Abstract
Objectives
Mexico has a rapidly aging population at risk for cognitive impairment. Social and leisure activities may protect against cognitive decline in older adults. The benefits of these behaviors may vary by patterns of cognitive impairment. The objectives of this study were to identify latent states of cognitive functioning, model the incidence of transitions between these states, and investigate how social and leisure activities were associated with state transitions over a 6-year period in Mexican adults aged 60 and older.
Methods
We performed latent transition analyses to identify distinct cognitive statuses in the 2012 and 2018 waves of the Mexican Health and Aging Study (N = 9,091). We examined the transition probabilities between these states and their associations with social and leisure activities.
Results
We identified 4 cognitive statuses at baseline: normal cognition (43%), temporal disorientation (30%), perceptual-motor function impairment (7%), and learning and memory impairment (20%). Various social and leisure activities were associated with reduced odds of death and disadvantageous cognitive transitions, as well as increased odds of beneficial transitions.
Discussion
Mapping the effects of popular social and leisure activities onto common patterns in cognitive functioning may inform the development of more enjoyable and effective health-protective behavioral interventions.
Keywords: Cognitive aging, Health behavior, Hispanic or Latino, Leisure activities, Longitudinal studies
Mexico has a rapidly aging population, with changing demographics and increasing disease burdens (Angel et al., 2017). As the Mexican population ages, the number of people at risk for cognitive decline and impairment will continue to increase. Older adults in Mexico have high rates of diabetes, obesity, and depression (Gutierrez et al., 2020; Wong et al., 2017), and low rates of formal education, each of which can increase the risk for cognitive decline and impairment (Baumgart et al., 2015; Wong et al., 2017). There is, however, increasing recognition that behavioral patterns and aspects of the social environment in later life may influence one’s trajectory of cognitive decline (Dominguez et al., 2021; Zhao et al., 2018).
Social and leisure activities in later life, including various types of social engagement and cognitively stimulating activities, appear to protect against cognitive decline and dementia (Bielak et al., 2019; Dekhtyar et al., 2019; Hu et al., 2012; Scarmeas et al., 2001; Verghese et al., 2003; Wang et al., 2002; Wilson et al., 2002). The cognitively protective effects of social and leisure activities in later life may be more pronounced in populations with risk factors prevalent in Mexico, such as low educational attainment (Bielak & Gow, 2023). Conceptually, as detailed by the Multi-level Leisure Mechanisms Framework, social and leisure activities may exert health-protective effects via numerous interrelated processes and mechanisms of action (Fancourt et al., 2021). For example, engaging in social and leisure activities may influence cognitive outcomes by developing health-protective social networks, or by altering brain physiology (Fancourt et al., 2021). There is a need for longitudinal research that better characterizes the nature of the effects that specific social and leisure activities may have on different domains of cognitive functioning and the potential mechanisms through which they may promote brain health (Bielak & Gow, 2023).
Previous studies investigating the effects of social and leisure activities on cognitive functioning have employed variable-centered analyses (e.g., investigating the relations between covariates and outcomes of interest; Howard & Hoffman, 2018). Person-centered analyses may provide additional insight. Person-centered analyses are concerned with (a) characterizing emergent subpopulations or states, (b) how individuals tend to transition between these states, and (c) how such transitions may be related to variables of interest. Person-centered analyses may be useful for understanding common patterns of cognitive decline, and how specific social and leisure activities predict changes in cognitive states (Howard & Hoffman, 2018). This knowledge could facilitate targeted, preventive interventions to help slow and prevent cognitive decline. The aims of this study were (a) to identify latent states of cognitive functioning and model the incidence of transitions between these states over a 6-year period in Mexican adults (60 and older), and (b) to investigate how social and leisure behaviors were associated with these states and state transitions.
Method
Data
We used data from the 2012 and 2018 waves of the Mexican Health and Aging Study (MHAS; Wong et al., 2017). MHAS is a longitudinal nationally representative study of adults aged 60 and older occurring in Mexico. It is conducted in Spanish. MHAS began in 2001, with participants born in 1951 or earlier. Follow-up interviews were conducted in 2003, 2012, 2015, and 2018. In 2012 and 2018, refresher samples were added to the study. The cognitive battery expanded as the study progressed. In the present study, we used data from the 2012 and 2018 waves. We used 2012 as the baseline because it was the first wave that included social and leisure activities, it included tasks that measured cognitive domains not included in earlier waves (e.g., date orientation), and because we expected that the 6-year period between 2012 and 2018 was sufficient to observe meaningful changes in cognitive functioning over time. All participants gave informed consent at the time of interview. For all waves, MHAS had high response and follow-up rates (85%–93%; Orozco-Rocha et al., 2018). If participants were unavailable or unable to complete the interview, proxy interviews were conducted with a spouse or other family member. Interviewers ascertained information pertaining to participants’ survival in these circumstances. The leading cause of attrition in MHAS was death (Orozco-Rocha et al., 2018). We excluded proxy interviews, as these did not include direct assessments of participants’ cognitive functioning or social and leisure activities.
Measures
Social and leisure activities
Social and leisure activities were measured in 2012. Participants were asked (translated from Spanish) whether they had participated in various activities during the past year (yes, no, don’t know, or refused to answer). These included: (a) “working as a volunteer”; (b) “attending a training session, information session, or class”; (c) “attending a sporting or social club”; (d) “reading a book, magazine, or newspaper”; (e) “doing crossword puzzles, a jigsaw puzzle, or games with numbers (e.g., sudoku)”; (f) “playing games like cards, dominoes, or chess”; (g) “using technology (i.e., phone or computer) to communicate with friends or relatives or access the internet”; (h) “doing activities having to do with maintenance, repairs, gardening, etc.” (i) “watching television,” and (j) “sewing, embroidering, knitting, or doing other crafts.” We created an additional variable reflecting a median split of the total number of above social and leisure activities that participants reported engaging in (0–3 vs 4–10; Scarmeas et al., 2001).
Cognitive functioning
The MHAS cognitive battery included a modified Cross-Cultural Cognitive Examination (Glosser et al., 1993). In the present study, we included all seven neuropsychological tasks that were administered in both the 2012 and 2018 waves of MHAS. These included tasks of constructional praxis (a timed drawing task to re-create a presented figure), verbal fluency (a timed task to name all the animals that one can think of), verbal learning (an immediate recall task requiring participants to remember and repeat a list of eight words), visual scanning (participants are shown a specific figure, then have 60 s to circle as many occurrences of that figure as possible on a page printed with an array of different figures), constructional praxis recall (drawing the figure presented in the constructional praxis task by memory), delayed word recall (verbally producing the list of words presented in the verbal learning task), and temporal orientation (correctly stating the current year, month, and day; Mejía-Arango et al., 2015). We used MHAS-provided variables that had missing data imputed (Downer et al., 2021). We dichotomized these variables according to whether participants’ responses reflected impairment, defined by the MHAS Cognitive Aging Group as ≥1.5 standard deviations (SD) below norms calculated by age and education level (Mejia-Arango et al., 2021; Mejía-Arango et al., 2015). We only included participants who had complete data for neuropsychological tasks in 2012.
Statistical Methods
We conducted latent transition analysis with random intercepts (RI-LTA) to identify latent statuses reflective of patterns of performance on cognitive tasks over the 2012 and 2018 MHAS waves (Muthén & Asparouhov, 2022). Latent transition analysis is a person-centered approach (Howard & Hoffman, 2018) used to identify the characteristics of qualitatively distinct latent states and the probabilities of transitioning among these states over time (Collins & Lanza, 2009). Patterns of participants’ scores on all cognitive tasks were used to delineate cognitive states at baseline as well as the incidence of transitions between these states over the study period. We included random intercepts to help parse out stable, between-subject (trait) differences and thus more accurately model status (state) transition probabilities; this modeling feature controls for time-invariant potentially confounding variables (Hertzog et al., 1999; Mund et al., 2021; Muthén & Asparouhov, 2022). Therefore, we did not include covariates in the models. We modeled death directly as an observed state occurring in the 2018 wave. We compared the fit of latent class analysis models with from two to six statuses at each wave (Collins & Lanza, 2009). We then fit a baseline RI-LTA model and n RI-LTA model assuming measurement invariance (i.e., constraining item response probabilities to equality between times). We chose among candidate models using Bayesian Information Criterion (BIC) values and considering the interpretability of solutions (Nasserinejad et al., 2017; Nylund et al., 2007). Finally, we evaluated how individual social and leisure activities, as well as an additional variable reflecting a median split of the total number of social and leisure activities that participants reported engaging in, were associated with participants’ latent statuses in 2012, and how each moderated subsequent transitions in latent cognitive status (Supplementary Figure A). RI-LTA models were estimated using maximum likelihood with robust standard errors. We used full information maximum likelihood to handle missing data. We set the nominal alpha for all analyses to p < .05. The latent class analyses and RI-LTA models were fit using Mplus version 8.3 (Muthén & Muthén, Los Angeles, CA). All other data analyses and procedures were conducted in R version 3.6.3.
Results
Participants
There were 14,448 participants who completed a direct interview in 2012 (Wong et al., 2017). After excluding those younger than 60 years of age, this number was 9,140. Forty-nine participants were excluded from the analysis due to missing data at baseline. The analytical sample thus consisted of 9,091 participants. Of those, 1,550 (17.0%) died by the 2018 wave (this was modeled directly as an observed state). Another 1,738 participants (19.1%) had missing data on at least one cognitive task at follow-up (missing data were handled using full information maximum likelihood). The average age of participants was 69.8 (SD = 7.5) years and participants were 54.7% female. The median number of social and leisure activities that participants reported engaging in was 3 (range = 0–10; interquartile range = 2–5).
RI-LTA
Model selection
The fit indices from latent class analyses are presented in Supplementary Table A. The BIC suggested that the four class solutions best fit the data for both the 2012 and 2018 waves. The Lo–Mendell–Rubin likelihood ratio test (LMR), and adjusted LMR suggested that the five class solution best fit the data. Favoring model parsimony and the interpretability of solutions, we fit an RI-LTA model with four latent classes at baseline. Results supported the assumption of measurement invariance (BIC = 102,452.907 vs 102,358.906). The entropy values of the latent class analyses (0.54–0.55) and the RI-LTA (0.61) suggested there was some uncertainty delineating the classes. This is not unexpected given that the data were at the population level; this inherent uncertainty is accounted for in latent transition analysis.
Prevalence of latent statuses at baseline and item response probabilities
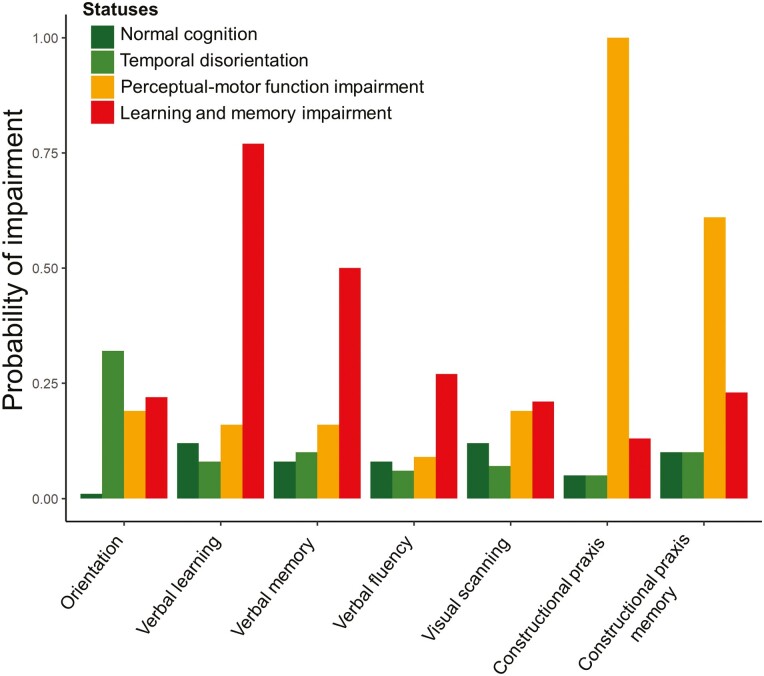
Figure 1 presents the item response probabilities that characterize the four RI-LTA classes (see Supplementary Table B for all parameter estimates). Our interpretation of the latent cognitive statuses was informed by the neurocognitive domains in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5; Sachdev et al., 2014). Based on the estimated model, the largest status was the normal cognition status with a prevalence of 43% in 2012. This latent status was characterized by relatively low probabilities of impairment on all seven of the cognition tasks (<12% for all cognitive tasks; Figure 1).
Figure 1.
Probabilities of impairment at baseline of latent statuses were identified in random intercept-latent transition analysis among participants from the Mexican Health and Aging Study included in these analyses (n = 9,091).
The second largest latent status in 2012 was the temporal disorientation status. This status comprised 30% of the sample in 2012. This status was similar to the normal cognition status, except that it had a comparatively greater probability of providing a response suggestive of cognitive impairment in the item reflecting date orientation (32% probability of impairment vs 0% in the normal cognition status; Figure 1).
With a prevalence of 7% in 2012, the perceptual-motor function impairment status was the least prevalent status. This status was characterized by a nearly 100% chance of impairment in the constructional praxis task (Figure 1).
Finally, the learning and memory impairment status had a 20% prevalence in 2012. This status was characterized by a relatively high probability of impairment in the verbal learning task (77%; Figure 1). Table 1 presents descriptive characteristics by participants’ most likely latent class at baseline.
Table 1.
Participant Characteristics by Most Likely Latent Class at Baseline Among Participants From the Mexican Health and Aging Study Included in These Analyses (n = 9,091)
| Characteristic | Category | Normal cognition (n = 5,376) | Temporal disorientation (n = 1,378) | Perceptual-motor function impairment (n = 736) | Learning and memory impairment (n = 1,601) | Total (N = 9,091) |
|---|---|---|---|---|---|---|
| Mean (SD) | ||||||
| Age | 68.4 (6.7) | 69.7 (6.9) | 70.5 (7.4) | 74.1 (8.9) | 69.8 (7.5) | |
| Count (%) | ||||||
| Sex/gender | Female | 2,967 (55.2) | 865 (62.8) | 465 (63.2) | 673 (42.0) | 4,970 (54.7) |
| Male | 2,409 (44.8) | 513 (37.2) | 271 (36.8) | 928 (58.0) | 4,121 (45.3) | |
| Education level | 0 years | 878 (16.3) | 449 (32.6) | 273 (37.1) | 366 (22.9) | 1,966 (21.6) |
| 1-6 years | 3,231 (60.1) | 503 (36.5) | 331 (45.0) | 914 (57.1) | 4,979 (54.8) | |
| 7+ years | 1,265 (23.5) | 426 (30.9) | 132 (17.9) | 320 (20.0) | 2,146 (23.6) | |
| Volunteering | Yes | 460 (8.6) | 121 (8.8) | 59 (8.0) | 90 (5.6) | 730 (8.0) |
| Attending a class | Yes | 782 (14.6) | 190 (13.8) | 87 (11.8) | 150 (9.4) | 1,209 (13.3) |
| Sports or social club | Yes | 378 (7.0) | 77 (5.6) | 36 (4.9) | 79 (4.9) | 570 (6.3) |
| Reading | Yes | 3,413 (63.6) | 678 (49.3) | 302 (41.4) | 764 (47.9) | 5,157 (56.9) |
| Puzzles | Yes | 922 (17.2) | 184 (13.4) | 74 (10.1) | 136 (8.5) | 1,316 (14.5) |
| Playing games | Yes | 824 (15.3) | 211 (15.3) | 76 (10.3) | 148 (9.3) | 1,259 (13.9) |
| Phone and internet | Yes | 3,799 (70.7) | 902 (65.5) | 439 (59.6) | 891 (55.8) | 6,031 (66.4) |
| Gardening/maintenance | Yes | 2,936 (54.6) | 717 (52.1) | 341 (47.4) | 691 (43.3) | 4,685 (51.6) |
| Watching television | Yes | 4,992 (92.9) | 1,221 (88.7) | 628 (85.3) | 1,364 (85.2) | 8,205 (90.3) |
| Sewing or other crafts | Yes | 1,558 (29.0) | 401 (29.1) | 181 (24.6) | 254 (15.9) | 2,394 (26.3) |
| At least four activities | Yes | 2,873 (53.7) | 626 (45.8) | 255 (35.2) | 256 (33.2) | 4,280 (47.4) |
Note: SD = standard deviation.
Latent status transition probabilities
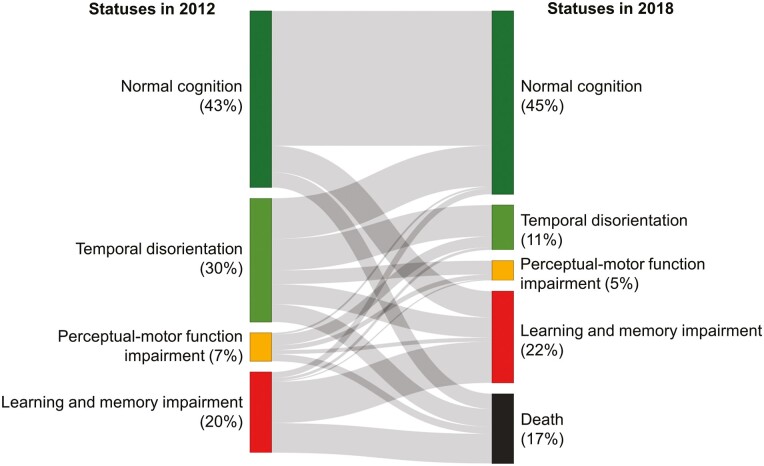
About 9% of participants with a normal cognition status in 2012 died by the 2018 wave (Figure 2). Based on the estimated model, participants with normal cognition in 2012 were most likely to remain in this status in 2018 (76%) and had a 15% probability of transitioning to the learning and memory impairment status. Participants with a normal cognition status in 2012 had a 0% chance of transitioning to either the temporal disorientation or the perceptual-motor function impairment status.
Figure 2.
Prevalence and transition probabilities associated with latent statuses identified in random intercept-latent transition analysis, based on the estimated model among participants from the Mexican Health and Aging Study included in these analyses (n = 9,091).
About 14% of participants with a temporal disorientation status in 2012 died by the 2018 wave. The most likely transition associated with this status was to the normal cognition status (34%). Only 25% of participants with this status in 2012 remained with this status by 2018. The probability of individuals with this status transitioning to the temporal disorientation and learning and memory impairment status was 11% and 16%, respectively.
About 25% of participants with a perceptual-motor function impairment in 2012 died by the 2018 wave. The most likely transition associated with this status was to the temporal disorientation status (38%). The probability of maintaining the same status was 17%. The probability of individuals with this status transitioning to the normal cognition status was 6%.
Finally, 37% of participants with a learning and memory impairment status in 2012 died by 2018. Participants with this status were most likely to remain in this status by 2018 (51%) and were unlikely to transition to the normal cognition (7%), temporal disorientation (3%), or perceptual-motor function impairment statuses (2%).
Association between baseline social and leisure activities and baseline cognition status
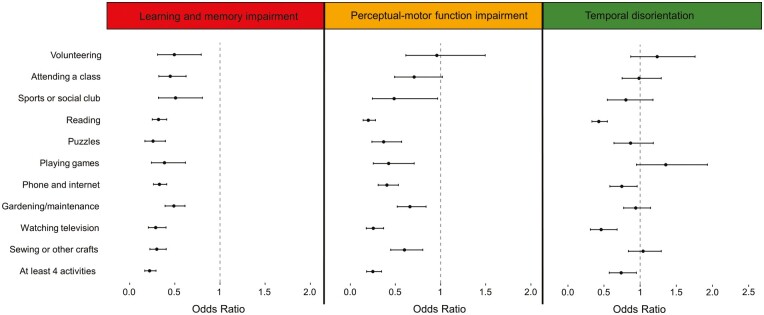
Each of the social and leisure activities and the additional variable reflecting a median split of the total number of social and leisure activities were individually associated with decreased odds of having a status of learning and memory impairment at baseline, relative to having a normal cognition status at baseline (Figure 3). Odds ratios (ORs) ranged from 0.26 (95% confidence interval [CI 0.17, 0.40]) for engaging in puzzles to 0.51 (95% CI [032., 0.80]) for participating in a sports or social club (Supplementary Table C). Engaging in at least four social and leisure activities was associated with 0.22 odds of having a status of learning and memory impairment at baseline, relative to having a normal cognition status (95% CI [0.17, 0.29]).
Figure 3.
Odds ratios with 95% confidence intervals of engaging in various social and leisure time activities on baseline cognitive statuses, relative to normal cognition status, based on the estimated model among participants from the Mexican Health and Aging Study included in these analyses (n = 9,091).
Participating in a sports or social club, reading, engaging in puzzles, engaging in games, phone or internet use, gardening and maintenance, watching television, and sewing and engaging in other crafts were associated with decreased odds of having a status of perceptual-motor function impairment at baseline, relative to having a normal cognition status (Figure 3). These odds ratios ranged from 0.20 (95% CI [0.14, 0.28]) for reading to 0.66 (95% CI [0.52, 0.84]) for gardening and maintenance (Supplementary Table C). Engaging in at least four social and leisure activities was associated with 0.25 odds of having a status of perceptual-motor function impairment at baseline, relative to having a normal cognition status (95% CI [0.18, 0.35]).
Reading (OR = 0.43, 95% CI [0.33, 0.55]), phone or internet use (OR = 0.75, 95% CI [0.58, 0.96]), and watching television (OR = 0.46, 95% CI [0.31, 0.68]) were associated with decreased odds of having a status of temporal disorientation at baseline, relative to having a normal cognition status (Figure 3; Supplementary Table C). Engaging in at least four social and leisure activities was associated with 0.74 (95% CI [0.57, 0.95]).
Predictive associations between baseline social and leisure activities and cognition status transitions
Table 2 presents the statistically significant associations observed between engaging in specific social and leisure activities at baseline (and an additional variable reflecting a median split of the total number of social and leisure activities) and subsequent cognition status transitions, relative to maintaining the same status (details presented in Supplementary Table D). Associations under the diagonal may generally be understood to be unfavorable transitions (e.g., transitioning from learning and memory impairment to death), although associations above the diagonal may generally be understood to be favorable transitions (e.g., transitioning from temporal disorientation impairment to normal cognition). For all statistically reliable transitions observed, engaging in the social/leisure activity was either associated with reduced odds of experiencing an unfavorable transition, or increased odds of a favorable transition.
Table 2.
Predictive Associations Between Engaging in Social and Leisure Activities at Baseline and Subsequent Cognition Status Transitions, Relative to Maintaining the Same Status, From Latent Transition Analyses Among Participants from the Mexican Health and Aging Study (odds ratios; n = 9,091)
| To: | ||||||
|---|---|---|---|---|---|---|
| Death | Learning and memory impairment | Perceptual-motor function impairment | Temporal disorientation | Normal cognition | ||
| From: | Learning and memory impairment | Gardening/maintenance (0.54) At least four activities (0.63) |
Ref. | Reading (11.59) | ||
| Perceptual-motor function impairment | Sewing or other crafts (0.38) At least four activities (0.47) |
Ref. | Reading (3.13) | |||
| Temporal disorientation | Sewing or other crafts (0.41) Attending a class (0.18) Watching television (0.43) |
Sewing or other crafts (0.37) | Ref. | Reading (8.25) Puzzles (5.10) phone and internet (5.26) Gardening/maintenance (2.20) At least four activities (5.70) |
||
| Normal cognition | At least four activities (0.61) | Reading (0.62) Phone and internet use (0.61) Sewing or other crafts (0.59) At least four activities (0.50) |
Ref. | |||
For individuals with a baseline status of normal cognition, engaging in at least four social and leisure activities was associated with decreased odds of death relative to keeping the same status (Table 2; Supplementary Table D). Reading, phone and internet use, sewing or engaging in other crafts, and engaging in at least four social and leisure activities were associated with decreased odds of transitioning to the learning and memory impairment cognitive status for this group, relative to keeping the same status. There were no other statistically significant moderating effects of social and leisure activities on latent status transition probabilities.
For individuals with a baseline cognition status of temporal disorientation, attending a class, watching television, and sewing and engaging in other crafts were associated with decreased odds of death, relative to keeping the same status (Table 2; Supplementary Table D). Reading, working on puzzles, phone and internet use, gardening/maintenance, and engaging in at least 4 social and leisure activities were associated with increased odds of transitioning to the normal cognition status, relative to keeping the same status.
For individuals with a baseline cognition status of perceptual-motor function impairment, sewing and engaging in other crafts, and engaging in at least four social and leisure activities were associated with decreased odds of death, relative to keeping the same status (Table 2; Supplementary Table D). Reading was associated with increased odds of transitioning to the normal cognition status, relative to keeping the same status.
For individuals with a baseline cognition status of learning and memory impairment, gardening/maintenance, watching television, and engaging in at least four social and leisure activities were significantly associated with decreased odds of death, relative to keeping the same status (Table 2; Supplementary Table D). Reading was associated with increased odds of transitioning to the temporal disorientation cognitive status, relative to keeping the same status. There were no other statistically significant moderating effects of any activities on latent status transition probabilities for individuals with this cognition status at baseline.
Discussion
We used RI-LTA to identify latent statuses reflective of cognitive functioning in longitudinal, nationally representative data from Mexico. We observed latent cognitive statuses that we termed normal cognition (43% of the sample at baseline), temporal disorientation (30%), perceptual-motor function impairment (7%), and learning and memory impairment (20%). Individuals with a status of perceptual-motor function impairment and learning and memory impairment were characterized by impairment in one or more cognitive tasks and were more likely to die during follow-up than those with a normal cognition status. Social and leisure activities were associated with reduced odds of death, reduced odds of disadvantageous cognitive statuses and transitions, and increased odds of beneficial cognitive statuses and transitions.
Our findings comport with investigations that have used similar analytic methods in other cohorts (Huang et al., 2019; Moorman et al., 2021; Zammit et al., 2020). The identification by Zammit and colleagues (2020) of a status reflecting frontal impairment (16% prevalence at baseline) is conceptually comparable to our perceptual-motor function impairment status—both reflect performance suggestive of impairment in perceptual speed and orientation (Zammit et al., 2020). Zammit et al. and Moorman et al. (2021) also identified cognitive statuses comparable to the status we termed learning and memory impairment (labeled memory-specific impairment and high fluency performance, respectively; Moorman et al., 2021; Zammit et al., 2020). Individuals with this status may be particularly at-risk, as a decline in learning and memory is a hallmark of Alzheimer’s disease progression (Sachdev et al., 2014). In our study, this status was associated with the highest odds of death.
Unlike the analyses conducted by Zammit et al. (2020), Moorman et al. (2021), and Huang et al. (2019), in our study we did not identify a status characterized by categorical cognitive impairment. This may be due to general cohort differences (e.g., our sample was younger than others) and/or because MHAS made use of abridged proxy interviews (e.g., with family members) that may have reduced direct participation from individuals with more severe cognitive impairment (proxy interviews were not included in the present study). Moorman et al. (2021) identified a status reflective of language impairment, and Huang et al. (2019) identified a status reflective of impairment in complex attention, but the items operationalizing these neurocognitive domains did not appear to differentiate the statuses we observed. The present study is unique given its focus on older adults in Mexico. Factors such as a lack of access to care and cultural differences (e.g., an inclination toward familial caregiving) may contribute to delayed contact with health care institutions and worse prognosis for cognitive outcomes in this population (Janevic & M Connell, 2001; Mausbach et al., 2004). More research is needed to better the understand unique challenges and opportunities older adults in Mexico may have as they relate to cognitive outcomes.
The Multi-level Leisure Mechanisms Framework identifies over 600 mechanisms of action in a conceptual hierarchy that maps out how social and leisure activities may affect health and cognition outcomes (Fancourt et al., 2021). The framework posits that one avenue through which social and leisure activities may affect health outcomes is by altering brain physiology. For example, social and leisure activities may help to increase cognitive reserve, the brain’s ability to engage in cognitive compensatory processes to circumvent neuropathological damage (Stern, 2009). Habitual reading has been theorized to increase cognitive reserve (Chan, 2021; Farina et al., 2018; Scarmeas & Stern, 2003), and in the present study reading was the strongest predictor of beneficial transitions in cognitive status. Our findings are consistent with other studies that have suggested reading may be beneficial for memory trajectories (Simon et al., 2022), and particularly protective against cognitive decline and dementia relative to other social and leisure activities (Scarmeas et al., 2001; Verghese et al., 2003; Wang et al., 2002). The results of the present study are consistent with the hypothesis that reading may help increase cognitive reserve.
In contrast to reading, which was a robust predictor of beneficial transitions in cognitive status, sewing or engaging in other crafts was associated with reduced odds of some of the less favorable transitions in cognitive status observed (i.e., reduced odds of death and learning and memory impairment). These findings align with previous studies, which found that productive activities, including sewing, knitting, crocheting, and weaving, are associated with a lower risk of dementia in older adults (Wang et al., 2002). Further, an intervention study found that lifestyle interventions centered on sewing and digital photography led to improvements in episodic memory in older adults (Park et al., 2014). Sewing or engaging in other crafts may exert protective effects on health and cognition by increasing activation in brain regions associated with attention and sensory processing (Fancourt et al., 2021). More research is needed to investigate the potential mechanisms through which specific social and leisure activities may influence health outcomes, and how this information may be incorporated into targeted health promotion efforts.
The Multi-level Leisure Mechanisms Framework also indicates that social and leisure activities may improve health and cognition outcomes via social processes (Fancourt et al., 2021). This aligns with the conceptual model put forth by Berkman, Glass, Brissette, and Seeman (2000), which suggests that increasing one’s social networks can facilitate health-protective social support, social influence, social engagement, and access to resources and material goods (Berkman et al., 2000). Inherently social activities may confer these benefits as well as provide opportunities for mental and physical exertion. In the present study, we did not observe evidence that some of the more apparently social activities (i.e., volunteering, attending a sports or social club, playing games) were predictive of beneficial transitions in cognitive states. This contrasts with literature that has identified marked cognitive benefits to be associated with varied social activities for older adults, such as high-intensity volunteering (Carlson et al., 2015; Fried et al., 2013; Gruenewald et al., 2016). It may be that more precise measures of social and leisure activities, perhaps including timeframe, variety, and frequency of social and leisure activities and/or the more granular processes that these activities entail (e.g., organizing, planning) may afford a more nuanced understanding of the effects social activities on cognitive outcomes (Bielak, 2017; Bielak & Gow, 2023). Given the importance of social processes in healthy aging, more research is needed to investigate how to help increase health-promoting social interactions and resources in older Mexican adults. These findings comport with other research that has suggested that social and physical activities may have less robust effects on cognitive functioning than mentally stimulating activities (Yates et al., 2016). More explicitly social activities may be relevant for certain aspects of cognitive functioning, such as they relate to speed and vocabulary, whereas mentally stimulating activities may be more strongly linked to memory-related outcomes (Simon et al., 2022). It may also be that in the present study, some of the more ostensibly individualistic leisure activities still affected cognitive outcomes via broadening social networks.
Engaging in gardening/maintenance activities was negatively associated with mortality for those with a Learning and memory impairment status. We did not, however, observe evidence that engaging in gardening/maintenance activities was protective against cognitive decline. These null findings comport with other studies that have examined the impact of physical activity on cognition in older adults in the context of social and leisure activities (Simon et al., 2022; Verghese et al., 2003; Wang et al., 2002). However, studies that have featured longer timelines and more direct measures of physical activity have found physical activity to protect against cognitive decline associated with pathology (Demurtas et al., 2020; Pisani et al., 2021). Physical activity may be more apt to building brain reserve (e.g., the number of neurons and synapses within certain structures of the brain [Stern, 2012]), whereas cognitively stimulating leisure and social activities may help to build cognitive reserve (Cheng, 2016). These distinct processes relevant to cognitive functioning may entail different time periods and benefits.
We found that engaging in at least four social and leisure activities was negatively associated with mortality for most cognitive statuses at baseline, relative to maintaining the same status. Higher overall engagement in social and leisure activities was also associated with decreased risk of learning and memory-related cognitive decline for those with a normal cognition status. These findings are in accord with previous studies that found that high engagement in varied social and leisure activities to be associated with a reduced risk of cognitive decline, Alzheimer’s disease, and dementia in older adults (Hu et al., 2012; Lee et al., 2021; Scarmeas et al., 2001; Verghese et al., 2003; Wang et al., 2002; Wilson et al., 2002). Engaging in a variety of social and leisure activities may be particularly beneficial for older adults, and perhaps even confer greater benefits than engaging in a higher frequency of a limited number of social and leisure activities (Carlson et al., 2012; Lee et al., 2021). This may be in part due to broadening social networks or building resilience (Berkman et al., 2000).
Finally, performance on the temporal orientation task seemed to serve as a differentiating factor between the normal cognition status and the temporal disorientation status, which were otherwise quite similar. About a third of individuals with a temporal disorientation status at baseline subsequently transitioned to a normal cognition status. Individuals with a temporal disorientation status, however, had a markedly increased probability of transitioning to one of the impaired statuses or death within the study period (greater than 40%). Temporal disorientation is common in older adults (Kington & Stewart, 2011), and may be an early indicator of impairment in memory domains and predictor of incident Alzheimer’s disease (Ashford et al., 1989; Guerrero-Berroa et al., 2009; Ryan et al., 2009). This may be a useful marker to help identify people who may be at risk of Alzheimer’s disease and related dementias for early intervention.
The conclusions that can be drawn from this study are limited by its observational design, which precludes causal inference. Although engaging in leisure behaviors in later life may preserve existing cognitive function (Hackett et al., 2019; Penninkilampi et al., 2018), an alternative explanation is that prodromal symptomatology instead preempts a reduction in social and leisure activities (Sommerlad et al., 2020). It may have been that in the present study cognitive impairment caused participants to withdraw from some or all of the activities investigated. More research designed to evaluate causality is needed to investigate the potentially protective effects of social and leisure activities on cognition in older Hispanic adults. Furthermore, practice effects may have influenced the longitudinal patterns of cognition we observed (Salthouse, 2019). This study is also limited by self-reported measurement of social and leisure activities; this form of measurement has well-documented limitations to construct validity (e.g., recall bias, social desirability bias). Lastly, the six-year follow-up period may have been too short to fully capture the dynamic relationships between cognitive aging, mortality, and engagement in social and leisure activities. Strengths of the study included objective measurement of cognitive functioning, the use of a nationally representative data set, advanced handling of missing data, and modeling death directly to limit survivorship bias.
Using RI-LTA of cognitive functioning in a longitudinal, nationally representative sample from Mexico we identified four discrete, latent cognitive statuses: normal cognition, temporal disorientation, perceptual-motor function impairment, and learning and memory impairment. Various social and leisure activities were associated with health-protective and cognitive benefits, and these relationships were moderated by cognitive status at baseline. Identifying commonly occurring patterns in cognitive functioning and mapping the effects of popular social and leisure activities onto them may inform the development of enjoyable lifestyle interventions.
Supplementary Material
Contributor Information
Michael C Robertson, Department of Nutrition, Metabolism & Rehabilitation Sciences; The University of Texas Medical Branch at Galveston, Galveston, Texas, USA.
Brian Downer, Department of Population Health & Health Disparities, The University of Texas Medical Branch at Galveston, Galveston, Texas, USA.
Paul E Schulz, Department of Neurology, The McGovern Medical School of UTHealth Houston, Texas, USA.
Rafael Samper-Ternent, Department of Management, Policy & Community Health, UTHealth Houston School of Public Health, Houston, Texas, USA.
Elizabeth J Lyons, Department of Nutrition, Metabolism & Rehabilitation Sciences; The University of Texas Medical Branch at Galveston, Galveston, Texas, USA.
Sadaf Arefi Milani, Department of Epidemiology, The University of Texas Medical Branch at Galveston, Galveston, Texas, USA.
Funding
This work was supported by the National Institute on Aging/National Institutes of Health (grant numbers K01AG075254, P30AG024832, P30AG059301, RF1AG068988), and the National Institutes of Health/Office of the Director (OD)/National Institute of Allergy and Infectious Diseases (NIAID)/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD; K12HD052023). MHAS funding statement: The MHAS is supported by the National Institutes of Health/National Institute on Aging (grant number R01 AG018016, R Wong, PI) in the United States and the Instituto Nacional de Estadística y Geografía (INEGI) in Mexico.
Conflict of Interest
P. E. Schulz serves as a consultant for Eli Lilly, Biogen, and Acadia Pharmaceuticals and gives speeches for them; and, he has contracts with multiple pharmaceutical companies to perform clinical trials. The other authors have no potential conflicts of interest to disclose.
Data Availability
The MHAS data used in these analyses are publicly available (https://mhasweb.org/Home/index.aspx). The code for the analysis will be made available to other researchers upon reasonable request. This secondary data analysis was not preregistered with an analysis plan in an independent, institutional registry.
References
- Angel, J. L., Vega, W., & López-Ortega, M. (2017). Aging in Mexico: Population trends and emerging issues. The Gerontologist, 57(2), 153–162. doi: 10.1093/geront/gnw136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford, J. W., Kolm, P., Colliver, J. A., Bekian, C., & Hsu, L.-N. (1989). Alzheimer patient evaluation and the mini-mental state: Item characteristic curve analysis. Journal of Gerontology, 44(5), P139–P146. doi: 10.1093/geronj/44.5.p139 [DOI] [PubMed] [Google Scholar]
- Baumgart, M., Snyder, H. M., Carrillo, M. C., Fazio, S., Kim, H., & Johns, H. (2015). Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective . Alzheimer’s & Dementia, 11(6), 718–726. doi: 10.1016/j.jalz.2015.05.016 [DOI] [PubMed] [Google Scholar]
- Berkman, L. F., Glass, T., Brissette, I., & Seeman, T. E. (2000). From social integration to health: Durkheim in the new millennium. Social Science & Medicine, 51(6), 843–857. doi: 10.1016/S0277-9536(00)00065-4 [DOI] [PubMed] [Google Scholar]
- Bielak, A. A. M. (2017). Different perspectives on measuring lifestyle engagement: A comparison of activity measures and their relation with cognitive performance in older adults. Aging, Neuropsychology, and Cognition, 24(4), 435–452. doi: 10.1080/13825585.2016.1221378 [DOI] [PubMed] [Google Scholar]
- Bielak, A. A. M., & Gow, A. J. (2023). A decade later on how to “use it” so we don’t “lose it”: An update on the unanswered questions about the influence of activity participation on cognitive performance in older age. Gerontology, 69(3), 336–355. doi: 10.1159/000524666 [DOI] [PubMed] [Google Scholar]
- Bielak, A. A. M., Mogle, J., & Sliwinski, M. J. (2019). What did you do today? Variability in daily activities is related to variability in daily cognitive performance. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 74(5), 764–771. doi: 10.1093/geronb/gbx145 [DOI] [PubMed] [Google Scholar]
- Carlson, M. C., Kuo, J. H., Chuang, Y.-F., Varma, V. R., Harris, G., Albert, M. S., Erickson, K. I., Kramer, A. F., Parisi, J. M., Xue, Q.-L., Tan, E. J., Tanner, E. K., Gross, A. L., Seeman, T. E., Gruenewald, T. L., McGill, S., Rebok, G. W., & Fried, L. P. (2015). Impact of the Baltimore Experience Corps Trial on cortical and hippocampal volumes . Alzheimer’s & Dementia, 11(11), 1340–1348. doi: 10.1016/j.jalz.2014.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson, M. C., Parisi, J. M., Xia, J., Xue, Q.-L., Rebok, G. W., Bandeen-Roche, K., & Fried, L. P. (2012). Lifestyle activities and memory: Variety may be the spice of life. The Women’s Health and Aging Study II. Journal of the International Neuropsychological Society, 18(2), 286–294. doi: 10.1017/S135561771100169X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, C. K. (2021). Can reading increase cognitive reserve? International Psychogeriatrics, 33(1), 15–17. doi: 10.1017/S1041610220001246 [DOI] [PubMed] [Google Scholar]
- Cheng, S.-T. (2016). Cognitive reserve and the prevention of dementia: The role of physical and cognitive activities. Current Psychiatry Reports, 18(9), 85. doi: 10.1007/s11920-016-0721-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, L. M., & Lanza, S. T. (2009). Latent class and latent transition analysis: With applications in the social, behavioral, and health sciences (Vol. 718). John Wiley & Sons. [Google Scholar]
- Dekhtyar, S., Marseglia, A., Xu, W., Darin-Mattsson, A., Wang, H.-X., & Fratiglioni, L. (2019). Genetic risk of dementia mitigated by cognitive reserve: A cohort study. Annals of Neurology, 86(1), 68–78. doi: 10.1002/ana.25501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demurtas, J., Schoene, D., Torbahn, G., Marengoni, A., Grande, G., Zou, L., Petrovic, M., Maggi, S., Cesari, M., Lamb, S., Soysal, P., Kemmler, W., Sieber, C., Mueller, C., Shenkin, S. D., Schwingshackl, L., Smith, L., & Veronese, N.; European Society of Geriatric Medicine Special Interest Group in Systematic Reviews and Meta-Analyses, Frailty, Sarcopenia, and Dementia. (2020). Physical activity and exercise in mild cognitive impairment and dementia: An umbrella review of intervention and observational studies. Journal of the American Medical Directors Association, 21(10), 1415–1422. doi: 10.1016/j.jamda.2020.08.031 [DOI] [PubMed] [Google Scholar]
- Dominguez, L. J., Veronese, N., Vernuccio, L., Catanese, G., Inzerillo, F., Salemi, G., & Barbagallo, M. (2021). Nutrition, physical activity, and other lifestyle factors in the prevention of cognitive decline and dementia. Nutrients, 13(11), 4080. doi: 10.3390/nu13114080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer, B., Avila, J., Chen, N.-W., & Wong, R. (2021). Imputation procedures for cognitive variables in the Mexican health and aging study: Evaluating the bias from excluding participants with missing data. Realidad, Datos y Espacio: Revista Internacional de Estadistica y Geografia, 12(2), 90–105. [PMC free article] [PubMed] [Google Scholar]
- Fancourt, D., Aughterson, H., Finn, S., Walker, E., & Steptoe, A. (2021). How leisure activities affect health: A narrative review and multi-level theoretical framework of mechanisms of action. Lancet Psychiatry, 8(4), 329–339. doi: 10.1016/S2215-0366(20)30384-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina, M., Paloski, L. H., de Oliveira, C. R., de Lima Argimon, I. I., & Irigaray, T. Q. (2018). Cognitive reserve in elderly and its connection with cognitive performance: A systematic review. Ageing International, 43(4), 496–507. doi: 10.1007/s12126-017-9295-5 [DOI] [Google Scholar]
- Fried, L. P., Carlson, M. C., McGill, S., Seeman, T., Xue, Q.-L., Frick, K., Tan, E., Tanner, E. K., Barron, J., Frangakis, C., Piferi, R., Martinez, I., Gruenewald, T., Martin, B. K., Berry-Vaughn, L., Stewart, J., Dickersin, K., Willging, P. R., & Rebok, G. W. (2013). Experience Corps: A dual trial to promote the health of older adults and children’s academic success. Contemporary Clinical Trials, 36(1), 1–13. doi: 10.1016/j.cct.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glosser, G., Wolfe, N., Albert, M. L., Lavine, L., Steele, J. C., Calne, D. B., & Schoenberg, B. S. (1993). Cross-cultural cognitive examination: Validation of a dementia screening instrument for neuroepidemiological research. Journal of the American Geriatrics Society, 41(9), 931–939. doi: 10.1111/j.1532-5415.1993.tb06758.x [DOI] [PubMed] [Google Scholar]
- Gruenewald, T. L., Tanner, E. K., Fried, L. P., Carlson, M. C., Xue, Q.-L., Parisi, J. M., Rebok, G. W., Yarnell, L. M., & Seeman, T. E. (2016). The Baltimore Experience Corps Trial: Enhancing generativity via intergenerational activity engagement in later life. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 71(4), 661–670. doi: 10.1093/geronb/gbv005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Berroa, E., Luo, X., Schmeidler, J., Rapp, M. A., Dahlman, K., Grossman, H. T., Haroutunian, V., & Beeri, M. S. (2009). The MMSE orientation for time domain is a strong predictor of subsequent cognitive decline in the elderly. International Journal of Geriatric Psychiatry, 24(12), 1429–37. doi: 10.1002/gps.2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez, S., Milani, S. A., & Wong, R. (2020). Is “busy” always better? Time-use activities and depressive symptoms among older Mexican adults. Innovation in Aging, 4(5), igaa030. doi: 10.1093/geroni/igaa030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett, R. A., Steptoe, A., Cadar, D., & Fancourt, D. (2019). Social engagement before and after dementia diagnosis in the English Longitudinal Study of Ageing. PLoS One, 14(8), e0220195. doi: 10.1371/journal.pone.0220195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog, C., Hultsch, D. F., & Dixon, R. A. (1999). On the problem of detecting effects of lifestyle on cognitive change in adulthood: Reply to Pushkar et al. (1999). Psychology and Aging, 14(3), 528–534. doi: 10.1037/0882-7974.14.3.528 [DOI] [Google Scholar]
- Howard, M. C., & Hoffman, M. E. (2018). Variable-centered, person-centered, and person-specific approaches: Where theory meets the method. Organizational Research Methods, 21(4), 846–876. doi: 10.1177/1094428117744021 [DOI] [Google Scholar]
- Hu, Y., Lei, X., Smith, J. P., & Zhao, Y. (2012). Effects of social activities on cognitive functions: Evidence from CHARLS. In Smith J. P. & Majmundar M. (Eds.), Aging in Asia: Findings From New and Emerging Data Initiatives (pp. 279–305). National Academies Press (US). https://www.ncbi.nlm.nih.gov/books/NBK109224/ [PubMed] [Google Scholar]
- Huang, F., Zhang, M., & Wang, S. (2019). Changes in cognitive function among older adults: A latent profile transition analysis. Archives of Gerontology and Geriatrics, 80, 12–19. doi: 10.1016/j.archger.2018.09.006 [DOI] [PubMed] [Google Scholar]
- Janevic, M. R., & M Connell, C. (2001). Racial, ethnic, and cultural differences in the dementia caregiving experience: Recent findings. The Gerontologist, 41(3), 334–347. doi: 10.1093/geront/41.3.334 [DOI] [PubMed] [Google Scholar]
- Kington, J., & Stewart, R. (2011). Temporal orientation in a national community sample of older people. International Journal of Geriatric Psychiatry, 26(2), 144–149. doi: 10.1002/gps.2505 [DOI] [PubMed] [Google Scholar]
- Lee, S., Charles, S. T., & Almeida, D. M. (2021). Change is good for the brain: Activity diversity and cognitive functioning across adulthood. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 76(6), 1036–1048. doi: 10.1093/geronb/gbaa020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mausbach, B. T., Coon, D. W., Depp, C., Rabinowitz, Y. G., Wilson-Arias, E., Kraemer, H. C., Thompson, L. W., Lane, G., & Gallagher-Thompson, D. (2004). Ethnicity and time to institutionalization of dementia patients: A comparison of Latina and Caucasian female family caregivers. Journal of the American Geriatrics Society, 52(7), 1077–1084. doi: 10.1111/j.1532-5415.2004.52306.x [DOI] [PubMed] [Google Scholar]
- Mejia-Arango, S., Avila, J., Downer, B., Garcia, M. A., Michaels-Obregon, A., Saenz, J. L., Samper-Ternent, R., & Wong, R. (2021). Effect of demographic and health dynamics on cognitive status in Mexico between 2001 and 2015: Evidence from the Mexican Health and Aging Study. Geriatrics, 6(3), 63. doi: 10.3390/geriatrics6030063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejía-Arango, S., Wong, R., & Michaels-Obregón, A. (2015). Normative and standardized data for cognitive measures in the Mexican Health and Aging Study. Salud Publica de Mexico, 57, s90–s96. doi: 10.21149/spm.v57s1.7594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman, S. M., Greenfield, E. A., & Carr, K. (2021). Using mixture modeling to construct subgroups of cognitive aging in the Wisconsin Longitudinal Study. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 76(8), 1512–1522. doi: 10.1093/geronb/gbaa191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mund, M., Johnson, M. D., & Nestler, S. (2021). Changes in size and interpretation of parameter estimates in within-person models in the presence of time-invariant and time-varying covariates. Frontiers in Psychology, 12. doi: 10.3389/fpsyg.2021.666928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén, B., & Asparouhov, T. (2022). Latent transition analysis with random intercepts (RI-LTA). Psychological Methods, 27(1), 1–16. doi: 10.1037/met0000370 [DOI] [PubMed] [Google Scholar]
- Nasserinejad, K., van Rosmalen, J., de Kort, W., & Lesaffre, E. (2017). Comparison of criteria for choosing the number of classes in Bayesian finite mixture models. PLoS One, 12(1), e0168838. doi: 10.1371/journal.pone.0168838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylund, K. L., Asparouhov, T., & Muthén, B. O. (2007). Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling: A Multidisciplinary Journal, 14(4), 535–569. doi: 10.1080/10705510701575396 [DOI] [Google Scholar]
- Orozco-Rocha, K., Wong, R., & Obregón, A. M. (2018). Attrition in panel surveys in Mexico: The Mexican Health and Aging Study (MHAS). Realidad, Datos y Espacio: Revista Internacional de Estadistica y Geografia, 9(1), 64. [PMC free article] [PubMed] [Google Scholar]
- Park, D. C., Lodi-Smith, J., Drew, L., Haber, S., Hebrank, A., Bischof, G. N., & Aamodt, W. (2014). The impact of sustained engagement on cognitive function in older adults: The Synapse Project. Psychological Science, 25(1), 103–112. doi: 10.1177/0956797613499592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninkilampi, R., Casey, A. -N., Singh, M. F., & Brodaty, H. (2018). The association between social engagement, loneliness, and risk of dementia: A systematic review and meta-analysis. Journal of Alzheimer’s Disease, 66(4), 1619–1633. doi: 10.3233/jad-180439 [DOI] [PubMed] [Google Scholar]
- Pisani, S., Mueller, C., Huntley, J., Aarsland, D., & Kempton, M. J. (2021). A meta-analysis of randomised controlled trials of physical activity in people with Alzheimer’s disease and mild cognitive impairment with a comparison to donepezil. International Journal of Geriatric Psychiatry, 36(10), 1471–1487. doi: 10.1002/gps.5581 [DOI] [PubMed] [Google Scholar]
- Ryan, J. J., Glass, L. A., Bartels, J. M., Bergner, C. M., & Paolo, A. M. (2009). Predicting neuropsychological test performance on the basis of temporal orientation. Aging, Neuropsychology, and Cognition, 16(3), 330–337. doi: 10.1080/13825580902741344 [DOI] [PubMed] [Google Scholar]
- Sachdev, P. S., Blacker, D., Blazer, D. G., Ganguli, M., Jeste, D. V., Paulsen, J. S., & Petersen, R. C. (2014). Classifying neurocognitive disorders: The DSM-5 approach. Nature Reviews Neurology, 10(11), 634–642. doi: 10.1038/nrneurol.2014.181 [DOI] [PubMed] [Google Scholar]
- Salthouse, T. A. (2019). Trajectories of normal cognitive aging. Psychology and Aging, 34(1), 17–24. doi: 10.1037/pag0000288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas, N., Levy, G., Tang, M. -X., Manly, J., & Stern, Y. (2001). Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology, 57(12), 2236–2242. doi: 10.1212/wnl.57.12.2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas, N., & Stern, Y. (2003). Cognitive reserve and lifestyle. Journal of Clinical and Experimental Neuropsychology, 25(5), 625–633. doi: 10.1076/jcen.25.5.625.14576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, S. S., Lee, S., Gu, Y., Mensing, A., Noofoory, D., Nazario, G. M. H., Babukutty, R. S., & Stern, Y. (2022). Leisure activity engagement across adulthood predicts cognitive change after five years: Do gender and age matter? Journal of the International Neuropsychological Society, 1–12. doi: 10.1017/S1355617722000510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerlad, A., Sabia, S., Livingston, G., Kivimäki, M., Lewis, G., & Singh-Manoux, A. (2020). Leisure activity participation and risk of dementia: An 18-year follow-up of the Whitehall II Study. Neurology, 95(20), e2803–e2815. doi: 10.1212/WNL.0000000000010966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, Y. (2009). Cognitive reserve. Neuropsychologia, 47(10), 2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, Y. (2012). Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurology, 11(11), 1006–12. doi: 10.1016/S1474-4422(12)70191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese, J., Lipton, R. B., Katz, M. J., Hall, C. B., Derby, C. A., Kuslansky, G., Ambrose, A. F., Sliwinski, M., & Buschke, H. (2003). Leisure activities and the risk of dementia in the elderly. New England Journal of Medicine, 348(25), 2508–2516. doi: 10.1056/nejmoa022252 [DOI] [PubMed] [Google Scholar]
- Wang, H. -X., Karp, A., Winblad, B., & Fratiglioni, L. (2002). Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: A longitudinal study from the Kungsholmen Project. American Journal of Epidemiology, 155(12), 1081–1087. doi: 10.1093/aje/155.12.1081 [DOI] [PubMed] [Google Scholar]
- Wilson, R. S., Mendes de Leon, C. F., Barnes, L. L., Schneider, J. A., Bienias, J. L., Evans, D. A., & Bennett, D. A. (2002). Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA, 287(6), 742–748. doi: 10.1001/jama.287.6.742 [DOI] [PubMed] [Google Scholar]
- Wong, R., Michaels-Obregon, A., & Palloni, A. (2017). Cohort profile: The Mexican Health and Aging Study (MHAS). International Journal of Epidemiology, 46(2), e2. doi: 10.1093/ije/dyu263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates, L. A., Ziser, S., Spector, A., & Orrell, M. (2016). Cognitive leisure activities and future risk of cognitive impairment and dementia: Systematic review and meta-analysis. International Psychogeriatrics, 28(11), 1791–1806. doi: 10.1017/S1041610216001137 [DOI] [PubMed] [Google Scholar]
- Zammit, A. R., Bennett, D. A., Hall, C. B., Lipton, R. B., Katz, M. J., & Muniz-Terrera, G. (2020). A latent transition analysis model to assess change in cognitive states over three occasions: Results from the rush memory and aging project. Journal of Alzheimer’s Disease, 73(3), 1063–1073. doi: 10.3233/jad-190778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, C., Noble, J. M., Marder, K., Hartman, J. S., Gu, Y., & Scarmeas, N. (2018). Dietary patterns, physical activity, sleep, and risk for dementia and cognitive decline. Current Nutrition Reports, 7(4), 335–345. doi: 10.1007/s13668-018-0247-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The MHAS data used in these analyses are publicly available (https://mhasweb.org/Home/index.aspx). The code for the analysis will be made available to other researchers upon reasonable request. This secondary data analysis was not preregistered with an analysis plan in an independent, institutional registry.