Abstract
Urinary tract infections affect more than 1 in 2 women during their lifetime. Among these, more than 10% of patients carry antibiotic-resistant bacterial strains, highlighting the urgent need to identify alternative treatments. While innate defense mechanisms are well-characterized in the lower urinary tract, it is becoming evident that the collecting duct (CD), the first renal segment encountered by invading uropathogenic bacteria, also contributes to bacterial clearance. However, the role of this segment is beginning to be understood. This review summarizes the current knowledge on CD intercalated cells in urinary tract bacterial clearance. Understanding the innate protective role of the uroepithelium and of the CD offers new opportunities for alternative therapeutic strategies.
Keywords: acidosis, cell and transport physiology, collecting ducts, renal tubular epithelial cells, water-electrolyte balance
Uropathogenic Escherichia coli Causes Urinary Tract Infections
Urinary tract infections (UTIs) can occur in the urethra (urethritis), bladder (cystitis), ureters, and kidneys (pyelonephritis)1 and predominantly affect women.2 Uropathogenic E. coli (UPEC) causes 70% of acute pyelonephritis in male patients and 80% of female patients.3 The remaining UTI cases are caused by other organisms, including Staphylococcus saprophyticus, Klebsiella, Enterobacter, Proteus species, and enterococci.3,4
UTIs can be classified as uncomplicated or complicated, depending on the probability for recurrence or progression to more severe infections.1,2 Uncomplicated UTIs occur in healthy individuals without urinary tract abnormalities and resolve with antibiotic treatment. Complicated UTIs can be due to abnormalities in the urinary tract or host defense. This review focuses on the most common cause of UTI, the UPEC.
Once UPEC reaches the bladder, its type 1 pilus adhesins will adhere to mannosylated uroplakins and integrin receptors expressed at the apical surface of the uroepithelium.5 UPEC can then be endocytosed and colonize the host cells, which may protect them from antibiotic treatments.
However, bladder cells have natural defense mechanisms to evade the infection. The UPEC internalization process triggers a Toll-Like Receptor 4 (TLR4)-dependent innate immune response and UPEC exocytosis. Alternatively, UPEC can escape to the cytoplasm where they can form quiescent, nondividing intracellular bacterial communities. UPEC also secretes toxins, such as α-hemolysis and proteases, that promote the release of cellular nutrients and siderophores to highjack the released cellular iron. α-Hemolysin also promotes epithelial exfoliation to allow further UPEC spreading. Cystitis pathogenesis has been recently reviewed in detail.6,7 On overcoming bladder natural defenses, UPEC can continue their ascension to the kidneys, further attaching through adhesins or pyelonephritis-associated pili to globoside-containing glycolipids at the apical surface of renal cells. Eventually, UPEC can overcome the tubular epithelial barrier to enter the blood stream, causing bacteremia.5
Mechanistic Insights into Intercalated Cells and Their Role in Host Defense against UTIs
Intercalated Cells in the Collecting Ducts
As host defense mechanisms in the bladder are evaded, UPEC can reach the collecting duct (CD). The CD contains a salt-and-pepper, highly plastic epithelium8 that is composed of two major cell types: intercalated cells (ICs) and principal cells (PCs) (Figure 1). Aquaporin-2–expressing cells serve as precursors for PCs, and IC subtypes and their differentiation is regulated by forkhead box protein I1 (Foxi1) transcription factor and JAG1 and NOTCH1/2 signaling.8 In the CD, ciliated PCs mediate sodium and water reabsorption and potassium secretion.9 Also located in the CD, ICs are dispersed throughout the renal cortex and medulla.10 Although some antibacterial molecules synthesized by PCs contribute to the innate immune response as stated below,11 this review focuses on the role of ICs in innate immunity.
Figure 1.
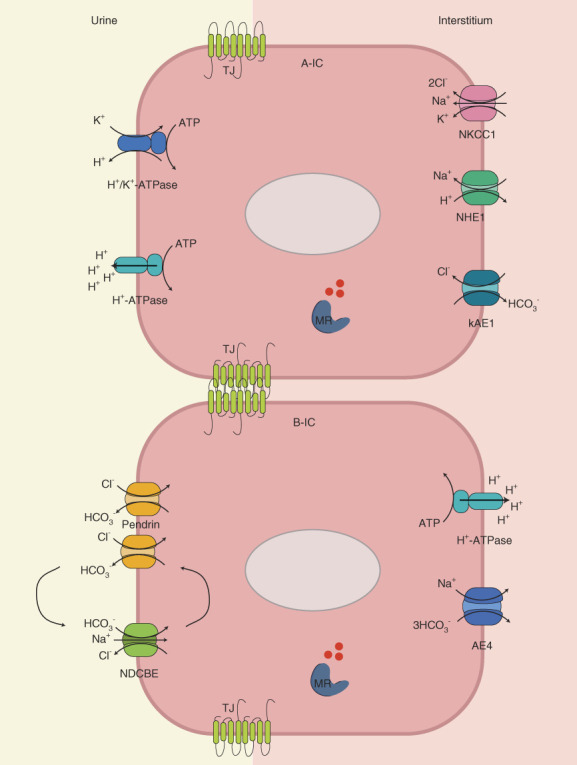
Diagram illustrating the various proteins in the IC involved in acid–base and salt homeostasis. (Top) A-ICs, (bottom) B-ICs. TJ claudins are represented in green between the two cells. TJ, tight junction; ATP, adenosine triphosphate; A-IC, type A-IC; B-IC, type B-IC; IC, intercalated cell; kAE1, kidney chloride/bicarbonate exchanger 1; MR, mineralocorticoid receptor; NDCBE, Na+-driven chloride/bicarbonate exchanger.
At least three types of ICs coexist: type A (A-IC), type B (B-IC), and non-A–non-B ICs. Although the role of non-A–non-B ICs remains unclear, recent RNA velocity studies showed that various IC subtypes and hybrid PCs–ICs coexist, suggesting that these cells may represent intermediate states between the two main CD cell types.12 Performing scRNA-seq on a resected kidney showed that six IC clusters exist. Marker genes differentiated the ICs into three A-ICs (subtype A, B, and C), one B-IC, one non-A–non-B-IC, and one hybrid PC–IC subtype.12 Although all A-ICs identified expressed SLC4A1, the A-IC subtypes differed in their expression of the heat shock protein HSPA1A and early growth response 1.
ICs Regulate Acid–Base Balance and Salt Homeostasis
Acid–Base Balance
Because natural defenses against UTIs involve the cell machinery responsible for acid–base homeostasis, we briefly outline the mechanisms involved in this process below.
In ICs, acidic conditions stimulate the diffusion of carbon dioxide across the membrane and the formation of carbonic acid, which is catalyzed by carbonic anhydrase II.10 Carbonic acid next ionizes into protons and bicarbonate. A-ICs excrete protons into the tubular lumen by the apical H+/K+-ATPase and the v-H+-ATPase (Figure 1).10 Heightened intracellular bicarbonate activates the bicarbonate-sensor soluble adenylate cyclase, which, in turn, results in Ser-175 phosphorylation and activation of the A subunit of the v-H+-ATPase.13
In the basolateral membrane, the kidney chloride/bicarbonate exchanger 1 and Slc26a1114 contribute to bicarbonate reabsorption into the interstitial fluid.10 During metabolic acidosis, both the v-H+-ATPase and the kidney chloride/bicarbonate exchanger 1 are upregulated to restore pH.15
A mirror mechanism occurs in type-B ICs during alkalosis by the now basolateral v-H+-ATPase and apical chloride/bicarbonate antiporter pendrin.16 Exclusively detected in the connecting tubule and initial CD, the role of non-A–non-B ICs remains unclear.17
Salt Homeostasis
The second main function of the CD is to fine-tune salt homeostasis, and recent evidence supports that the hyperosmolarity and, more specifically, medullary sodium chloride are essential in promoting an efficient immune response against bacterial infection.18,19 The role of electrogenic epithelial sodium channel ENaC in maintaining salt homeostasis is well-established. ENaC activity contributes to a lumen-negative transepithelial voltage, thereby promoting proton secretion in the CD, although this theory has been recently challenged.20
In B-ICs, the apical pendrin and the Na+-driven chloride/bicarbonate exchanger mediate thiazide-sensitive electroneutral NaCl reabsorption.21 Two cycles of pendrin exchange and one cycle of Na+-driven chloride/bicarbonate exchanger coordinated with basolateral AE4 result in the net uptake of one sodium and one chloride ion and secretion of two bicarbonate ions.22 However, the role of AE4 in salt homeostasis has been recently challenged.23 The essential contribution of tight junctions in electrolyte homeostasis has not been detailed in this study.24,25
ICs Sense and Trigger Inflammation
Recent studies have unveiled the role of ICs in innate immune reaction and in renal inflammation. The A-IC apical proinflammatory P2Y14 receptor (GPR105) triggers sterile inflammation.26 After an acute kidney injury, damaged cells in the proximal tubule release luminal UDP-Glucose, which is a DAMP molecule. When it reaches the CD A-ICs, UDP-Glucose binds to the apical P2Y14 receptors, leading to the activation of MEK1/2-extracellular signal-regulated kinase (ERK)1/2 pathways resulting in the production of proinflammatory chemokines, such as IL-8, CXCL1, CXCL2, CCL2, and CCL3. This secretion, in turn, attracts neutrophils and monocytes to the renal medulla.27,28
In Pyelonephritis, UPEC Preferentially Attaches to Desmoglein 2 in ICs and Activates TLR4
UPEC uses virulence factors to infiltrate and colonize the urinary system. Common UPEC cell surface factors include FimH and PapC, and common secreted factors include hemolysin (hly) and siderophores.29,30 The most prevalent virulence factor associated with UPEC, FimH, is present in over half of UPEC strains. Although the prevalence of virulence factors in cystitis and pyelonephritis varies depending on the demographic, the observed frequency of virulence factors in pyelonephritis and cystitis UPEC strains is not significantly different.29,30 In mice subjected to transurethral catheterization, UPEC uses its type 1 pili FimH adhesin to attach to the apical mannosylated desmoglein 2 receptor on the apical side of the renal medullary collecting duct (MCD).31 PapG adhesin of P pili also facilitates UPEC binding to kidney epithelial tissue.32 Although several TLRs, such as TLR2,33 5,34 and 11,35 have been implicated in the kidney response to UTIs, TLR4 is the one triggered in ICs.36,37 Knocking out murine TLR4 expression results in a lack of activation of proinflammatory mediators and a failure to clear UPEC.
In mice, two nonhemolytic UPEC strains triggered both NF-kB and MyD88-dependent and independent pathways, both resulting in the secretion of leukocyte chemoattractant macrophage inflammatory protein 2 (Figure 2).37 Noncytolytic apical UPEC was further shown to bind to inner medullary collecting duct (IMCD) cells through TLR4 and translocate transcellularly through caveolin-1 and clathrin-dependent pathways.36
Figure 2.
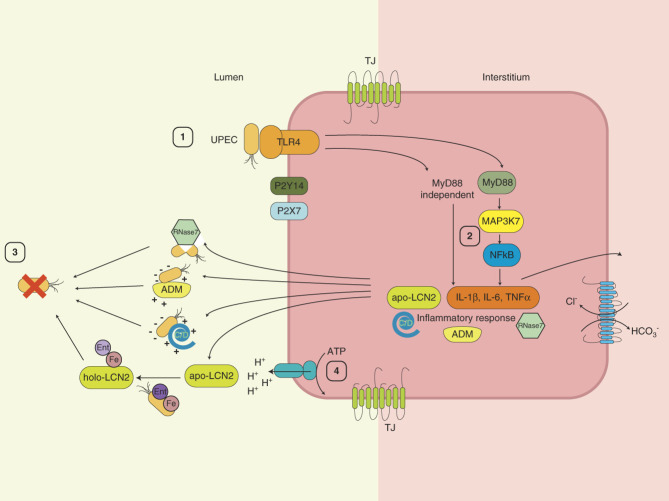
Schematic diagram summarizing the sequence of events from UPEC attachment to the apical surface of ICs to the multiple lines of defense, leading to UPEC clearance. Luminal UPEC attaches to TLR4 in ICs (1) and triggers downstream MyD88-dependent and -independent signaling, resulting in synthesis and secretion of inflammatory molecules; LCN2; and AMP, such as ADM, cathelicidins, and defensins (2). By various mechanisms, LCN2 and these AMPs result in bacterial clearance (3). Urinary acidification contributes to bacterial clearance as well (4). ADM, adrenomedullin; AMP, antimicrobial peptide; IC, intercalated cell; MAP3K7, mitogen-activated protein kinase kinase kinase 7; TLR4, toll-like receptor 4; UPEC, uropathogenic E. coli.
Additional pathways and markers are triggered on UTI. Isolated human and mouse ICs exposed to UPEC upregulate the expression of the Mitogen-activated protein kinase kinase kinase 7, as shown by both RNAseq and immunofluorescence on mouse kidney sections.38 Mitogen-activated protein kinase kinase kinase 7 is an essential component of the NF-kB signaling pathway, which results in the secretion of inflammatory molecules IL1, IL-6, and TNFα.39 Its activation is triggered by multiple stimuli, including LPS-mediated TLR4 activation (Figure 2).
Cellular Changes in ICs Promote an Innate Immune Response on UTIs
The abovementioned roles of ICs in UTI were further confirmed with flow cytometry cell sorting enrichment, followed by single-cell RNA sequencing of human and murine ICs.12 This study revealed the presence of six different subtypes of ICs, including two able to produce mature phagosomes in response to UTI. These cells also upregulate the v-H+-ATPase gene expression on UPEC exposure (Figure 2) and increase luminal acidification of phagosomes containing engulfed UPEC. In addition, a change in expression of genes involved in phagosome maturation was detected during UTI. IC subtypes A, B, and C showed that phagosome maturation becomes the predominant function after UPEC exposure. Thus, this groundbreaking study not only confirmed the role of ICs in UPEC internalization and degradation but also provided direct evidence of IC remodeling after UPEC exposure.
In Pyelonephritis, ICs Are Essential to Clear UPEC
Given the role of ICs in acid–base balance, the contribution of extracellular (luminal or interstitial) pH and the relationship between bacterial exposure and acid–base balance have been investigated. Indeed, studies in the medullary thick ascending limb support an ERK-mediated inhibitory effect of interstitial LPS on the sodium-proton exchanger 3, resulting in inhibition of bicarbonate absorption.40,41 In the CD, early in vitro studies supported that urinary acidification limits bacterial growth,42 suggesting that this may be a physiological defense mechanism for bacterial clearance. Patients with UTI who drank cranberry juice presented an increased urinary secretion of the organic hippuric acid, which inhibited bacterial growth.43 However, on the interstitial side, acidosis seems to have a detrimental effect on UPEC clearance. Despite an urine acidification, mice in metabolic acidosis and those exposed to UPEC display an increased accumulation of neutrophils and tissue resident macrophages; higher abundance of cytokines TNFα, IL-1β, and IL-6 as well as chemokines CXCL1, 2, and 5; and intensified pyelonephritis.44 These studies support that acidic urine plays a minimal role in bacterial clearance while acidosis results in a poorer outcome.
Further reports supported an essential role of ICs themselves in bacterial clearance. Knocking out CAII in mice (Car2−/− mice) results in metabolic acidosis, alkaline urine, and, importantly, a significant loss of ICs.45 Interestingly, after UTI, Car2−/− mice also have a heavier urinary bacterial load and are less able to clear UPEC bacteria compared with control littermates.46 In fact, the loss of CAII-positive ICs in Car2−/− mice is more detrimental to bacterial clearance than just the pharmacological inhibition of CAII activity.47 Similarly, knocking out murine ICs by deleting Tcfcp2l1 transcription factor showed that, while the other nephron segments and PC develop normally, the renal v-ATPase immunostaining is lost, supporting a loss of IC.42 On UTI, the knockout mice are unable to acidify their urine and to clear the bacterial invasion in comparison with their WT littermates.
The physiological mechanisms behind the role of acidic pH on IC-based bacterial clearance are starting to be deciphered from further in vitro studies. Research in the cancer field has demonstrated that cytosolic acidification upregulates 2-hydroxyglutarate, which, in turn, stabilizes the transcriptional regulator hypoxia-inducible factor 1-α subunit (HIF-1a).48 Growing immortalized CD cells M1 (that contain both PC and IC) in an acidic growth medium blunted UPEC growth because of HIF-1a upregulation, itself associated with a release of nitric oxide, and a TLR4-dependent upregulation of several antimicrobial peptides (AMPs), such as defensin b2 and cathelicidin (more details in the following sections).49 Mimicking acidosis by pharmacological stabilization of short-lived HIF-1a with AKB-4924 results in reduced UPEC-mediated epithelial cell death, lower UTI, an improved urinary bacterial clearance, and reduced inflammation in a UTI mouse model.50
Overall, these results support that metabolic acidosis or loss of ICs is detrimental to an efficient immune response and clearance of UPEC.
ICs Express Antimicrobial Proteins
Antimicrobial molecules are essential components of the urinary tract's response to UTIs. Like many epithelial cells, ICs express these molecules as eluded above. Flow cytometry cell sorting enrichment of isolated murine ICs has revealed that these cells endogenously express several types of antimicrobial molecules, including the bacteriostatic protein lipocalin 2 (LCN-2, also referred to as neutrophil gelatinase-associated lipocalin or NGAL); AMPs, such as adrenomedullin (ADM); and ribonucleases (RNAses).11 Some defensins, such as Defensin b1, Defensin b26, and antimicrobial peptide RNAse4, are enriched in both PCs and ICs compared with cells from other nephron segments. Cathelicidin, Calgranulin A (S100a8), and NGAL are specifically enriched in ICs versus other cell types.51 Importantly, urine composition, pH, and osmolality can affect AMP activity. For instance, high extracellular glucose treatment lowers the abundance of psoriasin (S100A7), RNAse7, and Defensin b4 in uroepithelial cells and urine exfoliated cells from patients with diabetes.52–54 In addition, peak performance of AMPs has been shown at urinary pH 5–6.5,55 although pH has variable effects. While alkaline extracellular pH reduces RNAse7 activity, β defensin 1 remains unaffected.56,57 Finally, UTIs typically cause a reduction in urine osmolality that may affect AMP activities.58,59
Together, these findings support that the urinary tract and in particular both ICs and PCs are equipped with antimicrobial proteins, with ICs encoding a specific pool of molecules to fight UTIs. These secreted AMPs are further described in the next paragraphs, with a focus on IC-produced AMPs.
CD Cells Secrete Antibacterial Peptides to Clear UPEC
IC-Produced LCN2/NGAL Chelates Luminal Iron and Impairs Bacterial Growth
UPEC relies on fimbrial adhesions, toxins, and nutrient-acquisition strategies for survival in the harsh conditions of the urinary tract.60 On TLR4 activation, UPEC-exposed A-ICs secrete the bacteriostatic protein LCN2 (Figure 2). LCN2 messenger levels increase by 27-fold in the mouse bladder and by six-fold in the kidney of mice and human urine on infection with Gram-negative UPEC.42,61
LCN2 exists in two forms: apo-LCN2 and holo-LCN2.62 Iron-free (Apo) LCN2 depletes cytosolic levels of iron and results in apoptosis induction while iron-bound (Holo) LCN2 increases cytosolic iron concentration and protects from apoptosis.62 By inoculating a UTI-susceptible mouse model with GFP-transfected UPEC, Paragas and colleagues showed that A-ICs specifically express LCN2 and that UPEC bacteria associate with A-ICs.42 After UPEC inoculation, mice lacking ICs display a blunted urinary acidification and urinary LCN2 expression compared with control mice.
LCN2 bactericidal mode of action is well-understood. UPEC bacteria capture iron from the host transferrin by releasing an enterochelin (Ent) siderophore with a high affinity for iron. A-ICs secrete LCN2 in the iron-free apo form,63 which also has a high affinity and is specific to the Ent:iron complex. The now holo-LCN2 dispels iron from bacterial use and reroutes it either for lysosomal degradation or for urinary excretion. In acidified lysosomes, the iron in the LCN2:Ent:iron complex is released and reduced while LCN2 is proteolytically cleaved.61,64,65 LCN2 was found to be necessary and sufficient to suppress UPEC activity.64,66
Cationic Host Defense Peptides Contribute to the Host Defense against UTI
Apart from starving bacteria from iron, the host organism secretes a variety of small cationic host defense peptides (CHDPs) that have microbicidal properties and/or enhance the host's immune response.67 Two classes of CHDPs, the cathelicidins and defensins, work in synergy to maximize their microbicidal activity (Figure 2). CHDP mode of action consists in interacting with the negatively charged bacterial membrane by an electrostatic interaction, permeation of the membrane, release of bacterial content, and bacterial death. CHDP can also alter bacterial cell wall biosynthesis and cell processes, such as replication, transcription, and translation. Furthermore, CHDP can have an immune-regulatory role on the host defense. Once uptaken into the host cells, CHDP can interact with a variety of host effectors, alter intracellular signaling pathways, and result in activation of specific transcription factors that modulate the inflammatory and immune system, as summarized in 67.
ADM Contributes to UTI Clearance
ADM is a multifunctional 52 residue, cationic peptide produced in many tissues and cells, such as macrophages and renal parenchymal cells.68 With ADM receptors' widespread expression, the peptide controls central body functions, including vascular tone regulation, fluid and electrolyte homeostasis, and inflammation.51
Children with acute pyelonephritis display lower plasma ADM but higher urine ADM concentrations compared with healthy participants.69 In rat IMCD at the basal state, ADM is expressed by both ICs and PCs and is enriched 19.44-fold compared with non-IMCD cells.70 Finally, in mice exposed to UPEC, ADM expression in both ICs and PCs is also upregulated.12
ADM disrupts the E. coli outer and cytoplasmic membrane through its conserved α-helical carboxyl terminus that carries the antimicrobial activity.71 The mechanism of action of ADM has been deciphered in other cell types. After LPS stimulation to rat macrophages, ADM gene expression and secretion is followed by the production of macrophage migration inhibitory factor, IL-1β, and IL-6.72 Opposingly, ADM also suppresses the secretion of the inflammatory mediator, TNFα.
In Humans, Ribonucleases (RNase) 7 and 4 Also Contribute to Bacterial Clearance
The RNase A superfamily encodes canonical peptides with antibacterial activity. Originally identified in the human epidermis,73 the 14.5-kD ribonuclease 7 (RNase 7) is found in the mature human kidney, ureter, and bladder, where it is the highest, and in the ICs and is secreted in urine in response to acute pyelonephritis.74 In keratinocytes, RNase 7 gene expression is triggered by TNFα, interferon gamma, and IL-1β73 and is regulated by insulin through a PI3K/AKT signaling pathway.75
Its broad-spectrum antimicrobial activity contributes to the sterility of the urinary tract against Gram-negative bacteria, Gram-positive bacteria, and yeast.74 Independent of its RNase catalytic activity, RNase 7 exerts its broad-spectrum antimicrobial role by permeating and disrupting the bacterial cell membranes, causing membrane splitting, bleb formation, and significant microbial cell death.76
Further supporting the role of RNase 7 in UPEC clearance, children and adolescent girls with a recurrent UTI history display a low urinary RNase 7 concentration.53 Female mice overexpressing RNase-7 and exposed to UTI showed reduced intracellular bacterial communities and UPEC titers in urine and the bladder. Conversely, knocking down RNase 7 activity results in a significant increase in bacterial growth.74
Expressed in the bladder, proximal tubule, ICs, and PCs, RNase 4 also seems to protect the urothelial barrier.77 Women with a history of UTIs produced urine with a lower RNase 4 concentration than healthy women, and neutralizing RNase 4 in urine promotes UPEC growth. RNase 4 antibacterial activity occurs through agglutination, permeabilization, and leakage of bacterial membranes.
In Humans, Secretion of Defensins Correlates with a Lower Recurrence of UTI
In the CHDP family, defensins are classified as a, b, and q defensins.67 Antimicrobial α-defensin 5 is a 32 amino acid peptide expressed in the human distal nephron, CD, and low urinary tract.78 On UTI, α-defensin 5 gene expression and protein abundance are upregulated and the peptide becomes abundantly secreted into the urine. Another class of antimicrobial α-defensin called human neutrophil peptides 1-3 (HNP1-3, encoded by DEFA1A3 gene) is also protective against UTIs in humans. A large study on children with vesicoureteral reflux showed that higher the number of DEFA1A3 gene copies, lower the risk of recurrent UTIs in patients receiving antibiotic prophylaxis, but not in untreated patients.79 More recently, mice expressing human DEFA1A3 defensin on UTI showed that DEFA1A3 is expressed by CD cells and is upregulated on UTI, and the mice display a better protection against kidney and bladder infection compared with control animals.80 Finally, this study demonstrated a synergistic effect of HNP1-3 with cathelicidin against UPEC and antibiotic-resistant strains of E. coli. Similarly, human b-defensin 1 is upregulated three times in pyelonephritis,81 and mice lacking this protein have higher UPEC titers in their urine.82 However, in uncomplicated UTIs, its concentration was similar between noninfected patients and patients with UTI.83 Whether this defensin is expressed in IC is unknown.
Cathelicidin is an α helical, small (23–37 amino acids) amphipathic peptide that is activated by serine protease cleavage.67 LL-37 is the only human cathelicidin and is upregulated in the urine of patients with UTI compared with controls.83 Cathelicidin is constitutively expressed in the epithelium of the urinary tract and of the kidney51 and prevents E. coli bacterial growth in UTIs, even in acidic urine conditions.49 In the initial stages of infection, bacterial contact leads to a rapid increase in synthesis and secretion of cathelicidin by epithelial cells to prevent bacterial attachment.
AMP Synergistically Contribute to the Host Defense
Bacterial clearance involves several physiological weapons that can synergistically contribute to bacterial clearance. Indeed, innate immune processes originating from different segments of the nephron contribute to the fight. For example, uromodulin (also called Tamm–Horsfall protein) is secreted into the TAL and contributes to the innate defenses by binding to ascending UPEC, acting as decoys for mannose-rich apical membrane proteins in epithelial cells.84 Hepcidin also contributes to UTI clearance by regulating iron availability, inflammation, and urinary acidification by a stimulatory action on Atp4a and Atp12a subunits.85 Finally, cathelicidin was found to have a synergistic effect with HNP1-3 while it has an additive effect with RNase 7.80 More synergistic effects may exist but have not been characterized yet.
Clinical Importance and Risk Factors
Chronic Kidney Diseases and Vesicourethral Reflux
As eluded above, the acid–base status of individuals may influence their UTI response. Children have a higher risk of developing acute pyelonephritis, in part, because of their high frequency of vesicourethral reflux (VUR).86–89 Metabolic acidosis in a mouse model of VUR exacerbates UTI severity and risk of kidney injury.44 Furthermore, type IV renal tubular acidosis, characterized by hyponatremia, hyperkalemia, and nonanion gap metabolic acidosis, can be a complication of UTIs in infants.90 In addition, the presence of renal tumors91 can influence the patient's response to UTIs, although these do not specifically affect ICs. Mutations in the PKD1 and PKD2 genes can cause polycystic kidney disease, a condition that can cause a reduction in the number of ICs and as a result may affect the patients' response to UTIs.92 Patients with autosomal dominant polycystic kidney disease are more prone to UTIs than healthy individuals.93–95
Diabetes and UTI
Type 2 diabetes is one well-understood example of a chronic disease that can influence the IC's innate immune response to UTIs. Patients with type 2 diabetes are at a higher risk of UTIs, pyelonephritis, and acute kidney injury,96,97 independently of glucosemia or glucosuria98; however, circulating levels of insulin regulate AMP production.75 Expressed in ICs, the insulin receptor forms tetramers consisting of two extracellular α subunits and two transmembrane β subunits. On insulin binding, the β subunits change conformation, which activates the receptor's kinase activity and triggers downstream signaling pathways involving PI3K and AKT kinase, which are essential for AMP synthesis.99 Type 2 diabetic and prediabetic mice both clear UTIs less efficiently than controls, have a decreased abundance of β subunits of insulin receptor, and have less AKT phosphorylation.98 Mice knocked out for the IC-insulin receptor appropriately acidify their urine but display reduced phosphorylated AKT kinase and fail to activate the secretion of AMP, such as LCN2 and RNase 4.98 Therefore, defective insulin receptor signaling in IC makes patients with diabetes more susceptible to UTIs.
Potential New Therapeutic Approaches
There is now evidence that ascending UPEC that have reached the CD is facing another layer of immune response predominantly triggered by ICs (Figure 2). Between sensing LPS, engaging in secretion of multiple AMPs, and triggering inflammation, these cells are clearly polyvalent. There is much work to do to fully understand the IC heterogeneity and specific function because no immortalized cell model fully recapitulates the physiology of CD cells. Mouse models are formidable tools to unveil the physiology of UTI defenses; however, they only partly recapitulate the human physiology. For example, RNase 7 is not present in the mouse genome. In addition, certain mouse strains are more susceptible to UTIs than others because of defective LPS signaling.100 Finally, a large amount of data have been generated in nonrefluxing mice that are not prone to pyelonephritis, although VUR is a common condition that could contribute to UTIs in children. Thus, many questions remain, including: what is the contribution of the abovementioned antimicrobial proteins in UTI defense in humans? Why are some individuals prone to recurrent UTIs? Do the mouse models really mirror spontaneous UTIs occurring in humans?
Despite these limitations and remaining questions, supporting the innate immune response by enhancing the secretion of various AMPs seems to be a promising treatment option. Given their nonspecific effect on pathogens, they may be less likely to cause bacterial resistance. Further understanding the physiology associated with innate antibacterial processes will be essential to treat UTIs in the future.
Acknowledgments
F.C. is supported by a Discovery Grant to E.C. from the Natural Sciences and Engineering Research Council (RGPIN-2017-06432). P.M. received a University of Alberta Faculty of Medicine and Dentistry Graduate Student Recruitment Studentship (GSRS), a Canada Graduate Scholarship Master's (CIHR), and a Walter H Johns Graduate Fellowship and is also supported by a Canadian Institutes of Health Research Project grant to E.C. (PS #168871). M.B. is supported by Alberta Innovates.
Footnotes
F.C. and P.M. contributed equally to this work.
Disclosures
All authors have nothing to disclose.
Funding
This work was supported by the Canadian Institutes of Health Research from PS 168871 and the Natural Sciences and Engineering Research Council of Canada from RGPIN-2017-06432 (E. Cordat).
Author Contributions
Conceptualization: Manav Batta, Forough Chelangarimiyandoab, Emmanuelle Cordat, Priyanka Mungara.
Data curation: Manav Batta, Forough Chelangarimiyandoab, Priyanka Mungara.
Resources: Emmanuelle Cordat.
Supervision: Emmanuelle Cordat.
Writing – original draft: Manav Batta, Forough Chelangarimiyandoab, Emmanuelle Cordat, Priyanka Mungara.
Writing – review & editing: Manav Batta, Forough Chelangarimiyandoab, Emmanuelle Cordat, Priyanka Mungara.
References
- 1.Wagenlehner FME Bjerklund Johansen TE Cai T, et al. Epidemiology, definition and treatment of complicated urinary tract infections. Nat Rev Urol. 2020;17(10):586–600. doi: 10.1038/s41585-020-0362-4 [DOI] [PubMed] [Google Scholar]
- 2.Geerlings SE. Clinical presentations and epidemiology of urinary tract infections. Microbiol Spectr. 2016;4(5). doi: 10.1128/microbiolspec.uti-0002-2012 [DOI] [PubMed] [Google Scholar]
- 3.Ronald A. The etiology of urinary tract infection: traditional and emerging pathogens. Dis Mon. 2003;49(2):71–82. doi: 10.1067/mda.2003.8 [DOI] [PubMed] [Google Scholar]
- 4.Desforges JF, Stamm WE, Hooton TM. Management of urinary tract infections in adults. N Engl J Med. 1993;329(18):1328–1334. doi: 10.1056/nejm199310283291808 [DOI] [PubMed] [Google Scholar]
- 5.Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13(5):269–284. doi: 10.1038/nrmicro3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lacerda Mariano L, Ingersoll MA. The immune response to infection in the bladder. Nat Rev Urol. 2020;17(8):439–458. doi: 10.1038/s41585-020-0350-8 [DOI] [PubMed] [Google Scholar]
- 7.Abraham SN, Miao Y. The nature of immune responses to urinary tract infections. Nat Rev Immunol. 2015;15(10):655–663. doi: 10.1038/nri3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner CA, Unwin R, Lopez-Garcia SC, Kleta R, Bockenhauer D, Walsh S. The pathophysiology of distal renal tubular acidosis. Nat Rev Nephrol. 2023;19(6):384–400. doi: 10.1038/s41581-023-00699-9 [DOI] [PubMed] [Google Scholar]
- 9.Prieto-Carrasquero MC, Botros FT, Kobori H, Navar LG. Collecting duct Renin: a major player in angiotensin II-dependent hypertension. J Am Soc Hypertens. 2009;3(2):96–104. doi: 10.1016/j.jash.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy A, Al-Bataineh MM, Pastor-Soler NM. Collecting duct intercalated cell function and regulation. Clin J Am Soc Nephrol. 2015;10(2):305–324. doi: 10.2215/CJN.08880914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saxena V Hains DS Ketz J, . Cell-specific qRT-PCR of renal epithelial cells reveals a novel innate immune signature in murine collecting duct. Am J Physiol Renal Physiol. 2018;315(4):F812–F823. doi: 10.1152/ajprenal.00512.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saxena V Gao H Arregui S, . Kidney intercalated cells are phagocytic and acidify internalized uropathogenic Escherichia coli. Nat Commun. 2021;12(1):2405. doi: 10.1038/s41467-021-22672-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alzamora R Thali RF Gong F, . PKA regulates vacuolar H+-ATPase localization and activity via direct phosphorylation of the a subunit in kidney cells. J Biol Chem. 2010;285(32):24676–24685. doi: 10.1074/jbc.m110.106278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, Barone S, Li H, Holiday S, Zahedi K, Soleimani M. Slc26a11, a chloride transporter, localizes with the vacuolar H(+)-ATPase of A-intercalated cells of the kidney. Kidney Int. 2011;80(9):926–937. doi: 10.1038/ki.2011.196 [DOI] [PubMed] [Google Scholar]
- 15.Bastani B, Purcell H, Hemken P, Trigg D, Gluck S. Expression and distribution of renal vacuolar proton-translocating adenosine triphosphatase in response to chronic acid and alkali loads in the rat. J Clin Invest. 1991;88(1):126–136. doi: 10.1172/jci115268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chambrey R Kurth I Peti-Peterdi J, . Renal intercalated cells are rather energized by a proton than a sodium pump. Proc Natl Acad Sci U S A. 2013;110(19):7928–7933. doi: 10.1073/pnas.1221496110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J, Kim YH, Cha JH, Tisher CC, Madsen KM. Intercalated cell subtypes in connecting tubule and cortical collecting duct of rat and mouse. J Am Soc Nephrol. 1999;10(1):1–12. doi: 10.1681/ASN.v1011 [DOI] [PubMed] [Google Scholar]
- 18.Goldspink A Schmitz J Babyak O, . Kidney medullary sodium chloride concentrations induce neutrophil and monocyte extracellular DNA traps that defend against pyelonephritis in vivo. Kidney Int. 2023;104(2):279–292. doi: 10.1016/j.kint.2023.03.034 [DOI] [PubMed] [Google Scholar]
- 19.Berry MR Mathews RJ Ferdinand JR, . Renal sodium gradient orchestrates a dynamic antibacterial defense zone. Cell. 2017;170(5):860–874.e19. doi: 10.1016/j.cell.2017.07.022 [DOI] [PubMed] [Google Scholar]
- 20.Ayasse N, de Bruijn PIA, Berg P, Sørensen MV, Leipziger J. Hydrochlorothiazide and acute urinary acidification: the “voltage hypothesis” of ENaC-dependent H + secretion refuted. Acta Physiol (Oxf). 2018;223(1):e13013. doi: 10.1111/apha.13013 [DOI] [PubMed] [Google Scholar]
- 21.Leviel F Hubner CA Houillier P, . The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest. 2010;120(5):1627–1635. doi: 10.1172/jci40145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eladari D, Chambrey R, Peti-Peterdi J. A new look at electrolyte transport in the distal tubule. Annu Rev Physiol. 2012;74(1):325–349. doi: 10.1146/annurev-physiol-020911-153225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vitzthum H Koch M Eckermann L, . The AE4 transporter mediates kidney acid-base sensing. Nat Commun. 2023;14(1):3051. doi: 10.1038/s41467-023-38562-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Günzel D, Yu ASL. Claudins and the modulation of tight junction permeability. Physiol Rev. 2013;93(2):525–569. doi: 10.1152/physrev.00019.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou J, Rajagopal M, Yu AS. Claudins and the kidney. Annu Rev Physiol. 2013;75(1):479–501. doi: 10.1146/annurev-physiol-030212-183705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azroyan A Cortez-Retamozo V Bouley R, et al. Renal intercalated cells sense and mediate inflammation via the P2Y14 receptor. PLoS One. 2015;10(3):e0121419. doi: 10.1371/journal.pone.0121419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Battistone MA Mendelsohn AC Spallanzani RG, et al. Proinflammatory P2Y14 receptor inhibition protects against ischemic acute kidney injury in mice. J Clin Invest. 2020;130(7):3734–3749. doi: 10.1172/jci134791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breton S, Battistone MA. Unexpected participation of intercalated cells in renal inflammation and acute kidney injury. Nephron. 2022;146(3):268–273. doi: 10.1159/000519265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah C, Baral R, Bartaula B, Shrestha LB. Virulence factors of uropathogenic Escherichia coli (UPEC) and correlation with antimicrobial resistance. BMC Microbiol. 2019;19(1):204. doi: 10.1186/s12866-019-1587-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghazvini H, Taheri K, Edalati E, Sedighi M, Mirkalantari S. Virulence factors and antimicrobial resistance in uropathogenic Escherichia coli strains isolated from cystitis and pyelonephritis. Turkish J Med Sci. 2019;49(1):361–367. doi: 10.3906/sag-1805-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLellan LK McAllaster MR Kim AS, . A host receptor enables type 1 pilus-mediated pathogenesis of Escherichia coli pyelonephritis. PLoS Pathog. 2021;17(1):e1009314. doi: 10.1371/journal.ppat.1009314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lund B, Lindberg F, Marklund BI, Normark S. The PapG protein is the alpha-D-galactopyranosyl-(1----4)-beta-D-galactopyranose-binding adhesin of uropathogenic Escherichia coli. Proc Natl Acad Sci U S A. 1987;84(16):5898–5902. doi: 10.1073/pnas.84.16.5898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Good DW, George T, Watts BA. Toll-like receptor 2 is required for LPS-induced Toll-like receptor 4 signaling and inhibition of ion transport in renal thick ascending limb. J Biol Chem. 2012;287(24):20208–20220. doi: 10.1074/jbc.m111.336255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersen-Nissen E Hawn TR Smith KD, . Cutting edge: Tlr5-/- mice are more susceptible to Escherichia coli urinary tract infection. J Immunol. 2007;178(8):4717–4720. doi: 10.4049/jimmunol.178.8.4717 [DOI] [PubMed] [Google Scholar]
- 35.Zhang D Zhang G Hayden MS, et al. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303(5663):1522–1526. doi: 10.1126/science.1094351 [DOI] [PubMed] [Google Scholar]
- 36.Chassin C Vimont S Cluzeaud F, et al. TLR4 facilitates translocation of bacteria across renal collecting duct cells. J Am Soc Nephrol. 2008;19(12):2364–2374. doi: 10.1681/ASN.2007121273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chassin C Goujon J-M Darche S, . Renal collecting duct epithelial cells react to pyelonephritis-associated Escherichia coli by activating distinct TLR4-dependent and -independent inflammatory pathways. J Immunol. 2006;177(7):4773–4784. doi: 10.4049/jimmunol.177.7.4773 [DOI] [PubMed] [Google Scholar]
- 38.Saxena V, Arregui S, Kamocka MM, Hains DS, Schwaderer A. MAP3K7 is an innate immune regulatory gene with increased expression in human and murine kidney intercalated cells following uropathogenic Escherichia coli exposure. J Cell Biochem. 2022;123(11):1817–1826. doi: 10.1002/jcb.30318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ajibade AA, Wang HY, Wang R-F. Cell type-specific function of TAK1 in innate immune signaling. Trends Immunol. 2013;34(7):307–316. doi: 10.1016/j.it.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 40.Good DW, George T, Watts BA. Lipopolysaccharide directly alters renal tubule transport through distinct TLR4-dependent pathways in basolateral and apical membranes. Am J Physiol Renal Physiol. 2009;297(4):866–874. doi: 10.1152/ajprenal.00335.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watts BA, George T, Sherwood ER, Good DW. Basolateral LPS inhibits NHE3 and HCO 3- absorption through TLR4/MyD88- dependent ERK activation in medullary thick ascending limb. Am J Physiol Cell Physiol. 2011;301(6):C1296–C1306. doi: 10.1152/ajpcell.00237.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paragas N Kulkarni R Werth M, . α-Intercalated cells defend the urinary system from bacterial infection. J Clin Invest. 2014;124(7):2963–2976. doi: 10.1172/jci71630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bodel P, Cotral R, Kass E. Cranberry juice and the antibacterial action of hippuric acid. J Lab Clin Med. 1959;54:881–888. PMID: 13801916. [PubMed] [Google Scholar]
- 44.Purkerson JM, Corley JL, Schwartz GJ. Metabolic acidosis exacerbates pyelonephritis in mice prone to vesicoureteral reflux. Physiol Rep. 2020;8(19):e14525. doi: 10.14814/phy2.14525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Breton S, Alper SL, Gluck SL, Sly WS, Barker JE, Brown D. Depletion of intercalated cells from collecting ducts of carbonic anhydrase II-deficient (CAR2 null) mice. Am J Physiol. 1995;269(6):F761–F774. doi: 10.1152/ajprenal.1995.269.6.f761 [DOI] [PubMed] [Google Scholar]
- 46.Hains DS Chen X Saxena V, et al. Carbonic anhydrase 2 deficiency leads to increased pyelonephritis susceptibility. Am J Physiol Renal Physiol. 2014;307(7):F869–F880. doi: 10.1152/ajprenal.00344.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ketz J Saxena V Arregui S, et al. Developmental loss, but not pharmacological suppression, of renal carbonic anhydrase 2 results in pyelonephritis susceptibility. Am J Physiol Renal Physiol. 2020;318(6):F1441–F1453. doi: 10.1152/ajprenal.00583.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nadtochiy SM, Schafer X, Fu D, Nehrke K, Munger J, Brookes PS. Acidic pH Is a metabolic switch for 2-hydroxyglutarate generation and signaling. J Biol Chem. 2016;291(38):20188–20197. doi: 10.1074/jbc.m116.738799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng H, Purkerson JM, Freeman RS, Schwaderer AL, Schwartz GJ. Acidosis induces antimicrobial peptide expression and resistance to uropathogenic E. coli infection in kidney collecting duct cells via HIF-1α. Am J Physiol Renal Physiol. 2020;318(2):F468–F474. doi: 10.1152/ajprenal.00228.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin AE Beasley FC Olson J, . Role of hypoxia inducible factor-1α (HIF-1α) in innate defense against uropathogenic Escherichia coli infection. PLoS Pathog. 2015;11(4):e1004818. doi: 10.1371/journal.ppat.1004818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chromek M Slamová Z Bergman P, . The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med. 2006;12(6):636–641. doi: 10.1038/nm1407 [DOI] [PubMed] [Google Scholar]
- 52.Brauner H Lüthje P Grünler J, . Markers of innate immune activity in patients with type 1 and type 2 diabetes mellitus and the effect of the anti-oxidant coenzyme Q10 on inflammatory activity. Clin Exp Immunol. 2014;177(2):478–482. doi: 10.1111/cei.12316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eichler T Bender K Murtha MJ, et al. Ribonuclease 7 shields the kidney and bladder from invasive uropathogenic escherichia coli infection. J Am Soc Nephrol. 2019;30(8):1385–1397. doi: 10.1681/ASN.2018090929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mohanty S Kamolvit W Scheffschick A, et al. Diabetes downregulates the antimicrobial peptide psoriasin and increases E. coli burden in the urinary bladder. Nat Commun. 2022;13(1):4983. doi: 10.1038/s41467-022-32636-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaye D. Antibacterial activity of human urine. J Clin Invest. 1968;47(10):2374–2390. doi: 10.1172/jci105921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valore EV, Park CH, Quayle AJ, Wiles KR, McCray PB, Ganz T. Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Invest. 1998;101(8):1633–1642. doi: 10.1172/jci1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang H, Schwaderer AL, Kline J, Spencer JD, Kline D, Hains DS. Contribution of structural domains to the activity of ribonuclease 7 against uropathogenic bacteria. Antimicrob Agents Chemother. 2013;57(2):766–774. doi: 10.1128/aac.01378-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaye D, Rocha H. Urinary concentrating ability in early experimental pyelonephritis. J Clin Invest. 1970;49(7):1427–1437. doi: 10.1172/jci106360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sterner G. Renal concentration capacity in adult patients with urinary tract infections. Scand J Urol Nephrol. 1991;25(3):219–222. doi: 10.3109/00365599109107950 [DOI] [PubMed] [Google Scholar]
- 60.Zagaglia C, Ammendolia MG, Maurizi L, Nicoletti M, Longhi C. Urinary tract infections caused by uropathogenic Escherichia coli strains-new strategies for an old pathogen. Microorganisms. 2022;10(7):1425. doi: 10.3390/microorganisms10071425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steigedal M Marstad A Haug M, . Lipocalin 2 imparts selective pressure on bacterial growth in the bladder and is elevated in women with urinary tract infection. J Immunol. 2014;193(12):6081–6089. doi: 10.4049/jimmunol.1401528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Devireddy LR, Gazin C, Zhu X, Green MR. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005;123(7):1293–1305. doi: 10.1016/j.cell.2005.10.027 [DOI] [PubMed] [Google Scholar]
- 63.Betten R Scharner B Probst S, . Tonicity inversely modulates lipocalin-2 (Lcn2/24p3/NGAL) receptor (SLC22A17) and Lcn2 expression via Wnt/β-catenin signaling in renal inner medullary collecting duct cells: implications for cell fate and bacterial infection. Cell Commun Signal. 2018;16(1):74. doi: 10.1186/s12964-018-0285-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10(5):1033–1043. doi: 10.1016/s1097-2765(02)00708-6 [DOI] [PubMed] [Google Scholar]
- 65.Bao G Clifton M Hoette TM, . Iron traffics in circulation bound to a siderocalin (Ngal)-catechol complex. Nat Chem Biol. 2010;6(8):602–609. doi: 10.1038/nchembio.402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Devireddy LR, Hart DO, Goetz DH, Green MR. A mammalian siderophore synthesized by an enzyme with a bacterial homolog involved in enterobactin production. Cell. 2010;141(6):1006–1017. doi: 10.1016/j.cell.2010.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mookherjee N, Anderson MA, Haagsman HP, Davidson DJ. Antimicrobial host defence peptides: functions and clinical potential. Nat Rev Drug Discov. 2020;19(5):311–332. doi: 10.1038/s41573-019-0058-8 [DOI] [PubMed] [Google Scholar]
- 68.Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev. 2000;21(2):138–167. doi: 10.1210/edrv.21.2.0396 [DOI] [PubMed] [Google Scholar]
- 69.Kalman S, Buyan N, Yurekli M, Ozkaya O, Bakkaloglu S, Soylemezoglu O. Plasma and urinary adrenomedullin levels in children with acute pyelonephritis. Nephrology. 2005;10(5):487–490. doi: 10.1111/j.1440-1797.2005.00468.x [DOI] [PubMed] [Google Scholar]
- 70.Uawithya P, Pisitkun T, Ruttenberg BE, Knepper MA. Transcriptional profiling of native inner medullary collecting duct cells from rat kidney. Physiol Genomics. 2008;32(2):229–253. doi: 10.1152/physiolgenomics.00201.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Allaker RP Grosvenor PW McAnerney DC, et al. Mechanisms of adrenomedullin antimicrobial action. Peptides. 2006;27(4):661–666. doi: 10.1016/j.peptides.2005.09.003 [DOI] [PubMed] [Google Scholar]
- 72.Wong LYF, Cheung BMY, Li YY, Tang F. Adrenomedullin is both proinflammatory and antiinflammatory: its effects on gene expression and secretion of cytokines and macrophage migration inhibitory factor in NR8383 macrophage cell line. Endocrinology. 2005;146(3):1321–1327. doi: 10.1210/en.2004-1080 [DOI] [PubMed] [Google Scholar]
- 73.Harder J, Schröder JM. RNase 7, a novel innate immune defense antimicrobial protein of healthy human skin. J Biol Chem. 2002;277(48):46779–46784. doi: 10.1074/jbc.m207587200 [DOI] [PubMed] [Google Scholar]
- 74.Spencer JD Schwaderer AL Dirosario JD, et al. Ribonuclease 7 is a potent antimicrobial peptide within the human urinary tract. Kidney Int. 2011;80(2):174–180. doi: 10.1038/ki.2011.109 [DOI] [PubMed] [Google Scholar]
- 75.Eichler TE Becknell B Easterling RS, et al. Insulin and the phosphatidylinositol 3-kinase signaling pathway regulate Ribonuclease 7 expression in the human urinary tract. Kidney Int. 2016;90(3):568–579. doi: 10.1016/j.kint.2016.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spencer JD Schwaderer AL Wang H, . Ribonuclease 7, an antimicrobial peptide upregulated during infection, contributes to microbial defense of the human urinary tract. Kidney Int. 2013;83(4):615–625. doi: 10.1038/ki.2012.410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bender K Schwartz LL Cohen A, . Expression and function of human ribonuclease 4 in the kidney and urinary tract. Am J Physiol Renal Physiol. 2021;320(5):F972–F983. doi: 10.1152/ajprenal.00592.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spencer JD Hains DS Porter E, . Human alpha Defensin 5 expression in the human kidney and urinary tract. PLoS One. 2012;7(2):e31712. doi: 10.1371/journal.pone.0031712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schwaderer AL Wang H Kim SH, . Polymorphisms in α-Defensin–encoding DEFA1A3 associate with urinary tract infection risk in children with vesicoureteral reflux. J Am Soc Nephrol. 2016;27(10):3175–3186. doi: 10.1681/ASN.2015060700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Canas JJ Liang D Saxena V, . Human neutrophil peptides 1-3 protect the murine urinary tract from uropathogenic Escherichia coli challenge. Proc Natl Acad Sci U S A. 2022;119(40):e2206515119. doi: 10.1073/pnas.2206515119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hiratsuka T Nakazato M Ihi T, et al. Structural analysis of human beta-defensin-1 and its significance in urinary tract infection. Nephron. 2000;85(1):34–40. doi: 10.1159/000045627 [DOI] [PubMed] [Google Scholar]
- 82.Morrison G, Kilanowski F, Davidson D, Dorin J. Characterization of the mouse beta defensin 1, Defb1, mutant mouse model. Infect Immun. 2002;70(6):3053–3060. doi: 10.1128/iai.70.6.3053-3060.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nielsen KL, Dynesen P, Larsen P, Jakobsen L, Andersen PS, Frimodt-Møller N. Role of urinary cathelicidin LL-37 and human β-defensin 1 in uncomplicated Escherichia coli urinary tract infections. Infect Immun. 2014;82(4):1572–1578. doi: 10.1128/iai.01393-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pak J, Pu Y, Zhang ZT, Hasty DL, Wu XR. Tamm-Horsfall protein binds to type 1 fimbriated Escherichia coli and prevents E. coli from binding to uroplakin Ia and Ib receptors. J Biol Chem. 2001;276(13):9924–9930. doi: 10.1074/jbc.m008610200 [DOI] [PubMed] [Google Scholar]
- 85.Houamel D Ducrot N Lefebvre T, . Hepcidin as a major component of renal antibacterial defenses against uropathogenic Escherichia coli. J Am Soc Nephrol. 2016;27(3):835–846. doi: 10.1681/ASN.2014101035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Keren R Shaikh N Pohl H, et al. Risk factors for recurrent urinary tract infection and renal scarring. Pediatrics. 2015;136(1):e13–e21. doi: 10.1542/peds.2015-0409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Garin EH. Primary vesicoureteral reflux; what have we learnt from the recently published randomized, controlled trials? Pediatr Nephrol. 2019;34(9):1513–1519. doi: 10.1007/s00467-018-4045-9 [DOI] [PubMed] [Google Scholar]
- 88.Maringhini S Cusumano R Corrado C, . Uromodulin and vesico-ureteral reflux: a genetic study. Biomedicines. 2023;11(2):509. doi: 10.3390/biomedicines11020509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lundstedt AC McCarthy S Gustafsson MCU, et al. A genetic basis of susceptibility to acute pyelonephritis. PLoS One. 2007;2(9):e825. doi: 10.1371/journal.pone.0000825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tseng MH Huang JL Huang SM, . Clinical features, genetic background, and outcome in infants with urinary tract infection and type IV renal tubular acidosis. Pediatr Res. 2020;87(7):1251–1255. doi: 10.1038/s41390-019-0727-7 [DOI] [PubMed] [Google Scholar]
- 91.Parker AS, Cerhan JR, Lynch CF, Leibovich BC, Cantor KP. History of urinary tract infection and risk of renal cell carcinoma. Am J Epidemiol. 2004;159(1):42–48. doi: 10.1093/aje/kwh014 [DOI] [PubMed] [Google Scholar]
- 92.Gao C Zhang L Zhang Y, . Insights into cellular and molecular basis for urinary tract infection in autosomal-dominant polycystic kidney disease. Am J Physiol Renal Physiol. 2017;313(5):F1077–F1083. doi: 10.1152/ajprenal.00279.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eroglu E Kocyigit I Cetin M, . Multiple urinary tract infections are associated with genotype and phenotype in adult polycystic kidney disease. Clin Exp Nephrol. 2019;23(10):1188–1195. doi: 10.1007/s10157-019-01752-3 [DOI] [PubMed] [Google Scholar]
- 94.Idrizi A Barbullushi M Koroshi A, . Urinary tract infections in polycystic kidney disease. Med Arh. 2011;65(4):213–215. doi: 10.5455/medarh.2011.65.213-215 [DOI] [PubMed] [Google Scholar]
- 95.Autosomal Dominant Polycystic Kidney Disease (ADPKD): Evaluation and Management Of Complicated Urinary Tract Infections—UpToDate. Accessed May 11, 2023. https://www.uptodate.com/contents/autosomal-dominant-polycystic-kidney-disease-adpkd-evaluation-and-management-of-complicated-urinary-tract-infections [Google Scholar]
- 96.Chiu PF, Huang CH, Liou HH, Wu CL, Wang SC, Chang CC. Long-term renal outcomes of episodic urinary tract infection in diabetic patients. J Diabetes Complications. 2013;27(1):41–43. doi: 10.1016/j.jdiacomp.2012.08.005 [DOI] [PubMed] [Google Scholar]
- 97.Patterson JE, Andriole VT. Bacterial urinary tract infections in diabetes. Infect Dis Clin North Am. 1997;11(3):735–750. doi: 10.1016/s0891-5520(05)70383-4 [DOI] [PubMed] [Google Scholar]
- 98.Murtha MJ Eichler T Bender K, . Insulin receptor signaling regulates renal collecting duct and intercalated cell antibacterial defenses. J Clin Invest. 2018;128(12):5634–5646. doi: 10.1172/jci98595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hale LJ, Coward RM. Insulin signalling to the kidney in health and disease. Clin Sci. 2013;124(6):351–370. doi: 10.1042/cs20120378 [DOI] [PubMed] [Google Scholar]
- 100.Poltorak A He X Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282(5396):2085–2088. doi: 10.1126/science.282.5396.2085 [DOI] [PubMed] [Google Scholar]