Abstract
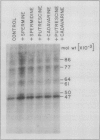
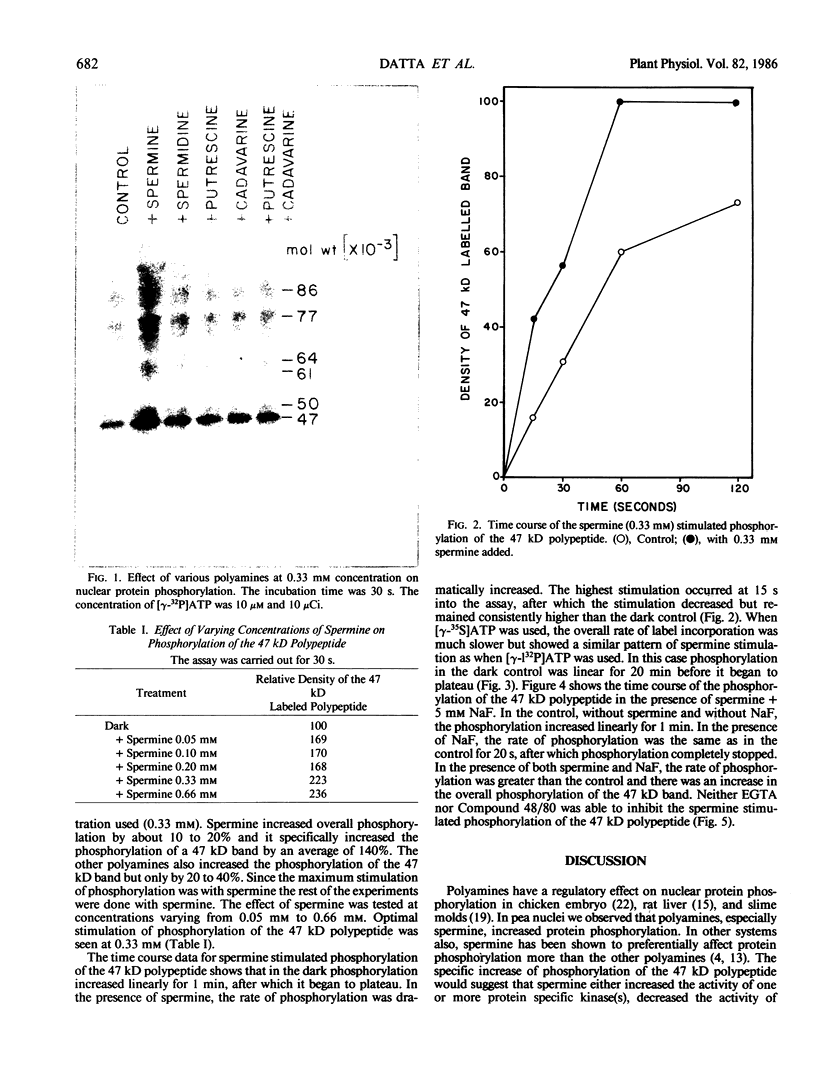
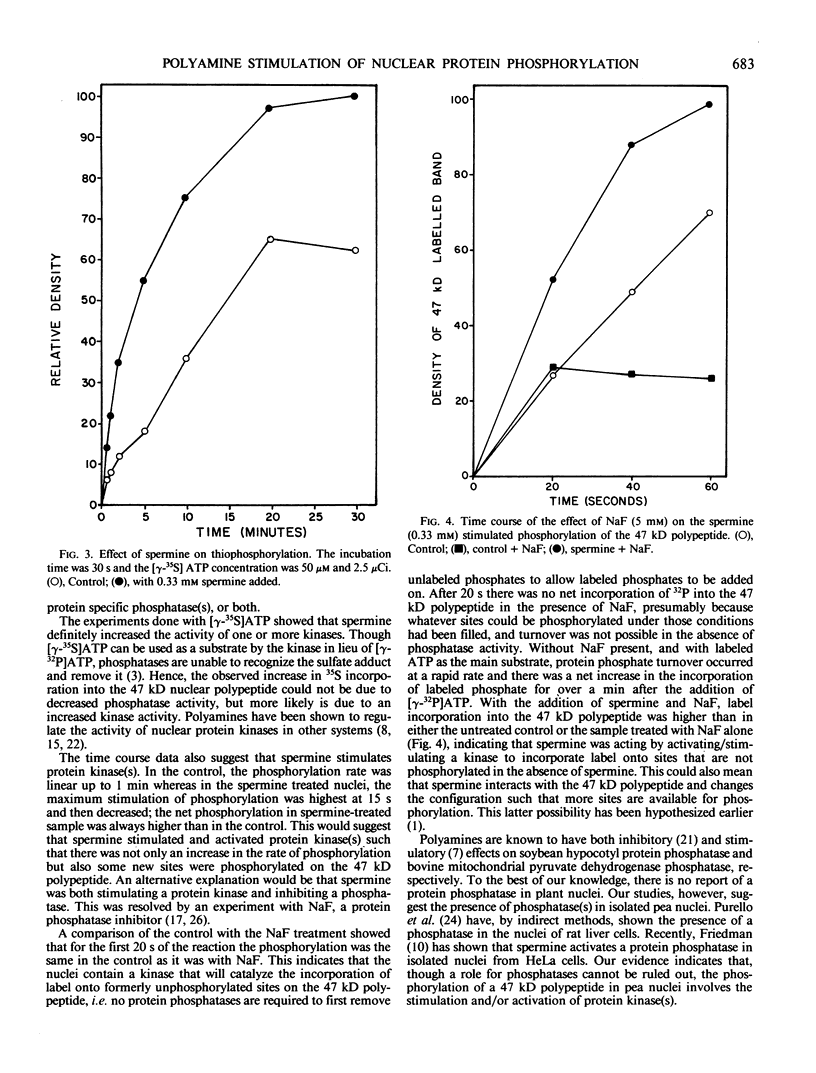
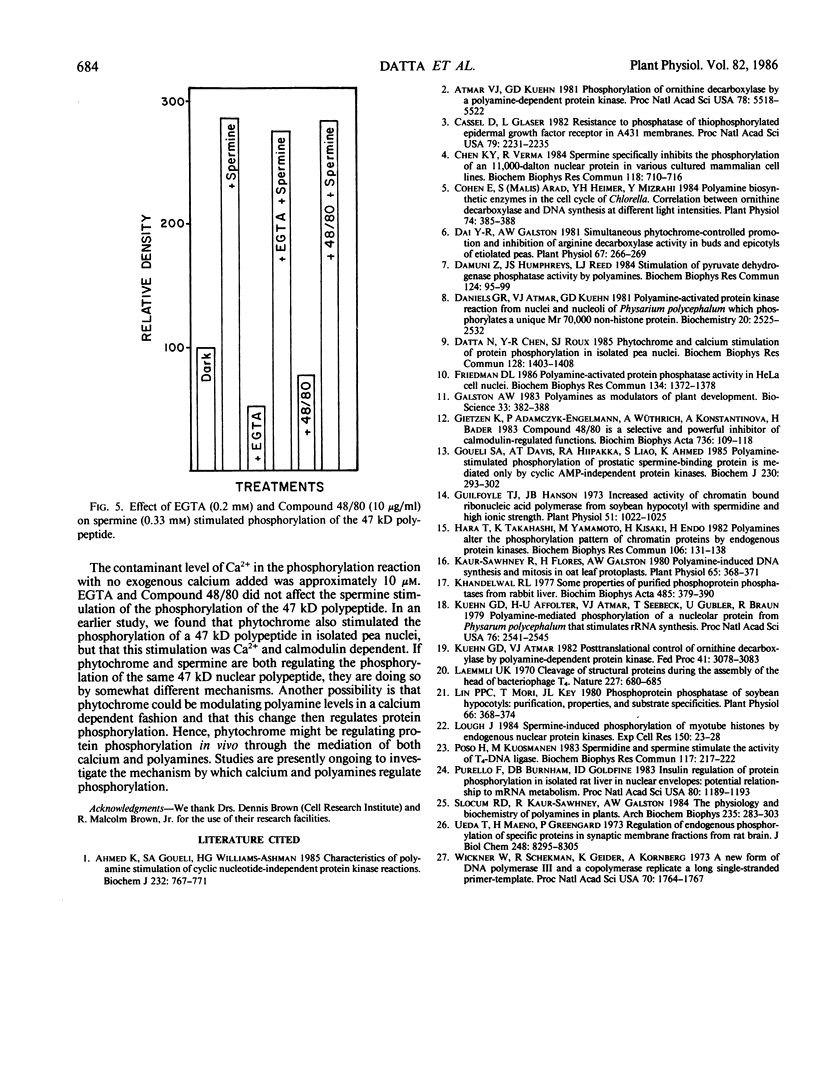
The phosphorylation of several proteins in isolated nuclei from Pisum sativum L. was stimulated by spermine. Although spermine increased the general protein phosphorylation by 10 to 20%, it increased the phosphorylation of a 47 kilodalton polypeptide by 150%. By comparison other polyamines, spermidine, putrescine, and cadavarine had far less effect on the phosphorylation of the 47 kilodalton or any other polypeptide. Sodium fluoride was able to inhibit the phosphorylation of the 47 kilodalton polypeptide in the control, implying the participation of protein phosphatase(s) in the phosphorylation of nuclear proteins. Spermine stimulated the phosphorylation of the 47 kilodalton polypeptide over the controls, even in the presence of NaF. This result indicates that spermine probably activates a nuclear kinase, a conclusion supported also by thiophosphorylation data. The inability of ethyleneglycol-bis (β-amino-ethyl ether)-N, N′-tetraacetic acid and Compound 48/80, a calmodulin antagonist, to inhibit this spermine stimulated phosphorylation renders improbable any role of calcium and calmodulin in mediating this response.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed K., Goueli S. A., Williams-Ashman H. G. Characteristics of polyamine stimulation of cyclic nucleotide-independent protein kinase reactions. Biochem J. 1985 Dec 15;232(3):767–771. doi: 10.1042/bj2320767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmar V. J., Kuehn G. D. Phosphorylation of ornithine decarboxylase by a polyamine-dependent protein kinase. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5518–5522. doi: 10.1073/pnas.78.9.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Glaser L. Resistance to phosphatase of thiophosphorylated epidermal growth factor receptor in A431 membranes. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2231–2235. doi: 10.1073/pnas.79.7.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. Y., Verma R. Spermine specifically inhibits the phosphorylation of an 11,000-dalton nuclear protein in various cultured mammalian cell lines. Biochem Biophys Res Commun. 1984 Feb 14;118(3):710–716. doi: 10.1016/0006-291x(84)91452-9. [DOI] [PubMed] [Google Scholar]
- Cohen E., Arad S. M., Heimer Y. H., Mizrahi Y. Polyamine Biosynthetic Enzymes in the Cell Cycle of Chlorella: Correlation between Ornithine Decarboxylase and DNA Synthesis at Different Light Intensities. Plant Physiol. 1984 Feb;74(2):385–388. doi: 10.1104/pp.74.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y. R., Galston A. W. Simultaneous Phytochrome-controlled Promotion and Inhibition of Arginine Decarboxylase Activity in Buds and Epicotyls of Etiolated Peas. Plant Physiol. 1981 Feb;67(2):266–269. doi: 10.1104/pp.67.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damuni Z., Humphreys J. S., Reed L. J. Stimulation of pyruvate dehydrogenase phosphatase activity by polyamines. Biochem Biophys Res Commun. 1984 Oct 15;124(1):95–99. doi: 10.1016/0006-291x(84)90921-5. [DOI] [PubMed] [Google Scholar]
- Daniels G. R., Atmar V. J., Kuehn G. D. Polyamine-activated protein kinase reaction from nuclei and nucleoli of Physarum polycephalum which phosphorylates a unique Mr 70 000 nonhistone protein. Biochemistry. 1981 Apr 28;20(9):2525–2532. doi: 10.1021/bi00512a025. [DOI] [PubMed] [Google Scholar]
- Datta N., Chen Y. R., Roux S. J. Phytochrome and calcium stimulation of protein phosphorylation in isolated pea nuclei. Biochem Biophys Res Commun. 1985 May 16;128(3):1403–1408. doi: 10.1016/0006-291x(85)91096-4. [DOI] [PubMed] [Google Scholar]
- Friedman D. L. Polyamine-activated protein phosphatase activity in HeLa cell nuclei. Biochem Biophys Res Commun. 1986 Feb 13;134(3):1372–1378. doi: 10.1016/0006-291x(86)90401-8. [DOI] [PubMed] [Google Scholar]
- Gietzen K., Adamczyk-Engelmann P., Wüthrich A., Konstantinova A., Bader H. Compound 48/80 is a selective and powerful inhibitor of calmodulin-regulated functions. Biochim Biophys Acta. 1983 Dec 7;736(1):109–118. doi: 10.1016/0005-2736(83)90175-x. [DOI] [PubMed] [Google Scholar]
- Goueli S. A., Davis A. T., Hiipakka R. A., Liao S., Ahmed K. Polyamine-stimulated phosphorylation of prostatic spermine-binding protein is mediated only by cyclic AMP-independent protein kinases. Biochem J. 1985 Sep 1;230(2):293–302. doi: 10.1042/bj2300293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilfoyle T. J., Hanson J. B. Increased Activity of Chromatin-bound Ribonucleic Acid Polymerase from Soybean Hypocotyl with Spermidine and High Ionic Strength. Plant Physiol. 1973 Jun;51(6):1022–1025. doi: 10.1104/pp.51.6.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T., Takahashi K., Yamamoto M., Kisaki H., Endo H. Polyamines alter the phosphorylation pattern of chromatin proteins by endogenous protein kinases. Biochem Biophys Res Commun. 1982 May 14;106(1):131–138. doi: 10.1016/0006-291x(82)92068-x. [DOI] [PubMed] [Google Scholar]
- Kaur-Sawhney R., Flores H. E., Galston A. W. Polyamine-induced DNA Synthesis and Mitosis in Oat Leaf Protoplasts. Plant Physiol. 1980 Feb;65(2):368–371. doi: 10.1104/pp.65.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal R. L. Some properties of purified phosphoprotein phosphatases from rabbit liver. Biochim Biophys Acta. 1977 Dec 8;485(2):379–390. doi: 10.1016/0005-2744(77)90173-5. [DOI] [PubMed] [Google Scholar]
- Kuehn G. D., Affolter H. U., Atmar V. J., Seebeck T., Gubler U., Braun R. Polyamine-mediated phosphorylation of a nucleolar protein from Physarum polycephalum that stimulates rRNA synthesis. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2541–2545. doi: 10.1073/pnas.76.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn G. D., Atmar V. J. Posttranslational control of ornithine decarboxylase by polyamine-dependent protein kinase. Fed Proc. 1982 Dec;41(14):3078–3083. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin P. P. Phosphoprotein Phosphatase of Soybean Hypocotyls: PURIFICATION, PROPERTIES, AND SUBSTRATE SPECIFICITIES . Plant Physiol. 1980 Sep;66(3):368–374. doi: 10.1104/pp.66.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lough J. Spermine-induced phosphorylation of myotube histones by endogenous nuclear protein kinases. Exp Cell Res. 1984 Jan;150(1):23–28. doi: 10.1016/0014-4827(84)90697-9. [DOI] [PubMed] [Google Scholar]
- Purrello F., Burnham D. B., Goldfine I. D. Insulin regulation of protein phosphorylation in isolated rat liver nuclear envelopes: potential relationship to mRNA metabolism. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1189–1193. doi: 10.1073/pnas.80.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pösö H., Kuosmanen M. Spermidine and spermine stimulate the activity of T4-DNA ligase. Biochem Biophys Res Commun. 1983 Nov 30;117(1):217–222. doi: 10.1016/0006-291x(83)91563-2. [DOI] [PubMed] [Google Scholar]
- Slocum R. D., Kaur-Sawhney R., Galston A. W. The physiology and biochemistry of polyamines in plants. Arch Biochem Biophys. 1984 Dec;235(2):283–303. doi: 10.1016/0003-9861(84)90201-7. [DOI] [PubMed] [Google Scholar]
- Ueda T., Maeno H., Greengard P. Regulation of endogenous phosphorylation of specific proteins in synaptic membrane fractions from rat brain by adenosine 3':5'-monophosphate. J Biol Chem. 1973 Dec 10;248(23):8295–8305. [PubMed] [Google Scholar]
- Wickner W., Schekman R., Geider K., Kornberg A. A new form of DNA polymerase 3 and a copolymerase replicate a long, single-stranded primer-template. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1764–1767. doi: 10.1073/pnas.70.6.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]