Abstract
PARP inhibitors (PARPi) have emerged as a promising targeted therapeutic intervention for metastatic castrate-resistant prostate cancer (mCRPC). However, the clinical utility of PARPi is limited to a subset of patients who harbor aberrations in the genes associated with the homologous recombination (HR) pathway. Here, we report that targeting metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), an oncogenic long noncoding RNA (lncRNA), contrives a BRCAness-like phenotype, and augments sensitivity to PARPi. Mechanistically, we show that MALAT1 silencing reprograms the homologous recombination (HR) transcriptome and makes prostate cancer cells more vulnerable to PARPi. Particularly, coinhibition of MALAT1 and PARP1 exhibits a decline in clonogenic survival, delays resolution of γH2AX foci, and reduces tumor burden in mice xenograft model. Moreover, we show that miR-421, a tumor suppressor miRNA, negatively regulates the expression of HR genes, while in aggressive prostate cancer cases, miR-421 is sequestered by MALAT1, leading to increased expression of HR genes. Conclusively, our findings suggest that MALAT1 ablation confers sensitivity to PARPi, thus highlighting an alternative therapeutic strategy for patients with castration-resistant prostate cancer (CRPC), irrespective of the alterations in HR genes.
Significance:
PARPi are clinically approved for patients with metastatic CRPC carrying mutations in HR genes, but are ineffective for HR-proficient prostate cancer. Herein, we show that oncogenic lncRNA, MALAT1 is frequently overexpressed in advanced stage prostate cancer and plays a crucial role in maintaining genomic integrity. Importantly, we propose a novel therapeutic strategy that emphasizes MALAT1 inhibition, leading to HR dysfunction in both HR-deficient and -proficient prostate cancer, consequently augmenting their susceptibility to PARPi.
Introduction
Prostate cancer is a molecularly heterogeneous and multifocal disease, with each foci exhibiting varying cellular disorganization and molecular alterations. Because of this heterogeneity, comprehending the molecular mechanisms underlying disease progression would help to develop effective screening, preventive, and treatment strategies (1, 2). Advancements in sequencing technologies revealed that prostate cancer progression is accompanied by the acquisition of multiple genomic aberrations, including somatic and/or germline mutations, copy-number alterations, microsatellite variations, and chromosomal alterations such as translocations, duplications, insertions, and deletions. Intriguingly, approximately 20%–30% of patients with advanced-stage prostate cancer harbor genomic aberrations in the genes associated with the DNA damage and repair (DDR) pathway, the most notable being loss-of-function mutations in BRCA1/2 (3, 4). Mutations in DDR genes alleviate the repair capacity of tumor cells and facilitate complex cancer-related genetic alterations (5). Nevertheless, several recent reports suggest that patients with castration-resistant prostate cancer (CRPC) exhibit higher expression of homologous recombination (HR) genes, including BRCA1, RAD54L, and RMI2, compared with localized cases (6). The mechanism(s) underlying their overexpression and the associated functional consequences are not yet established. Nevertheless, several chemotherapy drugs, including platinum-based medicines (7), radiation (8), and antiandrogens (9) kill cancer cells by inducing DNA damage. Consequently, cells that effectively repair DNA damage can withstand therapeutic drugs. Moreover, rapid DNA damage response activation and enhanced DNA repair capacity have been linked to therapeutic resistance in multiple cancer types (10, 11), suggesting that overexpression of the HR pathway might provide a survival advantage to CRPC cells and facilitate cancer progression.
Alternatively, long noncoding RNAs (lncRNA) have recently been demonstrated to be crucial players in the repair of DNA damage and offer novel targets for combating cancer resistance (12, 13). For instance, antisense RNA in the INK4 locus (ANRIL, ref. 14), DNA damage-sensitive RNA1 (DDSR1, ref. 15), and lncRNA radiation-induced regulator of PLK1 and RAD51 (lnc-RI, ref. 16) have been shown to modulate the DNA repair capacity by enhancing HR. Despite the intricate involvement of lncRNAs in maintaining genome integrity, thus far, only one study in prostate cancer has shown the direct interplay between lncRNAs and the DDR pathway, wherein they demonstrated that PCAT-1 induces functional deficiency in HR by repressing BRCA2 (17). Therefore, identifying lncRNAs that modulate genomic stability in prostate cancer and investigating their biological roles can aid in deciphering the mechanisms underlying the development of therapy-resistant prostate cancer.
Here, we unraveled a molecular network involving the lncRNA MALAT1 and the HR pathway in mCRPC. We showed that MALAT1 modulates DNA repair pathways and plays an essential role in maintaining genomic integrity in patients with advanced-stage prostate cancer. RNAi-mediated depletion of MALAT1 induces HR deficiency, which in turn enhances sensitivity to olaparib, a widely used PARP1 inhibitor. Collectively, this study provides strong evidence that MALAT1 is indispensable for maintaining genome integrity in advanced-stage prostate cancer, emphasizing a novel therapeutic approach wherein targeting MALAT1 augments sensitivity to PARP inhibition by inducing HR deficiency in prostate cancer.
Materials and Methods
In-Silico Data Processing and Computational Analysis
Microarray Analysis
The gene expression datasets, namely GSE35988 (18), GSE6919 (19), and GSE6752 (20), were downloaded from the GEO database (https://www.ncbi.nlm.nih.gov/geo/, RRID:SCR_005012) each of which comprises expression profiles for benign, localized prostate cancer, and CRPC samples. The differentially expressed genes (DEG) in patients with CRPC were identified using the “limma” package (RRID:SCR_010943) in R (21) with the cut-off criterion of an adjusted P value (Padj) <0.05 and log2 fold change |FC| > 0.6. Furthermore, the Venn analysis web tool at https://bioinformatics.psb.ugent.be/webtools/Venn was used to identify the commonly elevated genes in CRPC samples from the three groups. The samples were sorted on the basis of tissue type, and MALAT1 expression was plotted [log2 (normalized count)] using GraphPad Prism version 8.0 (RRID:SCR_002798). No cutoff was applied to the dataset.
Integrative Analyses for TCGA-PRAD Data
The HiSeq mRNA data for MALAT1 and clinical information for the TCGA-PRAD dataset were downloaded from the UCSC Xena browser (https://xenabrowser.net/, RRID:SCR_018938). To analyze the association of MALAT1 with the primary Gleason score, the samples were divided into three groups corresponding to primary Gleason scores 3, 4, and 5, and the data were plotted for MALAT1 expression (log2 (norm_count+1)). A similar analysis was performed for node status, response to therapy, and biochemical recurrence. For Kaplan–Meier survival analysis (RRID:SCR_021137), survival data of patients with primary tumors were retrieved from the UCSC Xena browser. Days to the first biochemical recurrence and up to the last follow-up were the two parameters considered for analysis. The samples were stratified into two groups based on the median expression value of MALAT1, wherein patients with expression values higher than the median were placed in the “MALAT1 high” group, while patients with expression values lower than the median were placed in the “MALAT1 low” group. Furthermore, Kaplan–Meier survival analysis was performed using GraphPad Prism 8.0 to calculate the 5-year survival probability.
To examine the association of MALAT1 with epithelial, mesenchymal, and stemness markers, we arranged the TCGA-PRAD patients’ samples in descending order of MALAT1 expression and divided the dataset into four “equal quartiles.” The top 25% of the patients (n = 125) corresponding to the upper quartile [QU, log2 (RPM+1) > 12.29] were assigned as MALAT1-high samples, while the lower quartile [QL, log2 (RPM+1) < 11.05] were considered MALAT1-low samples. The corresponding expression values for epithelial, mesenchymal, and stemness genes in MALAT1-high and MALAT1-low groups (without further cutoffs) were considered to examine their association with MALAT1.
Gene Coexpression Analysis
The pairwise Pearson correlation coefficient (ρ) between MALAT1 and DDR/cell-cycle genes in prostate cancer cohorts, namely GSE35988 (18), GSE3325 (22), and GSE77930 (23) was examined using the “corrplot” package (RRID:SCR_023081) in R. P < 0.05 was considered as a statistically significant threshold. Also, gene-set variation analysis (GSVA) was performed to investigate the variations in the activation status of the DDR pathway and G1–S phase transition in different clinical stages of prostate cancer. The gene sets for the “DDR pathway” and the “G1–S phase transition” were downloaded from the MSigDB database (RRID:SCR_016863). Ultimately, an R package named “complex heatmap” (RRID:SCR_017270) was used to demonstrate the enrichment of the genes mentioned above in each group and the expression of MALAT1 (24).
RNA-Sequencing Analysis
The transcriptome profiles for LNCaP-siNT and LNCaP-siMALAT1 (GSE72534; ref. 25) were downloaded from the sequence read archive (SRA) and analyzed in the galaxy (usegalaxy.org, RRID:SCR_006281). Raw sequencing FASTQ reads were prefetched and Fastq-dumped using the SRA-toolkit (http://ncbi.github.io/sra-tools/, RRID:SCR_004891). FastQC sequence quality checks were performed on the raw reads before mapping them to hg38 human using TopHat v2.1.0 (RRID:SCR_013035). Within the sample, FPKM normalization was performed for both conditions. The downregulated genes in LNCaP-abl-siMALAT1 cells (log2 FPKM difference ≤−0.6) were then subjected to the Database for Annotation, Visualization, and Integrated Discovery (DAVID, RRID:SCR_001881) bioinformatics platform (26) to identify deregulated biological processes (P < 0.05).
Cancer Cell Line Encyclopedia Analysis
MALAT1 mRNA expression in various cancer cell lines was retrieved from the Cancer Cell Line Encyclopedia (CCLE) database (RRID:SCR_013836) and was used to analyze its association with cancer type. The samples were sorted on the basis of tissue type, and MALAT1 expression was plotted using GraphPad Prism version 8.0.
Experimental Methods
Cell Line Culture Conditions and Authentication
The prostate cancer cell lines namely 22RV1 (RRID:CVCL_1045), VCaP (RRID:CVCL_2235), LNCaP (RRID:CVCL_0395), and PC3 (RRID:CVCL_0035) and benign prostate epithelial cells (PNT2, RRID:CVCL_2164) were sourced from the ATCC. DU145 (RRID:CVCL_0105) cells were generously gifted by Dr. Mohammad Asim, Department of Clinical and Experimental Medicine, Faculty of Health and Medical Sciences, University of Surrey (Guildford, United Kingdom). The cells were cultured according to ATCC-recommended guidelines in 37°C incubators with 5% CO2. Cell lines were typically cultured no longer than continuous 45 days upon thawing the frozen vials, and were grown up to 25 passages. Cell lines were periodically tested for Mycoplasma contamination (last tested: January 2021) using the PlasmoTest Mycoplasma Detection Kit (InvivoGen). The cell lines used in this study were authenticated by short tandem repeat profiling at the Life Code Technologies Private Limited, and DNA Forensics Laboratory (last authentication: December 2017).
Lentiviral Packaging
ViraPower Lentiviral Packaging Mix (Invitrogen) was used to generate viral particles for shSCRM and shMALAT1 (Supplementary Table S1) as previously described (27). Briefly, the shRNA constructs and packaging mix plasmids were transfected into HEK293FT (RRID:CVCL_6911) cells and incubated for 60–72 hours. The viral particles were then harvested and stored at −80°C for long-term storage. To create stable knockdown cell lines, the prostate cancer cells were infected with the collected lentiviral particles along with polybrene (hexadimethrine bromide; 8 µg/mL; Sigma-Aldrich). The culture medium was changed 24 hours after infection, and puromycin (Sigma-Aldrich) selection was started 72 hours later.
CRISPR-Cas9–Based MALAT1 Knockout
Lentiviral vectors containing a pair of guide RNAs (gRNA) targeting human MALAT1 (pDECKO_MALAT1_C, RRID:Addgene_72622, ref. 28), control gRNAs in pDECKO_GFP (RRID:Addgene_72619), and Cas9 (lentiCas9-Blast, RRID:Addgene_52962, ref. 29) were procured from Addgene, and lentiviral particles were produced. 22RV1 cells were then infected with the lenti-Cas9-Blast lentivirus and selected with blasticidin (5 µg/mL). Cas9-overexpressing cells were infected with the pDECKO_GFP and pDECKO_MALAT1_C lentiviruses and selected with puromycin (2 µg/mL). Single cells from a pooled population of pDECKO_MALAT1_C were injected into each well of a 96-well plate and cultured for another 2–3 weeks under puromycin selection to obtain single clones with MALAT1 knockout. For knockout validation, the isolated single clones were subjected to genomic PCR and qPCR (Supplementary Table S1).
HR Assay
MALAT1 knockout and control prostate cancer cells were nucleofected with 0.7 µg of pDR-GFP plasmid (RRID:Addgene_26475; ref. 30). Simultaneously RAD51-silenced and control prostate cancer cells were also nucleofected with 0.7 µg of pDR-GFP plasmid. Two days later, the cells were transfected with 2 µg of pCBASce (RRID:Addgene_26477; ref. 31). The cells were harvested 48 hours after transfection, and GFP expression was measured using flow cytometry. The percentage of GFP-positive cells was quantified to estimate the number of cells undergoing HR.
Chromatin Isolation by RNA Purification
The chromatin isolation by RNA purification (ChIRP) samples were prepared as described by Chu and colleagues (32). Briefly, 22RV1 cells were cross-linked using 1% formaldehyde for 10 minutes at room temperature. Next, formaldehyde was quenched using 125 mmol/L glycine for 5 minutes at room temperature. The cross-linked cells were washed twice with PBS and later lysed in lysis buffer consisting of Tris-Cl (50 mmol/L, pH 7.0), EDTA (10 mmol/L), and 1% SDS. Furthermore, the samples were sonicated to obtain DNA fragments of ∼500bp using the Bioruptor (Diagenode). Subsequently, a cocktail of biotin-labeled probes (25 pmol each; Supplementary Table S1) specific to MALAT1 was added to the fragmented chromatin and incubated for 4 hours at 37°C in a hybridization chamber. The hybridized content was then retrieved using streptavidin magnetic beads (Dynabeads Streptavidin, Invitrogen). A fraction of the sample was used for RNA extraction to validate MALAT1 pull-down by qRT-PCR, while the rest of the sample was used for protein sample preparation.
Mice Xenograft Study
All experimental procedures using mice were approved by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and abided by all regulatory standards of the Institutional Animal Ethics Committee of the Indian Institute of Technology Kanpur. Five- to 6-week-old male NOD.CB17-Prkdcscid/J (NOD/SCID; Jackson Laboratory, RRID:BCBC_4142) mice were anesthetized using a cocktail of ketamine and xylazine (50 mg/kg and 5 mg/kg, respectively) injected intraperitoneally. 22RV1-MALAT1-KO and 22RV1-SCR control cells were trypsinized and resuspended (2.5 × 106) in 100 µL of saline with 20% Matrigel and implanted subcutaneously at the dorsal flank on both sides of mice (n = 12 for each condition). On alternate days, tumor burden was measured with a digital Vernier caliper and tumor volume was calculated using the formula (π/6) (L × W2), (L = length; W = width). When the average tumor volume reached 100 mm3, the mice in each group were randomized into two groups (n = 6) and treated five times a week with either vehicle control or olaparib (50 mg/kg) diluted in 5% dimethyl sulfoxide (DMSO), 20% polyethylene glycol 400 (PEG-400; Sigma Aldrich) by oral gavage. To investigate spontaneous metastases to lungs and bone marrow of the xenografted mice, genomic DNA was isolated from these organs and assayed for the expression of human Alu-elements using TaqMan probe FAM-YB8-ALU-167 with sequence 5′-6-FAM-AGCTACTCGGGAGGCTGAGGCAGGA-TAMRA-3′, which specifically hybridizes to the human-specific YB8-Alu sequences (33). The standard curve for the TaqMan assay was generated by using serially diluted human genomic DNA spiked with mouse genomic DNA, and based on the Ct values, the number of metastasized cells was calculated.
IHC of Tumor Xenografts
Following the removal of tumor tissues from xenografted mice, they were fixed in 10% buffered formalin, paraffin-embedded, and sectioned at 3-µm thickness using a microtome (Leica). After tissue sections were deparaffinized and rehydrated, sodium citrate buffer was used for heat-induced antigen retrieval (pH 6.0). Furthermore, endogenous peroxidase activity was quenched with 3% hydrogen peroxide (H2O2) and blocked using 5% normal goat serum. The tissue sections were incubated with the Ki-67 (RRID:AB_2797703) antibody at 4°C overnight. Next morning, the sections were first washed with Tris-buffered saline (TBS) and then incubated with a biotinylated secondary antibody at room temperature for 1 hour, followed by incubation with an ABC (avidin–biotin complex, Vector Laboratories) solution for 30 minutes. Sections were then processed for detection of horseradish peroxidase (HRP) activity using 3, 3-diaminobenzidine (DAB) peroxidase (HRP) substrate kit (Vector Laboratories). Quantification for Ki-67 was performed using ImageJ software using 10 random histologic fields.
Statistical Analysis
For statistical analysis, unpaired two-tailed Student t test, one-way ANOVA, or two-way ANOVA were used unless otherwise specified in the respective figure legend. P ≤ 0.05 was considered significant. The error bars indicate SEM calculated from three independent experiments performed in triplicate.
Data Availability Statement
We have not performed global gene expression profiling or RNA-sequencing (RNA-seq) for this project nevertheless various publicly available datasets were used in this study, namely UCSC Xena (https://xenabrowser.net/) to retrieve the TCGA-PRAD dataset and GEO (www.ncbi.nlm.nih.gov/geo) to retrieve gene expression profiling in patients with prostate cancer: GSE35988, GSE6919, GSE6752, GSE3335, and GSE77930 while RNA-seq data for LNCaP-abl-siNT and -siMALAT1 was retrieved from GSE72534.
RESULTS
Higher MALAT1 Expression Associates with Advanced stage Prostate Cancer and Poor Prognosis
To identify the essential genes associated with advanced stage prostate cancer, we performed differential gene expression analysis using three publicly available microarray datasets comprising expression profiles of patients with prostate cancer, namely GSE35988 (18), GSE6919 (19), and GSE6752 (20). A total of 163 transcripts comprising 161 genes, a pseudogene (RPL32P3), and an oncogenic lncRNA (MALAT1) were found to be significantly elevated in patients with metastatic prostate cancer compared with localized cases (Fig. 1A; Supplementary Table S2). We observed that MALAT1 was highly upregulated in metastatic cases (∼1.5–4 times) compared to patients with localized prostate cancer (Fig. 1B–D). Analysis of transcriptome data from The Cancer Genome Atlas-Prostate Adenocarcinoma (TCGA-PRAD) cohort (n = 499; ref. 34) revealed that MALAT1 expression positively correlates with several clinicopathologic parameters such as Gleason score and node status (Supplementary Fig. S1A). Moreover, elevated levels of MALAT1 were observed in patients with progressive disease compared with those with complete response after primary therapy (Supplementary Fig. S1A), indicating its significance in predicting response to chemotherapy. Besides, higher MALAT1 levels were also noted in the patients who suffered from biochemical recurrence (BCR; Supplementary Fig. S1B). To further validate the same, we performed a Kaplan–Meier survival analysis by stratifying the TCGA-PRAD patients based on their MALAT1 median expression into high (≥median, n = 248) and low (≤median, n = 250) groups. Intriguingly, patients with elevated MALAT1 levels showed a higher probability of BCR compared with the MALAT1-low group (P = 0.0162, Fig. 1E). In addition, MALAT1-high patients in the TCGA-PRAD cohort showed higher chances of relapse compared with the MALAT1-low group (P = 0.0013, Fig. 1F), suggesting that MALAT1 levels could also predict the likelihood of disease recurrence.
FIGURE 1.
High MALAT1 expression is associated with poor prostate cancer prognosis. A, Venn diagram displaying genes upregulated in patients with metastatic prostate cancer compared with localized cases in three publicly available gene expression omnibus (GEO) datasets namely, GSE35988, GSE6919, and GSE6752. B, Dot plot with superimposed violin plot showing MALAT1 expression in patients with benign, primary, and metastatic prostate cancer in the GSE6919 dataset. MALAT1 transcript is reported as log2 median-centered ratio. C, Same as B, except GSE35988 dataset. D, Same as B, except GSE6752 dataset. E, Kaplan–Meier curves for BCR-free survival in the TCGA-PRAD dataset categorized as “MALAT1-high” (n = 223) and “MALAT1-low” (n = 219) groups based on the median expression of MALAT1. Blue line represents patients with higher expression of MALAT1 whereas the green line represents cases with patients with lower expression of MALAT1. F, Same as E, except relapse-free survival for the TCGA-PRAD dataset. Data represent mean ± SEM. For B and C, one-way ANOVA with Tukey multiple comparison test was applied, while for D, two-tailed unpaired Student t test was applied. The P values for E and F were computed by the log-rank test.
RNA-seq data analyses from the CCLE revealed high expression of MALAT1 in multiple human cancer cell lines, with the highest expression noted in prostate cancer (Supplementary Fig. S1C). Similarly, our quantitative PCR (qPCR) data with multiple prostate cancer cell lines (PC3, DU145, LNCaP, 22RV1, and VCaP) also exhibited higher expression of MALAT1 compared with PNT2, an immortalized nontumorigenic normal prostate epithelial cell line (Supplementary Fig. S1D). Taken together, these results indicate that MALAT1 positively correlates with the clinicopathologic features of aggressive prostate cancer and may serve as a promising prognostic marker.
MALAT1 Promotes Epithelial-to-Mesenchymal Transition, Stemness, and Chemoresistance in Prostate Cancer
MALAT1 has emerged as a metastasis-associated lncRNA in multiple malignancies and serves as a predictor in assessing response to cancer therapies (35–37). As indicated in Fig. 1, tumors with elevated levels of MALAT1 also show a higher propensity for lymph node metastasis, disease recurrence, and therapeutic failure in patients with prostate cancer. Therefore, we examined the association of MALAT1 with molecular factors allied with epithelial-to-mesenchymal transition (EMT) in the TCGA-PRAD cohort. Intriguingly, MALAT1-low patients showed increased levels of archetypal epithelial markers, such as CHD1, EPCAM, TJP1, CLDN7, and OCLN compared with MALAT1-high patients (Supplementary Fig. S2A). Concomitantly, patients with higher expression of MALAT1 showed elevated levels of mesenchymal markers, such as CTGF, KRT5, FOXC1, SNAI1, and FOXC2 (Supplementary Fig. S2B). To further investigate the functional significance of MALAT1 in EMT, we generated stable MALAT1-silenced (shMALAT1) and scrambled control (shSCRM) 22RV1 and LNCaP cells using lentivirus-based short hairpin RNAs (shRNA; Supplementary Fig. S2C and S2D). Characterization of these stable cells revealed a robust increase in the epithelial marker, E-cadherin, with a concomitant decrease in the mesenchymal marker (N-cadherin) in shMALAT1 cells compared with respective shSCRM control, emphasizing its importance in EMT (Fig. 2A). In accord with this, MALAT1 depletion remarkably reduced 3D cell migration in 22RV1 (∼60%) and LNCaP (∼70%) cells compared with shSCRM controls (Fig. 2B), suggesting that MALAT1 is required for prostate cancer cell migration. Metastatic prostate cancer cells frequently acquire cancer stem cell (CSC) properties, which result in self-renewal and chemoresistance (38, 39), therefore we examined the TCGA-PRAD cohort for any association of MALAT1 with self-renewal factors. Interestingly, MALAT1-high patients showed increased levels of key stemness factors, such as OCT4, NANOG, KLF4, ATP-binding cassette subfamily G member 2 (ABCG2), sex-determining region Y-Box 9 (SOX9) compared with MALAT1-low patients (Supplementary Fig. S2E), indicating a positive association of MALAT1 with stemness. To confirm this, we evaluated the expression of a few cell surface markers associated with CSCs, namely CD117 (c-KIT), a tyrosine kinase receptor; CD133 (prominin-1); and CD44 (HCAM), a cell-surface glycoprotein, in 22RV1-shMALAT1 and -shSCRM cells. Interestingly, shMALAT1 cells displayed a marked reduction in the expression of CD117 (∼50–70%), CD133 (∼90%), and CD44 (∼80%) compared with shSCRM control (Fig. 2C–E). Because self-renewal is an essential feature of stemness, we next examined the ability of 22RV1-shMALAT1 and -shSCRM cells to form prostatospheres. As anticipated, MALAT1 depletion abrogated the prostatosphere-forming ability of 22RV1 cells as well as their expansion in subsequent serial propagations (Fig. 2F and G). Moreover, the prostatospheres formed with MALAT1-deficient cells were significantly smaller compared with the SCRM control (Fig. 2G). Molecular characterization of 22RV1-shMALAT1 prostatospheres showed a significant reduction in the expression of pluripotency genes, namely C-KIT, OCT-4, NANOG, CD44, SOX2/9, and ABCG2 (Fig. 2H). Likewise, a robust decrease in the expression of CD338 (ABCG2), an ATP-binding cassette transporter, was also noted in 22RV1 shMALAT1 cells compared with 22RV1-shSCRM (Fig. 2I), suggesting that MALAT1 modulates stemness in prostate cancer cells. Aside from stem cell maintenance, several pluripotency factors, including CD117 and ABCG2, have been shown to confer chemotherapeutic resistance. In line with this, Liu and colleagues reported that a subpopulation of the 22RV1 cells that abundantly expressed CD117 and ABCG2; exhibited multidrug resistance (40). Hence, we next investigated the susceptibility of MALAT1-silenced cells to chemotherapeutic drugs, namely doxorubicin and 5-fluorouracil (5-FU). Interestingly, 22RV1-shMALAT1 cells exhibited enhanced sensitivity to chemotherapeutic drugs compared with 22RV1-shSCRM (Fig. 2J). These findings, thus, provide compelling evidence that the downregulation of MALAT1 effectively suppresses EMT as well as stemness and confers sensitivity to chemotherapeutic agents.
FIGURE 2.
MALAT1 promotes EMT, stemness, and chemoresistance in prostate cancer. A, Immunoblots showing the expression of EMT markers in shMALAT1 and shSCRM prostate cancer cells. β-Actin was used as an internal control. B, Boyden chamber Matrigel migration assay using the same cells as in A. Representative fields with the migrated cells are shown in the inset. The bar plot depicts the alteration in migratory potential of the prostate cancer cells upon MALAT1 knockdown. C, Flow cytometry analysis showing expression of CD117 (c-KIT) and CD133 in 22RV1-shMALAT1 and shSCRM control cells. D, Immunofluorescence images displaying the expression of CD117 and CD44 and in the same cells as in C. Scale bar, 20 µm. E, Dot plot represents quantification of CD117 and CD44 mean fluorescence intensity (MFI) per unit area shown as arbitrary units (AU). F, Representative phase-contrast images for the prostatospheres formed using 22RV1-shMALAT1 and shSCRM control cells on the indicated days. Scale bar, 100 µm. G, Bar plot superimposed with dots represents the mean area of the prostatospheres and percentage sphere formation efficiency. H, qPCR depicting the expression of stem cell markers in the prostatospheres derived from the same cells as in E. Expression level for each gene was normalized to GAPDH. I, Flow cytometry analysis showing expression of ATP-binding cassette superfamily G member 2 (CD338/ABCG2) using the same cells as in C. J, Cell cytotoxicity assay using chemotherapeutic drugs namely, doxorubicin and 5-fluorouracil using the same cells as in C. IC50 values were calculated by generating a dose–response curve using GraphPad Prism software. Experiments were performed with n = 3 biologically independent samples; the data represents mean ± SEM. The statistical difference among the groups was computed using one-way ANOVA with Dunnett multiple-comparison test for B, E, G, and H.
MALAT1 Modulates DNA Repair Pathways and Maintains Genome Integrity in Metastatic Prostate Cancer
To further delve into the molecular mechanism(s) underlying MALAT1-mediated carcinogenesis in the prostate gland, we analyzed the transcriptome profiles of MALAT1-silenced and control LNCaP-abl cells (LNCaP-derived castration-resistant cells) retrieved from a publicly available dataset, GSE72534 (25). Our analysis revealed 4,139 differentially expressed transcripts with a log2 FPKM difference (≤−0.6 or ≥0.6) that include 1,986 up- and 2,153 downregulated genes in MALAT1-silenced LNCaP-abl cells compared with the control cells. Functional annotation of the differentially downregulated genes using DAVID demonstrates several pathways associated with DDR, cell division, and cell cycle as the most significantly downregulated biological processes upon depletion of MALAT1 in LNCaP-abl cells (Fig. 3A; Supplementary Table S3). Consistent with these findings, GSVA using three prostate cancer cohorts (GSE35988, GSE3325, and GSE77930) exhibited significant enrichment (FDR < 0.05) of the gene signatures associated with DDR and G1-S phase transition in metastatic prostate cancer (Supplementary Fig. S3A). Notably, a strong positive correlation (ρ ≥ 0.35) between MALAT1 expression and the DDR gene signature (retrieved from mSigDB) was also observed in these prostate cancer datasets (Fig. 3B; Supplementary Fig. S3B). To examine the clinical significance of MALAT1-associated DDR genes, we performed Kaplan–Meier survival analysis for recurrence-free survival using the RNA-seq data of patients with prostate cancer from the TCGA-PRAD cohort categorized into two groups based on the median expression of MALAT1 and selected DDR genes. Intriguingly, patients with MALAT1-low and DDR-low signatures showed higher recurrence-free survival probability compared with MALAT1-high and DDR-high patients (Supplementary Fig. S3C).
FIGURE 3.
MALAT1 depletion impairs HR-mediated DSB repair in prostate cancer cells. A, DAVID analysis depicting biological pathways downregulated in LNCaP-abl-siMALAT1 cells relative to LNCaP-abl–siCTL. Bars represent -log10 (P) and the frequency polygon (line in orange) represents the number of genes. B, Correlogram depicting Pearson correlation coefficient (ρ) between DNA repair–associated genes and MALAT1 in prostate cancer patient samples from GSE35988 and GSE3325 datasets (FDR adjusted, P < 0.05). Correlation coefficients are expressed by the color from red to blue and the dot size is proportional to the strength of the correlation. Representative genes are marked on the sides of the correlogram. C, Representative confocal images for γH2AX foci (green) in control and MALAT1-ablated 22RV1 and LNCaP cells. The nucleus was visualized by DAPI (blue). Scale bar, 10 µm. D, Quantification of the number of γH2AX-positive foci in the indicated cells. Bar plot showing the percentage of cells with the indicated number of foci/nuclei in the same cells. The P value for the χ2 test is indicated. E, Immunoblots showing the expression of HR markers in MALAT1-silenced and shSCRM prostate cancer cells. β-Actin was used as a loading control. F, Same as C, except immunostaining for BRCA1. G, Same as C, except immuno-staining for RAD51. H, Schematic of the pDR-GFP reporter used to monitor HR activity in 22RV1 MALAT1-KO and shRAD51 cells. Bar plot exhibiting the percentage of GFP+ cells in 22RV1-MALAT1-KO and shRAD51 cells transfected with pDR-GFP reporter construct. Experiments were performed with n = 3 biologically independent samples; the data represent mean ± SEM. For D and F–H, one-way ANOVA with Dunnett multiple comparisons posthoc test was applied while the χ2 test was used for D.
We next examined the frequency of double-strand breaks (DSB) in MALAT1-silenced cells by evaluating the phosphorylation of H2AX on serine 139 residue (γH2AX). A marked increase in the abundance of γH2AX foci was observed in MALAT1-silenced 22RV1 and LNCaP cells (Fig. 3C and D), indicating that loss of MALAT1 results in the accumulation of damaged lesions. Notably, RNA-seq data of MALAT1-depleted LNCaP-abl cells show a marked decrease in the expression of genes that encode proteins involved in the HR pathway (Supplementary Fig. S3D). Likewise, 22RV1-shMALAT1 and LNCaP-shMALAT1 cells showed a significant reduction in the expression of major DNA damage effector proteins, including RAD51 and breast cancer gene 1/2 (BRCA1/2) both at the transcript (Supplementary Fig. S3E) and protein levels (Fig. 3E–G). Notably, a robust decrease in phosphorylation of CHEK2, a central kinase involved in HR signaling, was also observed in MALAT1-silenced 22RV1 and LNCaP cells (Fig. 3E). Because both 22RV1 and LNCaP cells harbor inactivating mutations in BRCA1/2 genes, we next examined whether MALAT1 can modulate the HR pathway in HR-proficient prostate cancer cells. Hence, we silenced MALAT1 using specific shRNAs in DU145 and PC3 cells (Supplementary Fig. S3F and S3G) and examined the expression of HR proteins. Interestingly, MALAT1 silencing led to reduced expression of RAD51, BRCA1, and BRCA2 (Fig. 3E). Concomitantly, a marked increase in the phosphorylation of H2AX foci was also noticed in MALAT1-silenced DU145 and PC3 cells (Fig. 3E), indicating the crucial role of MALAT1 in modulating the expression of several HR genes as well as genome integrity in both HR-proficient and -deficient prostate cancer cells.
To ascertain that MALAT1 modulates DNA repair by regulating HR activity, we sought to perform the direct repeat-GFP (DR-GFP) reporter assay, a prototypic assay to measure HR (30). In this vector system, a 24-bp I-SceI recognition site is integrated into the GFP gene that disrupts the open-reading frame (ORF), and a truncated GFP gene fragment with the correct ORF is inserted downstream in the construct. HR-mediated repair of the cleaved I-SceI site employing the downstream fragment would lead to a functional GFP with fluorescence, which could be measured by flow cytometry. Because the shRNAs used in this study are GFP positive and cannot be used for this experiment, CRISPR/Cas9–mediated gene knockout of MALAT1 was performed in 22RV1 cells (Supplementary Fig. S3H). Two independent MALAT1-KO clones with significantly reduced MALAT1 expression (Supplementary Fig. S3H) along with positive control 22RV1-shRAD51 cells were selected for the DR-GFP reporter assay. MALAT1 ablation resulted in a significant decrease in the GFP signal compared with the control 22RV1 cells (Fig. 3H), implying that MALAT1 ablation suppresses the HR pathway in shRNA.
MALAT1 Silencing Restrains Cell-Cycle Progression and Instigates Apoptosis In Prostate Cancer
On accumulation of DSBs, the cellular homeostatic mechanisms either obstruct the cell cycle to give cells more time to repair the lesions or initiate apoptosis if the damage is irreparable (41). Several HR proteins, like RAD51 and BRCA1, exhibit higher expression in the S or G2 phase, suggesting that a decrease in the expression of these HR proteins might influence cell-cycle progression (42, 43). Besides, phosphorylation of CHEK2, a critical player regulating the cell-cycle checkpoint, also decreased on MALAT1 knockdown (Fig. 3E). Moreover, Vidisha and colleagues previously demonstrated that MALAT1 depletion perturbs the cell-cycle machinery by suppressing the expression of genes involved in G1–S and mitotic progression (44). We, therefore, examined the cell-cycle distribution profile by performing propidium iodide (PI) staining. Intriguingly, a robust increase in the G1 cells with a concomitant reduction in the S-phase population was noted in MALAT1-silenced 22RV1 and LNCaP cells (Fig. 4A). Likewise, the EdU incorporation assay revealed a robust decrease in the number of S-phase cells upon MALAT1 depletion in 22RV1 and LNCaP cells (Fig. 4B). Intriguingly, the transcriptome profiles of MALAT1-silenced LNCaP-abl cells exhibited a marked decrease in the expression of key cell cycle–associated genes (Supplementary Fig. S4A). Corroborating with this, the expression of genes encoding for the proteins associated with G1–S and G2–M phase transition, such as CCNA2, CCNB, CDKs, centromere proteins, and mini-chromosome maintenance (MCM2–8) was significantly decreased in MALAT1-silenced prostate cancer cells in comparison with shSCRM cells (Fig. 4C; Supplementary Fig. S4B). Similarly, a strong positive correlation (ρ ≥ 0.4) between MALAT1 and cell cycle–associated genes was noted in prostate cancer patient specimens (GSE35988 and GSE3325; Supplementary Fig. S4C), suggesting that MALAT1 plays a pivotal role in cell-cycle progression. We next examined the expression of E2F1, the major determinant of the G1–S phase transition. Notably, a remarkable decrease in E2F1 levels was observed upon MALAT1 depletion in prostate cancer cells (Fig. 4D). In consonance with this, MALAT1 ablation markedly reduced cell proliferation in both 22RV1 and LNCaP cells (Fig. 4E).
FIGURE 4.
MALAT1 knockdown restrains cell-cycle progression and instigates apoptosis in prostate cancer cells. A, Flow cytometry analysis for accessing the cell-cycle distribution by propidium iodide (PI) DNA staining assay in MALAT1-silenced prostate cancer cells. The percentage of cells in each phase was calculated using FlowJo software. B, Representative images depicting EdU incorporation in the same cells as in A. Nuclei were stained with Hoechst 33342. Scale bar, 20 µm. Right, bar graph showing quantification of EdU uptake in the indicated cells. C, qRT-PCR analysis showing expression of genes associated with G1 and S-phase of the cell cycle in MALAT1-silenced 22RV1 cells. The expression level for each gene was normalized to GAPDH. D, Immunoblot showing the change in expression of E2F1 in the same cells as in A. β-Actin was used as an internal control. E, Line graph showing cell proliferation assay using the same cells as in A, at the indicated time points. F, Flow cytometry–based apoptosis assay using Annexin V-PE and 7-AAD staining in the same cells as in A. The percentage of apoptotic cells was calculated using FlowJo software. G, Immunoblots showing a change in the expression of apoptosis markers in the same cells as in A. β-Actin was used as an internal control. H, Schematic depicting that MALAT1 is a novel regulator of HR and plays an important role in the maintenance of genome stability in prostate cancer. MALAT1 depletion induces HR deficiency by decreasing the expression of several DDR genes and results in DSB accumulation which in turn induces cell-cycle arrest and instigates apoptosis. Experiments were performed with n = 3 biologically independent samples; the data represents mean ± SEM. For B and C, one-way ANOVA with Dunnett multiple comparisons posthoc test was applied, while for E, two-way ANOVA with Tukey multiple comparisons test was applied.
Because MALAT1 depletion dysregulates the cellular repair machinery, we speculated that the accumulation of DNA lesions might instigate apoptosis. Thus, we performed annexin-V and 7-AAD staining and noticed a robust increase in early apoptotic cells in MALAT1-depleted prostate cancer cells (Fig. 4F). Likewise, levels of cleaved PARP, an early hallmark of apoptosis, also increased upon silencing MALAT1 in both the cell lines (Fig. 4G). A robust decrease in the antiapoptotic Bcl-2 family proteins (BCL2 and Bcl-xL) was also observed (Fig. 4G), establishing that the loss of MALAT1 triggers apoptosis. Collectively, these findings establish that MALAT1 acts as a master regulator that modulates HR, cell-cycle progression, and apoptosis in prostate cancer (Fig. 4H).
MALAT1 Modulates the Expression of HR Genes by Sponging miRNA-421
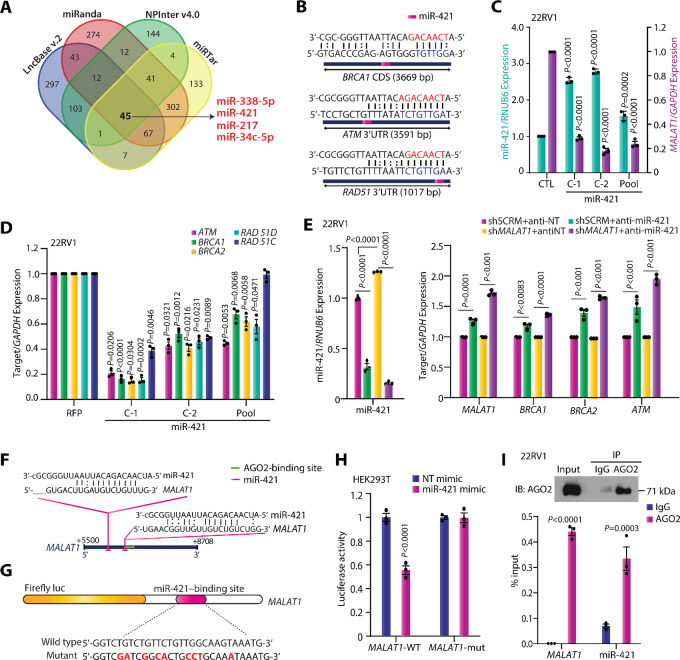
Having established that MALAT1 enhances the expression of HR genes, we next sought to explore the underlying mechanistic basis. To determine whether MALAT1 directly binds to HR proteins and modulates their expression, we performed ChIRP assay (32), wherein we used biotinylated antisense oligonucleotides targeting MALAT1 to retrieve the MALAT1-interacting proteins and examined the expression of critical DDR genes by immunoblotting. The enrichment of MALAT1 was confirmed by qRT-PCR (Supplementary Fig. S5A), while the interaction with HR proteins, namely, BRCA1 and RAD51, was examined by immunoblotting. However, to our surprise, immunoblot data using MALAT1-biotinylated probes failed to show direct interaction with HR proteins, in contrast, a remarkable interaction between MALAT1 and EZH2 was noted which was used as positive control (Supplementary Fig. S5B). Alternatively, we also cross-verified these results by performing RNA immunoprecipitation (RIP) using antibodies against BRCA1/2 and RAD51. Likewise, no interaction with BRCA1 or BRCA2 was observed in RIP assay, while a remarkable enrichment with the RAD51 antibody was noted, indicating that RAD51 directly interacts with MALAT1 (Supplementary Fig. S5C). Nevertheless, RIP assays often exhibit nonspecific interactions that may form after cell lysis, thus RNA-centric methods such as ChIRP are preferred to determine the protein interactome of lncRNAs (45, 46). Hence, we concluded that MALAT1 regulates the expression of HR proteins indirectly, either by modulating transcription factors or possibly by posttranscriptional regulation via miRNAs. Moreover, MALAT1 has been reported to function as a competing endogenous RNA (ceRNA) or miRNA sponge that interacts with miRNAs and in turn, enhances the expression of downstream target genes (47). In tandem with this, several miRNAs have been shown to modulate the expression of genes associated with the HR pathway (48). Therefore, we investigated whether MALAT1 could protect the proteins associated with the HR pathway by sponging the target miRNAs. To examine the miRNAs that might bind to the MALAT1 transcript, we used four miRNA-binding prediction tools, namely LncBase v.2 (49), miRanda (50), NPinter v4.0 (51), and miRTar (52). About 45 miRNAs were identified in common by all four tools (Fig. 5A; Supplementary Table S4). Surprisingly, miR-338–5p and miR-421, which we previously validated as tumor suppressor miRNAs in prostate cancer (53), were also present among the predicted miRNAs that interacted with MALAT1 (Fig. 5A). Moreover, miR-421 has been demonstrated to bind to the 3′ UTR of ATM and downregulate its expression in neuroblastoma, HeLa, and CRPC cells (54, 55). Besides, ectopic overexpression of miR-421 disrupts S-phase cell-cycle checkpoints and augments radiosensitivity (54). In addition, miRNA-binding prediction tools suggested that miR-421 can bind to several DDR genes, including BRCA1, RAD51 and ATM, suggesting that miR-421 acts as a crucial player in the HR pathway (Fig. 5B). Intrigued by this observation, we generated stable miR-421–overexpressing 22RV1 cells (Fig. 5C) and examined the expression of MALAT1 and major HR genes. Interestingly, overexpression of miR-421 in 22RV1 cells suppresses the expression of MALAT1 (Fig. 5C) as well as key HR genes including ATM, RAD51, BRCA1, and BRCA2 (Fig. 5D). Furthermore, to ascertain whether the decrease in expression of HR proteins in MALAT1-silenced cells is indeed due to miR-421–mediated posttranscriptional regulation, we transfected 22RV1-shMALAT1 and shSCRM cells with antagomiR (anti-miR) targeting miR-421. As expected, 22RV1-shSCRM transfected with anti-miR-421 showed a modest increase in the expression of HR genes while the MALAT1-depleted cells exhibited a robust increase, indicating that the decrease in the expression of HR genes upon MALAT1 silencing could be mediated by miR-421 (Fig. 5E).
FIGURE 5.
MALAT1 modulates the expression of HR genes by sponging tumor-suppressive miR-421. A, Venn diagram displaying the miRNAs predicted to bind to MALAT1 transcript using four miRNA prediction tools binding computational tools, namely LncBaseV.2, miRanda, NPInter v4.0, and miRTar. B, Mature miR-421 sequence and its seed sites within 3′UTR of BRCA1, ATM and RAD51. The seed sequence of miR-421 is shown in red while the target sequence is depicted in blue. C, Bar plot depicting the relative expression of miR-421 and MALAT1 in 22RV1-miR-421 cells and control cells. D, qPCR depicting relative expression of HR genes in the same cells as in C. E, Quantitative PCR depicting relative expression of HR genes in 22RV1-shSCRM and -shMALAT1 cells transfected with nontargeting antagomiR or antagomiR-421. F, Schematic illustrating the predicted miR-421–binding sites near the 3′ end of MALAT1. G, Illustration of luciferase reporter construct with the wild-type or mutated (transformed residues in red) miR-421–binding sites on MALAT1 3′ end downstream of the firefly luciferase reporter gene. H, Bar plots depicting the luciferase reporter activity in HEK293T cells cotransfected with MALAT1-WT or MALAT1-mut construct with nontargeting mimics or miR-421 mimic. I, RIP followed by real-time qPCR analyses demonstrating enrichment of MALAT1 and miR-421 with AGO2 antibody–bound beads in comparison to IgG (control antibody) in 22RV1 cells. The experiments were performed in triplicate with biologically independent samples (n = 3); the data represent mean ± SEM. The statistical analysis of differences was calculated using one-way ANOVA with Dunnett multiple-comparisons posthoc test for panels C–E and H–I.
Furthermore, to examine the possible interaction between MALAT1 and miR-421, we cloned the fragment of MALAT1 harboring putative miR-421 (+6,501–6,708 bp; Fig. 5F) binding sites in a luciferase reporter construct (Fig. 5G). Interestingly, the luciferase activity of MALAT1-WT reporter constructs was significantly restrained upon cotransfection with respective miR-421 mimics compared with the control (miR-NT; Fig. 5H). Moreover, to confirm the specificity of the interaction, key residues within the predicted seed sequence were mutated and luciferase assay was performed. As expected, the mutant constructs failed to show any noticeable response upon miR-421 mimic transfection (Fig. 5H), indicating that MALAT1 directly interacts with miR-421 via the predicted target site. Given that overexpression of miR-421 significantly suppresses the MALAT1 expression (Fig. 5C) and a miR-421–binding site on the MALAT1 transcript located adjacent to the AGO2-binding region (Fig. 5G), we speculate that miR-421 posttranscriptionally suppresses MALAT1 expression via RNA-induced silencing complex. To confirm this, we performed RIP using an AGO2 antibody and noticed a robust enrichment of MALAT1 as well as miR-421 with AGO2-bound beads in comparison with IgG (Fig. 5I). Collectively, these findings provide irrevocable evidence that a double-negative feedback loop between MALAT1 and miR-421 modulates the expression of HR genes in prostate cancer.
MALAT1 Knockdown Sensitizes Prostate Cancer Cells to PARPi
Cancer cells with a dysfunctional HR pathway heavily rely on PARP enzymes for removing damaged lesions and ensuring their survival. Any additional pharmacologic assault with PARPi or DNA-damaging drugs such as cisplatin, oxaliplatin, and carboplatin results in the accumulation of DNA lesions, eventually leading to cell death (56, 57). Because MALAT1 knockdown contrives HR deficiency in prostate cancer, we speculated that MALAT1-deficient cells would be vulnerable to chemotherapeutic agents that target DNA repair. To examine this, MALAT1-silenced prostate cancer cells were treated with varying concentrations of olaparib, an FDA-approved PARPi, and analyzed for any change in drug response. Interestingly, MALAT1 depletion in prostate cancer cells harboring mutations in BRCA1/2, namely 22RV1 and LNCaP showed a remarkable increase in drug sensitivity, while prostate cancer cells with no predicted biallelic mutations in canonical HR genes, that is, DU145 and PC3 cells (58), exhibited a modest increase in PARP sensitivity upon MALAT1 knockdown (Fig. 6A). MALAT1-silenced prostate cancer cells exhibited decreased cell proliferation compared with shSCRM cells, while the effect was more pronounced (∼80%) in olaparib-treated cells (Fig. 6B), indicating that MALAT1 depletion augments olaparib activity. In addition, the colony-forming ability of MALAT1-silenced prostate cancer cells was significantly impaired in the presence of olaparib in PARPi-sensitive (22RV1 and LNCaP) as well as PARPi-insensitive (DU145 and PC3) prostate cancer cell lines (Fig. 6C and D; Supplementary Fig. S6), suggesting that MALAT1 depletion could sensitize HR-deficient as well as HR-proficient prostate cancer cells to olaparib. As MALAT1 depletion markedly enhances cellular sensitivity to PARPi, we examined the effect of olaparib treatment on cell-cycle profiles in MALAT1-silenced prostate cancer cells by performing EdU staining. MALAT1-deficient prostate cancer cells upon olaparib treatment show a decrease in the S-phase population compared with the shSCRM control (Fig. 6E and F).
FIGURE 6.
MALAT1 depletion induces “BRCAness” and confers sensitivity to PARPi. A, Cell cytotoxicity assay for determining IC50 value of olaparib in SCRM control and MALAT1-silenced prostate cancer cells. The IC50 values were calculated by generating a dose–response curve using GraphPad Prism software. B, Line graph showing relative decrease in cell viability on olaparib treatment (10 µmol/L) in MALAT1-silenced 22RV1 and LNCaP cells as compared with scrambled control. The drug was replenished every 24 hours at the indicated time points. C, Foci formation assay in 22RV1-shSCRM and -shMALAT1 cells following treatment with olaparib (5 µmol/L) or vehicle control for 15 days. Inset showing representative images of foci. D, Foci formation assay in LNCaP-shSCRM and -shMALAT1 cells following treatment with olaparib (2 µmol/L) or vehicle control for 15 days. Inset showing representative images of foci. E, Representative confocal images for EdU uptake in MALAT1-deficient 22RV1 and LNCaP cells followed by olaparib (10 µmol/L) treatment for 48 hours. Scale bar, 50 µm. F, Bar graph showing quantification of EdU staining after 48-hour treatment with olaparib in the indicated cells. G, Representative confocal images for γH2AX foci (red) in the same cells as in C upon olaparib (10 µmol/L) treatment for 48 hours. The nucleus was visualized by Hoechst 33342 (blue). Scale bar, 10 µm. Quantification of the number of γH2AX-positive foci in the indicated cells. Bar plot showing the percentage of cells with the indicated number of foci/nuclei in the same cells. The P value for the χ2 test is indicated. H, Same as G, except LNCaP-shSCRM and LNCaP-shMALAT1 cells. I, Flow cytometry–based apoptosis assay using Annexin V-PE and 7-AAD staining in the same cells as in B upon olaparib (10 µmol/L) treatment for 48 hours. The percentage of the apoptotic cell population was calculated using FlowJo software. J, Same as I, except LNCaP-shSCRM and LNCaP-shMALAT1 cells. K, Immunoblot showing the change in expression of cleaved PARP in the same cells as in B upon olaparib (10 µmol/L) treatment for 48 hours. β-Actin was used as an internal control. The experiments were performed with n = 3 biologically independent samples; the data represents mean ± SEM. Extra sums of the square F-test was used to compute the statistical significance and compare the curves in A. The statistical analysis of difference was computed using two-way ANOVA with Tukey multiple-comparison posthoc test for B–D and F–H, while the χ2 test was used for G and H.
To assess whether MALAT1 depletion impairs the DNA repair system and sensitizes prostate cancer to olaparib, we examined the frequency of γH2AX foci in olaparib-treated MALAT1-silenced cells. Almost 2-fold higher γH2AX foci per cell were noted in olaparib-treated MALAT1-silenced cells compared with shSCRM control (Fig. 6G and H), suggesting that MALAT1 deficiency exacerbates olaparib-induced DNA damage in prostate cancer cells. Because the accumulation of DNA lesions instigates apoptosis, olaparib-treated shMALAT1 and shSCRM prostate cancer cells were further examined for cell death by AnnexinV-7AAD staining. A marginal increase in the early apoptotic cell population was observed in MALAT1-ablated prostate cancer cells compared with their respective shSCRM controls, while upon olaparib treatment the number of apoptotic cells was markedly increased (Fig. 6I and J). Consistent with this, the level of cleaved PARP was also increased in olaparib-treated shMALAT1 cells compared with shSCRM control (Fig. 6K). Furthermore, genetic depletion of PARP1 in HR-proficient as well as HR-deficient prostate cancer cells suppresses the expression of DDR genes in both, shSCRM as well as shMALAT1 cells, nevertheless, the shMALAT1 cells exhibit a more notable reduction in expression, suggesting that PARP1 depletion cooperatively suppress the expression of HR genes in MALAT1-silenced cells (Supplementary Fig. S7). Collectively, our results provide compelling evidence that MALAT1 depletion enhances the sensitivity to PARPi; hence, targeting both simultaneously will be a promising therapeutic approach for patients with advanced stage prostate cancer, who often develop resistance to conventional therapeutic strategies.
MALAT1 Ablation Enhances the Sensitivity of Prostate Cancer to PARPi
On the basis of our in vitro results and previous research emphasizing the role of MALAT1 in prostate tumorigenesis and metastasis, we next examined the impact of PARPi in MALAT1-high versus low prostate cancer tumors. For this, we implanted 22RV1-SCRM and 22RV1-MALAT1 knockout cells subcutaneously in athymic immunodeficient mice (n = 12 per group). The mice were randomly divided into two groups (n = 6) once the tumors reached a palpable size (average ∼100 mm3), the intended vehicle control and PARPi (50 mg/kg) were administered. A significant decrease in tumor volume was noted after treatment with PARPi in both 22RV1-SCRM and 22RV1 MALAT1 KO groups; however, the percentage reduction in tumor volume was more pronounced in the MALAT1-KO group (∼80%) compared with the 22RV1-SCRM (∼55%) at day 20 upon treatment with PARPi (Fig. 7A and B). This observation corroborates with our in vitro data, wherein the anticancer properties of PARPi were enhanced upon MALAT1 knockdown in prostate cancer cells. Subsequently, to examine the coinhibitory potential of MALAT1 and PARP1 in distant tumor metastasis, we determined the levels of human-specific Alu sequences in the lungs and bone marrow collected from xenografted mice. Mice implanted with MALAT1 KO cells showed a significant reduction in lung and bone metastases upon PARPi treatment, as compared with SCRM control cells (Fig. 7C and D). On the other hand, mice implanted with 22RV1 SCRM cells showed a significant reduction in bone metastasis (Fig. 7D) upon PARPi treatment while no significant change was observed in lung metastasis when compared with vehicle control (Fig. 7C). Furthermore, to investigate the effect of coinhibition of MALAT1 and PARP in tumor xenografts, we performed IHC staining for the cell proliferative marker Ki-67. As speculated, a significant decrease in Ki-67 levels was observed in mice implanted with MALAT1 KO cells upon PARPi treatment, as compared with SCRM control cells (Fig. 7E and F). Conclusively, our mice xenograft studies showed that olaparib-mediated reduction of prostate tumor growth and metastases is potentiated by inhibition of MALAT1. These findings suggest that targeting MALAT1 enhances the vulnerability of prostate cancer to PARPi therapy.
FIGURE 7.
MALAT1 ablation confers sensitivity to PARPi. A, Mean tumor volume of xenografts generated by implanting 22RV1-SCRM and 22RV1-MALAT1 KO cells in NOD-SCID mice and randomized into two groups (n = 6 each), namely, vehicle control and olaparib (50 mg/kg). B, Bar plot showing percent tumor reduction in the same mice as mentioned in A. C, Scatter dot plot showing the number of cells metastasized to the lungs in xenografted mice as mentioned in A. D, Same as C, except cells metastasized to bone marrow. E, Representative images depicting IHC staining for Ki-67 in formalin-fixed paraffin-embedded tumor xenograft specimens as in A. F, Scatter dot plot showing quantification of Ki-67 expression in the tumor tissue sections of the mice xenografts as in A. G, Schema depicting that MALAT1 inhibition perturbs HR machinery via miR-421 and this in turn enhances the sensitivity toward PARPi. MALAT1 sponges miR-421 which in turn enhances the DNA repair activity of prostate cancer cells and enables them to proliferate and survive even in the presence of therapy-induced damage. While MALAT1 depletion disrupts the HR machinery, the NHEJ pathway takes over and repairs the damage, and aids in the survival of prostate cancer cells. However, when, MALAT1-depleted cells are treated with pharmacologic inhibitors of PARP1, a type of enzyme that helps to repair damaged DNA via the NHEJ pathway, the cells are unable to repair the breaks brought on by treatment with PARP1 inhibitors and ultimately result in cell death. For A and B, the data represent mean ± SEM and the statistical difference was computed using two-way ANOVA with Tukey multiple comparison test while for C, D and F, the data are presented as median (middle line) with interquartile range and one-way ANOVA with Tukey multiple comparison test was applied.
Discussion
Advanced stage prostate cancer is characterized by higher metastatic potential, enhanced biochemical recurrence, and poor patient survival (59). Identification of molecular mechanisms orchestrating the progression to CRPC will facilitate development of novel targeted therapeutic strategies for the disease. In this study, we used an integrated bioinformatics approach to identify the molecular factor(s) associated with mCRPC and discovered MALAT1, an oncogenic lncRNA, is significantly upregulated in patients with mCRPC compared with localized cases. Consistent with this, previous studies also demonstrated frequent upregulation of MALAT1 in advanced stage prostate cancer which positively associates with an aggressive clinical phenotype (60, 61). Furthermore, MALAT1 upregulation has also been linked to poor prognosis in several malignancies (35, 37), suggesting that it may be associated with drug resistance.
Here, for the first time, we demonstrate that MALAT1 is essential for preserving the genomic integrity in advanced stage prostate cancer and protects tumor cells from the damage caused by anticancer agents by enhancing DNA repair pathways, which in turn induces resistance to chemotherapeutic drugs and facilitates the survival of cancer cells. Mechanistically, we show that targeting MALAT1 induces DSBs and apoptosis, which makes prostate cancer cells vulnerable to DNA repair inhibitors, such as olaparib, and DNA-damaging agents like doxorubicin. Contrary to the well-documented inactivating mutations in the HR pathway, herein we found that the core HR genes are upregulated in patients with CRPC and positively correlate with MALAT1 expression. In accord with this, several recent reports have also demonstrated that HR genes are frequently upregulated in lethal and advanced stage prostate cancer tumors, that is, CRPC (9) and neuroendocrine prostate cancer (6, 9). Furthermore, HR proteins are known to be elevated in several other therapy-resistant malignancies such as glioblastoma (11) and triple-negative breast cancer (62); however, the underlying biological mechanism(s) involved in their upregulation remain unknown and require extensive molecular characterization.
In this study, we found that MALAT1 modulates the expression of key HR proteins, namely BRCA1/2 and RAD51, but does not interact with them directly. Likewise, Huang and colleagues showed that MALAT1 indirectly modulates the expression of BRCA1 in non–small cell lung carcinoma (63). To delineate the possible connection between MALAT1 and HR genes, we explored the possibility of posttranscriptional regulation via miRNAs and found that MALAT1 sequesters miR-421, a tumor suppressor miRNA that regulates the expression of HR genes. In addition, our findings also demonstrate the presence of a double-negative feedback loop between MALAT1 and miR-421; wherein MALAT1 sequesters miR-421 and averts its binding to target proteins, while miR-421 posttranscriptionally suppresses MALAT1, suggesting that reciprocal regulation between MALAT1 and miR-421 regulates the expression of HR genes in prostate cancer. Nevertheless, further in-depth investigation is needed as we speculate that transcriptional and epigenetic alterations induced by silencing MALAT1, might also modulate the expression of HR proteins.
Collectively, our findings demonstrate that the HR deficiency induced by MALAT1 depletion phenocopies “BRCAness” and augments sensitivity to clinically approved DNA repair inhibitors such as olaparib (Fig. 7G). In consonance with our results, several recent reports also demonstrate that MALAT1 depletion enhances sensitivity toward chemotherapeutic drugs like docetaxel (64), oxaliplatin (65), cisplatin (66), and cytarabine (67) by sponging miR-200b, miR-324–3p, miR-145, and miR-96, respectively. MALAT1 also increases the transcriptional activity of YAP1, which can alter the HR as well as the non–homologous end joining (NHEJ) pathway, to confer radioresistance to colorectal cancer cells (68). Furthermore, MALAT1 has been shown to modulate the alternative NHEJ (A-NHEJ) pathway by directly interacting with LIG3 and PARP1 in multiple myeloma cells and targeting MALAT1 enhances sensitivity to PARPi or proteasomal inhibitors (69). These findings, in line with ours, allude to the fact that MALAT1 is a crucial molecular modulator of the DDR pathway that may augment chemoresistance in advanced stage cancer.
PARPi, such as olaparib and rucaparib have shown promising results in the clinical management of multiple malignancies, including mCRPC, particularly in patients harboring mutations in the HR genes (70). However, the proportion of patients with prostate cancer harboring HR mutations is less than 20%; hence, targeting alternative genetic and epigenetic factors that selectively regulate the HR pathway may sensitize HR-proficient prostate cancer to PARPi. Moreover, several preclinical studies have indicated that androgen receptor (AR) signaling antagonists may enhance the sensitivity of prostate cancer cells to PARPi. Specifically, AR antagonists demonstrate contextual synthetic lethality with PARPi by modulating the expression of key HR proteins such as BRCA1/2 and RAD51, and potentially enhance the vulnerability of prostate cancer cells on alternative DNA repair mechanisms, such as PARP-mediated repair (71). Considering this, the combination of enzalutamide and olaparib in patients with mCRPC, particularly those who have alterations in DNA repair genes was assessed in Profound clinical trial (NCT02987543). The results showed that patients who received the combination therapy had better overall, and radiographic progression-free survival (rPFS) compared with those receiving enzalutamide alone (72). Furthermore, recently the FDA-approved talazoparib in combination with enzalutamide for the treatment of mCRPC patients harboring HR gene mutations, which markedly improved the rPFS in comparison with placebo and enzalutamide in TALAPRO-2 clinical trial (73). In addition, recent reports also suggest that MALAT1 expression can be modulated by androgens, while it reciprocally enhances the transcriptional activity of AR by acting as its coactivator (74). However, the specific interactions and mechanisms are still under investigation, and we conjecture that there is a direct connection between MALAT1, AR signaling, and the HR pathway.
Furthermore, multiple lines of evidence show that small-molecule inhibitors against bromodomain and extra-terminal motif (BET) proteins (75), PI3K (PI3K/AKT; ref. 76), and histone deacetylases (HDAC; ref. 77) synergize with PARPi by suppressing the expression of BRCA1 and RAD51 while CDK1 inhibitors disrupt the recruitment of BRCA1 to DNA damage sites and in turn, enhance sensitivity to PARPi (78). Pharmacologic inhibition of DNA methyltransferases (DNMT) increases PARPi sensitivity by enhancing PARP1-DNA binding (79). Our data suggests the presence of a double-negative feedback loop between MALAT1 and PARP1 in both AR-negative and AR-positive prostate cancer cell lines (Supplementary Fig. S7). It has been shown that PARPi traps both PARP1 and PARP2 at the damaged DNA, where PARP–DNA complexes show more cytotoxicity than unrepaired SSBs caused by PARP inactivation (80). Recently, a competitive binding study showed that PAR binding releases PARP1 from DNA via its WGR domain (Trp-Gly-Arg rich), and PAR induces catalytic stimulation of PARP1, while impeding the DNA-dependent stimulation. This report stresses on the role of high-affinity PAR reader domains of PARP1 and proposes a novel mechanism of allosteric regulation of DNA-dependent and DNA-independent activities of PARP1 (81). Thus, considering these lines of evidence, we speculate that PARPi might be affecting other key enzymes involved in DNA repair in addition to PARP1.
On the basis of these preclinical studies, several drug combination approaches are being evaluated in clinical trials, for instance, the clinical trial TRAP (NCT03787680) is evaluating the efficacy and safety of olaparib in combination with an ATR inhibitor (AZD6738), while another clinical trial, NCT02893917 is investigating the efficacy of a combinatorial regimen including cediranib, a VEGF receptor inhibitor, and olaparib for the treatment of patients with mCRPC (82). Along similar lines, our findings provide a compelling rationale for conducting clinical trials in patients with advanced stage disease to investigate the safety and efficacy of combinatorial therapy using MALAT1 antisense oligonucleotides/GAPmers or small-molecule inhibitors against MALAT1 with PARPi or DNA-damaging agents like cisplatin. In addition, this study provides an important molecular connection between MALAT1, miR-421, and HR pathway in prostate cancer. Understanding the precise involvement of miR-421 in the HR pathway may contribute to the development of miR-421 as a DNA damage response signature and a possible therapeutic target for patients with prostate cancer. Conclusively, our findings indicate that oncogenic lncRNA MALAT1 protects prostate cancer tumor cells from anticancer agents by initiating the HR pathway, revealing a potential therapeutic vulnerability that can be exploited by targeting MALAT1 or overexpressing miR-421 in conjunction with PARPi.
Supplementary Material
Supplementary Methods
MALAT1 is upregulated in prostate cancer and positively associates with aggressive clinical phenotype.
MALAT1 positively associates with mesenchymal and stemness markers in prostate cancer patients.
MALAT1 regulates the DNA repair pathway in prostate cancer.
MALAT1 depletion restrains cell cycle progression in prostate cancer.
MALAT1 does not interact with HR proteins.
MALAT1 depletion enhances sensitivity to PARP inhibitors in HR proficient PCa cells.
MALAT1 and PARP depletion cooperatively suppress expression of HR genes in HR-deficient as well as HR-proficient PCa cells.
Supplementary Table S1: List of the Primers
Supplementary Table S2: Genes upregulated in metastatic prostate cancer patients in comparison to localized cases.
Supplementary Table S3: Biological pathways downregulated on MALAT1 ablation, predicted by DAVID analysis.
Supplementary Table S4: Computational prediction of microRNAs with putative binding sites on the MALAT1transcript.
Acknowledgments
This work is supported by the DBT/Wellcome Trust India Alliance fellowship (grant number: IA/S/19/2/504659; to B. Ateeq). B. Ateeq is a senior fellow of the DBT/Wellcome Trust India Alliance and acknowledges financial support from the SERB-POWER (grant number: SPG/2021/000851) and S. Ramachandran-National Bioscience Award for Career Development (grant number: BT/HRD/NBA/NWB/39/2020–21) from the Department of Biotechnology, Ministry of Education. The authors thank the Indian Institute of Technology, Kanpur for infrastructure support and the Fund for Improvement of S&T Infrastructure (FIST) by the Department of Science and Technology for BD FACSMelody cell sorter. We are grateful to Prof. Jonaki Sen and Prof. Pradip Sinha for extending the use of the microscope facility, and Prof. Dhirendra Katti for providing access to Synergy H1 Hybrid Multi-Mode Reader. We profusely thank Dr. Mohammad Asim, University of Surrey for providing DU145 cells, Dr. Abid Mattoo for pDR-GFP and pCBASce vectors, and Prof. Ganesh Nagaraju, IISc Bangalore, for providing the RAD51 antibody. The authors thank Mahendra Palecha for the technical assistance.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Communications Online (https://aacrjournals.org/cancerrescommun/).
Authors’ Disclosures
B. Ateeq reports a patent to Bushra Ateeq and Anjali Yadav [Indian Provisional Patent, Indian Institute of Technology, Kanpur; Chemosensitization of castrate-resistant prostate cancer to PARP inhibitors by MALAT ablation; application number: E-2/1119/2022/DEL (202111037231); patent pending]. No other disclosures were reported.
Authors’ Contributions
A. Yadav: Conceptualization, data curation, formal analysis, validation, investigation, visualization, methodology, writing-original draft, writing-review and editing. T. Biswas: Data curation, investigation, methodology, writing-review and editing. A. Praveen: Data curation, methodology, writing-review and editing. P. Ganguly: Data curation, investigation, methodology, writing-review and editing. A. Bhattacharyya: Investigation, methodology. A. Verma: Resources, investigation, writing-review and editing. D. Datta: Resources, investigation. B. Ateeq: Conceptualization, resources, formal analysis, supervision, funding acquisition, investigation, writing-original draft, project administration, writing-review and editing.
References
- 1. Boyd LK, Mao X, Lu YJ. The complexity of prostate cancer: Genomic alterations and heterogeneity. Nat Rev Urol 2012;9:652–64. [DOI] [PubMed] [Google Scholar]
- 2. Wang G, Zhao D, Spring DJ, Depinho RA. Genetics and biology of prostate cancer. Genes Dev 2018;32:1105–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Armenia J, Wankowicz SAM, Liu D, Gao J, Kundra R, Reznik E, et al. The long tail of oncogenic drivers in prostate cancer. Nat Genet 2018;50:645–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abida W, Cyrta J, Heller G, Prandi D, Armenia J, Coleman I, et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc Natl Acad Sci 2019;166:11428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andor N, Maley CC, Ji HP. Genomic instability in cancer: Teetering on the limit of tolerance. Cancer Res 2017;77:2179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang W, Liu B, Wu W, Li L, Broom BM, Basourakos SP, et al. Targeting the MYCN–PARP–DNA damage response pathway in neuroendocrine prostate cancer. Clin Cancer Res 2018;24:696–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Amable L. Cisplatin resistance and opportunities for precision medicine. Pharmacol Res 2016;106:27–36. [DOI] [PubMed] [Google Scholar]
- 8. Khanna KK, Jackson SP. DNA double-strand breaks: Signaling, repair and the cancer connection. Nat Genet 2001;27:247–54. [DOI] [PubMed] [Google Scholar]
- 9. Polkinghorn WR, Parker JS, Lee MX, Kass EM, Spratt DE, Iaquinta PJ, et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov 2013;3:1245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009;458:780–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006;444:756–60. [DOI] [PubMed] [Google Scholar]
- 12. Storici F, Tichon AE. RNA takes over control of DNA break repair. Nat Cell Biol 2017;19:1382. [DOI] [PubMed] [Google Scholar]
- 13. Michelini F, Pitchiaya S, Vitelli V, Sharma S, Gioia U, Pessina F, et al. Damage-induced lncRNAs control the DNA damage response through interaction with DDRNAs at individual double-strand breaks. Nat Cell Biol 2017;19:1400–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu L, Chen Y, Huang Y, Cao Y, Liu T, Shen H, et al. Long non-coding RNA ANRIL promotes homologous recombination-mediated DNA repair by maintaining ATR protein stability to enhance cancer resistance. Mol Cancer 2021;20:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharma V, Khurana S, Kubben N, Abdelmohsen K, Oberdoerffer P, Gorospe M, et al. A BRCA1-interacting lncRNA regulates homologous recombination. EMBO Rep 2015;16:1520–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shen L, Wang Q, Liu R, Chen Z, Zhang X, Zhou P, et al. LncRNA Inc-RI regulates homologous recombination repair of DNA double-strand breaks by stabilizing RAD51 mRNA as a competitive endogenous RNA. Nucleic Acids Res 2018;46:717–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prensner JR, Chen W, Iyer MK, Cao Q, Ma T, Han S, et al. PCAT-1, a long noncoding RNA, regulates BRCA2 and controls homologous recombination in cancer. Cancer Res 2014;74:1651–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012;487:239–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu YP, Landsittel D, Jing L, Nelson J, Ren B, Liu L, et al. Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J Clin Oncol 2004;22:2790–9. [DOI] [PubMed] [Google Scholar]
- 20. Chandran UR, Ma C, Dhir R, Bisceglia M, Lyons-Weiler M, Liang W, et al. Gene expression profiles of prostate cancer reveal involvement of multiple molecular pathways in the metastatic process. BMC Cancer 2007;7:65–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 2015;43:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Varambally S, Yu J, Laxman B, Rhodes DR, Mehra R, Tomlins SA, et al. Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell 2005;8:393–406. [DOI] [PubMed] [Google Scholar]
- 23. Kumar A, Coleman I, Morrissey C, Zhang X, True LD, Gulati R, et al. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat Med 2016;22:369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gu Z. Complex heatmap visualization. iMeta 2022;1:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang S, Zang C, Xiao T, Fan J, Mei S, Qin Q, et al. Modeling cis-regulation with a compendium of genome-wide histone H3K27ac profiles. Genome Res 2016;26:1417–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sherman BT, Hao M, Qiu J, Jiao X, Baseler MW, Lane HC, et al. DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res 2022;50:W216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goel S, Bhatia V, Kundu S, Biswas T, Carskadon S, Gupta N, et al. Transcriptional network involving ERG and AR orchestrates Distal-less homeobox-1 mediated prostate cancer progression. Nat Commun 2021;12:5325–4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aparicio-Prat E, Arnan C, Sala I, Bosch N, Guigó R, Johnson R. DECKO: Single-oligo, dual-CRISPR deletion of genomic elements including long non-coding RNAs. BMC Genomics 2015;16:846–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods 2014;11:783–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pierce AJ, Johnson RD, Thompson LH, Jasin M. XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev 1999;13:2633–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Richardson C, Moynahan ME, Jasin M. Double-strand break repair by interchromosomal recombination: suppression of chromosomal translocations. Genes Dev 1998;12:3831–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chu C, Qu K, Zhong FL, Artandi SE, Chang HY. Genomic maps of long noncoding RNA occupancy reveal principles of RNA-chromatin interactions. Mol Cell 2011;44:667–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Van Der Horst EH, Leupold JH, Schubbert R, Ullrich A, Allgayer H. TaqMan®-based quantification of invasive cells in the chick embryo metastasis assay. Biotechniques 2004;37:940-2, 944, 946. [DOI] [PubMed] [Google Scholar]
- 34. Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer. Cell 2015;163:1011–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ji P, Diederichs S, Wang W, Böing S, Metzger R, Schneider PM, et al. MALAT-1, a novel noncoding RNA, and thymosin β4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene 2003;22:8031–41. [DOI] [PubMed] [Google Scholar]
- 36. Gutschner T, Hämmerle M, Eissmann M, Hsu J, Kim Y, Hung G, et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res 2013;73:1180–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhao M, Wang S, Li Q, Ji Q, Guo P, Liu X. MALAT1: A long non-coding RNA highly associated with human cancers. Oncol Lett 2018;16:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Batlle E, Clevers H. Cancer stem cells revisited. Nat Med 2017;23:1124–34. [DOI] [PubMed] [Google Scholar]
- 39. Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: An evolving paradigm. Nat Rev Cancer 2013;13:714–26. [DOI] [PubMed] [Google Scholar]
- 40. Liu T, Xu F, Du X, Lai D, Liu T, Zhao Y, et al. Establishment and characterization of multi-drug resistant, prostate carcinoma-initiating stem-like cells from human prostate cancer cell lines 22RV1. Mol Cell Biochem 2010;340:265–73. [DOI] [PubMed] [Google Scholar]
- 41. Pucci B, Kasten M, Giordano A. Cell Cycle and Apoptosis. Neoplasia 2000;2:291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Deng CX. BRCA1: Cell cycle checkpoint, genetic instability, DNA damage response and cancer evolution. Nucleic Acids Res 2006;34:1416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yoon SW, Kim DK, Kim KP, Park KS. Rad51 regulates cell cycle progression by preserving G2–M transition in mouse embryonic stem cells. Stem Cells Dev 2014;23:2700–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tripathi V, Shen Z, Chakraborty A, Giri S, Freier SM, Wu X, et al. Long noncoding RNA MALAT1 controls cell cycle progression by regulating the expression of oncogenic transcription factor B-MYB. PLoS Genet 2013;9:e1003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McHugh CA, Russell P, Guttman M. Methods for comprehensive experimental identification of RNA-protein interactions. Genome Biol 2014;15:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barra J, Leucci E. Probing long non-coding RNA-protein interactions. Front Mol Biosci 2017;11:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhou Q, Liu L, Zhou J, Chen Y, Xie D, Yao Y, et al. Novel Insights in to MALAT1 Function as a MicroRNA Sponge in NSCLC. Front Oncol 2021;11:758653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang Y, Taniguchi T. MicroRNAs and DNA damage response: Implications for cancer therapy. Cell Cycle 2013;12:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Paraskevopoulou MD, Vlachos IS, Karagkouni D, Georgakilas G, Kanellos I, Vergoulis T, et al. DIANA-LncBase v2: indexing microRNA targets on non-coding transcripts. Nucleic Acids Res 2016;44:D231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Betel D, Koppal A, Agius P, Sander C, Leslie C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol 2010;11:R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Teng X, Chen X, Xue H, Tang Y, Zhang P, Kang Q, et al. NPInter v4.0: An integrated database of ncRNA interactions. Nucleic Acids Res 2020;48:D160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hsu JB, Chiu CM, Hsu SD, Huang WY, Chien CH, Lee TY, et al. MiRTar: An integrated system for identifying miRNA-target interactions in human. BMC Bioinf 2011;12:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bhatia V, Yadav A, Tiwari R, Nigam S, Goel S, Carskadon S, et al. Epigenetic silencing of miRNA-338–5p and miRNA-421 drives SPINK1-positive prostate cancer. Clin Cancer Res 2019;25:2755–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hu H, Du L, Nagabayashi G, Seeger RC, Gatti RA. ATM is down-regulated by N-Myc-regulated microRNA-421. Proc Natl Acad Sci USA 2010;107:1506–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yin Y, Xu L, Chang Y, Zeng T, Chen X, Wang A, et al. N-Myc promotes therapeutic resistance development of neuroendocrine prostate cancer by differentially regulating miR-421/ATM pathway. Mol Cancer 2019;18:11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kaelin WG Jr. The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer 2005;5:689–98. [DOI] [PubMed] [Google Scholar]
- 57. McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, Swift S, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res 2006;66:8109–15. [DOI] [PubMed] [Google Scholar]
- 58. Feiersinger GE, Trattnig K, Leitner PD, Guggenberger F, Oberhuber A, Peer S, et al. Olaparib is effective in combination with, and as maintenance therapy after, first-line endocrine therapy in prostate cancer cells. Mol Oncol 2018;12:561–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nakazawa M, Paller C, Kyprianou N. Mechanisms of therapeutic resistance in prostate cancer. Curr Oncol Rep 2017;19:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ren S, Liu Y, Xu W, Sun Y, Lu J, Wang F, et al. Long noncoding RNA MALAT-1 is a new potential therapeutic target for castration resistant prostate cancer. J Urol 2013;190:2278–87. [DOI] [PubMed] [Google Scholar]
- 61. Wang D, Ding L, Wang L, Zhao Y, Sun Z, Karnes RJ, et al. LncRNA MALAT1 enhances oncogenic activities of EZH2 in castration-resistant prostate cancer. Oncotarget 2015;6:41045–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhou J, Zhou JA, Zhang N, Wang J. Evolving insights: how DNA repair pathways impact cancer evolution. Cancer Biol Med 2020;17:805–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Huang J, Lin C, Dong H, Piao Z, Jin C, Han H, et al. Targeting MALAT1 induces DNA damage and sensitize non-small cell lung cancer cells to cisplatin by repressing BRCA1. Cancer Chemother Pharmacol 2020;86:663–72. [DOI] [PubMed] [Google Scholar]
- 64. Chen J, Liu X, Xu Y, Zhang K, Huang J, Pan B, et al. TFAP2C-Activated MALAT1 Modulates the Chemoresistance of Docetaxel-Resistant Lung Adenocarcinoma Cells. Mol Ther Nucleic Acids 2017;14:567–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Li P, Zhang X, Wang H, Wang L, Liu T, Du L, et al. MALAT1 is associated with poor response to oxaliplatin-based chemotherapy in colorectal cancer patients and promotes chemoresistance through EZH2. Mol Cancer Ther 2017;16:739–51. [DOI] [PubMed] [Google Scholar]
- 66. Wang H, Wang L, Zhang G, Lu C, Chu H, Yang R, et al. MALAT1/miR-101–3p/MCL1 axis mediates cisplatin resistance in lung cancer. Oncotarget 2017;9:7501–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hu N, Chen L, Wang C, Zhao H. MALAT1 knockdown inhibits proliferation and enhances cytarabine chemosensitivity by upregulating miR-96 in acute myeloid leukemia cells. Biomed Pharmacother 2019;112:108720. [DOI] [PubMed] [Google Scholar]
- 68. Yao P, Wu Y, Zhao K, Li Y, Cao J, Xing C. The feedback loop of ANKHD1/lncRNA MALAT1/YAP1 strengthens the radioresistance of CRC by activating YAP1/AKT signaling. Cell Death Dis 2022;13:103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hu Y, Lin J, Fang H, Fang J, Li C, Chen W, et al. Targeting the MALAT1/PARP1/LIG3 complex induces DNA damage and apoptosis in multiple myeloma. Leukemia 2108;32:2250–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wu K, Liang J, Shao Y, Xiong S, Feng S, Li X. Evaluation of the Efficacy of PARP Inhibitors in Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Front Pharmacol 2021;12:777663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Li L, Karanika S, Yang G, Wang J, Park S, Broom BM, et al. Androgen receptor inhibitor-induced “BRCAness” and PARP inhibition are synthetically lethal for castration-resistant prostate cancer. Sci Signal 2017;10:eaam7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. De Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med 2020;382:2091–102. [DOI] [PubMed] [Google Scholar]
- 73. Agarwal N, Azad A, Shore ND, Carles J, Fay AP, Dunshee C, et al. Talazoparib plus enzalutamide in metastatic castration-resistant prostate cancer: TALAPRO-2 phase III study design. Future Oncol 2022;18:425–36. [DOI] [PubMed] [Google Scholar]
- 74. Wang R, Sun Y, Li L, Niu Y, Lin W, Lin C, et al. Preclinical study using MALAT1 small interfering RNA or androgen receptor splicing variant 7 degradation enhancer ASC-J9® to suppress enzalutamide-resistant prostate cancer progression. Eur Urol 2017;72:835–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yang L, Zhang Y, Shan W, Hu Z, Yuan J, Pi J, et al. Repression of BET activity sensitizes homologous recombination–proficient cancers to PARP inhibition. Sci Transl Med 2017;9:eaal1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ibrahim YH, García-García C, Serra V, He L, Torres-Lockhart K, Prat A, et al. PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer Discov 2012;2:1036–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yin L, Liu Y, Peng Y, Peng Y, Yu X, Gao Y, et al. PARP inhibitor veliparib and HDAC inhibitor SAHA synergistically co-target the UHRF1/BRCA1 DNA damage repair complex in prostate cancer cells. J Exp Clin Cancer Res 2018;37:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Johnson N, Li YC, Walton ZE, Cheng KA, Li D, Rodig SJ, et al. Compromised CDK1 activity sensitizes BRCA-proficient cancers to PARP inhibition. Nat Med 2011;17:875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Muvarak NE, Chowdhury K, Xia L, Robert C, Choi EY, Cai Y, et al. Enhancing the cytotoxic effects of PARP inhibitors with DNA demethylating agents – A potential therapy for cancer. Cancer Cell 2016;30:637–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Murai J, Huang SY, Das BB, Renaud A, Zhang Y, Doroshow JH, et al. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res 2012;72:5588–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Deeksha W, Abhishek S, Rajakumara E. PAR recognition by PARP1 regulates DNA-dependent activities and independently stimulates catalytic activity of PARP1. FEBS J 2023 Jul 18 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 82. Kim JW, McKay RR, Radke MR, Zhao S, Taplin ME, Davis N, et al. Randomized trial of olaparib with or without cediranib for metastatic castration-resistant prostate cancer: the results from National Cancer Institute 9984. J Clin Oncol. 2023;41:871–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods
MALAT1 is upregulated in prostate cancer and positively associates with aggressive clinical phenotype.
MALAT1 positively associates with mesenchymal and stemness markers in prostate cancer patients.
MALAT1 regulates the DNA repair pathway in prostate cancer.
MALAT1 depletion restrains cell cycle progression in prostate cancer.
MALAT1 does not interact with HR proteins.
MALAT1 depletion enhances sensitivity to PARP inhibitors in HR proficient PCa cells.
MALAT1 and PARP depletion cooperatively suppress expression of HR genes in HR-deficient as well as HR-proficient PCa cells.
Supplementary Table S1: List of the Primers
Supplementary Table S2: Genes upregulated in metastatic prostate cancer patients in comparison to localized cases.
Supplementary Table S3: Biological pathways downregulated on MALAT1 ablation, predicted by DAVID analysis.
Supplementary Table S4: Computational prediction of microRNAs with putative binding sites on the MALAT1transcript.
Data Availability Statement
We have not performed global gene expression profiling or RNA-sequencing (RNA-seq) for this project nevertheless various publicly available datasets were used in this study, namely UCSC Xena (https://xenabrowser.net/) to retrieve the TCGA-PRAD dataset and GEO (www.ncbi.nlm.nih.gov/geo) to retrieve gene expression profiling in patients with prostate cancer: GSE35988, GSE6919, GSE6752, GSE3335, and GSE77930 while RNA-seq data for LNCaP-abl-siNT and -siMALAT1 was retrieved from GSE72534.