Abstract
The antifungal activity of the nucleoside analog 3′-deoxyadenosine (cordycepin) was studied in a murine model of invasive candidiasis. When protected from deamination by either deoxycoformycin or coformycin, both of which are adenosine deaminase inhibitors, cordycepin exhibited potent antifungal efficacy, as demonstrated by prolongation of survival and a decrease in CFU in the kidneys of mice treated with cordycepin plus an adenosine deaminase inhibitor. The antifungal effect was seen with three different Candida isolates: Candida albicans 64, a relatively fluconazole-resistant clinical isolate of C. albicans (MIC, 16 μg/ml), and the fluconazole-resistant Candida krusei. Cordycepin and related compounds may provide another avenue for the discovery of clinically useful antifungal drugs.
It has recently been shown that the nucleoside analog 3′-deoxyadenosine (also known as cordycepin), when protected against conversion to 3′-deoxyinosine by the enzyme adenosine deaminase (ADA), exhibits specific cytotoxic activity against leukemia cells expressing terminal deoxynucleotidyl transferase (1). Over 20 years ago, this nucleoside was studied as a potential antiparasitic agent and found to markedly inhibit the in vitro proliferation of Plasmodium species (8). However, in follow-up in vivo therapeutic studies in murine disease models, the need to protect cordycepin from deamination by ADA (thus preventing the formation of the cytotoxically inactive derivative 3′-deoxyinosine) was not appreciated, and because the in vitro antiproliferative activity was not seen in vivo, cordycepin was not advanced into clinical trials for treatment of parasitic disease. We have recently found that when cordycepin is protected from ADA deamination (by either coformycin or deoxycoformycin, both of which are ADA inhibitors), the potent in vitro antimalarial activity of cordycepin is retained in vivo (9).
This wide spectrum of antiproliferative activity, from mammalian cells to parasites, prompted us to assess the cytotoxic activity of this unique nucleoside in a well-described animal model of candidiasis, which we previously established for the assessment of antifungal drug activity (4–7).
(These data were presented in part at the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy, New Orleans, La., 15 to 18 September 1996.)
MATERIALS AND METHODS
Fungi.
Aliquots of cultures of Candida albicans 64 (6, 7), Candida krusei, and a relatively fluconazole-resistant C. albicans clinical isolate (MIC = 16 μg/ml, as determined by the broth microdilution modification of the National Committee for Clinical Laboratory Standards standardized method), stored as stocks at −70°C, were grown for 48 h on fresh Sabouraud dextrose agar slants, harvested, and washed with sterile saline. Suspensions consisting of 4 × 107 blastoconidia per ml were prepared in sterile saline for tail vein injection.
Mice.
Three-week-old male ICR mice (weight, 16 to 20 g) were obtained from Harlan Sprague-Dawley, Indianapolis, Ind. Mice were acclimatized for at least 2 days prior to infection. They were fed food and water ad libitum.
Reagents.
Cordycepin, 3′-deoxycitidine, 3′-deoxyguanosine, 3′-deoxyuridine, deoxycoformycin, and coformycin were obtained as dry powders from Sigma Chemical Company (St. Louis, Mo.). Fresh solutions of each nucleoside, sterilized by passage through a Millipore filter, were prepared daily in sterile saline at a concentration of 10 mg/ml. Amphotericin B was obtained from Sigma as the deoxycholate suspension. Fluconazole was obtained from Pfizer Central Research (Groton, Conn.) as a dry powder and was made up fresh daily as a sterile solution.
Invasive Candida infection.
As previously described (5, 6), mice were infected with approximately 4 × 106 blastoconidia (0.1 ml) by injection into the lateral tail vein at time zero. Agent administration was begun 24 h following infection (day 2) and continued for 10 consecutive days (days 2 to 12). There were 8 to 10 mice per group for survival studies, as indicated below. Additional mice were infected and sacrificed for the kidney culture component of the experiment.
Treatment of mice.
Treatment regimens were selected on the basis of experiments in a murine leukemia model (3). Amphotericin B (1 mg/kg of body weight/day), nucleosides (1.5 mg/kg/day), and ADA inhibitors (0.4 mg/kg/day) were administered daily by intraperitoneal injection. Fluconazole (80 mg/kg/day) was administered by oral gavage in two divided doses. One day following completion of therapy and on day 30 (the end of the experiment), two mice in each group were sacrificed for culture of the kidneys, liver, and spleen. The organs were homogenized with a Tekmar Tissumizer, serial dilutions were plated on blood agar plates, the plates were incubated for 48 h, and fungal colonies were counted.
Statistics.
Comparisons of the Kaplan-Meir survival curves for each treatment group were performed by using the log rank statistic. Colony counts in the kidneys were compared by using the Student t test. Significance was defined as a P value of ≤0.05. The analyses were performed on an IBM-compatible computer using the SPSS statistics program.
RESULTS
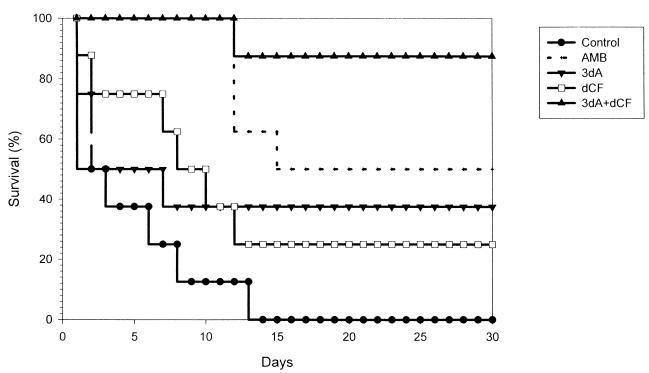
The results of a representative experiment (of a total of four separate studies employing 8 to 10 mice per treatment group per experiment) are shown in Fig. 1. It is evident from Fig. 1 that all control mice infected with C. albicans 64 (n = 8) died by day 14. In contrast, 88% of mice treated with the cordycepin-deoxycoformycin combination (n = 7) survived to the end of the experiment (day 30). Mice treated with either cordycepin alone or deoxycoformycin alone had survival rates (38 and 25%, respectively) significantly lower than those of mice treated with the nucleoside combination (P < 0.025 for all comparisons). In this experiment, mice treated with amphotericin B had a survival rate of 50% (P = 0.034 versus the control value; P = 0.12 versus the value for mice treated with the cordycepin-deoxycoformycin combination). The median survival times for the control group and for mice treated with amphotericin B, cordycepin, deoxycoformycin, or the cordycepin-deoxycoformycin combination were 2, 16, 3, 9, and >30 days, respectively. Similar results were obtained in the other three experiments.
FIG. 1.
Cordycepin therapy of murine candidiasis. Mice were infected with 4 × 106 blastoconidia of C. albicans strain 64. Treatment began 24 h postinfection and continued for 10 days. AMB, amphotericin B; 3dA, 3′-deoxyadenosine; dCF, deoxycoformycin.
In separate experiments, performed under identical conditions, in which C. albicans 64-infected mice were treated with deoxycytidine, deoxyguanidine, or deoxyuracil, either alone or in combination with deoxycoformycin, there was no effect on survival of treated mice in comparison to that of control mice (data not shown).
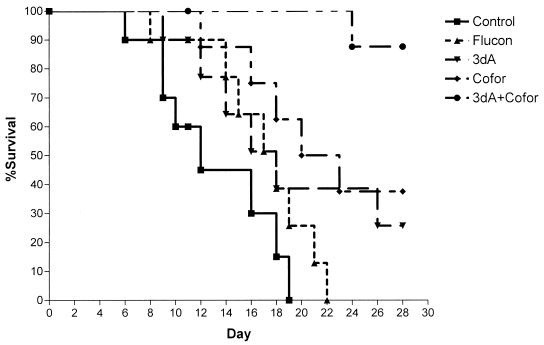
Mice infected with fluconazole-resistant C. albicans also responded to the cordycepin-deoxycoformycin combination. Mice infected with 107 CFU of a clinical isolate of C. albicans that was relatively resistant to fluconazole (MIC, 16 μg/ml) exhibited excellent protection against lethal infection when treated with the combination as shown in Fig. 2. The 30-day survival rate of mice treated with cordycepin-coformycin (88%) was significantly higher than those of untreated mice (0%) and of mice treated with either drug alone (cordycepin, 26%; coformycin, 38%; and fluconazole, 0%; P ≤ 0.023 for all comparisons). The median survival time in the cordycepin-coformycin combination treatment group was >30 days, whereas it was 12, 18, 18, or 20 days in mice treated with saline (controls), fluconazole, cordycepin alone, or coformycin alone, respectively.
FIG. 2.
Treatment of infection caused by relatively fluconazole-resistant C. albicans. Mice were infected with 107 blastoconidia of a relatively fluconazole-resistant clinical isolate of C. albicans. Treatment began 24 h postinfection and continued for 10 days. Flucon, fluconazole; 3dA, 3′-deoxyadenosine; Cofor, coformycin.
Cultures of the kidneys confirmed the survival results. Just prior to the first treatment dose and 24 h after infection, the mean CFU per kidney ± the standard deviation was 3.9 × 106 ± 0.1 × 106. As shown in Table 1, decreased numbers of C. albicans were recovered from the kidneys of mice treated with fluconazole, cordycepin, coformycin, or the cordycepin-coformycin combination compared to the numbers of colonies obtained for mice prior to therapy (P < 0.001). Of note, there were significantly fewer yeasts present in the mice treated with the combination of cordycepin and coformycin than in those treated with either drug alone. This was especially striking when day 30 colony counts were compared. However, the kidneys were not sterilized with the doses used in these experiments.
TABLE 1.
Infection of mice with relatively fluconazole-resistant C. albicans: kidney culture resultsa
| Treatment | No. of organisms recovered (103) on day:
|
|
|---|---|---|
| 12 | 30 | |
| Fluconazole | 4.5 ± 0.7 | NDb |
| Cordycepin | 5.2 ± 0.2 | 210 ± 1 |
| Coformycin | 4.1 ± 0.2 | 250 ± 70 |
| Cordycepin + coformycin | 2.2 ± 0.3c | 4.3 ± 0.7d |
Prior to therapy there were 3.9 × 106 CFU/kidney (n = 2).
ND, not done because no mice survived to this point.
P = 0.021 versus value for treatment with cordycepin alone; P = 13 versus value for treatment with coformycin alone.
P = 0.005 versus value for treatment with either agent alone.
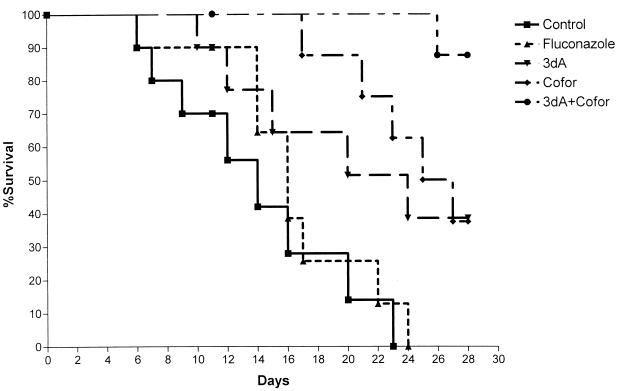
Experiments using a fluconazole-resistant C. krusei isolate (MIC, 64 μg/ml) provided similar results. As shown in Fig. 3, all mice treated with saline or fluconazole died, with median survival times of 14 and 16 days, respectively. Similarly, mice treated with cordycepin or coformycin alone had median survival times of 24 and 25 days, respectively. Mice treated with cordycepin and coformycin had a median survival time of >30 days and a cumulative 88% survival rate at day 30 (P < 0.035 for all comparisons).
FIG. 3.
Treatment of infection caused by fluconazole-resistant C. krusei. Mice were infected with 107 blastoconidia of a fluconazole-resistant isolate of C. krusei. Treatment began 24 h postinfection and continued for 10 days. Flucon, Fluconazole; 3dA, 3′-deoxyadenosine; Cofor, coformycin.
As in the previous experiment, the results of kidney cultures for C. krusei-infected mice were consistent with the survival results. Just prior to the first treatment dose and 24 h after infection, there were 4.1 × 106 ± 0.02 × 106 CFU per kidney. After 10 days of treatment and 30 days of observation, the kidneys of surviving mice were cultured; the results are shown in Table 2. The results are similar to those obtained in the previous experiment. Combination therapy was more effective at decreasing colony counts than was single-drug therapy or treatment with fluconazole (P < 0.035). On day 30, the results were even more pronounced, with no appreciable growth of fungi in the combination-treated group.
TABLE 2.
Infection of mice with C. krusei: kidney culture resultsa
| Treatment | No. of organisms recovered (103) on day:
|
|
|---|---|---|
| 12 | 30 | |
| Fluconazole | 5.8 ± 0.5 | NDb |
| Cordycepin | 29 ± 8 | 240 ± 50 |
| Coformycin | 33 ± 0.2 | 330 ± 20 |
| Cordycepin + coformycin | 3.2 ± 0.3c | 4.9 ± 0.1d |
Prior to therapy there were 4.1 × 106 CFU/kidney (n = 2).
ND, not done because no mice survived to this point.
P ≤ 0.035 versus value for treatment with either agent alone.
P = 0.013 versus value for treatment with cordycepin alone; P = 0.002 versus value for treatment with coformycin alone.
DISCUSSION
Cordycepin is clearly a nucleoside with a broad spectrum of biological activity. Its activity in terminal deoxynucleotidyl transferase-positive leukemia cells (1) has led to a phase I trial of the compound in patients with acute pre-B- and pre-T-cell leukemia (3). In addition, its in vitro antimalarial activity, which was first documented several decades ago (8), now has clinical significance with the recognition of the importance of inhibition of ADA for the maintenance of its antimalarial activity in vivo. In this communication, we add to its spectrum of biological activity by reporting on its efficacy in effectively treating ADA-inhibited mice infected with fluconazole-susceptible and -resistant Candida species.
The ADA inhibitors coformycin and deoxycoformycin were seen to have some antifungal effects when used individually in this model of murine candidiasis. However, given the immunosuppressive effects of ADA inhibition, it is not likely that the antifungal activity can translate into clinical use. The combination of cordycepin with either of these ADA inhibitors resulted in significant antifungal activity. However, the mechanism of action is not definitively known, and it is possible that it does not involve ADA inhibition. However, we have recent evidence that a cordycepin analog that is resistant to deamination possesses the same biological activities as the combination used in the studies reported herein. We therefore suspect that the antifungal activity of cordycepin can be expressed by either inhibition of ADA or stabilization of the molecule so that the amino group is protected from cleavage from the aromatic ring.
The results of the kidney cultures support the survival study data. In these experiments, no attempt was made to optimize dosing regimens. Rather, this work serves as a proof of principle and a demonstration of the antifungal efficacy of cordycepin. Therefore, it is not surprising that the kidneys were not sterilized, since doses were not adjusted for a maximal effect. It is striking, however, that while fungi were still cultured from the kidneys of mice on day 30, no appreciable growth occurred over the 18-day period from the day 12 cultures, 1 day following cessation of therapy, to day 30, the end of the experiment. This suggests that the residual fungi had not yet recovered from the damaging effects of the previous therapy. Thus, more-intense exposure to cordycepin or combinations of cordycepin with other antifungal drugs, such as azoles or amphotericin B, might be required to achieve sterilization of organs and a complete cure of the infection.
The mechanism of action of cordycepin as an antileukemic agent is not yet fully understood. Following exposure of ADA-inhibited leukemia cells to cordycepin, a series of early events associated with drug-induced apoptosis (i.e., protein kinase A activation, random DNA fragmentation, augmented p53 expression, caspase-3 activation, and poly[ADP-ribose] polymerase degradation) have been noted, and at 48 h the classic morphological and biochemical features of apoptotic cell death are present (2). We are currently exploring the possible existence of a related apoptotic cascade in Candida species following exposure to cordycepin.
Identification of useful antifungal drugs with unique modes of action that differ from those of currently available antifungal drugs is desirable since fungi resistant to available agents would not be likely to be resistant to these newer drugs, and the possibility of combining compounds with differing modes of action may lead to more-effective strategies for the treatment of invasive mycoses. The results of the experiments presented herein suggest that cordycepin may represent a new class of antifungal compounds and offer new options for the treatment of fungal infections.
ACKNOWLEDGMENT
We thank Xiu-Ping Liu for excellent technical assistance.
Footnotes
Publication no. 011 from the Collaborative Medical Mycology Program (Pfizer Inc., Roerig Division; Phytera; Scriptgen Pharmaceuticals; and Section of Infectious Diseases, Boston Medical Center).
REFERENCES
- 1.Koc Y, McCaffrey R. 2′,3′-Dideoxy killing of TdT-positive cells is due to a trace contaminant. Leukemia. 1995;9:53–57. [PubMed] [Google Scholar]
- 2.Koc Y, Urbano A, Sweeney E, McCaffrey R. Induction of apoptosis by cordycepin in ADA-inhibited TdT-positive leukemic cells. Leukemia. 1996;10:1019–1024. [PubMed] [Google Scholar]
- 3.Seldin, D., S. Lahey, A. Urbano, R. McCaffrey, and F. Foss. 1997. Phase 1 trial of cordycepin and deoxycoformycin in TdT-positive acute leukemia. Blood 90(Suppl.):246b.
- 4.Sugar A M. Interactions of amphotericin B and SCH 39304 in the treatment of experimental murine candidiasis: lack of antagonism of a polyene-azole combination. Antimicrob Agents Chemother. 1991;35:1669–1671. doi: 10.1128/aac.35.8.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugar A M, Goldani L Z, Picard M. Treatment of murine invasive candidiasis with amphotericin B and cilofungin: evidence for enhanced activity with combination therapy. Antimicrob Agents Chemother. 1991;35:2128–2130. doi: 10.1128/aac.35.10.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugar A M, Hitchcock C A, Troke P F, Picard M. Combination therapy of murine invasive candidiasis with fluconazole and amphotericin B. Antimicrob Agents Chemother. 1995;39:598–601. doi: 10.1128/AAC.39.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugar A M, Salibian M, Goldani L Z. Saperconazole therapy of murine disseminated candidiasis: efficacy and interactions with amphotericin B. Antimicrob Agents Chemother. 1994;38:371–373. doi: 10.1128/aac.38.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trigg P I, Gutteridge W E, Williamson J. The effects of cordycepin on malaria parasites. Trans R Soc Trop Med Hyg. 1971;65:514–520. doi: 10.1016/0035-9203(71)90162-3. [DOI] [PubMed] [Google Scholar]
- 9.Wigzell, H., R. P. McCaffrey, and A. M. Sugar. Unpublished data.