Abstract
Cellular stress granules arise in cells subjected to stress and promote cell survival. A cellular protein that localizes to stress granules is Z-DNA-binding protein 1 (ZBP1), which plays a major role in necroptosis, a programmed cell death pathway mediated by the kinase RIPK3. Here, we showed that the stress-granule inducer arsenite activated RIPK3-dependent necroptosis. This pathway required ZBP1, which localized to arsenite-induced stress granules. RIPK3 localized to stress granules in the presence of ZBP1, leading to the formation of ZBP1-RIPK3 necrosomes, phosphorylation of the RIPK3 effector MLKL, and execution of necroptosis. Cells that did not form stress granules did not induce necroptosis in response to arsenite. Together, these results show that arsenite induces ZBP1-mediated necroptosis in a manner dependent on stress granule formation.
INTRODUCTION
Various environmental stressors perturb cellular homeostasis (1). Exposure to a stressor activates an intracellular adaptive response, leading to expression of stress-induced genes, directing cellular metabolism to counteract the effects of the ongoing stress (2). This integrated stress response (ISR) results from activation of one of four stress-activated protein kinases: GCN2, HRI, PERK, or PKR (3–6). Each of these kinases phosphorylates the α subunit of eukaryotic translation initiation factor, eIF2, resulting in inhibition of the translation of housekeeping genes. Phosphorylation of eIF2α leads to the formation of stalled translation pre-initiation complexes containing the 40S ribosomal subunit and untranslated mRNA (1). Several RNA-binding proteins, including TIAR, TIA1, and G3BP, bind to the exposed mRNA and organize the stalled complexes into stress granules, which are cytoplasmic microdomains that store the stalled translational complexes until the stress ceases (7–9). Stress granules can then release the untranslated mRNA back into the polysomes, allowing cell homeostasis to occur. Alternatively, the mRNA can also be directed for degradation (10, 11). If the cellular stress persists, it can result in programmed apoptotic cell death (12).
In contrast to accidental cell death, programmed cell death pathways are highly regulated pathways that are controlled by multiple cellular regulatory proteins (13, 14) and include caspase-dependent apoptosis and pyroptosis and caspase-independent necroptosis [reviewed in (15–17)]. Necroptosis is a receptor-interacting protein kinase 3 (RIPK3)-dependent programmed cell death (18, 19) typically activated through stimulation of a death receptor, such as the tumor necrosis factor receptor (TNFR) or Fas/CD95 (20–22). Upon stimulation, the death receptor recruits the protein kinase RIPK1, which interacts with RIPK3 in a manner dependent on their RIP homotypic interaction motif (RHIM) domains (23, 24). Activated RIPK3 undergoes autophosphorylation and phosphorylates the mixed lineage kinase-like (MLKL) (25, 26). Subsequently, phosphorylated MLKL (pMLKL) trimerizes and forms pores in the plasma membrane, leading to ionic imbalance and water flow into the cells, resulting in cellular swelling and final rupture of the plasma membrane (27, 28). The explosive rupture of cells during necroptotic cell death leads to a release of damage-associated molecular patterns (DAMPs) that act in a pro-inflammatory manner [reviewed in (29)].
In addition to death receptors, necroptosis is also induced by the cellular Z-DNA-binding protein 1 (ZBP1), also known as DNA-dependent activator of IFN-regulatory factors (DAI) or DLM-1 (30–32). ZBP1 can interact with Z-form DNA as well as Z-form RNA and, similar to RIPK1, contains RHIM domains, allowing it to interact with RIPK3 (33–35). ZBP1-mediated necroptosis is activated during infection with influenza A virus (IAV), murine cytomegalovirus (mCMV), and vaccinia virus (VACV) (36–39). The exact mechanism of ZBP1-mediated necroptosis activation, however, has remained enigmatic. In addition to activating RIPK3, ZBP1 is recruited to arsenite-induced stress granules (40, 41), the physiological relevance of which has been unclear, especially because the cells used to show recruitment of ZBP1 to stress granules do not express RIPK3, the key mediator of necroptotic cell death. Therefore, it has remained undetermined whether stress granules play a role in necroptosis.
Here, we investigated cell death induced by an oxidative stressor, sodium arsenite. We showed that arsenite induced necroptosis that is dependent on ZBP1. Because arsenite induces stress granules (4), we evaluated the role of stress granules in the necroptosis pathway. Our data demonstrated that stress granules were required for arsenite-induced necroptosis because blocking stress granule formation reduced necroptotic death. We identified the granules as canonical stress granules and showed that both ZBP1 and RIPK3 localized to stress granules under conditions of arsenite-induced necroptosis. RIPK3 localized to stress granules only in the presence of ZBP1, suggesting that ZBP1 recruited RIPK3 to stress granules where the two proteins form necrosomes. This study demonstrates that stress granules mediate necroptotic cell death and provides insight into the mechanism of ZBP1-mediated necroptosis.
RESULTS
Arsenite induces RIPK3-dependent necroptosis that is primed by interferon
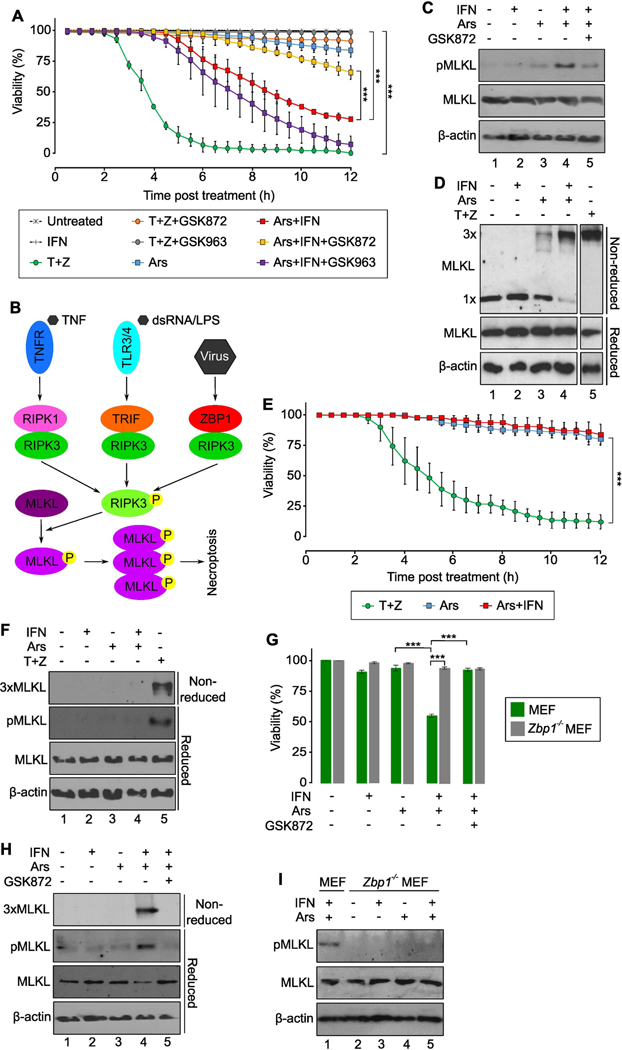
Because ZBP1 is recruited to arsenite-induced stress granules and because ZBP1 can bind to and activate RIPK3, leading to necroptotic cell death (32, 40), we asked whether arsenite could lead to necroptotic cell death. Treatment of murine L929 cells with arsenite alone did not substantially increase cell death (Fig. 1A, blue and table S1). ZBP1 is not constitutively expressed in untreated L929 cells but is inducible by treatment with type I interferon (IFN) (36). Priming with mouse IFNα significantly increased cell death after arsenite treatment, with 50% of the cells dying at 7 hours post-arsenite treatment and 75% of the cells dead at 11 hours (Fig. 1A, red). IFN treatment alone did not lead to significant cell death. Although we used high concentrations of sodium arsenite to induce necroptosis in the majority of cells, we detected significant induction of cell death at concentrations as low as 1 μM (Fig. S1, A and B), closer to the EPA limit for arsenite in drinking water of just below 0.1 μM (42). Arsenite at concentrations of 1 to 50 μM, to which different populations throughout the world are exposed, has been associated with cancer, cardiovascular diseases, hypertension, and diabetes mellitus [reviewed in (43)]. These data showed that physiologically relevant concentrations of arsenite can induce death in IFN-primed L929 cells.
Fig. 1. Arsenite induces necroptosis mediated by ZBP1.
(A) Viability assay in mouse IFNα (IFN)-primed and arsenite (Ars)-treated L929 cells as measured by SYTOX dye exclusion. N=3 sets of cells per group. See table S1 for the complete list of p-values. (B) Schematic of RIPK3 activation and execution of necroptosis. (C) Phosphorylation of MLKL (pMLKL) in L929 cells. N=4 independent experiments. (D) Trimerization (3x) of MLKL in L929 cells. TNFα+zVAD (T+Z) treatment was visualized on the same blot but with a shorter exposure time. N=3 independent experiments. (E) Viability assay in ZBP1 KO L929 cells as measured by SYTOX dye exclusion. N=3 sets of cells per group. (F) Phosphorylation and trimerization of MLKL in ZBP1 KO L929 cells. N=3 independent experiments. (G) Viability assay in primary MEFs as measured by propidium iodide dye exclusion. N=5 sets of cells per group. See table S2 for complete list of p-values. (H) Phosphorylation and trimerization of MLKL in primary MEFs. N=2 independent experiments. (I) Phosphorylation of MLKL in primary ZBP1 KO MEFs. N=2 independent experiments. Quantified data are presented as means ± SD. ***P < 0.001.
Unlike apoptosis, necroptosis occurs within a rapid time frame. Death in IFN-primed L929 cells was observed within a few hours following arsenite treatment. Cell viability was significantly increased when L929 cells were treated with the RIPK3-specific inhibitor GSK872 (Fig. 1A, yellow). Necroptosis is characterized by activation of RIPK3 and subsequent phosphorylation of MLKL (25, 26) Phosphorylated MLKL (pMLKL) inserts into the plasma membrane and trimerizes to form a pore, leading to ionic imbalance and water flow into cells and resulting in cellular swelling and plasma membrane rupture (27, 28) (Fig. 1B). Treatment with arsenite alone did not result in high levels of pMLKL, but when combined with pretreatment with mouse IFNα, arsenite induced pMLKL, which was inhibited by GSK872 (Fig. 1C, lanes 3–5). Moreover, whereas L929 cells treated with arsenite alone showed low levels of trimerized MLKL, likely due to low levels of endogenous IFN, arsenite treatment of IFN-primed cells resulted in high levels of trimerized MLKL (Fig. 1D, lanes 3 and 4). Trimerization of MLKL is implicated only in necroptosis (44, 45). Finally, to confirm dependence of arsenite-activated death on MLKL, we generated Mlkl knockout (Mlkl−/−) L929 cells using CRISPR/Cas-9 technology. Treatment of MLKL-deficient L929 cells with arsenite did not lead to significant cell death, even in IFN-primed cells (Fig. S2, A and B), demonstrating that arsenite-induced death depended on MLKL. Together, these results reveal that arsenite induces necroptosis in IFN-primed L929 cells in a RIPK3-dependent manner that leads to phosphorylation and trimerization of MLKL.
ZBP1 mediates arsenite-induced necroptosis
Activation of RIPK3 occurs through its interaction with a mammalian RHIM-containing proteins: TRIF, RIPK1, or ZBP1 (21, 32, 36, 46). TRIF-mediated necroptosis resulting from activation of Toll-like receptor 3 (TLR3) or TLR4 has been most frequently associated only with immune cells, where TLR3 and TLR4 are primarily found (46–48). To test for the requirement of RIPK1, we utilized the RIPK1 inhibitor GSK963, which did not rescue viability in IFN-primed arsenite-treated cells (Fig. 1A, purple). In contrast, necroptosis induced by treatment with TNFα and zVAD, which progresses through RIPK1, was inhibited by GSK963 (Fig. 1A, compared green and gray). These data demonstrated that RIPK1 is not involved in arsenite-induced necroptosis.
We next tested whether arsenite-induced death depended on ZBP1. ZBP1 is a reasonable candidate as the mediator of arsenite-induced necroptosis because the arsenite-driven death was observed only in IFN-primed L929 cells and expression of ZBP1 in these cells is IFN-dependent (36). ZBP1 knockout (Zbp1−/−) L929 cells showed little arsenite-induced death, even with mouse IFNα (Fig. 1E, blue and red). In contrast, Zbp1−/− L929 cells were sensitive to death induced by TNFα and zVAD (Fig. 1E, green and table S2), which progresses through RIPK1 but not ZBP1 (Fig. 1B). Additionally, MLKL phosphorylation and trimerization were inhibited in arsenite-treated Zbp1−/− L929 cells, but not in cells treated with TNFα and zVAD (Fig. 1F, lanes 4 and 5). Collectively, these data demonstrated that arsenite-induced necroptosis depends on ZBP1.
To confirm that arsenite-induced necroptosis was not an artifact of the L929 cell line, we performed the same experiments in primary MEFs. Arsenite treatment did not lead to substantial death unless MEFs were primed with mouse IFNα (Fig. 1G, green). This death pathway depended on RIPK3 because treatment with GSK872 rescued cell viability (Fig. 1G, green). Treatment of IFN-primed MEFs with arsenite led to phosphorylation and trimerization of MLKL (Fig 1H, lane 4), which was inhibited by GSK872 (lane 5). As in L929 cells, ZBP1 was an IFN-inducible protein in primary MEFs (Fig. S2C). As opposed to WT cells, arsenite did not induce death in primary Zbp1−/− MEFs, even after priming with IFNα (Fig. 1G, gray). Furthermore, phosphorylation of MLKL was not detected in these cells following arsenite treatment (Fig. 1I). Collectively, these data demonstrated that arsenite induces ZBP1-mediated necroptosis in MEFs.
Stress granules are required for arsenite-induced necroptosis in MEFs and human U2OS cells
ZBP1 is recruited to arsenite-induced stress granules (40). Because arsenite-induced necroptosis is mediated by ZBP1, we asked whether stress granules were required for this pathway using MEFs lacking a crucial stress granule protein, TIA1 (8). These cells showed reduced ability to form granules following arsenite treatment compared to WT MEFs (Fig. 2, A and B). Arsenite treatment alone did not lead to significant loss of cell viability in WT or TIA1-deficient MEFs (Fig. 2, C and D). Arsenite treatment of IFN-primed WT MEFs, which induced the expression of ZBP1 (Fig. S3A), led to significant cell death that was inhibited by GSK872 and was therefore RIPK3 dependent (Fig. 2, C and D). However, arsenite treatment of Tia1−/− MEFs did not cause cell death, even after priming with IFN (Fig. 2, C and D). Thus, ZBP1- and RIPK3-dependent cell death induced by arsenite is inhibited in cells with reduced ability to form stress granules. We detected phosphorylated MLKL (Fig. 2E) and trimerized MLKL (Fig. 2F) in IFN-primed WT MEFs following arsenite treatment, suggesting that arsenite-induced cell death was necroptotic in nature. There was little phosphorylated MLKL (Fig. 2E) and no MLKL trimerization (Fig. 2F) in IFN-primed TIA1-deficient MEFs, further demonstrating arsenite-induced necroptosis depended on stress granules. Together, our data in MEFs show that formation of stress granules is required for arsenite-induced necroptosis.
Fig. 2. Arsenite-induced necroptosis depends on formation of stress granules.
(A and B) Stress granule formation assessed by immunofluorescence analysis for G3BP1 in MEFs 3 hours after arsenite (Ars) treatment (A). Quantification of stress granule formation (B). N=3 sets of cells per group. Scale bar, 10 μm. (C and D) Viability assay in MEFs primed with mouse IFNα (IFN) and treated with arsenite as measured by propidium iodide dye exclusion (C). Quantification of cell viability (D). N=6 sets of cells per group. Scale bar, 100 μm. (E) Phosphorylation of MLKL (pMLKL) in MEFs and L929 cells. N=3 independent experiments. (F) Trimerization (3x) of MLKL in MEFs. N=3 independent experiments. (G and H) Viability assay in U2OS cells transfected with the indicated plasmids as measured by propidium iodide dye exclusion at 14 hours following arsenite treatment (G). Quantification of cell viability (H). N=3 sets of cells per group. Scale bar, 20 μm. (I) Stress granule formation assessed by immunofluorescence analysis for TIAR in U2OS cells at 2 hours following arsenite treatment. Cells were transfected with the indicated plasmids and treated with arsenite 24 hours later. Red arrows indicate cells with stress granules. N=3 sets of cells per group. Scale bars, 20 μm. (J) Viability assay in U2OS cells measured as in (G). Cells were transfected with the indicated plasmids. Quantified data are presented as means ± SD. ***P < 0.001.
To expand our studies beyond murine cells, we utilized the human U2OS bone osteosarcoma epithelial cell line. These cells are ZBP1- and RIPK3-deficient and therefore unable to undergo necroptosis (39, 49, 50). Ectopic expression of both ZBP1 and RIPK3, but not expression of either protein by itself, rendered U2OS cells susceptible to arsenite-induced death (Fig. 2, G and H, and Fig. S3, B and C). To test whether this ZBP1- and RIPK3-mediated cell death depended on the formation of stress granules, we assessed the viability of U2OS cells deficient in stress granule proteins G3BP1 and G3BP2. G3bp1/2−/− U2OS cells could not form stress granules under many conditions, including following arsenite treatment (Fig. 2I) (7). In contrast to WT U2OS cells, ectopic expression of ZBP1 and RIPK3 in G3bp1/2−/− U2OS cells did not render the cells susceptible to arsenite-induced death. Furthermore, stress granule formation was restored in G3bp1/2−/− U2OS cells reconstituted with G3BP1 and ectopically expressing ZBP1 and RIPK3 (Fig. 2I and Fig. S3D). Moreover, arsenite treatment of such cells resulted in cell death (Fig. 2J). These results showed that arsenite-induced necroptosis is dependent on stress granules.
Abrogation of canonical stress granules inhibits necroptosis
Arsenite induces oxidative stress, which leads to the activation of the kinase heme-regulated inhibitor (HRI), phosphorylation of eIF2α, inhibition of translation initiation and formation of stress granules (51–53). We showed that reduction of reactive oxygen species (ROS) levels by treatment of cells with L-glutathione inhibited arsenite-induced necroptosis (Fig. S4, A and B), which identified arsenite-induced oxidative stress as the basis for arsenite-induced necroptosis.
Several types of granules have been identified in cells [reviewed in (10)]. Arsenite induces so-called canonical stress granules, which form as a result of inhibition of translation initiation during the integrated stress response (Fig. 3A). We utilized cycloheximide (CHX) and integrated stress response inhibitor (ISRIB) to inhibit canonical stress granule formation. CHX interferes with the translocation step of protein synthesis, which inhibits translation elongation. This leads to polysome stalling, which masks Tia1, G3bp, and Tiar mRNAs and effectively inhibits stress granule formation (Fig. 3A). Pretreating L929 cells with CHX inhibited granule formation following arsenite treatment (Fig. 3, B and C). Similar results were obtained when cells were pretreated with ISRIB, which interferes with the ability of phosphorylated eIF2α to inhibit translation initiation, leading to the formation of stress granules (Fig. 3A). Pretreating cells with ISRIB inhibited arsenite-induced granules (Fig. 3, B and C). Thus, the data obtained with CHX and ISRIB confirms the canonical nature of stress granules formed in response to arsenite treatment. We then asked if arsenite-induced necroptosis depended on canonical stress granules. Arsenite-induced cell death was significantly inhibited by treatment with either CHX or ISRIB (Fig. 3, D and E). Furthermore, arsenite treatment of IFN-primed L929 cells did not result in phosphorylation or trimerization of MLKL if cells were also treated with CHX or ISRIB (Fig. 3F). Together, these data show that arsenite-induced necroptosis depends on canonical stress granules.
Fig. 3. Canonical stress granules are required for necroptosis activated by arsenite.
(A) Schematic of the ISR leading to stress granule formation. Inhibitors of the pathway, integrated stress response inhibitor (ISRIB) and cycloheximide (CHX), are indicated. (B and C) Stress granule formation assessed by immunofluorescence analysis for G3BP1 in CHX- and ISRIB-treated L929 cells at 1 hour post-arsenite (Ars) treatment, with or without mouse IFNα (IFN) priming (B). Quantification of stress granule formation (C). N=6 sets of cells per group. Scale bar, 20 μm. (D and E) Viability assay in L929 cells measured by propidium iodide dye exclusion (D). Quantification of cell viability (E). N=3 sets of cells per group. Scale bar, 100 μm. (F) Phosphorylation (pMLKL) and trimerization (3x) of MLKL in L929 cells. N=2 independent experiments. Quantified data are represented as means ± SD. ***P < 0.001.
ZBP1 localizes to stress granules to form necrosomes with RIPK3 during arsenite-induced necroptosis
In IFN-primed cells, ZBP1 was distributed throughout the cytoplasm with perinuclear concentration (Fig. 4A, IFN). Treatment of cells with arsenite alone resulted in formation of stress granules that were G3BP1+ (Fig. 4A, Ars) but that lacked ZBP1. In IFN-primed L929 cells treated with arsenite to induce necroptosis, ZBP1 localized to stress granules (Fig. 4A, Ars+IFN and Fig. 4, B and C). When necroptosis was inhibited by treatment with CHX or ISRIB to abrogate granule formation, ZBP1 remained dispersed in the cytoplasm (Fig. 4, B and D). Similarly, arsenite treatment of Tia1−/− MEFs led to cytoplasmic ZBP1, in contrast to the stress granule localization of ZBP1 in WT MEFs (Fig. 4, E to G). These results demonstrate that necroptosis induces the localization of ZBP1 to stress granules.
Fig. 4. ZBP1 localizes to stress granules during arsenite-induced necroptosis.
(A and D) Protein localization assessed by immunofluorescence analysis for G3BP1 and ZBP1 in unprimed or mouse IFNα (IFN)-primed (A) or IFN-primed cycloheximide (CHX) or integrated stress response inhibitor (ISRIB)-treated (D) L929 cells at 1 hour following arsenite (Ars) treatment. N=6 sets of cells per group. Scale bars, 30 μm in (A) and 50 μm in (D). (B) Quantification of the formation of ZBP1-containing stress granules in IFN-primed L929 cells from assays in (A) and (D). (C) Quantification of colocalization of stress granules containing G3BP1 and ZBP1 in cells shown in (A). (E to G) Protein localization assessed by immunofluorescence analysis for ZBP1 and G3BP1 in IFN-primed WT MEF or Tia1−/− MEFs at 3 hours following arsenite treatment (E). Quantification of ZBP1-containing stress granules (F) and of colocalization of G3BP1 and ZBP1 (G). N=3 sets of cells per group. Scale bar, 50 μm. Quantified data are presented as means ± SD. ***P < 0.001.
Necroptosis is executed by a complex referred to as the necrosome (54). Necrosomes comprise multiple copies of RIPK3 and of a RHIM-domain containing protein (Fig. 1B) to form a macromolecular complex of fibrillar structure (Fig. 5A) (21, 32, 36, 46). Because arsenite-induced necroptosis depended on ZBP1, we asked whether we could detect necrosomes containing high molecular weight complexes of ZBP1 and RIPK3. (24, 55). We treated cells with a cell-permeant disulfide-containing chemical crosslinker, DSP, after arsenite treatment to covalently crosslink proteins in complexes (Fig. 5B). We detected ZBP1 and RIPK3 in macromolecular complexes in cells treated with arsenite and IFN (Fig. 5C, lane 4), but not in cells treated with arsenite (Fig. 5C, lane 3) or IFN (Fig. 5C, lane 2) alone. These complexes were detectable only under necroptotic conditions and therefore, we believe that they represent ZBP1-RIPK3 necrosomes. To confirm that ZBP1 and RIPK3 formed a complex, we analyzed ZBP1 immunoprecipitates. RIPK3 precipitated with ZBP1 only in IFN-primed cells treated with arsenite (Fig. 5D, lane 4), and not in cells treated with arsenite (Fig. 5D, lane 3) or IFN (Fig. 5D, lane 2) alone. Together, these data demonstrated that ZBP1 and RIPK3 form the necrosomes in arsenite-treated cells undergoing necroptosis. Furthermore, we detected G3BP1 in ZBP1 immunoprecipitates from cells undergoing necroptosis, which is consistent with the localization of ZBP1 to stress granules during arsenite-induced necroptosis (Fig. 5D, lane 4).
Fig. 5. ZBP1 recruits RIPK3 to stress granules to form necrosomes.
(A) Schematic of necrosome components from previous studies (24). (B) Schematic of DSP crosslinking method used to detect necrosome formation. (C) Necrosome formation assessed after DSP crosslinking in mouse IFNα (IFN)-primed and arsenite (Ars)-treated L929 cells. *indicates an aggregated form of the protein. N=2 independent experiments. (D) Protein complex formation assessed by Western blot analysis of ZBP1 immunoprecipitates from L929 cells for RIPK3 and G3BP1. N=2 independent experiments. (E) Protein localization assessed by immunofluorescence analysis for G3BP1 and RIPK3 in L929 cells at 2 hours following arsenite treatment. N=3 sets of cells per group. Scale bar, 30 μm. (F) Protein complex formation assessed by Western blot analysis of G3BP1 immunoprecipitates from L929 and ZBP1 KO L929 cells for ZBP1 and RIPK3. N=2 independent experiments. (G) Necrosome formation assessed after DSP crosslinking in L929 and ZBP1 KO L929 cells. *indicates an aggregated form of the protein. G3BP1* blot shows bands on the same gel. N=2 independent experiments. (H) Protein localization assessed by immunofluorescence analysis for G3BP1 and RIPK3 in IFN-primed L929 and ZBP1 KO L929 cells treated with arsenite for 2 hours. N=3 sets of cells per group. Scale bar, 25 μm.
RIPK3 forms necrosomes only upon ZBP1 localization to stress granules
We sought to determine whether necrosome formation takes place in stress granules. Immunofluorescence analysis showed that RIPK3 was distributed throughout the cytoplasm and G3BP1 localized to stress granules in arsenite-treated cells (Fig. 5E, Ars), but that RIPK3 localized to stress granules in IFN-primed cells treated with arsenite (Fig. 5E, Ars+IFN). These results imply that ZBP1-RIPK3 necrosomes form in stress granules. Because RIPK3 localized to stress granules only in IFN-primed cells when ZBP1 was present and localized to stress granules as well (Fig. 4A), we wondered whether ZBP1 localization to granules was required to recruit RIPK3. ZBP1 coprecipitated with the stress granule protein G3BP1 only in IFN-primed L929 cells treated with arsenite (Fig. 5F, lane 8), consistent with ZBP1 localizing to stress granules during necroptosis (Fig. 4A). Furthermore, RIPK3 coprecipitated with G3BP1 only in IFN-primed L929 cells treated with arsenite (Fig. 5F, lane 8), but not in cells that had not been primed with IFN (Fig. 5F, lane 7). In the absence of ZBP1, RIPK3 did not coprecipitate with G3BP1 from IFN-primed L929 cells following arsenite treatment (Fig. 5F, lane 4). These data imply that ZBP1 recruits RIPK3 to stress granules.
DSP crosslinking revealed high-molecular-weight complexes formed by ZBP1 and RIPK3 in IFN-primed WT L929 cells treated with arsenite (Fig. 5G, lane 4), whereas RIPK3 was not detected in macromolecular complexes in Zbp1−/− L929 cells (Fig. 5G, lane 8). G3BP1 formed high-molecular-weight complexes in WT L929 cells treated with arsenite, regardless of whether the cells were primed with IFN or not (Fig. 5G, lanes 3, 4, 7, and 8). Because granule formation required only arsenite treatment and not IFN treatment (Fig. 4A and 5E), we believe that these complexes represent stress granules. Immunofluorescence analysis showed that stress granules formed in arsenite-treated cells both in presence and absence of ZBP1 (Fig. 5H), but that RIPK3 localized to stress granules only in the presence of ZBP1 (Fig. 5H, L929). Together, these data show that ZBP1 localizes to stress granules where it recruits RIPK3 to form necrosomes to execute arsenite-induced necroptosis.
DISCUSSION
Stress granules are non-membranous ribonucleoprotein aggregates that assemble rapidly in the cytoplasm under stress conditions (56). Stress granules serve as storage sites for untranslated mRNAs, which can be returned to the cytoplasm within a few minutes after the stress has ceased and the level of free mRNAs has decreased. Alternatively, unused mRNA can be degraded (57–59). Cellular stress can result in apoptotic cell death and stress granules harbor some pro-apoptotic proteins, potentially contributing to inhibition of the pathway (60–62). Thus, stress granules can promote cell survival during as well as homeostasis after cellular stress. Here, we demonstrated that stress granules do not always have a pro-survival function and were required for activation of necroptotic cell death.
We showed that the canonical stress granule inducer sodium arsenite triggered rapid cell death in IFN-primed cells in a manner that depended on the protein kinase RIPK3. The presence of the phosphorylated and trimerized form of MLKL identified the arsenite-induced cell death pathway as necroptosis. Arsenite-induced necroptosis was ZBP1-dependent. During necroptosis, ZBP1 localized to G3BP1-containing stress granules where it formed a necrosome with RIPK3. RIPK3 localized to stress granules only in the presence of ZBP1, suggesting that ZBP1 recruited RIPK3 to stress granules to form necrosomes. Reducing granule formation by genetic deficiency or knockdown of proteins necessary for the process prevented arsenite from inducing necroptosis and leading to MLKL phosphorylation and trimerization, showing that stress granules were required for necroptosis. Furthermore, inhibition of canonical stress granules resulted in inhibition of arsenite-induced necroptosis. Together, our data show that arsenite leads to ZBP1-dependent necroptosis, which depends on the formation of canonical stress granules (Figure 6).
Fig. 6. Schematic model of stress-granule mediated necroptosis.
(A) In cells treated with arsenite, the formation of stress granules is induced. Stress granules are formed as result of HRI-mediated integrated stress response (4). When stress granules are formed, ZBP1 localizes to stress granules, where it is likely to bind to Z-RNA (34, 40, 41). Binding to Z-RNA may result in a conformational change in ZBP1, exposing its RHIM. ZBP1 in stress granules recruits RIPK3 by RHIM-RHIM interactions and the two proteins will form necrosomes, leading to the execution of necroptosis. Red arrows indicate translocation of proteins. (B) When formation of stress granules is inhibited, whether chemically (CHX, ISRIB) or genetically (Tia1−/−, G3bp1/2−/−), ZBP1 remains localized in the cytoplasm and is unable to form necrosomes. Necroptosis is not activated.
For these studies, we used high concentrations of arsenite (up to 500 μM), which are typically required for stress granule induction in the majority of cells. We also observed that arsenite caused IFN-dependent death at more physiologically relevant concentrations as low as 1 μM (Fig. S1, A and B). It is unknown if at such low concentrations arsenite can induce stress granule formation in a smaller population of cells, or if the observed death is stress granule-independent. Arsenite induces oxidative stress, which leads to production of reactive oxygen species (ROS) (63, 64). Production of ROS has been previously detected during necroptosis (65) and production of mitochondrial ROS promotes necroptosis (66).
All known ZNA-binding proteins localize to stress granules (41) and the presence of a ZNA-binding domain (Zα) is sufficient to localize a protein into stress granules. ZBP1 contains two Zα domains and localizes to stress granules (40). ZNAs can emerge in cells as a result of oxidative stress. For instance, oxidation of RNA leads to the formation of 8-oxoguanosine from guanosine (67). 8-oxoguanosine has a higher propensity to adopt an anti configuration as opposed to the syn configuration adopted by most bases in nucleic acids. Because purines in Z form nucleic acids adopt an anti configuration, oxidation of RNA may induce flip into a Z configuration. 8-oxoguanosine has been detected in arsenite-treated cells (68). We hypothesize that oxidative stress leads to formation of 8-oxoguanosine and hence Z-RNA, which localizes to stress granules where it is bound by the Zα domain of ZBP1, leading to activation of RIPK3, phosphorylation of MLKL, and necroptotic cell death (Fig. 6). Indeed, ZBP1 binds to Z-RNA and activates necroptosis in cells infected with a vaccinia virus mutant unable to bind Z-RNA (34).
Necroptosis has been implicated in multiple diseases, including neurodegenerative diseases (such as multiple sclerosis) (69), cancer (70), cardiovascular diseases (71, 72), ethanol-induced liver damage (73), and inflammaging (74) as well as viral and bacterial infections [reviewed in (75)]. Neuronal loss in Alzheimer’s disease (AD) has been attributed to necroptosis (76, 77). The brains of AD patients harbor an increased number of stress granules (78, 79). Furthermore, AD is characterized by IFN-mediated inflammation of the brain (80–82). ZBP1 is an IFN-inducible protein and our data showed that ZBP1 localized to stress granules to activate necroptosis. Therefore, it would be interesting to investigate whether ZBP1 might be involved in necroptosis that is responsible for neurodegradation in AD. Finally, 8-oxoguanosine accumulates in DNA and RNA with age and elevated levels of RNA oxidation are detected in AD and related neurodegenerative disorders (83, 84). Our studies give new insight into stress granule function as well as mechanisms of necroptosis induction, which we hope will contribute to better understanding of innate immunity and etiology of human diseases.
MATERIALS AND METHODS
Cells
Cells were grown at 37oC in atmosphere supplemented with 5% CO2. Murine fibroblast L929 cells were maintained in the Minimum Essential Media (MEM) (Corning) supplemented with 5% fetal bovine serum (FBS). Mouse embryonic fibroblasts (MEF) and human bone osteosarcoma epithelial cells (U2OS) cells were a kind gift from Dr. Nancy Kedersha (Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts) (7, 85). These cells were maintained in Dulbecco’s Modification of MEM media (DMEM) (Corning) supplemented with 10% FBS and 2 μM L-glutamine. Primary MEFs were mechanically isolated from WT or knock-out C57BL/6 mouse embryos collected at day 13 post coitum. These cells were maintained in DMEM supplemented with 10% heat-inactivated FBS and 1X antibiotic-antimycotic cocktail (100 U/mL penicillin, 100 μg/mL streptomycin, 0.25 μg/mL amphotericin B) (Gibco). Primary cells were maintained for up to 5 passages.
Cell treatments
Cells were pretreated with recombinant mouse interferon alpha (IFNα, Calbiochem) at 100 U/mL (L929) or 1000 U/mL (MEF and primary MEF) for 18 hours before being treated with sodium arsenite at 500 μM (L929) or 50 μM (MEF and primary MEF) unless stated otherwise. To induce the canonical necroptosis pathway, cells were pretreated with carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone (Z-VAD-FMK, pan-caspase inhibitor, ApexBio) at 100 μM, one hour before being treated with 20 ng/mL mouse tumor necrosis factor alpha (TNF-α, Sigma), and continuously treated thereafter. To determine the requirement for the activity of the RIP proteins, cells were pretreated with N-(6-(isopropylsulfonyl)quinolin-4-yl)benzo[d]thiazol-5-amine (GSK872, RIPK3 inhibitor; GlaxoSmithKline) at 3 μM and 2,2-dimethyl-1-(5(S)-phenyl-4,5-dihydro-pyrazol1-yl)-propan-1-one (GSK963, RIPK1 inhibitor; GlaxoSmithKline) at 5 μM, one hour before being treated with sodium arsenite in the presence of the RIPK1 or RIPK3 inhibitor. To inhibit canonical stress granule formation, cells were pretreated with cycloheximide (CHX) (Sigma) at 20 μg/mL for 2 hours or with integrated stress response inhibitor (ISRIB) (Sigma) at 1 μM overnight (14 to 16 hours) before being treated with sodium arsenite.
SYTOX nuclear stain exclusion assay
The viability of L929 cells seeded in 12-well CytoOne tissue culture-treated plates (USA Scientific) was assessed by Hoechst 33342 (H3570, Thermo Fisher) and SYTOX Green Nucleic Acid Stain (S7020, ThermoScientific), which were applied for 15 min before arsenite treatment and during arsenite treatment. Cells were incubated at 37°C with 5% CO2-supplemented air on an EVOS FL auto imaging system (Invitrogen) with an onstage incubator system and an image was taken every 30 min for 12 hours.
Protein extraction and Western immunoblot analysis
Cells seeded in 6-well tissue culture-treated dishes (VWR) were lysed in RIPA lysis buffer (150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 25 mM Tris pH 7.4) with 1X Halt Protease and Phosphatase Inhibitor Cocktail (Pierce Thermo Scientific) by 10-min incubation on ice. A standard protocol for SDS-PAGE was followed. Proteins were transferred on a nitrocellulose membrane in 10 mM 3-(cyclohexylamino)-1-propane sulfonic acid (CAPS), 20% v/v methanol, pH 11. Antibodies used for immunoblotting were specific for ZBP1 (mAb, Zippy-1, AG-20B-0010-C100, Adipogen), RIPK3 (D4G2A, mAb, #95702, Cell Signaling Technology), or G3BP1 (pAb, 13057–2-AP, Proteintech). Secondary antibodies used were goat anti-rabbit IgG or goat anti-mouse IgG, both HRP-linked (Cell Signaling Technology). Antibodies specific for β-actin were HRP-linked (sc-47778, Santa Cruz Biotechnology). Immunoreactive bands were visualized by chemiluminescence with SuperSignal West Pico PLUS or Dura Extended Duration substrate (ThermoFisher).
MLKL phosphorylation assay
Cells seeded in 6-well tissue culture-treated dishes (VWR) were treated with arsenite for 5 hours and lysed in RIPA lysis buffer as described above. Lysates were centrifuged in QIAshredder columns (79654, Qiagen) to aid in extracting MLKL from membrane-bound trimers. Western immunoblot analysis (as described above) was followed using primary antibodies specific for phosphorylated MLKL (mAb, S345, ab196436, abcam) and total MLKL (mAb, D6W1K, Cell Signaling Technology).
MLKL trimerization assay
Cells were seeded, treated, and lysed in RIPA lysis buffer as described for the MLKL phosphorylation assay. Western immunoblot analysis was performed as described above with the following exceptions: SDS-PAGE was run in non-reducing conditions and proteins were transferred overnight (16 hours) onto a nitrocellulose membrane at low voltage (20 V). To detect MLKL trimers, primary antibodies specific for total MLKL (mAb, D6W1K, Cell Signaling Technology) were used. SDS-PAGE under reducing conditions was performed to detect MLKL protein monomers.
DSP crosslinking
Cells seeded in 6-well tissue culture-treated dishes (VWR) were treated with arsenite for 2 hours, scraped, pelleted, and resuspended in phosphate-buffered saline (PBS). To crosslink proteins, dithiobis(succinimidyl propionate) (Lomant’s Reagent, DSP) (22586, ThermoFisher Scientific) was added at 1 mM and cell suspensions were incubated for 30 min at 25oC. Cells were pelleted, resuspended in RIPA lysis buffer (as described above) and lysed for 10 min on ice. Crosslinked protein complexes were subjected to a non-reducing SDS-PAGE and transferred overnight (20 hours) to nitrocellulose membranes at 20V. Western immunoblot analysis followed the protocol described above. To analyze protein monomers, DSP-crosslinked samples were reduced by addition of 1,4-dithiothreitol (DTT, Sigma-Aldrich) at 50 mM and incubation for 60 min at 37oC. Samples were denatured for 15 min at 95oC prior to Western immunoblot analysis.
Creation of Zbp1−/− L929 cells and Mlkl−/− L929 cells
L929 cells at 50% confluency were infected with Edit-R Lentiviral particles containing Cas9 sgRNA (#VCAS10133, Dharmacon) at MOI 0.3 and incubated for 4 hours at 37°C, after which L929 growth medium was added. Following 24-hour incubation, the medium was replaced with L929 growth medium containing 8 μg/mL blasticidin. Following selection, expression of Cas9 in cells was confirmed by Western immunoblot (#NBP2–36440L, Novus Biologicals). To knock out the Zbp1 gene, Cas9-expressing L929 cells at 50% confluency were infected with a mixture of three Edit-R Lentiviral ZBP1-specific sgRNAs (sgRNA1: VSGM10144–246664134, sgRNA2:−246664138, sgRNA3:−246664139, Dharmacon) at an MOI of 0.3 and incubated for 4 hours at 37°C, after which L929 growth medium was added. After 24 hours, the medium was replaced with L929 growth medium containing 10 μg/mL puromycin. Following selection, surviving cells were seeded into a 96-well dish with one cell per well and were clonally expanded. The successful knock-out of Zbp1 was confirmed by Western immunoblot analysis (mAb, Zippy-1, AG-20B-0010-C100, Adipogen). To create Mlkl−/− L929 cells, the same protocol was followed, except a single Edit-R Lentiviral MLKL-specific sgRNA was used (VSGM10144–246701514, Dharmacon). The successful knock-out of Mlkl was confirmed by Western immunoblot analysis (mAb, D6W1K, Cell Signaling Technology).
Co-immunoprecipitation
Cells seeded in a 6-well tissue culture-treated dish (VWR) were treated with arsenite for 2 hours and lysed in a non-denaturing lysis buffer [10 mM Tris-HCl, pH 7.5, 1 mM DTT, 2 mM MgCl2, 50 mM KCl, 1% Triton X-100, 1X HALT Protease/ Phosphatase Inhibitor Cocktail (78446, ThermoFisher)] for 10 min on ice. Equal volumes of Buffer I (20 mM Tris-HCl, pH 7.5, 0.4 mM NaCl, 1 mM DTT, 1 mM EDTA, 1% Triton X-100, 20% glycerol, 2 mM Na3VO4) were added. Lysates were precleared by incubation with Protein A/G PLUS-Agarose (sc-2003, Santa Cruz) for 30 min at 4oC with rotation. To capture protein complexes, the lysates were incubated with antibodies specific for ZBP1 (mAb, Zippy-1, AG-20B-0010-C100, Adipogen) or G3BP1 (mAb, 66486–1-Ig, Proteintech) overnight at 4oC with rotation. Protein A/G PLUS-Agarose (sc-2003, Santa Cruz) was added to lysates, incubated for 90 min at 4oC with rotation, and washed three times in Buffer I for 5 min. Proteins bound to agarose beads were released by the addition of 4x Laemmli (250 mM Tris, pH 6.8, 8% SDS, 50% glycerol, 0.04% bromophenol blue) with 50 mM DTT and boiling for 15 min at 95oC. Western immunoblot analysis as described above was followed using the antibody NBP1–76854 (Novus Biologicals) to detect ZBP1.
Immunofluorescence
Cells seeded in a 48-well CytoOne tissue culture-treated plate (USA Scientific) were treated with arsenite, fixed in 2% paraformaldehyde for 15 min. at room temperature, and washed twice with PBS for 5 min. The following steps were performed at room temperature unless specified otherwise. Cells were permeabilized with 0.2% Triton X-100 (in PBS) for 10 min and washed twice with PBS for 5 min. To limit nonspecific antibody binding, cells were blocked with 5% bovine serum albumin (BSA) (in PBS) overnight at 4oC. Cells were incubated for two hours with primary antibodies specific for G3BP1 (1:200, Proteintech, 66486–1-Ig or 13057–2-AP), ZBP1 (1:200, Novus Biologicals, NBP1–76854), TIAR (1:100, Santa Cruz Biotechnology, sc-398372), and RIPK3 (1:50, ProScience, 2293) diluted in 2% BSA (in PBS) as indicated and washed twice with PBS for 10 min. Cells were incubated for 1 hour in the dark with secondary antibodies (1:500, Thermo Fisher, T862, T2767, A11001 or 1:1000, ThermoFisher, A32731) diluted in 2% BSA (in PBS) as indicated and washed twice with PBS for 10 min. Cells were stained with DAPI (4′,6-diamidino-2-phenylindole) (in PBS) for 15 min and washed twice with PBS for 10 min. Cells were imaged and quantified using EVOS FL auto imaging system (Invitrogen).
Propidium iodide (PI) nuclear stain exclusion assay
Cells seeded in 12-well CytoOne tissue culture-treated plates (USA Scientific) were treated with arsenite for 8 hours (unless specified otherwise) and incubated with Hoechst 33342 (H3570, Thermo Fisher) and propidium iodide (P1304MP, Thermo Fisher) for 15 min before imaging on an EVOS FL auto imaging system (Invitrogen).
Cell transfections
U2OS cells seeded in 48-well CytoOne tissue culture-treated plates (USA Scientific) were transfected the day after seeding with a total of 0.3 μg DNA encoding ZBP1, RIPK3, or G3BP1 using X-tremeGENE HP DNA Transfection Reagent (06 366 244 001, Roche) according to the manufacturer’s recommendations with the following exceptions: 2 μL of the transfection reagent were used per every μg of DNA (2:1 ratio) and the transfection complex was incubated at room temperature for 30 min. At 24 hours post-transfection, cells were treated with 500 μM sodium arsenite and cell viability was assessed 14 hours later with the PI nuclear stain exclusion assay. To assess protein expression, the same protocol was followed, except cells were seeded in 6-well tissue culture-treated dishes (VWR), transfected with a total of 2 μg DNA, and lysed in RIPA buffer 24 hours post-transfection.
Statistical analysis
Normal-based 95% confidence interval (CI): mean ± 2 standard deviations (SD) was used as the basis for statistical analysis. All statistical analysis was performed in Stata 17 (College Station, TX: StataCorp LLC). Two-sided, two-sample z-test of equality of proportions with the significance level (α) of 0.05 was performed to assess statistical significance of the observed differences between samples.
Supplementary Material
Acknowledgments:
We thank Dr. Nancy Kedersha from Brigham and Women’ Hospital and Harvard Medical School, Boston, Massachusetts for providing MEFs, Tia1−/− MEFs, U2OS cells, and G3bp1/2−/− U2OS cells. We thank Dr. Samantha Cotsmire for assistance in isolating primary MEFs from murine embryos.
Funding:
This work was supported by grants from the National Institute of Allergy and Infectious Diseases (NIAID) (R01AI095394) and the National Institute of Environmental Health Sciences (NIEHS) (R21ES030838).
Footnotes
Competing interests: Authors declare that they have no competing interests. Disclaimer: This article was prepared while Brian Johnson was employed at Arizona State University. The opinions expressed in this article are the author’s own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
Data and materials availability:
All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials. Zbp1−/− and Mlkl−/− L929 cells are available from BLJ through a material transfer agreement with Arizona State University.
References and Notes:
- 1.Wek RC, Jiang HY, Anthony TG, Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans 34, 7–11 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Harding HP et al. , Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 6, 1099–1108 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Liu Z, Lv Y, Zhao N, Guan G, Wang J, Protein kinase R-like ER kinase and its role in endoplasmic reticulum stress-decided cell fate. Cell Death Dis 6, e1822 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McEwen E. et al. , Heme-regulated inhibitor kinase-mediated phosphorylation of eukaryotic translation initiation factor 2 inhibits translation, induces stress granule formation, and mediates survival upon arsenite exposure. J Biol Chem 280, 16925–16933 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Wek SA, Zhu S, Wek RC, The histidyl-tRNA synthetase-related sequence in the eIF-2 alpha protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids. Mol Cell Biol 15, 4497–4506 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams BR, PKR; a sentinel kinase for cellular stress. Oncogene 18, 6112–6120 (1999). [DOI] [PubMed] [Google Scholar]
- 7.Kedersha N. et al. , G3BP-Caprin1-USP10 complexes mediate stress granule condensation and associate with 40S subunits. J Cell Biol 212, 845–860 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kedersha NL, Gupta M, Li W, Miller I, Anderson P, RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J Cell Biol 147, 1431–1442 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solomon S. et al. , Distinct structural features of caprin-1 mediate its interaction with G3BP-1 and its induction of phosphorylation of eukaryotic translation initiation factor 2alpha, entry to cytoplasmic stress granules, and selective interaction with a subset of mRNAs. Mol Cell Biol 27, 2324–2342 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson P, Kedersha N, RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat Rev Mol Cell Biol 10, 430–436 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Kedersha N, Anderson P, Regulation of translation by stress granules and processing bodies. Prog Mol Biol Transl Sci 90, 155–185 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas MG, Loschi M, Desbats MA, Boccaccio GL, RNA granules: the good, the bad and the ugly. Cell Signal 23, 324–334 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krysko DV, Vanden Berghe T, Parthoens E, D’Herde K, Vandenabeele P, Methods for distinguishing apoptotic from necrotic cells and measuring their clearance. Methods Enzymol 442, 307–341 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Lockshin RA, Williams CM, Programmed cell death—II. Endocrine potentiation of the breakdown of the intersegmental muscles of silkmoths. Journal of Insect Physiology 10, 643–649 (1964). [Google Scholar]
- 15.D’Arcy MS, Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int 43, 582–592 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Shi J, Gao W, Shao F, Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem Sci 42, 245–254 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P, Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol 15, 135–147 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Cho YS et al. , Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137, 1112–1123 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He S. et al. , Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 137, 1100–1111 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Holler N. et al. , Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol 1, 489–495 (2000). [DOI] [PubMed] [Google Scholar]
- 21.Vercammen D. et al. , Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med 187, 1477–1485 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang DW et al. , RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325, 332–336 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV, TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity 4, 387–396 (1996). [DOI] [PubMed] [Google Scholar]
- 24.Wu XN et al. , Distinct roles of RIP1-RIP3 hetero- and RIP3-RIP3 homo-interaction in mediating necroptosis. Cell Death Differ 21, 1709–1720 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun L. et al. , Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148, 213–227 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Wang H. et al. , Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell 54, 133–146 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Dondelinger Y. et al. , MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep 7, 971–981 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Hildebrand JM et al. , Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proc Natl Acad Sci U S A 111, 15072–15077 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dhuriya YK, Sharma D, Necroptosis: a regulated inflammatory mode of cell death. J Neuroinflammation 15, 199 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu Y. et al. , Cloning of DLM-1, a novel gene that is up-regulated in activated macrophages, using RNA differential display. Gene 240, 157–163 (1999). [DOI] [PubMed] [Google Scholar]
- 31.Takaoka A. et al. , DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448, 501–505 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Yang D. et al. , ZBP1 mediates interferon-induced necroptosis. Cell Mol Immunol 17, 356–368 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaiser WJ, Upton JW, Mocarski ES, Receptor-interacting protein homotypic interaction motif-dependent control of NF-kappa B activation via the DNA-dependent activator of IFN regulatory factors. J Immunol 181, 6427–6434 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koehler H. et al. , Vaccinia virus E3 prevents sensing of Z-RNA to block ZBP1-dependent necroptosis. Cell Host Microbe 29, 1266–1276.e1265 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rebsamen M. et al. , DAI/ZBP1 recruits RIP1 and RIP3 through RIP homotypic interaction motifs to activate NF-kappaB. EMBO Rep 10, 916–922 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koehler H. et al. , Inhibition of DAI-dependent necroptosis by the Z-DNA binding domain of the vaccinia virus innate immune evasion protein, E3. Proc Natl Acad Sci U S A 114, 11506–11511 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuriakose T. et al. , ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci Immunol 1, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thapa RJ et al. , DAI Senses Influenza A Virus Genomic RNA and Activates RIPK3-Dependent Cell Death. Cell Host Microbe 20, 674–681 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Upton JW, Kaiser WJ, Mocarski ES, DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe 11, 290–297 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deigendesch N, Koch-Nolte F, Rothenburg S, ZBP1 subcellular localization and association with stress granules is controlled by its Z-DNA binding domains. Nucleic Acids Res 34, 5007–5020 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng SK, Weissbach R, Ronson GE, Scadden AD, Proteins that contain a functional Z-DNA-binding domain localize to cytoplasmic stress granules. Nucleic Acids Res 41, 9786–9799 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drinking Water Requirements for States and Public Water Systems. United States Environmental Protection Agency; Accessed: 03/01/2022, URL: https://www.epa.gov/dwreginfo/drinking-water-arsenic-rule-history#Review. [Google Scholar]
- 43.Druwe IL, Vaillancourt RR, Influence of arsenate and arsenite on signal transduction pathways: an update. Arch Toxicol 84, 585–596 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dovey CM et al. , MLKL Requires the Inositol Phosphate Code to Execute Necroptosis. Mol Cell 70, 936–948.e937 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Najafov A. et al. , TAM Kinases Promote Necroptosis by Regulating Oligomerization of MLKL. Mol Cell 75, 457–468.e454 (2019). [DOI] [PubMed] [Google Scholar]
- 46.He S, Liang Y, Shao F, Wang X, Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci U S A 108, 20054–20059 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsumoto M, Funami K, Tatematsu M, Azuma M, Seya T, Assessment of the Toll-like receptor 3 pathway in endosomal signaling. Methods Enzymol 535, 149–165 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Vaure C, Liu Y, A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front Immunol 5, 316 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coupienne I, Fettweis G, Piette J, RIP3 expression induces a death profile change in U2OS osteosarcoma cells after 5-ALA-PDT. Lasers Surg Med 43, 557–564 (2011). [DOI] [PubMed] [Google Scholar]
- 50.Stöhr N. et al. , ZBP1 regulates mRNA stability during cellular stress. J Cell Biol 175, 527–534 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen JJ, Regulation of protein synthesis by the heme-regulated eIF2alpha kinase: relevance to anemias. Blood 109, 2693–2699 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kedersha N. et al. , Evidence that ternary complex (eIF2-GTP-tRNA(i)(Met))-deficient preinitiation complexes are core constituents of mammalian stress granules. Mol Biol Cell 13, 195–210 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sudhakar A. et al. , Phosphorylation of serine 51 in initiation factor 2 alpha (eIF2 alpha) promotes complex formation between eIF2 alpha(P) and eIF2B and causes inhibition in the guanine nucleotide exchange activity of eIF2B. Biochemistry 39, 12929–12938 (2000). [DOI] [PubMed] [Google Scholar]
- 54.Zhang J, Yang Y, He W, Sun L, Necrosome core machinery: MLKL. Cell Mol Life Sci 73, 2153–2163 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li J. et al. , The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 150, 339–350 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Banani SF, Lee HO, Hyman AA, Rosen MK, Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol 18, 285–298 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cougot N, Babajko S, Séraphin B, Cytoplasmic foci are sites of mRNA decay in human cells. J Cell Biol 165, 31–40 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jain S. et al. , ATPase-Modulated Stress Granules Contain a Diverse Proteome and Substructure. Cell 164, 487–498 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kedersha N. et al. , Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J Cell Biol 151, 1257–1268 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arimoto K, Fukuda H, Imajoh-Ohmi S, Saito H, Takekawa M, Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nat Cell Biol 10, 1324–1332 (2008). [DOI] [PubMed] [Google Scholar]
- 61.Buchan JR, Parker R, Eukaryotic stress granules: the ins and outs of translation. Mol Cell 36, 932–941 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsai NP, Wei LN, RhoA/ROCK1 signaling regulates stress granule formation and apoptosis. Cell Signal 22, 668–675 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruiz-Ramos R, Lopez-Carrillo L, Rios-Perez AD, De Vizcaya-Ruíz A, Cebrian ME, Sodium arsenite induces ROS generation, DNA oxidative damage, HO-1 and c-Myc proteins, NF-kappaB activation and cell proliferation in human breast cancer MCF-7 cells. Mutat Res 674, 109–115 (2009). [DOI] [PubMed] [Google Scholar]
- 64.Shi H. et al. , Arsenite causes DNA damage in keratinocytes via generation of hydroxyl radicals. Chem Res Toxicol 17, 871–878 (2004). [DOI] [PubMed] [Google Scholar]
- 65.Vanlangenakker N. et al. , cIAP1 and TAK1 protect cells from TNF-induced necrosis by preventing RIP1/RIP3-dependent reactive oxygen species production. Cell Death Differ 18, 656–665 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y. et al. , RIP1 autophosphorylation is promoted by mitochondrial ROS and is essential for RIP3 recruitment into necrosome. Nat Commun 8, 14329 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kong Q, Lin CL, Oxidative damage to RNA: mechanisms, consequences, and diseases. Cell Mol Life Sci 67, 1817–1829 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhan Y. et al. , Localized control of oxidized RNA. J Cell Sci 128, 4210–4219 (2015). [DOI] [PubMed] [Google Scholar]
- 69.Ofengeim D. et al. , Activation of necroptosis in multiple sclerosis. Cell Rep 10, 1836–1849 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Su Z, Yang Z, Xie L, DeWitt JP, Chen Y, Cancer therapy in the necroptosis era. Cell Death Differ 23, 748–756 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang T. et al. , CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat Med 22, 175–182 (2016). [DOI] [PubMed] [Google Scholar]
- 72.Zhe-Wei S, Li-Sha G, Yue-Chun L, The Role of Necroptosis in Cardiovascular Disease. Front Pharmacol 9, 721 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roychowdhury S, McMullen MR, Pisano SG, Liu X, Nagy LE, Absence of receptor interacting protein kinase 3 prevents ethanol-induced liver injury. Hepatology 57, 1773–1783 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Royce GH, Brown-Borg HM, Deepa SS, The potential role of necroptosis in inflammaging and aging. Geroscience 41, 795–811 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sridharan H, Upton JW, Programmed necrosis in microbial pathogenesis. Trends Microbiol 22, 199–207 (2014). [DOI] [PubMed] [Google Scholar]
- 76.Caccamo A. et al. , Necroptosis activation in Alzheimer’s disease. Nat Neurosci 20, 1236–1246 (2017). [DOI] [PubMed] [Google Scholar]
- 77.Xu C. et al. , TNF-α-dependent neuronal necroptosis regulated in Alzheimer’s disease by coordination of RIPK1-p62 complex with autophagic UVRAG. Theranostics 11, 9452–9469 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ash PE, Vanderweyde TE, Youmans KL, Apicco DJ, Wolozin B, Pathological stress granules in Alzheimer’s disease. Brain Res 1584, 52–58 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wolozin B, Ivanov P, Stress granules and neurodegeneration. Nat Rev Neurosci 20, 649–666 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jayaraman A, Htike TT, James R, Picon C, Reynolds R, TNF-mediated neuroinflammation is linked to neuronal necroptosis in Alzheimer’s disease hippocampus. Acta Neuropathologica Communications 9, 159 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roy ER et al. , Type I interferon response drives neuroinflammation and synapse loss in Alzheimer disease. J Clin Invest 130, 1912–1930 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu Z, Jiang N, Su W, Zhuo Y, Necroptosis: A Novel Pathway in Neuroinflammation. Frontiers in Pharmacology 12, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nie B. et al. , Age-dependent accumulation of 8-oxoguanine in the DNA and RNA in various rat tissues. Oxid Med Cell Longev 2013, 303181 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nunomura A. et al. , RNA oxidation in Alzheimer disease and related neurodegenerative disorders. Acta Neuropathol 118, 151–166 (2009). [DOI] [PubMed] [Google Scholar]
- 85.Gilks N. et al. , Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol Biol Cell 15, 5383–5398 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials. Zbp1−/− and Mlkl−/− L929 cells are available from BLJ through a material transfer agreement with Arizona State University.