Abstract
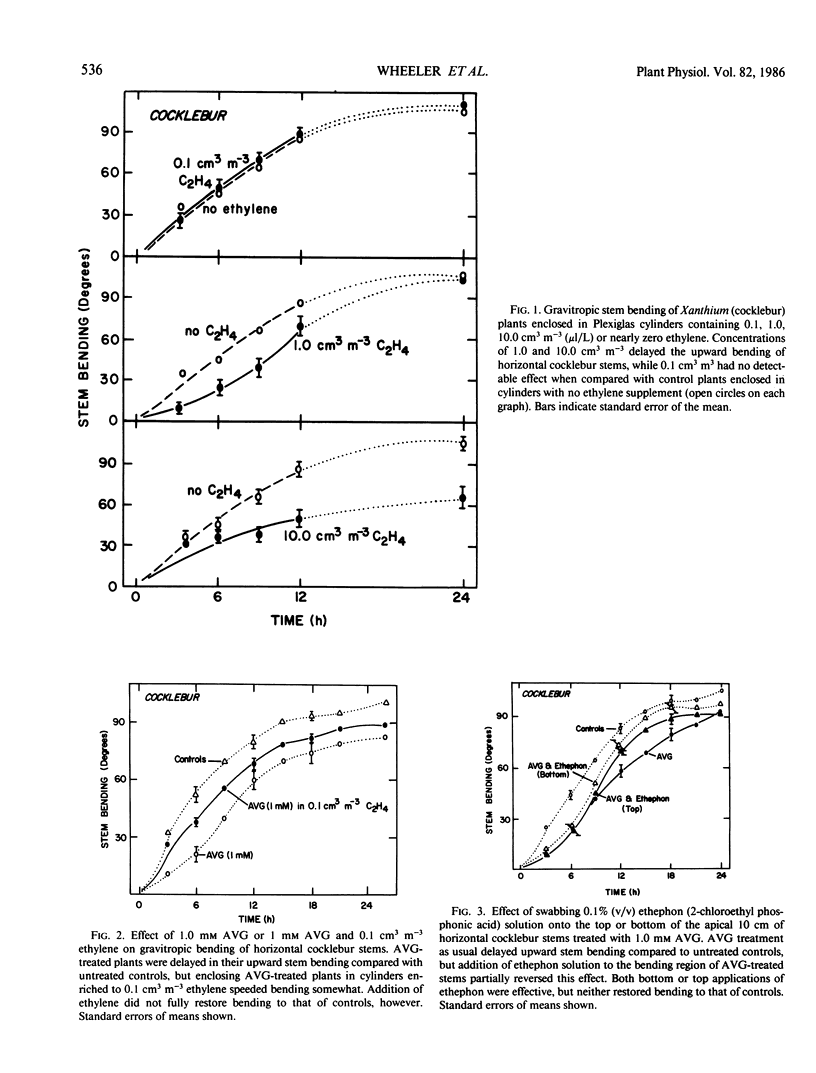
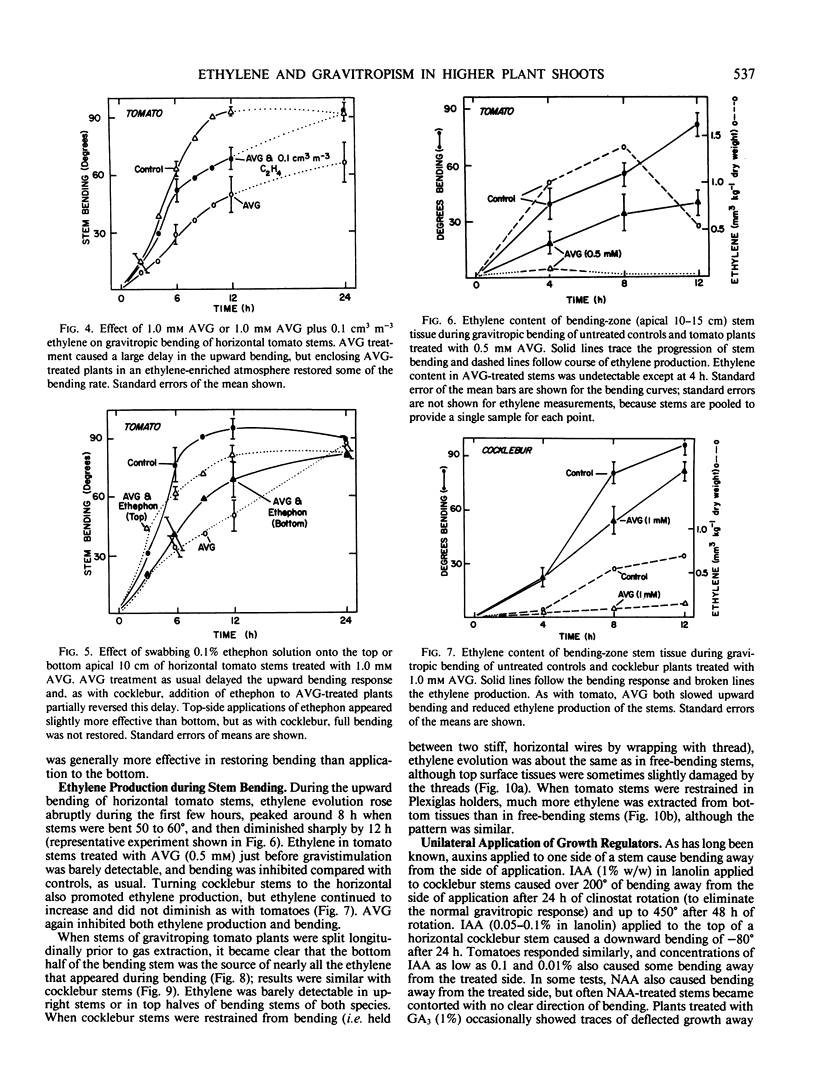
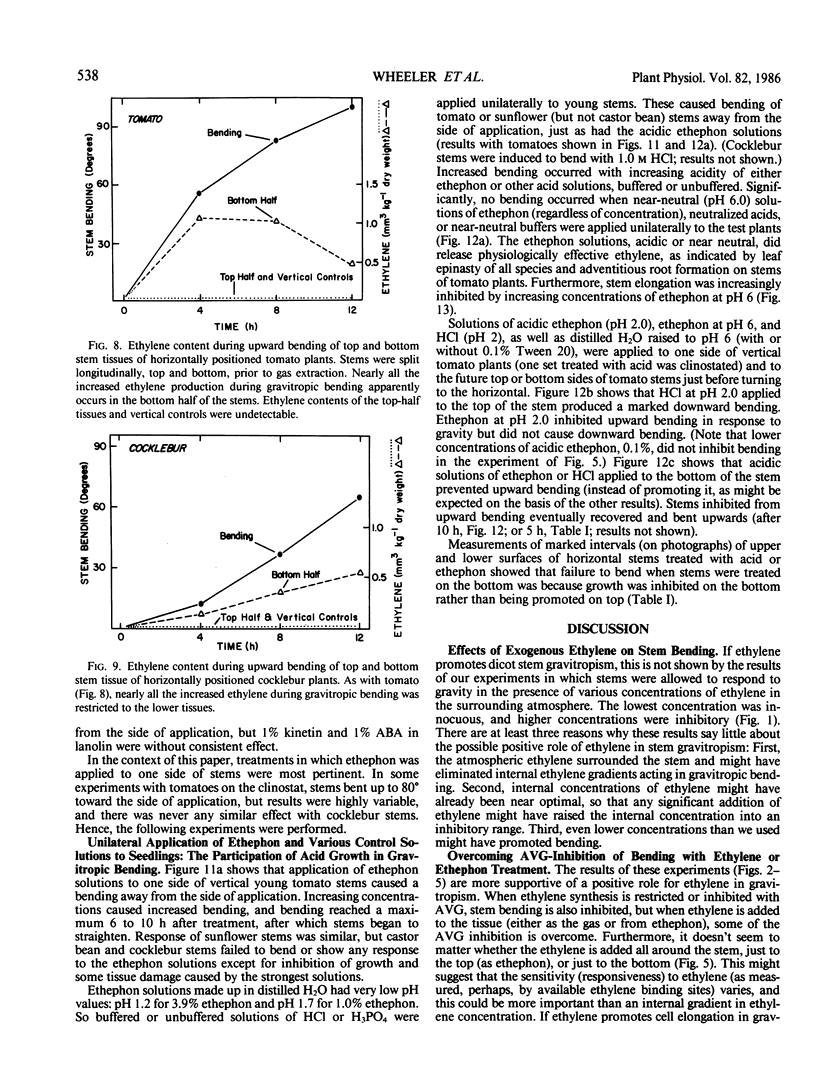
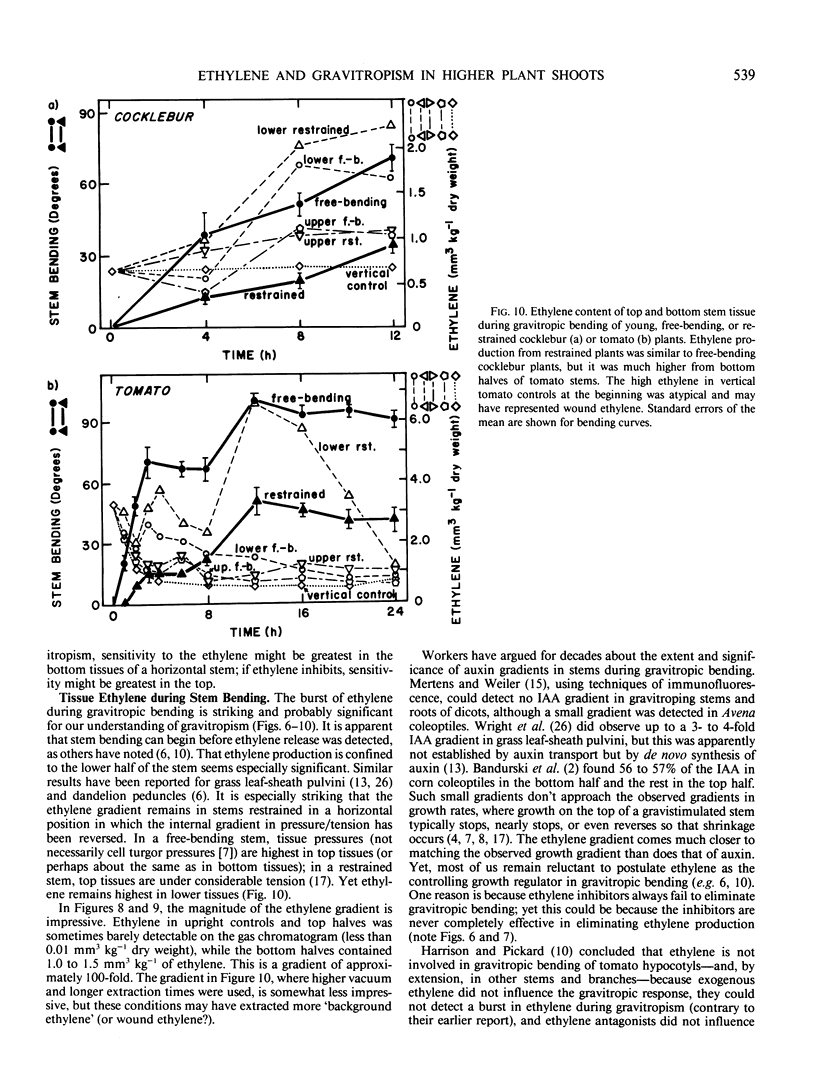
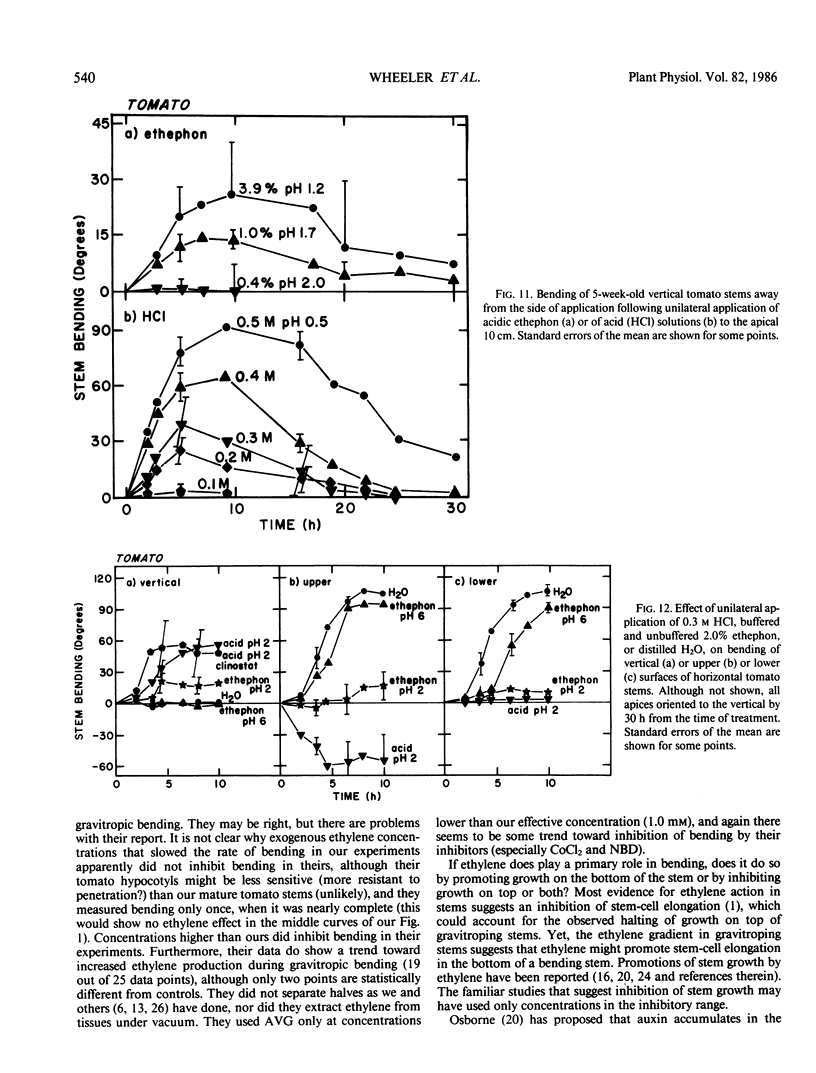
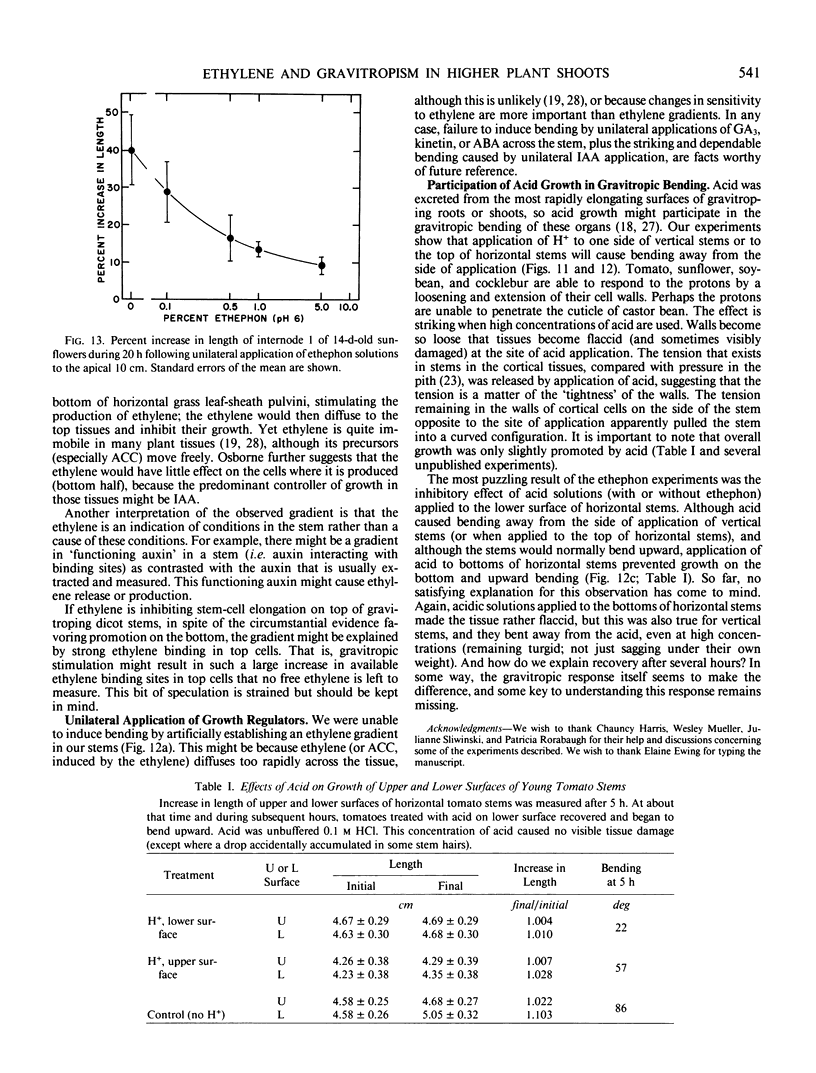
Ethylene at 1.0 and 10.0 cubic centimeters per cubic meter decreased the rate of gravitropic bending in stems of cocklebur (Xanthium strumarium L.) and tomato (Lycopersicon esculentum Mill), but 0.1 cubic centimeter per cubic meter ethylene had little effect. Treating cocklebur plants with 1.0 millimolar aminoethoxyvinylglycine (AVG) (ethylene synthesis inhibitor) delayed stem bending compared with controls, but adding 0.1 cubic centimeter per cubic meter ethylene in the surrounding atmosphere (or applying 0.1% ethephon solution) partially restored the rate of bending of AVG-treated plants. Ethylene increases in bending stems, and AVG inhibits this. Virtually all newly synthesized ethylene appeared in bottom halves of horizontal stems, where ethylene concentrations were as much as 100 times those in upright stems or in top halves of horizontal stems. This was especially true when horizontal stems were physically restrained from bending. Ethylene might promote cell elongation in bottom tissues of a horizontal stem or indicate other factors there (e.g. a large amount of `functioning' auxin). Or top and bottom tissues may become differentially sensitive to ethylene. Auxin applied to one side of a vertical stem caused extreme bending away from that side; gibberellic acid, kinetin, and abscisic acid were without effect. Acidic ethephon solutions applied to one side of young seedlings of cocklebur, tomato, sunflower (Helianthus annuus L.), and soybean (Glycine max [L.] Merr.) caused bending away from that side, but neutral ethephon solutions did not cause bending. Buffered or unbuffered acid (HCl) caused similar bending. Neutral ethephon solutions produced typical ethylene symptoms (i.e. epinasty, inhibition of stem elongation). HCl or acidic ethephon applied to the top of horizontal stems caused downward bending, but these substances applied to the bottom of such stems inhibited growth and upward bending—an unexpected result.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandurski R. S., Schulze A., Dayanandan P., Kaufman P. B. Response to gravity by Zea mays seedlings. I. Time course of the response. Plant Physiol. 1984;74:284–288. doi: 10.1104/pp.74.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. M., Morgan P. W. A method for determining the concentration of ethylene in the gas phase of vegetative plant tissues. Plant Physiol. 1970 Aug;46(2):352–354. doi: 10.1104/pp.46.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick A. V., Burg S. P. An explanation of the inhibition of root growth caused by indole-3-acetic Acid. Plant Physiol. 1967 Mar;42(3):415–420. doi: 10.1104/pp.42.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D. C., Macdonald I. R., Hart J. W., Berg A. Image Analysis of Geo-Induced Inhibition, Compression, and Promotion of Growth in an Inverted Helianthus annuus L. Seedling. Plant Physiol. 1984 Nov;76(3):589–594. doi: 10.1104/pp.76.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen D. J., Bendixen L. E. Ethylene-induced Tropism of Trifolium fragiferum L. Stolons. Plant Physiol. 1974 Jan;53(1):80–82. doi: 10.1104/pp.53.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison M. A., Pickard B. G. Evaluation of ethylene as a mediator of gravitropism by tomato hypocotyls. Plant Physiol. 1986;80:592–595. doi: 10.1104/pp.80.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe M. J. Physiological Studies on Pea Tendrils: VII. Evaluation of a Technique for the Asymmetrical Application of Ethylene. Plant Physiol. 1970 Oct;46(4):631–633. doi: 10.1104/pp.46.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leather G. R., Forrence L. E. Increased Ethylene Production during Clinostat Experiments May Cause Leaf Epinasty. Plant Physiol. 1972 Feb;49(2):183–186. doi: 10.1104/pp.49.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller W. J., Salisbury F. B., Blotter P. T. Gravitropism in Higher Plant Shoots : II. Dimensional and Pressure Changes during Stem Bending. Plant Physiol. 1984 Dec;76(4):993–999. doi: 10.1104/pp.76.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métraux J. P., Kende H. The role of ethylene in the growth response of submerged deep water rice. Plant Physiol. 1983 Jun;72(2):441–446. doi: 10.1104/pp.72.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury F. B., Wheeler R. M. Interpreting Plant Responses to Clinostating: I. MECHANICAL STRESSES AND ETHYLENE. Plant Physiol. 1981 Apr;67(4):677–685. doi: 10.1104/pp.67.4.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler R. M., Salisbury F. B. Gravitropism in Higher Plant Shoots: I. A ROLE FOR ETHYLENE. Plant Physiol. 1981 Apr;67(4):686–690. doi: 10.1104/pp.67.4.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright L. Z., Rayle D. L. Evidence for a Relationship between H Excretion and Auxin in Shoot Gravitropism. Plant Physiol. 1983 May;72(1):99–104. doi: 10.1104/pp.72.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel R. W. Some Physiological Characteristics of the Ethylene-requiring Tomato Mutant Diageotropica. Plant Physiol. 1973 Oct;52(4):385–389. doi: 10.1104/pp.52.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]


