Abstract
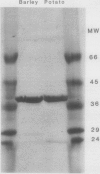
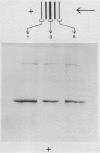
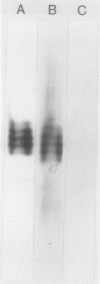
Using Affigel Blue and oxamate-agarose affinity chromatography, lactate dehydrogenase (LDH) was purified 2000-fold from hypoxically induced barley roots. Molecular weights of the native and sodium dodecyl sulfate-denatured LDH protein were 157 and 40 kilodaltons, respectively, indicating a tetramer. Purified barley LDH was very similar in size and kinetic properties to potato LDH. However, their amino acid compositions differed substantially and antibodies raised against barley LDH did not cross-react with potato LDH on immunoblots, implying that the barley and potato LDHs are not closely related proteins. In vivo [35S] methionine labeling and immunoprecipitation experiments indicated that hypoxia increased the rate of LDH protein synthesis, and immunoblot analysis showed that LDH protein levels rose during hypoxia. We conclude that increased enzyme synthesis plays a major part in the induction of LDH enzyme activity by low O2 levels in barley roots.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betsche T. L-Lactate dehydrogenase from leaves of higher plants. Kinetics and regulation of the enzyme from lettuce (Lactuca sativa L). Biochem J. 1981 Jun 1;195(3):615–622. doi: 10.1042/bj1950615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Everse J., Kaplan N. O. Lactate dehydrogenases: structure and function. Adv Enzymol Relat Areas Mol Biol. 1973;37:61–133. doi: 10.1002/9780470122822.ch2. [DOI] [PubMed] [Google Scholar]
- Hanson A. D., Jacobsen J. V. Control of lactate dehydrogenase, lactate glycolysis, and alpha-amylase by o(2) deficit in barley aleurone layers. Plant Physiol. 1984 Jul;75(3):566–572. doi: 10.1104/pp.75.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Hoffman N. E., Bent A. F., Hanson A. D. Induction of lactate dehydrogenase isozymes by oxygen deficit in barley root tissue. Plant Physiol. 1986 Nov;82(3):658–663. doi: 10.1104/pp.82.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jervis L. Characterization of potato (Solanum tuberosum)lactate dehydrogenase isoenzymes by affinity chromatography and hybridization. Biochem J. 1981 Sep 1;197(3):755–758. doi: 10.1042/bj1970755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley P. M., Freeling M. Anaerobic expression of maize fructose-1,6-diphosphate aldolase. J Biol Chem. 1984 Nov 25;259(22):14180–14183. [PubMed] [Google Scholar]
- Kelley P. M., Freeling M. Anaerobic expression of maize glucose phosphate isomerase I. J Biol Chem. 1984 Jan 10;259(1):673–677. [PubMed] [Google Scholar]
- Kiltz H. H., Keil W., Griesbach M., Petry K., Meyer H. The primary structure of porcine lactate dehydrogenase: isoenzymes M4 and H4. Hoppe Seylers Z Physiol Chem. 1977 Jan;358(1):123–127. doi: 10.1515/bchm2.1977.358.1.123. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leland T. J., Hanson A. D. Induction of a Specific N-Methyltransferase Enzyme by Long-Term Heat Stress during Barley Leaf Growth. Plant Physiol. 1985 Oct;79(2):451–457. doi: 10.1104/pp.79.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. S., Fitch W. M., Pan Y. C., Sharief F. S. Evolutionary relationships of vertebrate lactate dehydrogenase isozymes A4 (muscle), B4 (heart), and C4 (testis). J Biol Chem. 1983 Jun 10;258(11):7029–7032. [PubMed] [Google Scholar]
- Markert C. L., Shaklee J. B., Whitt G. S. Evolution of a gene. Multiple genes for LDH isozymes provide a model of the evolution of gene structure, function and regulation. Science. 1975 Jul 11;189(4197):102–114. doi: 10.1126/science.1138367. [DOI] [PubMed] [Google Scholar]
- Poerio E., Davies D. D. A comparison of potato and vertebrate lactate dehydrogenases. Biochem J. 1980 Nov 1;191(2):341–348. doi: 10.1042/bj1910341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. K., Callis J., Wemmer D., Walbot V., Jardetzky O. Mechanisms of cytoplasmic pH regulation in hypoxic maize root tips and its role in survival under hypoxia. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3379–3383. doi: 10.1073/pnas.81.11.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs M. M., Freeling M., Okimoto R. The anaerobic proteins of maize. Cell. 1980 Jul;20(3):761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallevik K., Jensenius J. C. A simple and reliable method for the drying of polyacrylamide slab gels. J Biochem Biophys Methods. 1982 Apr;6(1):17–21. doi: 10.1016/0165-022x(82)90021-5. [DOI] [PubMed] [Google Scholar]