;352:9–18. [DOI] [PubMed] [Google Scholar]
- 19.Mesnier J, Ducrocq G, Danchin N, et al. International observational analysis of evolution and outcomes of chronic stable angina: the multinational CLARIFY study. Circulation. 2021;144:512–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dourado LOC, Poppi NT, Adam EL, et al. The effectiveness of intensive medical treatment in patients initially diagnosed with refractory angina. Int J Cardiol. 2015;186:29–31. [DOI] [PubMed] [Google Scholar]
- 21.Boden WE, Kaski JC, Al-Lamee R, Weintraub WS. What constitutes an appropriate empirical trial of antianginal therapy in patients with stable angina before referral for revascularisation? Lancet. 2022;399:691–694. [DOI] [PubMed] [Google Scholar]
- 22.Boden WE, Stone PH. To stent or not to stent? Treating angina after ISCHEMIA—why a conservative approach with optimal medical therapy is the preferred initial management strategy for chronic coronary syndromes: insights from the ISCHEMIA trial. Eur Heart J. 2021;42:1394–1400. [DOI] [PubMed] [Google Scholar]
- 23.Mason CM. Preventing coronary heart disease and stroke with aggressive statin therapy in older adults using a team management model. J Am Acad Nurse Pract. 2009;21:47–53. [DOI] [PubMed] [Google Scholar]
- 24.Brener MI, Tung J, Stant J, et al. An updated healthcare system–wide clinical pathway for managing patients with chest pain and acute coronary syndromes. Crit Pathw Cardiol. 2019;18:167–175. [DOI] [PubMed] [Google Scholar]
- 25.Brummel A, Carlson AM. Comprehensive medication management and medication adherence for chronic conditions. J Manag Care Spec Pharm. 2016;22:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie JX, Gunzburger EC, Kaun L, et al. Medical therapy utilization and long-term outcomes following percutaneous coronary intervention: five-year results from the veterans affairs clinical assessment, reporting, and tracking system program. Circ Cardiovasc Qual Outcomes. 2019;12:e005455. [DOI] [PubMed] [Google Scholar]
- 27.Iqbal J, Zhang Y-J, Holmes DR, et al. Optimal medical therapy improves clinical outcomes in patients undergoing revascularization with percutaneous coronary intervention or coronary artery bypass grafting: insights from the Synergy Between Percutaneous Coronary Intervention With TAXUS and Cardiac Surgery (SYNTAX) trial at the 5-year follow-up. Circulation. 2015;131:1269–1277. [DOI] [PubMed] [Google Scholar]
- 28.Khattab AA, Knecht M, Meier B, et al. Persistence of uncontrolled cardiovascular risk factors in patients treated with percutaneous interventions for stable coronary artery disease not receiving cardiac rehabilitation. Eur J Prev Cardiol. 2013;20:743–749. [DOI] [PubMed] [Google Scholar]
- 29.Investigators SCOT-HEART. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385:2383–2391. [DOI] [PubMed] [Google Scholar]
- 30.Taqueti VR, di Carli MF. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72:2625–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah NR, Hulten EA, Tandon S, Murthy VL, Dorbala S, Thompson RC. Recent clinical trials support continued emphasis on patient-first over modality-first approaches to initial test selection in patients with stable ischemic heart disease. J Nucl Cardiol. Published online February 11, 2022. 10.1007/s12350-022-02908-7 [DOI] [PubMed] [Google Scholar]
- 32.Hobson P, Bakker J. How the heart attack gender gap is costing women’s lives. Br J Card Nurs. 2019;14:1–3. [Google Scholar]
- 33.Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suda A, Takahashi J, Hao K, et al. Coronary functional abnormalities in patients with angina and nonobstructive coronary artery disease. J Am Coll Cardiol. 2019;74:2350–2360. [DOI] [PubMed] [Google Scholar]
- 35.Taqueti VR, Hachamovitch R, Murthy VL, et al. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation. 2015;131:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ford TJ, Stanley B, Sidik N, et al. 1-Year outcomes of angina management guided by invasive coronary function testing (CorMicA). J Am Coll Cardiol Intv. 2020;13:33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knuuti J, Wijns W, Saraste A, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. [DOI] [PubMed] [Google Scholar]
- 38.Ong P, Camici PG, Beltrame JF, et al. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16–20. [DOI] [PubMed] [Google Scholar]
- 39.Sechtem U, Brown D, Godo S, Lanza GA, Shimokawa H, Sidik N. Coronary microvascular dysfunction in stable ischaemic heart disease (nonobstructive coronary artery disease and obstructive coronary artery disease). Cardiovasc Res. 2020;116:771–786. [DOI] [PubMed] [Google Scholar]
- 40.Writing Committee Members, Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;78:e187–285. [DOI] [PubMed] [Google Scholar]
- 41.Bradley C, Berry C. Definition and epidemiology of coronary microvascular disease. J Nucl Cardiol. 2022;29:1763–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta A, Taqueti VR, van de Hoef TP, et al. Integrated noninvasive physiological assessment of coronary circulatory function and impact on cardiovascular mortality in patients with stable coronary artery disease. Circulation. 2017;136:2325–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacobs M, Benovoy M, Chang L-C, et al. Automated segmental analysis of fully quantitative myocardial blood flow maps by first-pass perfusion cardiovascular magnetic resonance. IEEE Access. 2021;9:52796–52811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kotecha T, Martinez-Naharro A, Boldrini M, et al. Automated pixel-wise quantitative myocardial perfusion mapping by CMR to detect obstructive coronary artery disease and coronary microvascular dysfunction: validation against invasive coronary physiology. J Am Coll Cardiol Img. 2019;12:1958–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serruys PW, Hara H, Garg S, et al. Coronary computed tomographic angiography for complete assessment of coronary artery disease: JACC state-of-the-art review. J Am Coll Cardiol. 2021;78:713–736. [DOI] [PubMed] [Google Scholar]
- 46.Marzilli M, Merz CNB, Boden WE, et al. Obstructive coronary atherosclerosis and ischemic heart disease: an elusive link. J Am Coll Cardiol. 2012;60:951–956. [DOI] [PubMed] [Google Scholar]
- 47.de Caterina R, Boden WE. The nomenclature vagaries for the clinical manifestations of myocardial ischemic syndromes—a call to action. Int J Cardiol. 2020;304:5–7. [DOI] [PubMed] [Google Scholar]
- 48.Weintraub WS, Boden WE, Zhang Z, et al. Cost-effectiveness of percutaneous coronary intervention in optimally treated stable coronary patients. Circ Cardiovasc Qual Outcomes. 2008;1:12–20. [DOI] [PubMed] [Google Scholar]
- 49.Weintraub WS, Boden WE. Making cardiovascular care more responsive to societal needs. Am J Med. 2017;130:1259–1261. [DOI] [PubMed] [Google Scholar]
- 50.Diamond GA, Kaul S. Evidence-based financial incentives for healthcare reform: putting it together. Circ Cardiovasc Qual Outcomes. 2009;2:134–140. [DOI] [PubMed] [Google Scholar]
- 51.Bucholz EM, Rodday AM, Kolor K, Khoury MJ, de Ferranti SD. Prevalence and predictors of cholesterol screening, awareness, and statin treatment among US adults with familial hypercholesterolemia or other forms of severe dyslipidemia (1999–2014). Circulation. 2018;137:2218–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cardoso R, Abovich A, Boden WE, Arbab-Zadeh A, Blankstein R, Blumenthal RS. The 2021 AHA/ACC/SCAI coronary artery revascularization recommendations. JACC Adv. 2022;1:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Writing Committee Members, Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79:e21–e129. [DOI] [PubMed] [Google Scholar]


