Abstract
Background:
We assessed the efficacy and safety of mechanical thrombectomy (MT) in adult stroke patients with anterior circulation large vessel occlusion presenting in the late time window not fulfilling the DEFUSE-3 (Thrombectomy for Stroke at 6 to 16 Hours With Selection by Perfusion Imaging trial) and DAWN (Thrombectomy 6 to 24 Hours After Stroke With a Mismatch Between Deficit and Infarct trial) inclusion criteria.
Methods:
Cohort study of adults with anterior circulation large vessel occlusion admitted between 6 and 24 hours after last-seen-well at 5 participating Swiss stroke centers between 2014 and 2021. Mismatch was assessed by computer tomography or magnetic resonance imaging perfusion with automated software (RAPID or OLEA). We excluded patients meeting DEFUSE-3 and DAWN inclusion criteria and compared those who underwent MT with those receiving best medical treatment alone by inverse probability of treatment weighting using the propensity score. The primary efficacy end point was a favorable functional outcome at 90 days, defined as a modified Rankin Scale score shift toward lower categories. The primary safety end point was symptomatic intracranial hemorrhage within 7 days of stroke onset; the secondary was all-cause mortality within 90 days.
Results:
Among 278 patients with anterior circulation large vessel occlusion presenting in the late time window, 190 (68%) did not meet the DEFUSE-3 and DAWN inclusion criteria and thus were included in the analyses. Of those, 102 (54%) received MT. In the inverse probability of treatment weighting analysis, patients in the MT group had higher odds of favorable outcomes compared with the best medical treatment alone group (modified Rankin Scale shift: acOR, 1.46 [1.02–2.10]; P=0.04) and lower odds of all-cause mortality within 90 days (aOR, 0.59 [0.37–0.93]; P=0.02). There were no significant differences in symptomatic intracranial hemorrhage (MT versus best medical treatment alone: 5% versus 2%, P=0.63).
Conclusions:
Two out of 3 patients with anterior circulation large vessel occlusion presenting in the late time window did not meet the DEFUSE-3 and DAWN inclusion criteria. In these patients, MT was associated with higher odds of favorable functional outcomes without increased rates of symptomatic intracranial hemorrhage. These findings support the enrollment of patients into ongoing randomized trials on MT in the late window with more permissive inclusion criteria.
Keywords: acute stroke, patient selection, reperfusion, thrombectomy
Large vessel occlusions of the anterior circulation (LVO) account for a substantial proportion of ischemic stroke cases.1 They are associated with poor functional outcomes in the absence of fast reperfusion.2 Mechanical thrombectomy (MT) has become the standard of care for LVO patients within the first 6 hours after symptom onset.3–7 The time window for MT has been extended up to 24 hours after the randomized trials DEFUSE-3 (Thrombectomy for Stroke at 6 to 16 Hours With Selection by Perfusion Imaging) and DAWN (Thrombectomy 6 to 24 Hours After Stroke With a Mismatch Between Deficit and Infarct) were published.8,9 This has contributed to the increased use of MT, but evidence for patients with LVO not satisfying DEFUSE-3 and DAWN criteria is limited.10,11
See related article, p 731
The inclusion criteria of both trials were strict. In DEFUSE-3, patients were selected based on results of perfusion imaging between 6 and 16 hours after last-known-well. The DAWN trial enrolled patients up to 24 hours after symptom onset. A mismatch between clinical severity and volume of ischemic core was required to participate in the study. Exclusion criteria in both trials were mild clinical severity (defined as a National Institutes of Health Stroke Scale [NIHSS] <6 points in DEFUSE-3 or as a NIHSS <10 points in DAWN), isolated occlusion of the M2 segment of the middle cerebral artery and large infarct cores on baseline perfusion imaging. Reflecting these selective enrollment criteria, recent retrospective studies showed that a relevant proportion of patients with LVO presenting in the late time window did not meet DEFUSE-3 or DAWN inclusion criteria (Non-DEFUSE-Non-DAWN patients [NDND patients]), underlining that a large part of our patients in every day clinical practice are not represented in these recent clinical trials.12,13 Little is known about the safety and benefit of MT in NDND patients.
In our analyses of adult patients with LVO presenting between 6 and 24 hours, we sought to: (i) determine the proportion of NDND patients, (ii) compare the efficacy of MT with best medical treatment alone (BMT), and (iii) assess the treatment safety for both procedures (ie, symptomatic intracranial hemorrhage [sICH] within 7 days of stroke onset; and all-cause mortality within 90 days).
Methods
The Ethics Committee of North-Western Switzerland approved this study (EKNZ No. 2020-00552). Anonymized data may be provided by the corresponding authors upon reasonable request.
Study Design, Patients, and Data Collection
This multicenter cohort study was performed using data from 5 certified Swiss stroke centers capable of MT from the prospective Swiss Stroke Registry (EKNZ UBE 15/30). Outcome measures are prospectively recorded by qualified personnel. Inclusion criteria were a LVO diagnosis confirmed by computer tomography (CT) or magnetic resonance imaging (MRI) angiography (ie, internal carotid artery, M1 or M2 segment of the middle cerebral artery), admission between 6 and 24 hours after last-seen-well, and availability of perfusion imaging source data (CT or MRI). We excluded patients who had no available perfusion imaging and those with withdrawn research consent.
We assessed demographic (eg, age), clinical (eg, modified Rankin Scale [mRS] score before the event, NIHSS score on admission, prior medication, medical history), laboratory (eg, international normalized ratio), treatment (eg, intravenous tissue-type plasminogen activator, endovascular treatment), and outcome data (eg, mRS at 90 days) of all consecutive adult patients (ie, age ≥18 years) admitted between January 2014 and June 2021. Follow-up assessment at 90 days was routinely performed using different approaches (either in-person at the clinic, by telephone, or through medical records), with the choice left to each participating center.
Baseline Imaging Evaluation
LVO was verified in the acute phase by a neuroradiologist at the participating site. We then evaluated following imaging parameters: (i) Two experienced neuroradiologists visually assessed the Alberta Stroke Program Early CT Score (ASPECTS) based on non-contrast CT. The evaluators were blinded to clinical outcomes and medical history; (ii) Broadly used automated software detected mismatches on CT or MRI perfusion (for CT perfusion: RAPID [iSchemaView Inc, Menlo Park, CA]; for MRI perfusion: Olea [Olea Medical Solutions, La Ciotat, France]).14,15 For detailed baseline imaging evaluation, see the Supplemental Material.16–20
DEFUSE-3 and DAWN Ineligibility
We assessed ineligibility for both trials based on the original published in- and exclusion criteria.8,9
Mechanical Thrombectomy
The MT group included patients who underwent mechanical endovascular treatment either by stent retriever and/or aspiration technique at the discretion of the treating interventionalist. The treating physician determined the reperfusion status according to the modified Treatment in Cerebral Ischemia (mTICI) classification based on the angiographic images after the intervention.
Best Medical Treatment
BMT included intravenous administration of intravenous tissue-type plasminogen activator as indicated by the treating team, following current guidelines.11,21,22 For the BMT group, modified Treatment in Cerebral Ischemia status was unavailable.
Outcome Measures
Efficacy outcomes were defined as a shift in the mRS score toward lower categories (primary efficacy end point) and mRS score of 0–2 points (secondary efficacy end point) at 90 days. Safety outcomes included the rates of symptomatic intracranial hemorrhage (primary safety end point; defined as confirmed by CT or MRI and occurring within 7 days of acute ischemic stroke and associated with a NIHSS worsening of ≥4 points) and all-cause mortality (secondary safety end point) within 90 days.23,24
Statistical Analyses
Only NDND patients with LVO were included in our analyses. Univariable comparisons of the MT and BMT groups regarding clinical characteristics and outcome parameters were performed using the χ2 and Mann-Whitney U test for categorical and continuous variables, respectively. The results are presented as medians (interquartile ranges [IQR]) or numbers (%).
We performed inverse probability of treatment weighting using the propensity score to assess the primary and secondary end points, controlling for confounding factors described in the literature as influencing functional outcome (see text section S2).3,4,25–31 These variables are: age, hypertension, diabetes, atrial fibrillation, occlusion site, NIHSS score at admission, premorbid mRS score, intravenous thrombolysis, time from onset to admission, blood glucose level at admission, systolic and diastolic blood pressure, ischemic core volume, and hypoperfusion lesion volume. In contrast to propensity score matching, inverse probability of treatment weighting does not lead to sample size loss, and yields valid results even with small sample sizes.32 The diagnostic plots used to test the underlying assumptions of logistic regression for propensity score estimation were added as Figure S1A–C. An overview of the distribution of standardized mean differences before and after the introduction of weights is given in Figure S2A–C.
Only cases with complete information for the predefined covariable set were used. Imputations were omitted due to: (i) the presumed missingness at random, since all missing data concerned routinely determined variables (eg, serum glucose at admission), and (ii) the limited number of cases with missing values (N=18).
There is evidence that the RAPID and Olea packages may provide different results.33 Therefore, we performed additional sensitivity analyses considering only patients with CT perfusion evaluated with RAPID.
P<0.05 were considered significant. We report 95% CIs. The statistical software used was RStudio, version 1.4.1106 (R Foundation for Statistical Computing, Vienna, Austria).34
Results
Baseline Characteristics
Between January 2014 and June 2021, 278 patients with LVO presented 6 to 24 hours after last-seen-well at 1 of the 5 participating Swiss stroke centers (Figure 1 and Figure S3). Of these, 190 (68%) were NDND patients and were thus included in the analysis. Detailed reasons for ineligibility for DEFUSE-3 and DAWN are presented in Figure 1 for the MT and BMT groups separately.
Figure 1.
Flowchart of included patients with large-vessel occlusion in the anterior circulation in the 6- to 24-h time window. DEFUSE-3 indicates Thrombectomy for Stroke at 6 to 16 Hours With Selection by Perfusion Imaging trial; DAWN, Thrombectomy 6 to 24 Hours After Stroke With a Mismatch Between Deficit and Infarct trial; LVO, large vessel occlusion of the anterior circulation; mTICI, modified Treatment in Cerebral Ischemia classification (ranges from 0 to 3, with successful recanalization indicated by grade 2b or higher); mRS, modified Rankin Scale (mRS), 0 to 6 points, with higher scores indicating more severe neurological disability; NIHSS, National Institutes of Health Stroke Scale, 0 to 42 points, with higher scores indicating more severe deficits; and tPA, tissue-type plasminogen activator.
Among the NDND patients, 102 (54%) received MT. Within the MT group, successful reperfusion (ie, modified Treatment in Cerebral Ischemia score of 2b or 3) was achieved in 82 (80%), and incomplete reperfusion in 20 (20%) cases. Compared with the BMT group, the MT group had: higher NIHSS on admission (12 [IQR, 5–19] versus 10 [5–18], P=0.22), shorter times from admission to imaging (min; 25 [16–33] versus 32 [24–47], P=0.01) and from admission to bolus (in the case of pretreatment with intravenous tissue-type plasminogen activator; 45 [33–60] versus 59 [49–95], P=0.04), more often proximal LVO (M1 occlusion: 53% versus 40%, P=0.10), larger hypoperfusion volume (81 mL [IQR, 52–127] versus 52 mL [IQR, 25–118], P=0.01), and larger core-to-perfusion mismatch volume (69 mL [IQR, 43–100] versus 29 mL [IQR, 14–55], P≤0.001). All baseline characteristics are reported in Table 1.
Table 1.
Conventional Group Comparisons of Admission Characteristics of Non-DEFUSE-Non-DAWN Patients Presenting 6 to 24 Hours After Symptom Onset
Efficacy Outcomes
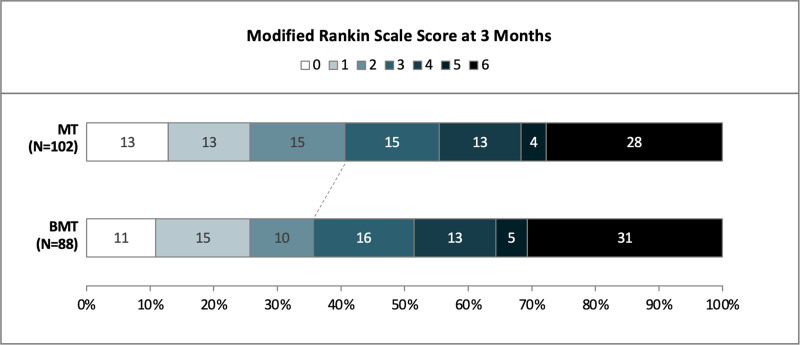
In the univariable analysis, favorable functional outcome at 90 days was numerically more frequent in the MT than BMT group without reaching statistical significance (mRS 0–2: 40% versus 36%, P=0.70; Figure 2Table 2 and Figure 2, Table S1).
Table 2.
Conventional Group Comparisons of Efficacy and Safety Outcomes in Non-DEFUSE-Non-DAWN Patients Presenting 6 to 24 Hours After Symptom Onset
Figure 2.
Functional outcome at 90 d in Non-DEFUSE-Non-DAWN patients receiving mechanical thrombectomy vs best medical treatment alone. BMT indicates best medical treatment alone; and MT, mechanical thrombectomy.
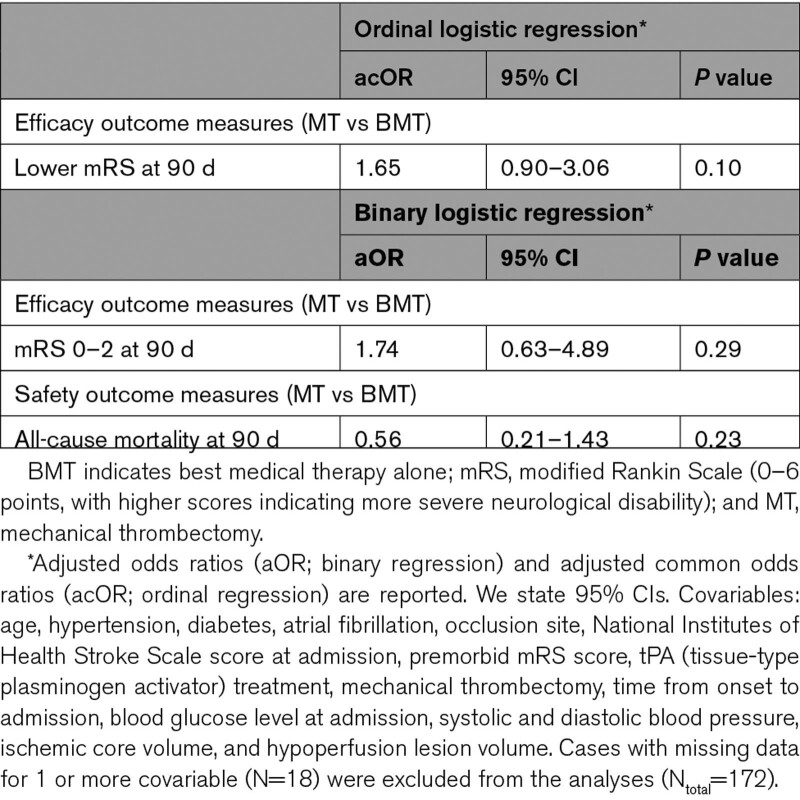
Unweighted multivariable regression analysis revealed no statistically significant difference in efficacy measures (Table 3). Inverse probability of treatment weighting analysis using ordinal regression showed that patients receiving MT had 46% higher adjusted odds for a lower mRS category (acOR, 1.46 [1.02–2.10]; P=0.04) (Table 4). In binary regression, MT was associated with higher odds for functional independence (ie, mRS 0–2) at 90 days, although not reaching statistical significance (aOR, 1.40 [0.91–2.17]; P=0.12).
Table 3.
Unweighted Multivariable Regression Analysis of Efficacy and Safety Outcomes of Non-DEFUSE-Non-DAWN Patients Presenting 6 to 24 Hours After Symptom Onset
Table 4.
IPTW Analysis of Efficacy and Safety Outcomes of Non-DEFUSE-Non-DAWN Patients Presenting 6 to 24 Hours After Symptom Onset
Safety Outcomes
The rates of sICH within 7 days and all-cause mortality at 90 days were comparable between MT and BMT groups (sICH: 5% versus 2%, P=0.63; all-cause mortality: 28% versus 31%, P=0.86, respectively; Table 2). For sICH, regression models were not used due to low case numbers across both treatment groups. For all-cause mortality at 90 days, unweighted binary regression showed no difference between groups, whereas inverse probability of treatment weighting analysis demonstrated lower odds for all-cause mortality for the MT group (aOR, 0.59 [0.37–0.93]; P=0.02; Tables 3 and 4).
Sensitivity Analysis
In DEFUSE-3 and DAWN, the RAPID software was used to determine the mismatch and core volume, respectively. Due to technical constraints, CT and MRI perfusion images were evaluated using different automated software in our cohort (RAPID for CT perfusion; Olea for MRI perfusion). Of the 190 NDND patients, 13% received MRI perfusion. Thus, we performed a sensitivity analysis in which we adjusted for the type of perfusion imaging in the regression models. Our analysis provided findings consistent with the main ones (mRS shift toward lower categories for the MT group: acOR, 1.45 [1.00–2.13]; P=0.049; mRS 0–2 points: aOR, 1.43 [0.93–2.21]; P=0.11; all-cause mortality: acOR, 0.58 [0.37–0.92]; P=0.02).
Discussion
Our 3 main findings are: (i) most adult patients presenting with LVO in the late time window do not meet DEFUSE-3 or DAWN inclusion criteria, (ii) approximately half of the NDND patients nevertheless receive MT in clinical practice, (iii) in NDND patients, after adjustment for confounders, MT was associated with higher odds for favorable functional outcome and lower odds for all-cause mortality at 90 days without an increased rate of sICH as compared to patients with BMT.
Both DEFUSE-3 and DAWN trials had very restrictive inclusion criteria. In our MT group, the main reasons for DEFUSE-3 ineligibility were distal occlusion site (ie, isolated occlusion of the M2 segment), low baseline NIHSS score (ie, <6 points), premorbid functional disability (ie, mRS score >2 points), and treatment with intravenous tissue-type plasminogen activator >4.5 hours after last-known-well. Main reasons for DAWN ineligibility were the absence of clinical-imaging-mismatch, low baseline NIHSS score (ie, <10 points), and premorbid functional disability (ie, mRS score >1 point). Comparing the exclusion criteria between the MT and BMT groups, there were differences in the frequency of exclusion based on noncompliance with perfusion imaging criteria (ie, core volume, mismatch volume, and mismatch ratio). In the MT group, 20 patients (20%) were ineligible because of noncompliance with imaging criteria, whereas 43 patients (49%) were ineligible in the BMT group.
Only a few studies have examined the efficacy and safety of MT in DEFUSE-3 and/or DAWN ineligible patients.12,13 A recent study looked at patients who would have been excluded from DAWN but were DEFUSE-3 eligible.12 Here, patients with ischemic infarct core volumes between 50 and 70 mL (ie, too large for inclusion in DAWN but small enough for DEFUSE-3) still had a clinical benefit related to MT without demonstrating an excess of sICH, as compared with patients with BMT. However, the cohort was small (N=33), and these patients were clinically severely affected (median NIHSS score 18 points). Desai et al investigated NDND patients. In their cohort, about 70% were NDND patients, which is consistent with the finding of our study. However, their NDND patients (N=37) were clinically more severely affected (median NIHSS score 18 points) than those in our present study. The authors concluded that MT can be safely offered to trial ineligible patients, particularly those with an ischemic core volume of <70 mL, baseline mRS of 0–2, and age ≤80 years.
Our third main finding was that, despite no significant difference in functional outcomes between MT and BMT in the univariable comparisons, MT was associated with better functional outcomes and lower all-cause mortality after considering baseline variables likely to have influenced the decision in favor of MT. While this indicates a selection bias (for which patients with M1 occlusion, higher NIHSS and larger core-to-perfusion mismatch volume were more likely to be treated with MT), this finding also suggests that a substantial proportion of NDND patients in the 6–24 hour window benefit of MT. Regarding mortality, our results are in line with those of a recent case series.35 This showed that even patients with minimal penumbra (ie, relative mismatch ≤20%) on perfusion imaging may still benefit from MT in terms of reduced 90-day mortality. The selection criteria of the DEFUSE-3 and DAWN trials were chosen to prove the concept of MT in a later time window but might be too restrictive in clinical practice. Also, while the selection bias would have argued, a priori, toward a worse outcome in the MT arm, the opposite was true, further underscoring the potential therapeutic benefit of MT. In this cohort, acute treatment decisions for MT based on the ensemble of clinical and radiological characteristics seem to have improved functional outcomes compared with BMT. This was consistent with the sensitivity analysis results. However, it remains unclear whether some of the patients with BMT would have benefited more from MT.
In our study, 40% of the patients in the MT group achieved a 90-day mRS of 0–2, a lower proportion than in DEFUSE-3 (45%) and DAWN (49%).8,9 The reason for this discrepancy most likely is that we did not exclude the one-fifth of our study population with premorbid disability (ie, mRS 3–5) that would have been excluded from DEFUSE-3 and DAWN. Other possible explanations for the relatively high proportion of functional independence observed in our NDND cohort are the low ischemic core volumes at admission and the high recanalization rates. The recanalization rate of 80% was in between that achieved in DEFUSE-3 (76%) and DAWN (84%).8,9 Remarkably, the overall sICH rate was low in our cohort (MT versus BMT: 5% versus 2%). It was slightly lower than those in DEFUSE-3 (MT versus BMT: 7% versus 4%) and DAWN (MT versus BMT: 6% versus 3%).8,9
Notably, 38% of patients in our cohort had isolated M2 occlusions of the middle cerebral artery. This is interesting since these patients are ineligible for MT according to DEFUSE-3 and DAWN criteria. In line with our findings, current evidence supports that these patients benefit from MT within 12 hours.36 The ongoing DISTAL trial (NCT05029414) has now extended the comparison of MT versus BMT to more distal occlusion sites of the anterior (ie, M2-4 of the middle cerebral artery, A1-3 of the anterior cerebral artery) and posterior circulation (ie, P1-2 of the posterior cerebral artery) for up to 24 hours after last-seen-well.
In our view, these real-life–derived data provide evidence that the interdisciplinary collaboration of acute neurological teams and experienced interventionalists can be associated with favorable functional outcomes in trial-ineligible patients. However, the restrictive inclusion criteria that most patients in clinical practice do not fulfill, together with safety concerns (ie, increased sICH rates), might still lead to ambiguity regarding appropriate patient selection and emphasize the urgent need for further research in this field.37–42 Several randomized trials with more permissive inclusion criteria regarding the infarct core size at admission are currently being performed (TESLA [NCT03805308], TENSION [NCT03094715], SELECT 2 [NCT03876457], IN EXTREMIS [Large Stroke Therapy Evaluation]/LASTE [NCT03811769], MR CLEAN-LATE [ISRCTN19922220]) and will hopefully expand our knowledge. The recent RESCUE-Japan LIMIT study has demonstrated that patients with large ischemic cores indicated by low ASPECTS values (mainly evaluated on the basis of MRIs) had better functional outcomes at 90 days with MT compared to BMT.43 Regardless of the pending study results, our study contributes important complementary findings as the pending trials focus on patients with ASPECTS ≤5 and in our NDND cohort 89% of patients had an ASPECTS >5. Thus, our findings will be of relevance even after the publication of these trials.
Strengths and Limitations
Our study has several strengths: (i) it is based on large, prospectively curated data of consecutive patients from several Swiss stroke centers who received either MT or BMT in clinical practice without predefined inclusion criteria, (ii) the centralized reevaluation of initial perfusion imaging ensures reader-independent comparability, (iii) the application of propensity score based weighting corrects for observed confounders and accounts for potential selection bias.
We acknowledge limitations: (i) the lack of randomization. Although we adjusted for the most important known confounders for outcome, there is still a risk of residual confounding (by unobserved or unmeasured confounders) and by the fact that outcome assessment was not blinded. In addition, although weighting resulted in a substantial improvement in the balance between treatment groups, ischemic core and hypoperfusion volumes were marginally outside the targeted standardized mean difference range of –0.10 to 0.10 (Figure S2A), (ii) we used naive variance estimators instead of robust or bootstrap-based estimators due to the previously reported potential overestimation of variance, (iii) the high proportion of isolated distal occlusions. The site of vessel occlusion impacts the functional outcome, with patients with distal occlusions having higher rates of favorable outcomes.44 However, we tried to address this issue by including the site of occlusion in the propensity score estimation and the weighted models, (iv) the rare occurrence of sICH prevents conclusive safety comparisons between the MT and BMT groups.
Conclusions
Two out of 3 adult patients with LVO presenting in the 6–24 hours window did not meet the DEFUSE-3 and DAWN inclusion criteria. Yet, MT was associated with higher odds of 90-day favorable functional outcomes without increased rates of sICH. These findings support the enrollment of patients into ongoing randomized trials on MT in the late time window with more permissive inclusion criteria.
Article Information
Acknowledgments
We thank Inga Lappe (Department of Neuroradiology, University Hospital Basel) and Malin Zahn (Department of Neurology, University Hospital Zurich) for their assistance in analyzing the CT and MRI images.
Sources of Funding
The study was funded by the University Hospital Basel and the Propatient Research Foundation (PRF). The funders (University Hospital Basel, PRF) did not influence the design of the study, data collection, or analysis of the data.
Disclosures
MK received speaker honoraria from Medtronic. LHB received personal fees from Claret Medical and InnovHeart. UF reports research grants from Medtronic for the SWIFT DIRECT trial (Solitaire With the Intention for Thrombectomy Plus Intravenous t-PA Versus DIRECT Solitaire Stent-Retriever Thrombectomy in Acute Anterior Circulation Stroke) and BEYOND SWIFT registry (Registry for Evaluating Outcome of Acute Ischemic Stroke Patients Treated With Mechanical Thrombectomy); consulting fees from Medtronic, Stryker and CSL Behring (fees paid to institution); membership of a Data Safety Monitoring Board for the IN EXTREMIS trial (Large Stroke Therapy Evaluation) and TITAN trial (Thrombectomy in Tandem Occlusion). GMDM received speaker honoraria from Medtronic. The other authors report no conflicts of interest.
Supplemental Material
STROBE Checklist
Text section S1–S2
Table S1
Figures S1–S3
Supplementary Material
Nonstandard Abbreviations and Acronyms
- BMT
- best medical treatment alone
- CT
- computed tomography
- DAWN
- Thrombectomy 6 to 24 Hours After Stroke With a Mismatch Between Deficit and Infarct
- DEFUSE-3
- Thrombectomy for Stroke at 6 to 16 Hours With Selection by Perfusion Imaging
- IQR
- interquartile range
- LVO
- anterior circulation large vessel occlusion
- MRI
- magnetic resonance imaging
- mRS
- modified Rankin Scale
- MT
- mechanical thrombectomy
- NDND
- Non-DEFUSE-Non-DAWN patients
- NIHSS
- National Institutes of Health Stroke Scale
- sICH
- symptomatic intracranial hemorrhage
T.D. Dittrich and P.B. Sporns contributed equally.
G.M. De Marchis and M. Psychogios contributed equally.
This article was sent to Hanne Christensen, Senior Guest Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 729.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.122.039793.
Contributor Information
Peter B. Sporns, Email: peter.sporns@hotmail.de.
Lilian F. Kriemler, Email: lilian.kriemler@spitaeler-sh.ch.
Salome Rudin, Email: Salome.Rudin@usb.ch.
Anh Nguyen, Email: Anh.Nguyen@usb.ch.
Annaelle Zietz, Email: annaelle.zietz@usb.ch.
Alexandros A. Polymeris, Email: alexandros.polymeris@usb.ch.
Christopher Tränka, Email: Christopher.Traenka@usb.ch.
Sebastian Thilemann, Email: Thilemann@gmx.de.
Benjamin Wagner, Email: benjamin.wagner@usb.ch.
Valerian L. Altersberger, Email: valerian.altersberger@usb.ch.
Ines Piot, Email: inesarmance.piot@usb.ch.
Filip Barinka, Email: filipbarinka@yahoo.co.uk.
Susanne Müller, Email: Susanne.Wegener@usz.ch.
Martin Hänsel, Email: martin.haensel@usz.ch.
Henrik Gensicke, Email: henrik.gensicke@usb.ch.
Stefan T. Engelter, Email: stefan.engelter@usb.ch.
Philippe A. Lyrer, Email: philippe.lyrer@usb.ch.
Raoul Sutter, Email: raoul.sutter@usb.ch.
Christian H. Nickel, Email: christian.nickel@usb.ch.
Mira Katan, Email: mira.katan@usb.ch.
Nils Peters, Email: nils.peters@hirslanden.ch.
Zsolt Kulcsár, Email: zsolt.kulcsar@usz.ch.
Grzegorz M. Karwacki, Email: grzegorz.karwacki@luks.ch.
Marco Pileggi, Email: marco.pileggi@gmail.com.
Carlo Cereda, Email: carlo.cereda@eoc.ch.
Susanne Wegener, Email: Susanne.Wegener@usz.ch.
Leo H. Bonati, Email: leo.bonati@usb.ch.
Urs Fischer, Email: Urs.Fischer@usb.ch.
Marios Psychogios, Email: marios.psychogios@usb.ch.
Gian Marco De Marchis, Email: gian.demarchis@usb.ch.
References
- 1.Sweid A, Hammoud B, Ramesh S, Wong D, Alexander TD, Weinberg JH, Deprince M, Dougherty J, Maamari DJ, Tjoumakaris S, et al. Acute ischaemic stroke interventions: large vessel occlusion and beyond. Stroke Vasc Neurol. 2020;5:80–85. doi: 10.1136/svn-2019-000262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ribo M, Molina CA, Cobo E, Cerdà N, Tomasello A, Quesada H, De Miquel MA, Millan M, Castaño C, Urra X, et al. Association between time to reperfusion and outcome is primarily driven by the time from imaging to reperfusion. Stroke. 2016;47:999–1004. doi: 10.1161/strokeaha.115.011721 [DOI] [PubMed] [Google Scholar]
- 3.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 4.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–2295. doi: 10.1056/nejmoa1415061 [DOI] [PubMed] [Google Scholar]
- 5.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/nejmoa1414792 [DOI] [PubMed] [Google Scholar]
- 6.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, San Román L, Serena J, Abilleira S, Ribó M, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. doi: 10.1056/nejmoa1503780 [DOI] [PubMed] [Google Scholar]
- 7.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/nejmoa1414905 [DOI] [PubMed] [Google Scholar]
- 8.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart RA, Torbey MT, Kim-Tenser M, Leslie-Mazwi T, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–718. doi: 10.1056/nejmoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, et al. Thrombectomy 6 to 24 Hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 10.Williams MM, Leslie-Mazwi T, Hirsch JA, Kittel C, Spiotta A, De Leacy R, Mocco J, Albuquerque FC, Ducruet AF, Goyal N, et al. Real-world effects of late window neurothrombectomy: procedure rates increase without night-time bias. J Neurointerv Surg. 2020;12:460–464. doi: 10.1136/neurintsurg-2019-015223 [DOI] [PubMed] [Google Scholar]
- 11.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2019;50:e344–e418. doi: 10.1161/str.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 12.Leslie-Mazwi TM, Hamilton S, Mlynash M, Patel AB, Schwamm LH, Lansberg MG, Marks M, Hirsch JA, Albers GW. DEFUSE 3 Non-DAWN patients. Stroke. 2019;50:618–625. doi: 10.1161/strokeaha.118.023310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai SM, Rocha M, Molyneaux BJ, Starr M, Kenmuir CL, Gross BA, Jankowitz BT, Jovin TG, Jadhav AP. Thrombectomy 6–24 hours after stroke in trial ineligible patients. J Neurointerv Surg. 2018;10:1033–1037. doi: 10.1136/neurintsurg-2018-013915 [DOI] [PubMed] [Google Scholar]
- 14.Maegerlein C, Fischer J, Mönch S, Berndt M, Wunderlich S, Seifert CL, Lehm M, Boeckh-Behrens T, Zimmer C, Friedrich B. Automated calculation of the Alberta stroke program early CT score: feasibility and reliability. Radiology. 2019;291:141–148. doi: 10.1148/radiol.2019181228 [DOI] [PubMed] [Google Scholar]
- 15.Xiong Y, Huang CC, Fisher M, Hackney DB, Bhadelia RA, Selim MH. Comparison of automated CT perfusion softwares in evaluation of acute ischemic stroke. J Stroke Cerebrovasc Dis. 2019;28:104392. doi: 10.1016/j.jstrokecerebrovasdis.2019.104392 [DOI] [PubMed] [Google Scholar]
- 16.Sakai Y, Delman BN, Fifi JT, Tuhrim S, Wheelwright D, Doshi AH, Mocco J, Nael K. Estimation of Ischemic core volume using computed tomographic perfusion. Stroke. 2018;49:2345–2352. doi: 10.1161/strokeaha.118.021952 [DOI] [PubMed] [Google Scholar]
- 17.Wouters A, Christensen S, Straka M, Mlynash M, Liggins J, Bammer R, Thijs V, Lemmens R, Albers GW, Lansberg MG. A comparison of relative time to peak and tmax for mismatch-based patient selection. Front Neurol. 2017;8:539. doi: 10.3389/fneur.2017.00539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neumann-Haefelin T, Wittsack HJ, Wenserski F, Siebler M, Seitz RJ, Mödder U, Freund HJ. Diffusion- and perfusion-weighted MRI. The DWI/PWI mismatch region in acute stroke. Stroke. 1999;30:1591–1597. doi: 10.1161/01.str.30.8.1591 [DOI] [PubMed] [Google Scholar]
- 19.Albers GW, Wald MJ, Mlynash M, Endres J, Bammer R, Straka M, Maier A, Hinson HE, Sheth KN, Taylor Kimberly W, et al. Automated calculation of Alberta stroke program early CT Score: validation in patients with large hemispheric infarct. Stroke. 2019;50:3277–3279. doi: 10.1161/strokeaha.119.026430 [DOI] [PubMed] [Google Scholar]
- 20.Mokin M, Primiani CT, Siddiqui AH, Turk AS. ASPECTS (Alberta Stroke Program Early CT Score) measurement using hounsfield unit values when selecting patients for stroke thrombectomy. Stroke. 2017;48:1574–1579. doi: 10.1161/strokeaha.117.016745 [DOI] [PubMed] [Google Scholar]
- 21.Sandset EC, Anderson CS, Bath PM, Christensen H, Fischer U, Gąsecki D, Lal A, Manning LS, Sacco S, Steiner T, et al. European Stroke Organisation (ESO) guidelines on blood pressure management in acute ischaemic stroke and intracerebral haemorrhage. Eur Stroke J. 2021;6:Ii. doi: 10.1177/23969873211026998 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.BergeWhiteley E, Audebert W, De Marchis H, Fonseca GM, Padiglioni AC, de la Ossa C, Strbian NP, Tsivgoulis D, Turc G. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. 2021;6:I–lxii. doi: 10.1177/2396987321989865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Kummer R, Broderick JP, Campbell BC, Demchuk A, Goyal M, Hill MD, Treurniet KM, Majoie CB, Marquering HA, Mazya MV, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46:2981–2986. doi: 10.1161/strokeaha.115.010049 [DOI] [PubMed] [Google Scholar]
- 24.Whiteley WN, Emberson J, Lees KR, Blackwell L, Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G, et al. Risk of intracerebral haemorrhage with alteplase after acute ischaemic stroke: a secondary analysis of an individual patient data meta-analysis. Lancet Neurol. 2016;15:925–933. doi: 10.1016/s1474-4422(16)30076-x [DOI] [PubMed] [Google Scholar]
- 25.Olmos AG. P. A Practical guide for using propensity score weighting in R. Pract Assess, Res Eval. 2015;20 25. doi: 10.7275/jjtm-r398 [Google Scholar]
- 26.Flint AC, Faigeles BS, Cullen SP, Kamel H, Rao VA, Gupta R, Smith WS, Bath PM, Donnan GA, Lees KR, et al. THRIVE score predicts ischemic stroke outcomes and thrombolytic hemorrhage risk in VISTA. Stroke. 2013;44:3365–3369. doi: 10.1161/strokeaha.113.002794 [DOI] [PubMed] [Google Scholar]
- 27.Sojka M, Szmygin M, Pyra K, Tarkowski P, Luchowski P, Wojczal J, Drelich-Zbroja A, Jargiełło T. Predictors of outcome after mechanical thrombectomy for acute ischemic stroke in patients aged ≥90 years. Clin Neurol Neurosurg. 2021;200:106354. doi: 10.1016/j.clineuro.2020.106354 [DOI] [PubMed] [Google Scholar]
- 28.Mocco J, Zaidat OO, von Kummer R, Yoo AJ, Gupta R, Lopes D, Frei D, Shownkeen H, Budzik R, Ajani ZA, et al. Aspiration thrombectomy after intravenous alteplase versus intravenous alteplase alone. Stroke. 2016;47:2331–2338. doi: 10.1161/strokeaha.116.013372 [DOI] [PubMed] [Google Scholar]
- 29.Alaka SA, Menon BK, Brobbey A, Williamson T, Goyal M, Demchuk AM, Hill MD, Sajobi TT. Functional outcome prediction in ischemic stroke: a comparison of machine learning algorithms and regression models. Front Neurol. 2020;11:889. doi: 10.3389/fneur.2020.00889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Z, Li J, Zhao M, Zhang M, Wang T, Chen L, Liu Q, Wang H, Lu J, Zhao X. Baseline cerebral ischemic core quantified by different automatic software and its predictive value for clinical outcome. Front Neurosci. 2021;15:608799. doi: 10.3389/fnins.2021.608799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarraj A, Hassan AE, Savitz S, Sitton C, Grotta J, Chen P, Cai C, Cutter G, Imam B, Reddy S, et al. Outcomes of endovascular thrombectomy vs medical management alone in patients with large ischemic cores: a secondary analysis of the optimizing patient’s Selection for Endovascular Treatment in Acute Ischemic Stroke (SELECT) study. JAMA Neurol. 2019;76:1147–1156. doi: 10.1001/jamaneurol.2019.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pirracchio R, Resche-Rigon M, Chevret S. Evaluation of the propensity score methods for estimating marginal odds ratios in case of small sample size. BMC Med Res Methodol. 2012;12:70. doi: 10.1186/1471-2288-12-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deutschmann H, Hinteregger N, Wießpeiner U, Kneihsl M, Fandler-Höfler S, Michenthaler M, Enzinger C, Hassler E, Leber S, Reishofer G. Automated MRI perfusion-diffusion mismatch estimation may be significantly different in individual patients when using different software packages. Eur Radiol. 2021;31:658–665. doi: 10.1007/s00330-020-07150-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2020.
- 35.Hoelter P, Schmidt M, Breuer L, Kallmünzer B, Schwab S, Doerfler A, Engelhorn T. Endovascular treatment in patients with large vessel occlusion: reduced mortality despite minimal penumbra. Neuroradiology. 2019;61:1469–1476. doi: 10.1007/s00234-019-02280-3 [DOI] [PubMed] [Google Scholar]
- 36.Menon BK, Hill MD, Davalos A, Roos Y, Campbell BCV, Dippel DWJ, Guillemin F, Saver JL, van der Lugt A, Demchuk AM, et al. Efficacy of endovascular thrombectomy in patients with M2 segment middle cerebral artery occlusions: meta-analysis of data from the HERMES collaboration. J Neurointerv Surg. 2019;11:1065–1069. doi: 10.1136/neurintsurg-2018-014678 [DOI] [PubMed] [Google Scholar]
- 37.Goyal M, Ospel JM, Menon B, Almekhlafi M, Jayaraman M, Fiehler J, Psychogios M, Chapot R, van der Lugt A, Liu J, et al. Challenging the ischemic core concept in acute ischemic stroke imaging. Stroke. 2020;51:3147–3155. doi: 10.1161/strokeaha.120.030620 [DOI] [PubMed] [Google Scholar]
- 38.Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, Schellinger PD, Toni D, de Vries J, White P, et al. European Stroke Organisation (ESO) – European Society for Minimally Invasive Neurological Therapy (ESMINT) Guidelines on mechanical thrombectomy in acute ischaemic stroke endorsed by Stroke Alliance for Europe (SAFE). Eur Stroke J. 2019;4:6–12. doi: 10.1177/2396987319832140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X, Xie Y, Wang H, Yang D, Jiang T, Yuan K, Gong P, Xu P, Li Y, Chen J, et al. Symptomatic intracranial hemorrhage after mechanical thrombectomy in Chinese ischemic stroke patients: the ASIAN score. Stroke. 2020;51:2690–2696. doi: 10.1161/strokeaha.120.030173 [DOI] [PubMed] [Google Scholar]
- 40.Venditti L, Chassin O, Ancelet C, Legris N, Sarov M, Lapergue B, Mihalea C, Ozanne A, Gallas S, Cortese J, et al. Pre-procedural predictive factors of symptomatic intracranial hemorrhage after thrombectomy in stroke. J Neurol. 2021;268:1867–1875. doi: 10.1007/s00415-020-10364-x [DOI] [PubMed] [Google Scholar]
- 41.Schröder J, Thomalla G. A critical review of Alberta stroke program early CT score for evaluation of acute stroke imaging. Front Neurol. 2016;7:245. doi: 10.3389/fneur.2016.00245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Charbonnier G, Bonnet L, Biondi A, Moulin T. Intracranial bleeding after reperfusion therapy in acute ischemic stroke. Front Neurol. 2020;11:629920. doi: 10.3389/fneur.2020.629920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshimura S, Sakai N, Yamagami H, Uchida K, Beppu M, Toyoda K, Matsumaru Y, Matsumoto Y, Kimura K, Takeuchi M, et al. Endovascular therapy for acute stroke with a large ischemic region. N Engl J Med. 2022;386:1303. doi: 10.1056/NEJMoa2118191 [DOI] [PubMed] [Google Scholar]
- 44.Tian H, Parsons MW, Levi CR, Lin L, Aviv RI, Spratt NJ, Butcher KS, Lou M, Kleinig TJ, Bivard A. Influence of occlusion site and baseline ischemic core on outcome in patients with ischemic stroke. Neurology. 2019;92:e2626–e2643. doi: 10.1212/wnl.0000000000007553 [DOI] [PubMed] [Google Scholar]