Abstract
Purpose:
Exposures to ambient air pollutants may prime the lung enhancing risk of acute respiratory distress syndrome (ARDS) in sepsis. Our objective was to determine the association of short-, medium-, and long-term pollutant exposures and ARDS risk in critically ill sepsis patients.
Methods:
We analyzed a prospective cohort of 1,858 critically ill patients with sepsis, and estimated short- (3-day), medium- (6-weeks), and long- (5-years) term exposures to ozone, nitrogen dioxide (NO2), sulfur dioxide (SO2), carbon monoxide (CO), particulate matter <2.5 μm (PM2.5), and PM<10 μm (PM10) using weighted averages of daily levels from monitors within 50 km of subjects’ residences. Subjects were followed for 6 days for ARDS by the Berlin Criteria. The association between each pollutant and ARDS was determined using multivariable logistic regression adjusting for preselected confounders. In 764 subjects, we measured plasma concentrations of inflammatory proteins at presentation and tested for an association between pollutant exposure and protein concentration via linear regression.
Results:
ARDS developed in 754 (41%) subjects. Short- and long-term exposures to SO2, NO2, and PM2.5 were associated with ARDS risk (SO2: OR for the comparison of the 75th to 25th long term exposure percentile 1.43(95% CI 1.16, 1.77);p<0.01; NO2: 1.36(1.06, 1.74);p=0.04, PM2.5: 1.21(1.04, 1.41);p=0.03). Long-term exposures to these three pollutants were also associated with plasma interleukin-1 receptor antagonist and soluble tumor necrosis factor receptor-1 concentrations.
Conclusion:
Short and long-term exposures to ambient SO2, PM2.5, and NO2 are associated with increased ARDS risk in sepsis, representing potentially modifiable environmental risk factors for sepsis-associated ARDS.
Keywords: Acute Respiratory Distress Syndrome, Acute Lung Injury, Sepsis, Air Pollution
TWEET:
Short and long-term exposures to the ambient air pollutants SO2, NO2, and PM2.5 are associated with increased risk of ARDS in sepsis.
INTRODUCTION
Acute Respiratory Distress Syndrome (ARDS) is a common cause of respiratory failure characterized by the acute onset of diffuse bilateral non-cardiogenic pulmonary edema and severe hypoxemia occurring in the setting of a significant precipitating insult.(1, 2) Unfortunately, the syndrome carries a mortality greater than 30% and lacks pharmacologic therapies that target underlying biologic mechanisms.(3, 4) The most common precipitating insult for ARDS is sepsis, the dysregulated host response to infection.(5) Sepsis can result in ARDS either from a pulmonary or non-pulmonary source of infection,(6) is the sixth leading cause of hospitalization in the United States (US), and is the leading cause of death among the critically ill.(7) Environmental factors that alter ARDS risk in sepsis are incompletely understood;(8-11) however, there is an urgent need to identify modifiable ARDS risk factors and their underlying mechanisms in order to develop strategies to reduce sepsis-associated ARDS risk and mortality.
Components of ambient air pollution, including particulate matter (PM), ozone (O3), nitrogen dioxide (NO2), sulfur dioxide (SO2), and carbon monoxide (CO), have been linked to the development of lung disease, exacerbations of lung disease, and respiratory mortality.(12-19) The Industrial Revolution led to dramatic spikes in ambient air pollution that were linked to increases in acute respiratory hospitalizations and mortality, eventually spurring the development of air quality standards in the US and elsewhere.(20, 21) Concordant with these reports, healthy rodents exposed to high levels of O3 and certain types of PM develop acute lung injury by the induction of oxidative stress, increased alveolar capillary barrier permeability, and recruitment of inflammatory cells to the lung.(22) In healthy humans, even low to moderate exposure to air pollutants results in a transient decline in lung function and recruitment of inflammatory cells.(23, 24) Additionally, exposure to ambient air pollution is not uniform across regions of the US, with individuals of lower socioeconomic status and racial minorities burdened by higher exposure, potentially contributing to health outcome disparities.(25, 26)
We previously reported associations between exposure to components of ambient air pollution and risk of ARDS in two geographically diverse cohorts.(10, 11) In our first study of 1,558 critically ill patients at risk for ARDS presenting to Vanderbilt University Medical Center, we identified an independent association between long-term O3 exposure and risk of ARDS.(11) This association was strongest among the subgroup of patients whose ARDS risk factor was traumatic injury; however, the sepsis subgroup may have been underpowered to identify independent associations. In our second study, we identified associations between O3, fine PM < 2.5 μm in diameter (PM2.5), NO2, SO2, and CO with risk of ARDS in a prospective cohort of 996 critically ill patients presenting to the Penn Medicine Trauma Center after traumatic injury.(10)
The primary objective of the current study was to determine if our previous findings in trauma patients are also evident in the largest group of patients at risk for ARDS, those with sepsis. We aimed to determine the independent association of short-, medium-, and long-term exposure to the ambient air pollutants O3, NO2, SO2, CO, PM2.5 and PM10 with the risk of developing ARDS in a large well-phenotyped prospective cohort study of sepsis. Additionally, we aimed to determine the association of short-, medium-, and long-term exposure to ambient air pollutants and biomarkers of inflammation measured early after presentation with sepsis. Globally, we hypothesized that short-, medium-, and long-term exposure to elevated ambient air pollutants would prime the lung for injury, resulting in an increased risk of sepsis-associated ARDS. Some of the results of this study have been previously reported in the form of an abstract.(27)
METHODS
Additional methods are provided in the online data supplement.
Study Population
We included patients enrolled in the Molecular Epidemiology of SepSis in the Intensive care unit (MESSI) prospective cohort study between 2008 and 2018. MESSI enrollment criteria have been previously described.(28-31) Briefly, all patients admitted to a single-center medical intensive care unit (ICU) are screened for sepsis according to the sepsis-2 criteria for severe sepsis or septic shock and enrolled if they had a primary indication for ICU admission of sepsis. MESSI exclusion criteria included admission from a long-term acute care facility and an active do-not-intubate order at the time of enrollment. For the current study, we excluded patients without an available residential address and those who did not live within 50 km of an EPA air quality monitor. The cohort protocol is approved by the Institutional Review Board of the University of Pennsylvania (Protocol #808542) with a waiver of timely informed consent with consent obtained at the earliest feasible timepoint.
Air Pollution Exposure
Exposures to Environmental Protection Agency (EPA) criteria pollutants, O3, NO2, SO2, CO, PM2.5, and PM10, were estimated using the EPA’s publicly available Aerometric Information Retrieval System. An individual patient’s average exposure was assessed using the inverse-distance squared weighted average of daily levels from all EPA air quality monitors within 50 km of the geocoded location of the patient’s residential address.(10, 11) Ozone levels were only measured in summer months. We defined short, medium, and long-term exposure based on prior literature as the average exposure over the 3 days, 6 weeks, and 5 years prior to presentation with sepsis, respectively.
ARDS Outcome
Enrolled patients were followed for 6 days from presentation for the development of ARDS based on the Berlin definition, with the added requirement for intubation and invasive mechanical ventilation.(2, 32) We follow patients for 6 days for ARDS to identify ARDS directly related to sepsis. Prior studies demonstrate that nearly 100% of ARDS that occurs directly related to a precipitating factor develops within 5 days.(33, 34) All chest radiographs ordered for clinical purposes were reviewed by trained physician investigators for ARDS with consensus review for any discordant radiographs.
Plasma Biomarker Measurements
Residual plasma initially drawn for clinical testing at emergency department, or, for transfers from the hospital ward, ICU presentation, was obtained by research personnel for biomarker testing. This approach allows us to obtain plasma drawn as close to admission as possible. Plasma concentrations of inflammatory proteins Interleukin (IL)-8, IL-6, IL-1 receptor antagonist (IL-1RA), and soluble tumor necrosis factor receptor-1 (sTNFR1) were measured in duplicate in a subset of 764 subjects enrolled in the larger cohort selected based on availability of plasma and cost using commercially available Meso Scale Discovery assays optimized for human plasma.
Statistical Analysis
Our primary objective was to determine the association between long-term air pollutant exposure and ARDS risk, and our secondary objectives were to determine the association of short- and medium-term air pollutant exposure and ARDS risk. Patient characteristics were compared across quartiles of air pollutant exposure using the Pearson Chi-square test or Kruskal-Wallis test, as appropriate. We then estimated the association of short, medium, and long-term exposure and risk of ARDS via multivariable logistic regression models adjusting for prespecified confounders chosen based on a directed acyclic graph including covariates hypothesized to confound the relationship between air pollutant exposure and ARDS.(35) Specifically, we adjusted for age, sex, self or surrogate reported race, smoking history, alcohol use, severity of illness based on the Acute Physiology and Chronic Health Evaluation (APACHE) III score, distance to the hospital, month of admission, and median household income of the patients’ home addresses’ 5-digit zip code. We a priori elected not to adjust for source of sepsis or initial management decisions as we hypothesized that these variables may be in the causal pathway linking air pollution to ARDS risk. Additionally, we conducted an a priori defined sensitivity analysis in which we limited derivation of the exposure estimates to EPA monitors within 15 km of patients’ residences to reduce exposure misclassification. We also examined for interactions between pollutant levels and smoking history, age, and race using the likelihood ratio test. Statistical significance was considered for two-sided p-values less than 0.05.
The correlation between short-, medium-, and long-term exposure to air pollutants and plasma biomarker concentrations was first estimated and tested using Spearman’s rho. We then fit multivariable linear regression models for each biomarker as the outcome adjusting for the pre-specified confounders. We did not adjust for sepsis severity or occurrence of ARDS in these models as we hypothesized these variables also exist in the causal pathway. Plasma biomarker concentrations were log transformed to improve model fit. Logistic regression was then used to determine the association of the biomarkers with ARDS risk.
RESULTS
Cohort Characteristics
We enrolled 1,858 septic patients with available residential addresses, 754 (41%) of whom developed ARDS within 6 days of presentation (Supplemental Figure E1). The source of sepsis was pulmonary in 34% of subjects, 58% of subjects were male, the median age was 61 years (interquartile range (IQR) 51 - 69), and the racial distribution was similar to the population of the Philadelphia Metropolitan Area that presents to the Hospital of the University of Pennsylvania (Table 1). All enrolled patients lived within 50 km of at least one air quality monitor, with the majority living within 50 km of multiple air quality monitors. The median number of air quality monitors within 50 kms ranged from 12 to 25 depending on the pollutant (Supplemental Table E1). Median (IQR) short-, medium-, and long-term concentrations of each air pollutant were largely lower than US air quality standards and are provided in Supplemental Table E2. Long-term concentrations of SO2, NO2, and PM2.5 were moderately correlated with one another. Ozone demonstrated a negative correlation with NO2 and PM10, and a weak correlation with CO (Supplemental Table E3). Short-, medium-, and long-term concentrations of each individual pollutant were moderately to highly correlated (Supplemental Table E4). Supplemental Table E5 provides patient characteristics and ARDS cases by quartiles of long-term 5-year average air pollutant concentrations. Notably, patients of non-white race and lower socioeconomic status were more likely to have a higher exposure to NO2, SO2, CO, PM2.5, and PM10. Additionally, pulmonary source of sepsis was more common in the higher exposure quartiles for all air pollutants except PM10.
Table 1.
Patient Characteristics
| Patient Characteristic | Sepsis Population (n=1858) |
|---|---|
| Age | 61 (51, 69) |
| Male Sex | 1078 (58%) |
| Race | |
| White | 1119 (60%) |
| Black | 586 (32%) |
| Asian | 66 (4%) |
| Multiple/Unknown | 87 (5%) |
| APACHE III | 90 (66, 120) |
| Pulmonary Source of Sepsis | 552 (34%) |
| Current smoker | 203 (12%) |
| Alcohol Use | 192 (11%) |
| Distance to Penn (km) | 19.8 (4.7, 45.5) |
| Median household income (x 1000) | 55 (35, 77) |
| Admission SOFA Score | 10 (7, 13) |
| Administered Corticosteroids | 628 (34%) |
| Intravenous Fluids in first 24 hours (L) | 3.5 (1.9, 6.0) |
| ARDS | 754 (41%) |
| Mild ARDS | 76 (4%) |
| Moderate ARDS | 204 (11%) |
| Severe ARDS | 474 (26%) |
| Hypertension | 951 (51%) |
| Diabetes Mellitus | 544 (29%) |
| Chronic Renal Disease | 288 (16%) |
| Cirrhosis | 193 (10%) |
| Active Malignancy | 741 (40%) |
Data are reported as median (IQR) or n (%).
APACHE = acute physiology and chronic health evaluation.
Pollutant Exposure and ARDS
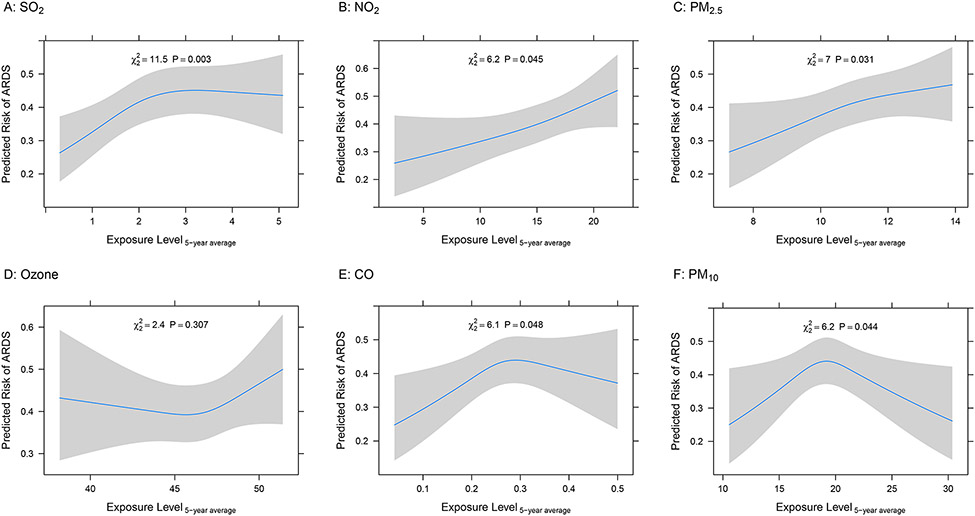
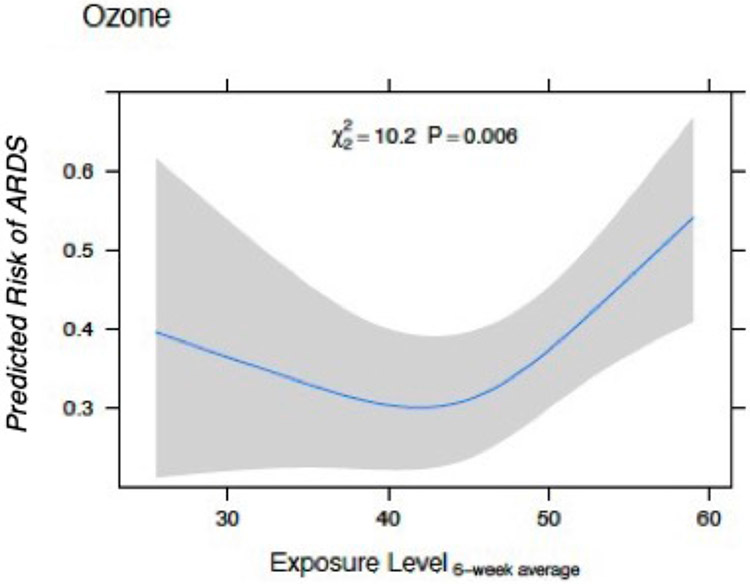
We identified several statistically significant associations between short-, medium-, and long-term air pollutant exposures and ARDS risk in sepsis (Table 2). Exposure to elevated SO2 demonstrated the strongest association with ARDS risk at each time window of exposure (Figure 1a). Short- and long-term exposures to NO2 were also associated with ARDS risk (Figure 1b). Results for medium-term exposure to NO2 and ARDS risk were not statistically significant. Exposure to PM2.5 demonstrated an association with ARDS risk with each time window of exposure (Figure 1c). Exposure to O3 was significantly associated with ARDS risk only for medium-term exposure estimates; however, the relationship was non-linear (Figures 1d, Figure 2). We did not identify linear associations between increased exposure to CO or PM10 and ARDS risk (Figure 1e-f).
Table 2.
Logistic regression analysis for the association of exposure to individual air pollutants and ARDS risk
| Pollutant | Three-day Average Exposure |
Six-week Average Exposure |
Five-Year Average Exposure |
|||
|---|---|---|---|---|---|---|
| OR* (95% CI) | P | OR* (95% CI) | P | OR* (95% CI) | P | |
| Ozone | 1.22 (0.98, 1.52) | 0.12 | 1.24 (1.00, 1.54) | 0.01 | 1.09 (0.96, 1.24) | 0.31 |
| NO2 | 1.37 (1.10, 1.71) | <0.01 | 1.18 (0.93, 1.51) | 0.08 | 1.36 (1.06, 1.74) | 0.04 |
| SO2 | 1.31 (1.06, 1.63) | 0.04 | 1.45 (1.19, 1.77) | <0.01 | 1.43 (1.16, 1.77) | <0.01 |
| CO | 0.99 (0.88, 1.12) | 0.29 | 0.94 (0.81, 1.09) | 0.71 | 1.25 (1.03, 1.52) | 0.05 |
| PM2.5 | 1.07 (0.90, 1.28) | 0.03 | 1.17 (1.00, 1.39) | 0.01 | 1.21 (1.04, 1.41) | 0.03 |
| PM10 | 0.99 (0.85, 1.17) | 0.12 | 0.95 (0.81, 1.13) | 0.72 | 1.04 (0.94, 1.14) | 0.04 |
The ORs are for the comparison of the 75th to the 25th percentile of exposure for each air pollutant adjusted for pre-specified confounders in multivariable logistic regression. The single air pollutant models included restricted cubic splines including 3 knots to assess for non-linear associations. Some 95% confidence intervals cross 1.0 despite a p-value <0.05 due to non-linear associations.
Figure 1.
Standardized Risk Curves for the association of Long-Term Air Pollution Exposure (5-years) and Acute Respiratory Distress Syndrome (ARDS) Risk. The predicted risk of ARDS was estimated across air pollution exposures using logistic regression models adjusting for age, sex, race, smoking history, alcohol use, APACHE III score, distance to the hospital, month of admission, and median household income of the patients’ home addresses’ 5-digit zip code. Restricted cubic splines with three knots were used for each individual air pollutant to evaluate for non-linear associations with ARDS. A. SO2 parts per billion (ppb), B. NO2 ppb, C. PM2.5 ug/m3 D. Ozone ppb, E. CO parts per million (ppm), F. PM10 ug/m3. Gray bars represent 95% confidence intervals around the predicted risks.
Figure 2.
Standardized Risk Curves for Ozone Medium-Term Exposure (6-weeks) and Acute Respiratory Distress Syndrome (ARDS) Risk. The predicted risk of ARDS was estimated across parts per billion ozone exposures using a logistic regression model adjusting for age, sex, race, smoking history, alcohol use, APACHE III score, distance to the hospital, month of admission, and median household income of the patients’ home addresses’ 5-digit zip code. Restricted cubic splines with three knots were used for each individual air pollutant to evaluate for non-linear associations with ARDS. Gray bars represent 95% confidence intervals around the predicted risks.
Pollutant Exposure and Plasma Biomarkers
Short-, medium-, and long-term exposure to the three air pollutants associated with ARDS, SO2, NO2, and PM2.5, were not positively correlated with any biomarker of inflammation (Supplemental Table E6) in unadjusted analyses. However, after adjustment for sex, race, and APACHE III score, increasing exposure to SO2 was strongly associated with higher plasma concentrations of IL-1RA and sTNFR1 for short-, medium-, and long-term exposure windows (Table 3, Supplemental Table E6). In multivariable linear regression models, long-term exposures to PM2.5 and NO2 were also associated with plasma concentrations of IL-1RA and sTNFR1 (Table 3). Short- and medium-term exposures to PM2.5 and NO2 were not positively associated with any biomarker level (Supplemental Table E6). Plasma concentrations of IL1RA and sTNFR1 were strongly associated with ARDS (IL1RA: Odds Ratio (OR) per log increase 1.13 (95% CI 1.07, 1.18); p<0.001, sTNFR1: OR per log increase 1.36 (95% CI 1.16, 1.60); p<0.001).
Table 3.
Association of Long-Term Exposure to SO2. NO2, and PM2.5 with plasma biomarkers of inflammation adjusting for sex, race, and APACHE III score
| Pollutant | IL1RA | sTNFR1 | IL-8 | IL-6 | ||||
|---|---|---|---|---|---|---|---|---|
| Beta* (95% CI) |
p | Beta* (95% CI) |
p | Beta#* (95% CI) |
p | Beta* (95% CI) |
p | |
| SO2 (ppb) | 0.35 (0.18, 0.52) | <0.01 | 0.09 (0.03, 0.16) | <0.01 | 0.07 (−0.05, 0.18) | 0.08 | 0.10 (−0.02, 0.22) | 0.11 |
| NO2 (ppb) | 0.07 (0.01, 0.13) | 0.03 | 0.03 (0.01, 0.05) | 0.01 | 0.03 (−0.01, 0.07) | 0.21 | 0.03 (−0.01, 0.08) | 0.11 |
| PM2.5 (ug/m3) | 0.19 (0.04, 0.34) | 0.01 | 0.06 (0.01, 0.11) | 0.04 | 0.02 (−0.09, 0.12) | 0.74 | 0.05 (−0.05, 0.17) | 0.30 |
Multivariable linear regression models adjusting for sex, race, and APACHE III score. The natural log for the biomarkers was used as the outcome to approximate normality. The beta represents the increase in natural log of the biomarker per increase in ppb or ug/m3 of the air pollutant.
Sensitivity Analysis
Limiting exposure data to only air quality monitors within 15 km of a patient’s geocoded address did not substantially change our results (Supplemental Table E7). We did not identify significant statistical interaction between air pollutant exposures and smoking status, age, or race.
DISCUSSION
We identified strong and independent associations between short- and long- term exposure to SO2, NO2, and PM2.5 with risk of ARDS in a large cohort of patients admitted to the ICU with sepsis. These findings are consonant with those of our previous study in trauma patients, and expand the link between ambient air pollution and ARDS to the largest at-risk population, those with sepsis.(10) Notably, as subjects who traveled further to the hospital had a higher severity of illness and lower air pollution exposure, adjustment for severity of illness and distance to the hospital revealed associations between air pollution and ARDS risk that might not have been seen without robust confounder ascertainment. These associations represented an approximately 15 to 20% increases in adjusted ARDS risk between subject in the highest and lowest quartile of exposure and appeared linear for NO2 and PM2.5. We did not identify associations between PM10 or CO exposure and ARDS. The lack of an association between PM10 may not be surprising as larger particulate matter cannot deposit in the most distal airways and alveoli where the alveolar-capillary barrier dysfunction of ARDS occurs. Additionally, CO is unlike the other air pollutants as it has anti-inflammatory and antioxidant effects and is being investigated as a potential therapy for ARDS.(36, 37)
Similar to our previous study in trauma patients, O3 exposure had a non-linear, U-shaped associated with ARDS risk, and was significant at the 6-week exposure window.(10) It is possible that this relationship results from the complex interaction of O3 with other pollutants on determining ARDS risk. Ozone is formed from the interaction of sunlight with volatile organic compounds and nitrogen oxides rather than being directly emitted as a pollutant. This reaction can also be reversed, whereby O3 reacts with nitrogen oxide (NO) to generate NO2 resulting in a local depletion of O3. This reverse reaction occurs in areas of the highest NO emissions such as near roadways and industrial plants. Therefore, areas with the highest pollution from these sources often have the lowest O3 as demonstrated by the inverse relationship between NO2 and O3 exposure seen in our data.
There is now a growing body of literature linking acute and chronic exposure to air pollutants with ARDS, since the first published association of chronic O3 exposure and ARDS risk in the Nashville region.(11) Lin and colleagues identified an association between exposure to PM and emergency ambulance dispatches for ARDS in Guangzhou, China,(38) and Rhee and colleagues identified an association between PM2.5 and O3 and hospitalization for ARDS among individuals >65 years old in a Medicare administrative database.(39) Our study adds to these findings by including a prospective cohort rather than an administrative dataset, with robust ARDS phenotyping that did not rely on comparatively insensitive classification based on clinician recognition and documentation.(3, 40) We additionally include robust collection of potential confounders and limit our study population to those at risk for ARDS from sepsis. Further supporting a potential link between air pollution and ARDS, Rush and colleagues identified an association between O3 exposure and ARDS mortality,(41) and DeWeerdt and colleagues identified associations between exposures to multiple air pollutants and duration of mechanical ventilation in a mixed population of acute respiratory failure.(42) Chronic exposure to air pollution has also been associated with sepsis mortality, but it is unknown if increased ARDS risk mediates this increased mortality.(43) Additionally, in COVID-19, severe air pollution exposure has been associated with cumulative SARS-CoV-2 cases and higher mortality.(44, 45)
A major finding in our study and others is that the burden of elevated air pollutant exposure is higher among racial minorities and individuals living in areas with a lower median household income.(25) Recent large epidemiologic studies have demonstrated marked improvement in air quality over the last several decades; however, there has not been an equivalent reduction in disparities of exposure between communities.(25) Lower income communities and communities of color, particularly Black communities, continue to experience disproportionately higher levels of air pollution.(46, 47) Elevated air pollution exposure has also been linked to the development of severe COVID-19, a form of sepsis-associated ARDS, and has been hypothesized to explain some of the racial and economic disparities observed in COVID-19 outcomes.(48) While our study was conducted prior to the COVID-19 pandemic, our findings suggest that structural racial and economic inequalities impact ARDS risk in the larger sepsis population.(49, 50) It is likely that targeted interventions to reduce air pollution in these communities may have the greatest potential to reduce risk of ARDS in individuals who develop sepsis.
Ambient air pollutants have long been linked to respiratory and cardiovascular disease, such as ARDS; however, the exact pathologic mechanism underlying this link in each individual disease is incompletely understood. Exposure to elevated PM2.5, O3, SO2, and NO2 has been linked to chronic lung inflammation, generation of reactive oxygen species, and alveolar capillary barrier leakiness in healthy humans and murine models.(51, 52) In a mouse model of nebulized lipopolysaccharide (LPS) induced acute lung injury, additional exposure to PM2.5 was associated with increased broncho-alveolar lavage fluid leukocytes, increased cytokines, and impaired tissue remodeling compared to LPS exposure alone.(51) Our findings suggest that similar mechanisms may be relevant in human sepsis. Specifically, we identified associations between chronic air pollutant exposure and levels of IL1RA and sTNFR1, both markers of inflammation. It is possible that exposure to air pollutants primes the lung for injury in the setting of an exacerbating factor such as sepsis. Future research aimed at understanding the mechanisms of this lung priming could potentially lead to therapeutics to prevent or treat ARDS in those with high air pollution exposure.
Our study has several strengths. First, we studied sepsis-associated ARDS in a large cohort of patients with significant racial, socioeconomic, comorbidity, and geographical diversity in a large metropolitan area with extensive EPA monitoring of air pollutants. Second, all admissions to the medical ICU of the Hospital of the University of Pennsylvania were screened for sepsis and if enrolled, were followed for ARDS via individual chest radiograph and chart review by trained physician investigators. Third, we prospectively collected extensive confounders, including measures of severity of illness, and constructed adjusted regression models to reduce the effects of confounding. Finally, we conducted sensitivity analyses and examined for associations between air pollutant exposure and biomarkers of inflammation measured early after presentation with sepsis.
Our study has several limitations. Exposure misclassification is possible as we relied on estimates of air pollutant exposure based on subjects’ zip code from their most recent address. Occupational exposures, time spent indoors versus outdoors, apartment floor, and time spent driving on highways were unavailable. Our study is single-centered and conducted prior to the COVID-19 pandemic which limits generalizability; however, it was conducted over 10 years at a large referral center with a population that is diverse in exposures, demographics, and comorbidities. Unmeasured confounding is possible; however, the prospective nature of our cohort allows us to measure potential confounders that would be unavailable in administrative datasets. Given the number of related analyses performed, there is risk for type 1 error; however, I findings are consistent with previous literature. Additionally, our biomarker analyses are exploratory, were measured in a random subset of subjects, and utilized residual blood initially drawn for clinical purposes. Lastly, collinearity of some air pollutants as well as interaction between air pollutants makes it difficult to determine if one specific pollutant or multiple pollutants impact ARDS risk.
In conclusion, we have identified associations between short- and long-term exposure to PM2.5, NO2, and SO2 and ARDS development in the largest at-risk population, patients critically ill with sepsis. Additionally, we have identified a non-linear association between medium-term O3 exposure and ARDS risk in sepsis. Importantly, the overall exposure estimates for all the pollutants are largely lower than current EPA air quality standards, suggesting that even low to moderate exposure levels may have impact on the risk of ARDS in sepsis. Elevated exposure to air pollution represents a potentially modifiable risk factor for ARDS.
Supplementary Material
TAKE HOME MESSAGE.
Air pollution exposure has long been linked to acute and chronic respiratory disease, but its role in sepsis-related ARDS risk is largely unknown. We identified associations between short- and long- term exposure to sulfur dioxide, nitrogen dioxide, and PM2.5 with ARDS risk in sepsis, suggesting that short- and long- term exposures to ambient air pollutants are potentially modifiable environmental risk factors with implication for public health and environmental justice.
Funding:
The reported study was funded by the National Institutes of Health grants HL155159 (JPR), HL125723 (JPR), HL161196 (NJM), HL137915 (NJM), S10OD025172 (NJM), HL103836 (LBW), HL158906 (LBW), HL140026 (CSC).
Footnotes
Conflicts of Interest: Dr. Ware reports consulting fees from Akebia, Santhera, Global Blood Therapeutics, and Boerhinger Ingelheim and research contract support (paid to institution) from Genentech, Boehringer Ingelheim, and CSL Behring all unrelated to the topic of this article. Dr. Meyer reports consulting fees from Endpoint Health, Inc and research contract support to her institution from Quantum Leap Healthcare Collaborative and Biomarck, Inc. Dr. Calfee reports consulting fees from Vasomune, Gen1e Life Sciences, Cellenkos, NGMBio, and Janssen, and research support to her institution from Roche-Genentech and Quantum Leap Healthcare Collaborative, all unrelated to this article. Dr. Matthay reports research grant funding from Roche-Genentec and Quatum Therapeutics, and consultation for Novartis, Citius Pharmaceuticals, Johnson and Johnson, Gilead Pharmaceuticals, and ElifeScience.
REFERENCES
- 1.Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–33. [DOI] [PubMed] [Google Scholar]
- 3.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315(8):788–800. [DOI] [PubMed] [Google Scholar]
- 4.Matthay MA, McAuley DF, Ware LB. Clinical trials in acute respiratory distress syndrome: challenges and opportunities. Lancet Respir Med. 2017;5(6):524–34. [DOI] [PubMed] [Google Scholar]
- 5.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheu CC, Gong MN, Zhai R, Chen F, Bajwa EK, Clardy PF, et al. Clinical characteristics and outcomes of sepsis-related vs non-sepsis-related ARDS. Chest. 2010;138(3):559–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med. 2016;193(3):259–72. [DOI] [PubMed] [Google Scholar]
- 8.Calfee CS, Matthay MA, Eisner MD, Benowitz N, Call M, Pittet JF, et al. Active and passive cigarette smoking and acute lung injury after severe blunt trauma. Am J Respir Crit Care Med. 2011;183(12):1660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simou E, Leonardi-Bee J, Britton J. The Effect of Alcohol Consumption on the Risk of ARDS: A Systematic Review and Meta-Analysis. Chest. 2018;154(1):58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reilly JP, Zhao Z, Shashaty MGS, Koyama T, Christie JD, Lanken PN, et al. Low to Moderate Air Pollutant Exposure and Acute Respiratory Distress Syndrome after Severe Trauma. Am J Respir Crit Care Med. 2019;199(1):62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ware LB, Zhao Z, Koyama T, May AK, Matthay MA, Lurmann FW, et al. Long-Term Ozone Exposure Increases the Risk of Developing the Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2016;193(10):1143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, et al. Air Pollution and Mortality in the Medicare Population. N Engl J Med. 2017;376(26):2513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jerrett M, Burnett RT, Pope CA 3rd, Ito K, Thurston G, Krewski D, et al. Long-term ozone exposure and mortality. N Engl J Med. 2009;360(11):1085–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295(10):1127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet. 2014;383(9928):1581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pirozzi CS, Jones BE, VanDerslice JA, Zhang Y, Paine R 3rd, Dean NC. Short-Term Air Pollution and Incident Pneumonia. A Case-Crossover Study. Ann Am Thorac Soc. 2018;15(4):449–59. [DOI] [PubMed] [Google Scholar]
- 17.Balmes JR, Cisternas M, Quinlan PJ, Trupin L, Lurmann FW, Katz PP, et al. Annual average ambient particulate matter exposure estimates, measured home particulate matter, and hair nicotine are associated with respiratory outcomes in adults with asthma. Environ Res. 2014;129:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kariisa M, Foraker R, Pennell M, Buckley T, Diaz P, Criner GJ, et al. Short- and long-term effects of ambient ozone and fine particulate matter on the respiratory health of chronic obstructive pulmonary disease subjects. Arch Environ Occup Health. 2015;70(1):56–62. [DOI] [PubMed] [Google Scholar]
- 19.Winterbottom CJ, Shah RJ, Patterson KC, Kreider ME, Panettieri RA Jr., Rivera-Lebron B, et al. Exposure to Ambient Particulate Matter Is Associated With Accelerated Functional Decline in Idiopathic Pulmonary Fibrosis. Chest. 2018;153(5):1221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Logan WP. Mortality in the London fog incident, 1952. Lancet. 1953;1(6755):336–8. [DOI] [PubMed] [Google Scholar]
- 21.Dockery DW, Pope CA 3rd, Xu X, Spengler JD, Ware JH, Fay ME, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329(24):1753–9. [DOI] [PubMed] [Google Scholar]
- 22.Hollingsworth JW, Maruoka S, Li Z, Potts EN, Brass DM, Garantziotis S, et al. Ambient ozone primes pulmonary innate immunity in mice. J Immunol. 2007;179(7):4367–75. [DOI] [PubMed] [Google Scholar]
- 23.Mudway IS, Kelly FJ. An investigation of inhaled ozone dose and the magnitude of airway inflammation in healthy adults. Am J Respir Crit Care Med. 2004;169(10):1089–95. [DOI] [PubMed] [Google Scholar]
- 24.Huang W, Wang G, Lu SE, Kipen H, Wang Y, Hu M, et al. Inflammatory and oxidative stress responses of healthy young adults to changes in air quality during the Beijing Olympics. Am J Respir Crit Care Med. 2012;186(11):1150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colmer J, Hardman I, Shimshack J, Voorheis J. Disparities in PM2.5 air pollution in the United States. Science. 2020;369(6503):575–8. [DOI] [PubMed] [Google Scholar]
- 26.Bell ML, Ebisu K. Environmental inequality in exposures to airborne particulate matter components in the United States. Environ Health Perspect. 2012;120(12):1699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reilly JP, Zhao Z, Shashaty MGS, Koyama T, Anderson BJ, Jones TK, et al. Short and Long Term Exposure to Ambient Air Pollutants and Increased Risk of Acute Respiratory Distress Syndrome in Sepsis. American Journal of Respiratory and Critical Care Medicine. 2021;A13. A013 ARDS IN THE TIME OF COVID-19(203):A1055. [Google Scholar]
- 28.Reilly JP, Wang F, Jones TK, Palakshappa JA, Anderson BJ, Shashaty MGS, et al. Plasma angiopoietin-2 as a potential causal marker in sepsis-associated ARDS development: evidence from Mendelian randomization and mediation analysis. Intensive Care Med. 2018;44(11):1849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reilly JP, Anderson BJ, Hudock KM, Dunn TG, Kazi A, Tommasini A, et al. Neutropenic sepsis is associated with distinct clinical and biological characteristics: a cohort study of severe sepsis. Crit Care. 2016;20(1):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reilly JP, Meyer NJ, Shashaty MG, Anderson BJ, Ittner C, Dunn TG, et al. The ABO histo-blood group, endothelial activation, and acute respiratory distress syndrome risk in critical illness. J Clin Invest. 2021;131(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miano TA, Hennessy S, Yang W, Dunn TG, Weisman AR, Oniyide O, et al. Association of vancomycin plus piperacillin-tazobactam with early changes in creatinine versus cystatin C in critically ill adults: a prospective cohort study. Intensive Care Med. 2022;48(9):1144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah CV, Lanken PN, Localio AR, Gallop R, Bellamy S, Ma SF, et al. An alternative method of acute lung injury classification for use in observational studies. Chest. 2010;138(5):1054–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gajic O, Dabbagh O, Park PK, Adesanya A, Chang SY, Hou P, et al. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2011;183(4):462–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151(2 Pt 1):293–301. [DOI] [PubMed] [Google Scholar]
- 35.Lederer DJ, Bell SC, Branson RD, Chalmers JD, Marshall R, Maslove DM, et al. Control of Confounding and Reporting of Results in Causal Inference Studies. Guidance for Authors from Editors of Respiratory, Sleep, and Critical Care Journals. Ann Am Thorac Soc. 2019;16(1):22–8. [DOI] [PubMed] [Google Scholar]
- 36.Motterlini R, Otterbein LE. The therapeutic potential of carbon monoxide. Nat Rev Drug Discov. 2010;9(9):728–43. [DOI] [PubMed] [Google Scholar]
- 37.Otterbein LE, Mantell LL, Choi AM. Carbon monoxide provides protection against hyperoxic lung injury. Am J Physiol. 1999;276(4 Pt 1):L688–94. [DOI] [PubMed] [Google Scholar]
- 38.Lin H, Tao J, Kan H, Qian Z, Chen A, Du Y, et al. Ambient particulate matter air pollution associated with acute respiratory distress syndrome in Guangzhou, China. J Expo Sci Environ Epidemiol. 2018;28(4):392–9. [DOI] [PubMed] [Google Scholar]
- 39.Rhee J, Dominici F, Zanobetti A, Schwartz J, Wang Y, Di Q, et al. Impact of Long-Term Exposures to Ambient PM2.5 and Ozone on ARDS Risk for Older Adults in the United States. Chest. 2019;156(1):71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weissman GE, Harhay MO, Lugo RM, Fuchs BD, Halpern SD, Mikkelsen ME. Natural Language Processing to Assess Documentation of Features of Critical Illness in Discharge Documents of Acute Respiratory Distress Syndrome Survivors. Ann Am Thorac Soc. 2016;13(9):1538–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rush B, McDermid RC, Celi LA, Walley KR, Russell JA, Boyd JH. Association between chronic exposure to air pollution and mortality in the acute respiratory distress syndrome. Environ Pollut. 2017;224:352–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Weerdt A, Janssen BG, Cox B, Bijnens EM, Vanpoucke C, Lefebvre W, et al. Pre-admission air pollution exposure prolongs the duration of ventilation in intensive care patients. Intensive Care Med. 2020;46(6):1204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rush B, Wiskar K, Fruhstorfer C, Celi LA, Walley KR. The Impact of Chronic Ozone and Particulate Air Pollution on Mortality in Patients With Sepsis Across the United States. J Intensive Care Med. 2020;35(10):1002–7. [DOI] [PubMed] [Google Scholar]
- 44.Frontera A, Cianfanelli L, Vlachos K, Landoni G, Cremona G. Severe air pollution links to higher mortality in COVID-19 patients: The "double-hit" hypothesis. J Infect. 2020;81(2):255–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lembo R, Landoni G, Cianfanelli L, Frontera A. Air pollutants and SARS-CoV-2 in 33 European countries. Acta Biomed. 2021;92(1):e2021166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tessum CW, Paolella DA, Chambliss SE, Apte JS, Hill JD, Marshall JD. PM2.5 polluters disproportionately and systemically affect people of color in the United States. Sci Adv. 2021;7(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Josey KP, Delaney SW, Wu X, Nethery RC, DeSouza P, Braun D, et al. Air Pollution and Mortality at the Intersection of Race and Social Class. N Engl J Med. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katoto P, Brand AS, Bakan B, Obadia PM, Kuhangana C, Kayembe-Kitenge T, et al. Acute and chronic exposure to air pollution in relation with incidence, prevalence, severity and mortality of COVID-19: a rapid systematic review. Environ Health. 2021;20(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McGowan SK, Sarigiannis KA, Fox SC, Gottlieb MA, Chen E. Racial Disparities in ICU Outcomes: A Systematic Review. Crit Care Med. 2022;50(1):1–20. [DOI] [PubMed] [Google Scholar]
- 50.Mein SA, Annesi-Maesano I, Rice MB. COVID-19 Pandemic: A Wake-Up Call for Clean Air. Ann Am Thorac Soc. 2021;18(9):1450–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Souza Xavier Costa N, Ribeiro Junior G, Dos Santos Alemany AA, Belotti L, Schalch AS, Cavalcante MF, et al. Air pollution impairs recovery and tissue remodeling in a murine model of acute lung injury. Sci Rep. 2020;10(1):15314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pope CA 3rd, Bhatnagar A, McCracken JP, Abplanalp W, Conklin DJ, O'Toole T. Exposure to Fine Particulate Air Pollution Is Associated With Endothelial Injury and Systemic Inflammation. Circ Res. 2016;119(11):1204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.