Abstract
Objectives
A meta-analytic review was conducted to assess the effects of healthy aging, amnestic mild cognitive impairment (MCI), and Alzheimer’s disease (AD) on naturalistic autobiographical memory using the Autobiographical Interview, a widely used, standardized assessment that derives measures of internal (episodic) and external (nonepisodic) details from freely recalled autobiographical narratives.
Methods
A comprehensive literature search identified 21 aging, 6 MCI, and 7 AD studies (total N = 1,556 participants). Summary statistics for internal and external details for each comparison (younger vs older or MCI/AD vs age-matched comparison groups) and effect size statistics were extracted and summarized using Hedges’ g (random effects model) and adjusted for the presence of publication bias.
Results
The pattern of reduced internal and elevated external details in aging was robust and consistent across nearly all 21 studies. MCI and—to a greater extent—AD were associated with reduced internal details, whereas the external detail elevation faded with MCI and AD. Although there was evidence of publication bias on reporting of internal detail effects, these effects remained robust after correction.
Discussion
The canonical changes to episodic memory observed in aging and neurodegenerative disease are mirrored in the free recall of real-life events. Our findings indicate that the onset of neuropathology overwhelms the capacity of older adults to draw upon distributed neural systems to elaborate on past experiences, including both episodic details specific to identified events and nonepisodic content characteristic of healthy older adults’ autobiographical narratives.
Keywords: Aging, Alzheimer’s disease, Autobiographical memory, Episodic memory, Meta-analysis, Mild cognitive impairment
Memory change is the chief cognitive complaint in aging and neurodegenerative disease, especially Alzheimer’s disease (AD) and its prodrome, mild cognitive impairment (MCI). Although standardized assessments are effective in detecting impairment in the context of neuropathology, they lack ecological validity and sensitivity for subtle memory impairment. Naturalistic measures have been increasingly employed to assess memory for real-life experiences with a range of personal relevance, goal-directedness, emotionality, and temporal scale that cannot be achieved with standardized measures that use laboratory stimuli.
One such naturalistic measure—the Autobiographical Interview (Levine et al., 2002)—extracts reliable measures of internal (contextually specific or episodic) and external (nonepisodic) details of personal past events within a single freely recalled narrative. The inception of the Autobiographical Interview was motivated by observations of impaired remote memory in patients with preserved laboratory task performance (Levine et al., 1998). In the original Autobiographical Interview study (Levine et al., 2002), older adults showed reduced production of internal details for autobiographical events selected from five periods across the life span, replicating age-related effects on contextual recall (e.g., McIntyre & Craik, 1987). Older adults also produced more external details relative to younger adults, consistent with the notion that they have trouble suppressing off-target information during recall (Arbuckle & Gold, 1993; Hasher & Zacks, 1988) potentially due to impaired cognitive control capacity (Amer et al., 2016). Alternatively, external detail production may be due to compensation for reduced internal detail production (Devitt et al., 2017; but see Grilli & Sheldon, 2022). The Autobiographical Interview has since been used in over 300 studies in various clinical and healthy samples. Neuroimaging studies have demonstrated key relationships between internal detail recovery on the Autobiographical Interview and key brain regions implicated in autobiographical memory. Individuals with focal hippocampal damage show a profound reduction in internal detail recall, whereas external details are relatively spared (Addis et al., 2007; Miller et al., 2020; Rosenbaum et al., 2008). When damage is distributed, as in neurodegenerative disease or traumatic brain injury, internal details are selectively associated with the integrity of the medial temporal lobes. These associations are echoed in neuroimaging studies with healthy adults. In these cases, internal details are related to structural and functional measures of medial temporal lobe regions (e.g., the hippocampus; Hodgetts et al., 2017; Palombo et al., 2018; Thakral et al., 2020) that are situated in a larger left-lateralized network that includes posterior and anterior medial regions and lateral temporoparietal regions (e.g., Cabeza & St Jacques, 2007; Setton et al., 2022; Svoboda et al., 2006; Thakral et al., 2017). Conversely, elevated external details are observed in cases with damage to the prefrontal cortex (Levine, 2004), more distributed cortical damage (Esopenko & Levine, 2017; McKinnon et al., 2008), or as a result of reduced cognitive control secondary to mental health disorders such as posttraumatic stress disorder (e.g., McKinnon et al., 2015).
The purpose of the present study is to synthesize the literature on the Autobiographical Interview across the spectrum of healthy to pathological aging. Healthy aging is associated with subtle changes in the medial temporal lobes and cortical regions that are involved in event reconstruction and detail recovery. AD is characterized by medial temporal lobe and lateral temporoparietal atrophy. Amnestic MCI is a transitional category with elevated risk of AD (Albert et al., 2011). By the time memory impairment is diagnosed, it is often too late to test interventions for slowing disease progression. While more sensitive measures of memory decline are needed, the heterogeneity of normal aging and disease expression can make it difficult to detect pathological performance. In the current meta-analysis, we assessed the sensitivity of the Autobiographical Interview in 34 studies across these three diagnostic groups. This work provides profiles of impairment across internal and external details, indicating how dissociable mnemonic processes are affected by healthy aging and age-related impairment.
Method
Study Selection and Eligibility
The Autobiographical Interview solicits narrative descriptions of personally experienced events from a specific time and place in the past. In the original protocol (Levine et al., 2002), lifetime periods (e.g., childhood, teenage years, etc.) were used to cue events, but other cues may be used depending on the goals of the study. Additional information beyond the initial recall phase may be elicited through general and specific probing. Transcribed protocols are segmented into detail categories according to a manualized procedure. Details that appear to relate to specific events (e.g., unique events, perceptual, temporal, spatial, or thought/emotion details) are classified as “internal” (episodic). All other details (e.g., semantic, metacognitive, other statements) are classified as “external” (nonepisodic). A more detailed description of this protocol is included in Supplementary Material.
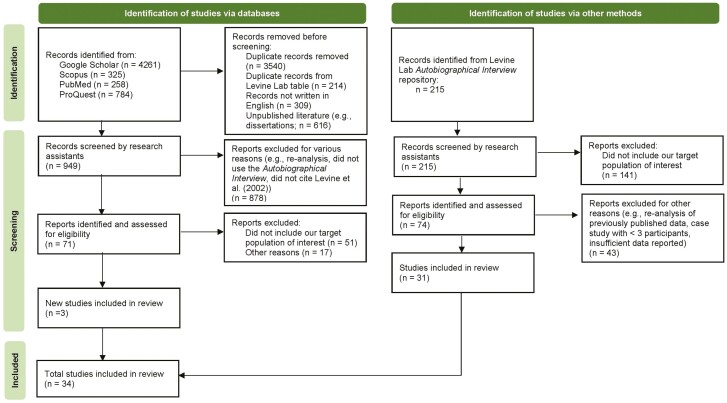
ProQuest, PubMed, Scopus, and Google Scholar databases were searched using the phrase “autobiographical interview” up until May 2021. The Publish or Perish software was used to download Google Scholar entries. Following screening for duplicates, 949 articles were then screened for use of the “standard” Autobiographical Interview (i.e., adhering to the administration and scoring instructions as specified by Levine et al., 2002, allowing for the use of any event cues, probing levels, or recall duration), contrasting young versus old or disorder (MCI, neurodegeneration) versus a healthy age-matched comparison group, and the use of previously unpublished data (verified with the corresponding author when necessary). All retrieved articles were confined to amnestic (as opposed to nonamnestic or mixed) MCI patients. One study not identified in the initial search—published in the Open Science Collaboration Framework’s Reproducibility Project—was included (Vásquez Echeverría, 2015), bringing the total to 34 studies with 1,556 participants included in the final analyses (Figure 1; see Supplementary Material for complete list).
Figure 1.
PRISMA flow diagram of articles searched in the systematic review process.
Data Collection Process
Summary statistics for internal and external details for the identified groups along with inferential statistics concerning group differences (e.g., t, p, and Cohen’s d) were extracted from each article by M. Eskandaripour and S. Simpson. When the required data were unavailable, we contacted the corresponding author(s). In two cases where we did not receive a response, data were extracted from graphical displays using DataThief III (https://www.datathief.org).
We also recorded study design characteristics, such as duration of recall (see Supplementary Material). Memory ages were heterogeneous but largely focused on remote memories of greater than 1 year in age. When different levels of probing were reported, we selected counts from the “highest” level (free recall only: 11%, free recall + general probe: 56%; free recall + general probe + specific probe: 11%; unspecified: 22%).
Outcome Variables
The dependent variables of interest were internal and external detail counts. Some protocols have also used the internal-to-total detail ratio as a proxy of memory “episodicity.” Given that the proportional measure was not always available and that our interest was in the patterns of internal versus external details, we analyzed these measures separately. The Autobiographical Interview manual provides instructions for segregating subcategories of internal and external details that can aid interpretation (see Supplementary Table S1) but these subcategories were not consistently reported across papers. While finer-grain categorization of external detail content can be conducted (e.g., Renoult et al., 2020; Strikwerda-Brown et al., 2019), the “standard” external detail composite unifies nonepisodic content for more reliable comparison to internal detail production (Renoult et al., 2020). As a result, this meta-analysis focused on total internal and external detail counts averaged across event cues.
Statistical Analyses
The Comprehensive Meta-Analysis (CMA) Software Version 3 (https://www.meta-analysis.com/) was used to extract Hedges’ g and standard error (SE) for each study and for combined studies using a random effects model. As the CMA software reports p-values to three decimal places, p-values reported as “p < 000” were written as “p < .001” in the text. The p-values listed in the tables generated by CMA were retained as-is. Publication bias was calculated using the classic fail-safe N (Nfs), which denotes the number of additional “negative” studies needed to bring the p value to greater than alpha (α = 0.05), and computed Egger’s regression intercept, which should be equal to zero in the absence of publication bias (please see Supplementary Material for more information about these meta-analysis measures). Funnel plots, whereby effect size is plotted against SE, were produced for the aging and the combined MCI/AD samples, which could not be processed separately due to the small number of studies. Asymmetrical distribution of studies within the funnel, with studies missing on the left side or near the bottom, indicates publication bias. Figures were produced using the CMA software and R (https://www.R-project.org/).
Results
Healthy Aging
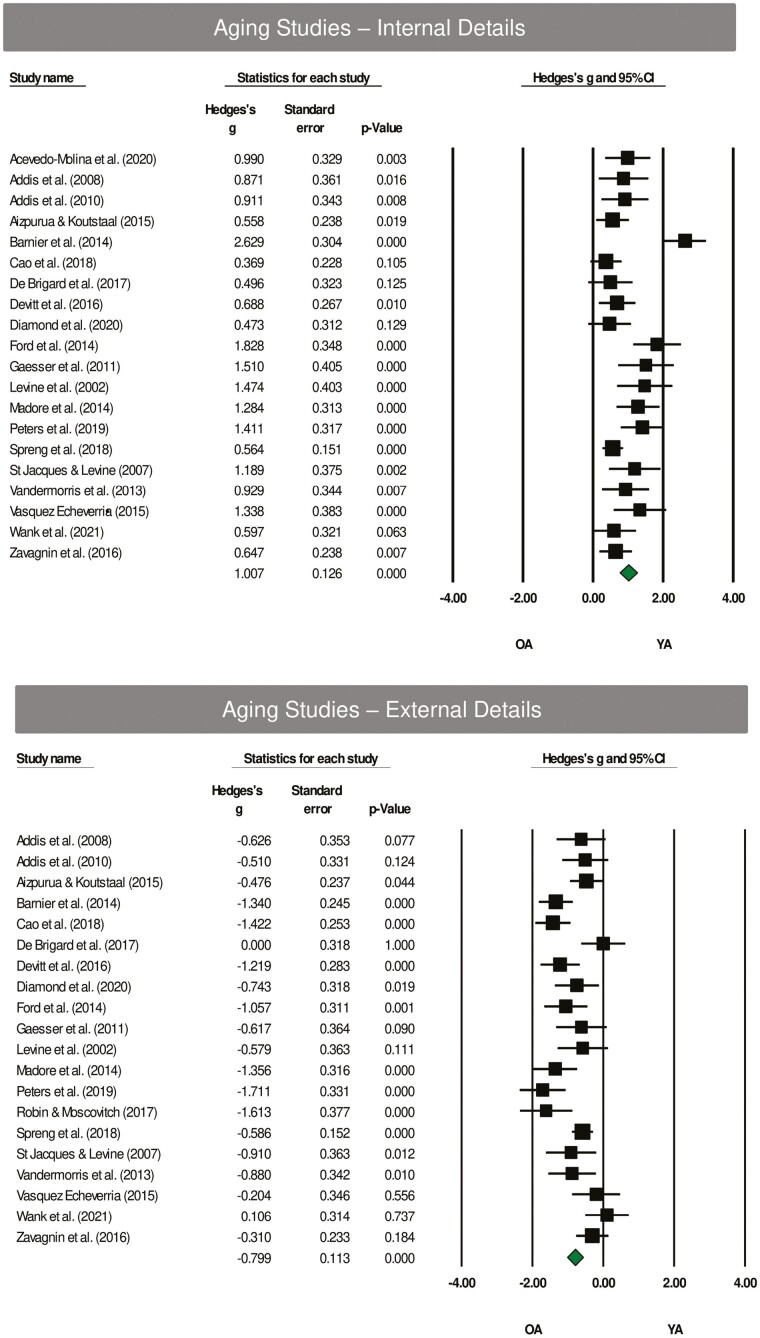
Very large effect sizes were noted for internal details (older < younger; Hedges’ g = 1.007, SE = 0.126, p < .001; Figure 2) and external details (older > younger; Hedges’ g = −0.799, SE = 0.113, p < .001; Figure 2). There were no exceptions to the directionality of the effect for internal details, with the lower-bound confidence intervals greater than 0 for 17/20 studies. The effect for external details was evident in 18/20 studies, with lower-bound confidence intervals less than 0 for 12/20 studies. Interpretation was unaffected by the exclusion of a potential outlier (Barnier et al., 2014).
Figure 2.
Top panel: Forest plot of internal detail effects using the Autobiographical Interview in aging studies. On average, younger adults (YA) generated more internal details relative to older adults (OA), Hedges’ g = 1.007, SE = 0.126, p < .001; Hedges’ g range = 0.369–2.629. Bottom panel: Forest plot of external detail effects using the Autobiographical Interview in aging studies. On average, OA generated more external details relative to YA, Hedges’ g = −0.799, SE = 0.113, p < .001; Hedges’ g range = −1.711 to 0.106. Solid squares = effect size for each study; size of squares = study weight (weighted by sample size); horizontal lines = 95% confidence intervals; diamond = summary effect; width of diamond = precision.
Mild Cognitive Impairment
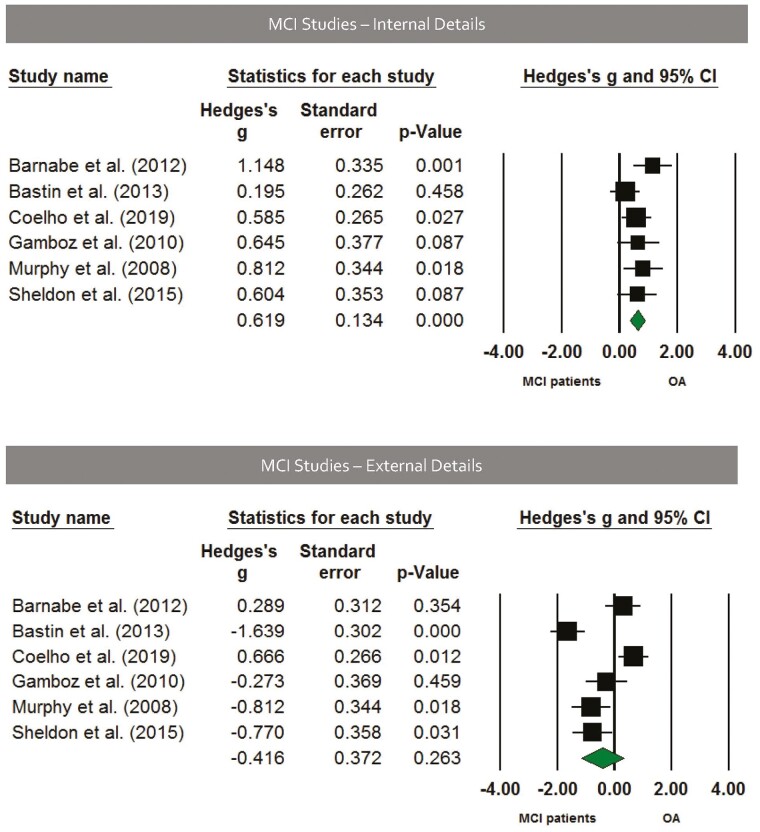
Six studies used the Autobiographical Interview to assess group differences in healthy older adults and patients with MCI. These results show that patients with MCI produced significantly fewer internal details (Hedges’ g = 0.619, SE = 0.134, p < .001; Figure 3) but a similar amount of external details (Hedges’ g = −0.416, SE = 0.372, p = .263; Figure 3) compared to healthy older adults.
Figure 3.
Top panel: Forest plot of internal detail effects using the Autobiographical Interview in mild cognitive impairment (MCI). On average, MCI groups generated fewer details than age-matched comparison groups, Hedges’ g = 0.619, SE = 0.134, p < .001; Hedges’ g range = 0.195–1.148. Bottom panel: Forest plot of external detail effects using the Autobiographical Interview in MCI. On average, patients with MCI generated a similar amount of external details relative to age-matched comparison groups, Hedges’ g = −0.416, SE = 0.372, p = .263; Hedges’ g range = −1.639 to 0.666. Solid squares = effect size for each study; size of squares = study weight (weighted by sample size); horizontal lines = 95% confidence intervals; diamond = summary effect; width of diamond = precision.
Alzheimer’s Disease
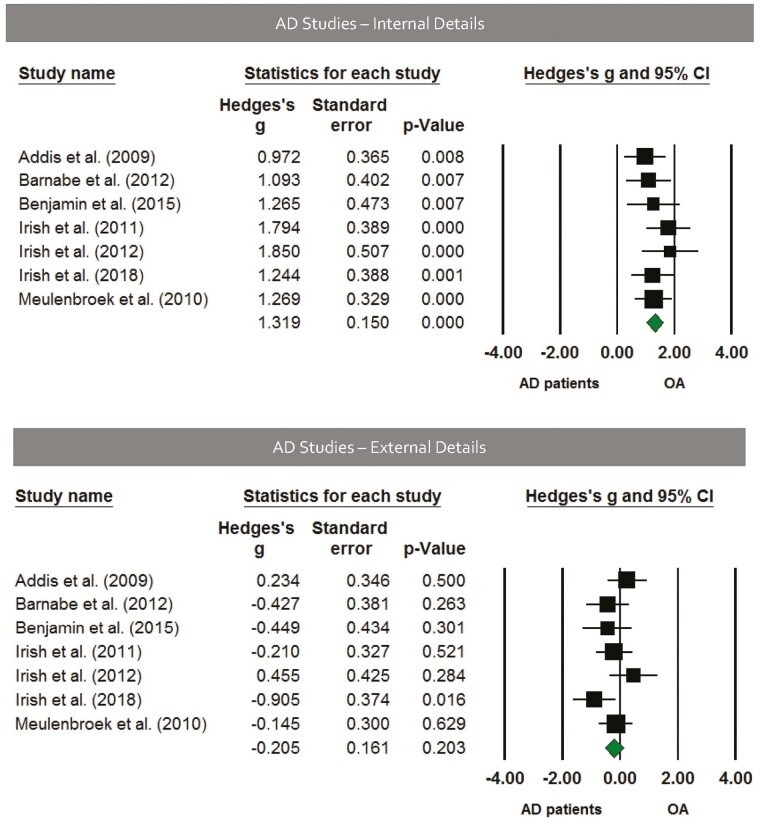
Seven studies used the Autobiographical Interview to assess group differences in healthy older adults and patients with AD. We observed that patients with AD produced fewer internal details (Hedges’ g = 1.319, SE = 0.15, p < .001; Figure 4) but a similar amount of external details compared to their healthy older adult counterparts (Hedges’ g = −0.205, SE = 0.161, p = .203; Figure 4).
Figure 4.
Top panel: Forest plot of internal detail effects using the Autobiographical Interview in Alzheimer’s disease (AD). On average, patients with AD produced fewer internal details than age-matched comparison groups, Hedges’ g = 1.319, SE = 0.15, p < .001; Hedges’ g range = 0.972–1.850. Bottom panel: Forest plot of external detail effects using the Autobiographical Interview in AD. On average, there was no difference between AD groups and age-matched comparison groups, Hedges’ g = −0.205, SE = 0.161, p = .203; Hedges’ g range = −0.905 to 0.455. Solid squares = effect size for each study; size of squares = study weight (weighted by sample size); horizontal lines = 95% confidence intervals; diamond = summary effect; width of diamond = precision.
Autobiographical Interview Effects Withstand Publication Bias
Significant Egger’s regression intercepts were observed for internal details (ps = .016 and .003 for the aging and MCI/AD samples, respectively), consistent with rightward asymmetry in funnel plots (see Supplementary Material), yet the fail-safe numbers were large (Nfs = 1,087, 316 for the aging and MCI/AD samples, respectively) indicating that hundreds of studies would be needed to nullify the observed internal detail effects. The effect size for aging remained large after using Duval and Tweedie’s Trim and Fill adjustment, Hedges’ g = 1.007. The observed value for the MCI/AD group (g = 0.970) remained medium-to-large (Hedges’ g = 0.770) after adjustment. For external details, no bias was observed in either the aging or combined MCI/AD analyses. All Egger regressions were nonsignificant (all ps > .200; see Supplementary Material for complete results).
Discussion
We examined the effects of aging, amnestic MCI, and AD on autobiographical memory as assessed by the Autobiographical Interview (Levine et al., 2002). Effects on internal (episodic) and external (nonepisodic) detail retrieval were extracted from 34 studies comprising 1,556 participants. Overall results are summarized in Figure 5. The observed effects held after adjustment for publication bias. There was a robust aging effect such that older adults recalled fewer internal details but an excess of external details relative to younger adults. The juxtaposition of healthy and pathological aging provides insight into the evolution of changes in autobiographical memory with disease progression. There was a graded effect on internal details, with magnification of impairment from MCI to AD, whereas the effect of increased external details was reduced in the MCI and AD samples. Our findings indicate that the onset of neuropathology overwhelms the capacity of healthy older adults to draw upon distributed neural systems to elaborate on past experiences, including both episodic details specific to identified events and nonepisodic content characteristic of healthy older adults’ autobiographical narratives. Results from this meta-analysis provide a general benchmark of effect size which will guide prospective research in the area of aging and cognition.
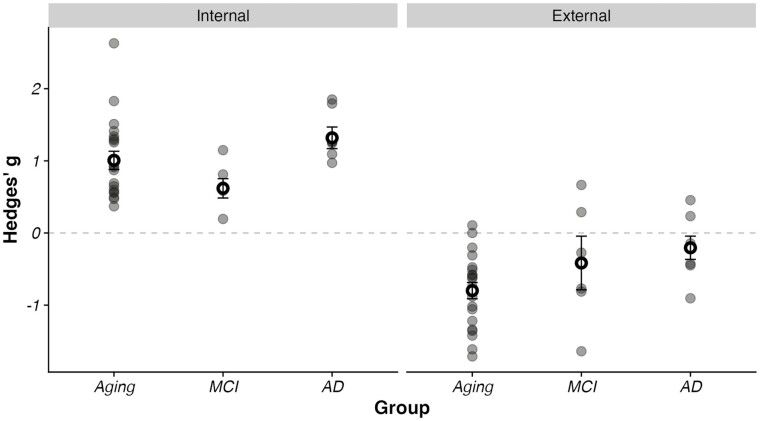
Figure 5.
Observed effect sizes for each individual study by internal details (left) and external details (right) for aging, mild cognitive impairment (MCI), and Alzheimer’s disease (AD). Each study is represented by a filled dot. Circles reflect group-level average Hedges’ g with standard error bars represented. There were large effects for internal details in all three comparisons, with a graded increase in effect size evident for AD versus MCI. External details were elevated in aging, MCI, and eliminated in AD. (Note that effects for MCI and AD are relative to age-matched comparison groups, isolating the effects of clinical classification from aging effects. In other words, MCI and AD effects would appear larger against a younger demographic, as is the case for our aging analysis.)
Healthy Aging
Aging is associated with reduced internal and increased external details on the Autobiographical Interview (Levine et al., 2002). This finding of impoverished internal detail generation is remarkably robust even after correction for publication bias, with no exceptions to this pattern across 20 studies. As the pattern reflects both decreases and increases in internal and external detail counts, respectively, it cannot be attributed to overall changes in retrieval output between young and older adults.
In retrospective tests of autobiographical memory, events are self-selected, introducing bias and lack of control over event characteristics, such as frequency of rehearsal, emotionality, and importance to self. Recovered details are presumed to reflect memory processing, yet they are unverified. However, the same aging pattern is evident with experimenter-controlled staged events in which memory accuracy is confirmed against the ground truth (Diamond, Armson, et al., 2020). These findings bolster interpretations of group differences in internal details as pertaining to memory processes and representations corresponding to actual experiences and not extraneous factors, such as differences in event selection, retention interval, or narrative style (Aizpurua & Koutstaal, 2015).
The profile of attenuated internal and elevated external detail recall in older adults can be viewed as a joint expression of attenuated episodic memory retrieval capacity and reduced filtering or compensatory production of nonepisodic content relative to younger adults. There is a wealth of evidence to suggest that subtle age-related changes in medial temporal lobe structure and function (Dickerson et al., 2009; Head et al., 2008) hinder older adults’ ability to retrieve highly specific details. External details are incidental to the identified main task in the Autobiographical Interview (i.e., to describe details of specific events). Elevated external detail production is observed in association with prefrontal cortex damage and associated executive functioning impairment (Levine, 2004) as well as with reduced cognitive control that accompanies certain mental health disorders (McKinnon et al., 2015). Accordingly, external detail production in healthy aging has been related to changes in the functional dynamics across networks implicated in executive and memory function (Fenerci et al., 2022). Lacking efficient, high-fidelity access to episodic details, older adults rely upon preserved accumulated knowledge such as that represented in lateral temporal structures (Maguire & Frith, 2003), potentially as a compensatory mechanism given their low internal detail recall (Devitt et al., 2017).
When categories of external details have been reported, these are predominantly semantic (e.g., Bastin et al., 2013; Levine et al., 2002), particularly personal semantics (facts about oneself) as opposed to general semantics (facts about the world; Acevedo-Molina et al., 2020; Renoult et al., 2020; but see Coelho et al., 2019 and Meulenbroek et al., 2010). However, external details should not be held as a measure of functional capacity in the semantic or any other system because they are incidental to the main task. For example, younger adults’ low production of external details should not be held as evidence of impairment. When older adults were prompted to provide personal and general semantic—and not episodic—content, they generated an excess of personal semantic details (autobiographical facts) regardless of instructions (Melega et al., submitted). Rather than interpret older adults’ pattern of internal and external detail recall solely through the lens of impairment or compensation for impairment, it could be that older adults broaden their narratives with personal semantic content because that is what matters to them (Grilli & Sheldon, 2022).
MCI and AD
Age-related reduction in internal detail production is magnified in people with MCI and AD. The effect is graded: variable for the MCI studies (Hedges’ g 0.195–1.148; Figure 4) but consistent and large for AD (Hedges’ g 0.972–1.850). While the effect size for MCI was smaller than that observed for healthy younger versus older adults, the comparison group for MCI was age-matched, meaning that the effect size is isolated to MCI effects that are presumed to be additive to those of aging. The AD effect was very large even in comparison to age-matched controls—similar to that observed in patients with hippocampal lesions (Miller et al., 2020). Together, these findings suggest that the age-related impairment in recalling internal details is intensified in people with MCI and AD, while their capacity to recover external details is preserved.
Given the known medial temporal lobe pathology that characterizes MCI and AD (e.g., Killiany et al., 2000; Petersen & Negash, 2008), these data support the claim that internal detail production on the Autobiographical Interview is sensitive to pathology in these areas. Early signs of AD-related network-level dysfunction are evident in the posterior medial region of the default mode network (Jones et al., 2016), a crucial hub in the construction of multidimensional event representations that form episodic memories (e.g., Ranganath & Ritchey, 2012). Thus, the loss of highly contextualized memory recall in MCI and AD corresponds to major changes in the anatomy and functionality of core brain regions that support episodic memory processing and may help explain the evolution from healthy to pathological aging (see Peterson & Negash, 2008 for further discussion).
There was weak evidence for external detail elevation in MCI relative to age-matched controls. While individuals with MCI may draw upon intact semantic function to augment their narratives as in healthy aging, they do so less consistently. In MCI, atrophy is primarily restricted to the medial temporal lobes (Petersen & Negash, 2008), while areas that support semantic processing (e.g., lateral temporal cortices, temporal pole; Svoboda et al., 2006) are still intact. In AD, however, the effect size for external details was close to zero, with only one of seven studies showing a significant effect. The presence of normal temporal pole activation during autobiographical memory recall in AD (Meulenbroek et al., 2010) supports the finding here that AD patients have the capacity to draw on semantic content, but this is insufficient to produce a greater shift toward nonepisodic processing as seen in healthy aging and, to a lesser extent, MCI.
Methodological Considerations
Approximately a quarter of the studies in the meta-analysis used the original lifetime periods from Levine et al. (2002), more than 50% probed time periods in the 1- to 10-year range, and the remainder were either unrestricted (6%) or did not report a time frame (14%; see Supplementary Material). Only a handful of the studies in the present sample considered memory recency, contrasting events occurring within the past year to more remote events that are expected to have lower internal detail scores. Remoteness effects indexed by lifetime period (e.g., childhood, early adulthood, etc.) cannot be independently assessed in aging, due to the confound with participant age (Levine et al., 2002). Nevertheless, there was no evidence to suggest that remoteness interacts with group in any study in which both of these effects were probed (e.g., Acevedo-Molina et al., 2020; Addis et al., 2008; Aizpurua & Koutstaal, 2015). In fact, we showed that age effects appeared even after a 2-day delay (Diamond, Abdi, et al., 2020). Similarly, there was no evidence for differential recency by group effects in the MCI (Barnabe et al., 2012; Bastin et al., 2013) or AD samples (e.g., Addis et al., 2009; Barnabe et al., 2012; Meulenbroek et al., 2010). More data with consistently controlled time period cues are required to formally assess the effects of recency across groups.
Recency effects are of theoretical interest in studies of patients with medial temporal lobe damage (e.g., Gilboa & Moscovitch, 2021), who tend to remember recent experiences with less specificity than remote memories. The Autobiographical Interview has been used to test competing theories concerning hippocampal involvement in recent versus remote memory (Kirwan et al., 2008; Rosenbaum et al., 2008). In the standard Autobiographical Interview administration, however, memory accessibility is confounded with recency. That is, only the most accessible remote events are selected because inaccessible events would not come to mind upon cueing. These more accessible remote events are likely well-rehearsed and therefore supported by semantic memory. Some researchers have used event cues selected by others to reduce such accessibility effects (e.g., Gilboa, 2004). The use of prospective memory paradigms for testing at specific delay periods can also address this confound (e.g., Diamond, Armson, et al., 2020), although it is impractical for questions concerning memory for very remote events across the life span.
Limitations
Our analysis of amnestic MCI and AD assumes homogeneity of patient characteristics across studies, which may not be justified given variability in diagnostic criteria and disease stage. Inferences of neural atrophy were derived from clinical diagnosis and not direct measurements. That said, it is safe to assume that pathology is greatest in AD, followed by MCI, then healthy aging. The effect of AD on internal details was very robust, falling well into the large range for all eight studies, indicating that the catastrophic mnemonic systems failure in this disease overshadows any noise owing to heterogeneous clinical samples.
Due to the limitations of the available data, we analyzed the effects for internal and external detail composites separately. It is generally more effective to analyze internal and external detail production jointly in a single analysis (e.g., given two groups, a 2 × 2 general linear model) or an internal-to-total detail ratio to control for protocol length (see Miloyan et al., 2019). One might predict that joint consideration of internal and external details would enhance effect size expression in aging (Levine et al., 2002), but we were unable to directly test this prediction given the available data. While the incorporation of total detail counts (either as a full factorial analysis of internal and external details or as a ratio) is recommended, correction using word counts (e.g., Setton et al., 2022) is not because it is the detail and not the word that is the unit of analysis in the Autobiographical Interview. The correction of composites by word count assumes equal scaling of verbosity across internal and external details, which is not the case (i.e., external details contain more words than internal details; Genugten & Schacter, 2022).
Finally, many studies included in this meta-analysis relied on relatively small sample sizes that could have inflated effect sizes and increased the risk of Type I error. Indeed, there was evidence of publication bias for internal details. Under the assumption that researchers were primarily concerned with internal details, this suggests that smaller effect sizes for internal, but not external details, were unpublished, remaining in the “file drawer.” Nonetheless, internal detail effect sizes remained robust after correction for publication bias (see Supplementary Materials).
Conclusion
The loss of memory for real-life events is one of the most devastating consequences of neurodegenerative disease, as these memories serve to strengthen our identity. The Autobiographical Interview is an effective tool for assessing episodic and nonepisodic elements of these events that are not readily captured via existing standard laboratory tests of memory. This review indicates the extent to which the long-established episodic memory impairment in MCI and AD extends to autobiographical memory, and further illustrates how profiles of recovered episodic and nonepisodic content can differentiate groups across the spectrum of age-related impairment. Autobiographical memory, assessed via internal details, progressively declines across healthy aging through to the stages of neurodegeneration. These findings reflect structural and functional neuroanatomical changes that accompany normal aging and pathology in the medial temporal lobes (particularly the hippocampus) and connected structures. Age-related enhancement of nonepisodic (external) details—be it either a sign of reduced cognitive control over memory or an indicator of compensation—is eclipsed by progressive neuropathology affecting event recovery, further reducing patients’ expression of their personal past events. The implications of these findings extend beyond aging and neurodegeneration, as autobiographical memory is affected in many other neurological and mental health conditions. Overall, this work advocates for the joint consideration of internal and external detail composites in differentiating between healthy and pathological aging profiles.
Supplementary Material
Acknowledgments
We are grateful to Catherine Le and Tolu Faromika for technical assistance. Finally, we thank the researchers who shared data for use in this meta-analysis.
Contributor Information
Stephanie Simpson, Rotman Research Institute at Baycrest Health Sciences, Toronto, Ontario, Canada; Department of Psychology, University of Toronto, Toronto, Ontario, Canada.
Mona Eskandaripour, Rotman Research Institute at Baycrest Health Sciences, Toronto, Ontario, Canada; Department of Psychology, University of Toronto, Toronto, Ontario, Canada.
Brian Levine, Rotman Research Institute at Baycrest Health Sciences, Toronto, Ontario, Canada; Department of Psychology, University of Toronto, Toronto, Ontario, Canada.
Funding
This work was supported by a Canadian Institutes of Health Research—Aging grant (MOP-148940 to B. Levine). S. Simpson was supported by a Natural Sciences and Engineering Research Council of Canada Postgraduate Scholarship-Doctoral award. This funding body had no role in design of the study, data collection, analysis, and/or interpretation of data, nor writing this manuscript.
Conflict of Interest
None declared.
Data Availability
Data used to conduct this meta-analysis are available on request. This study was not preregistered.
References
- Acevedo-Molina, M. C., Matijevic, S., & Grilli, M. D. (2020). Beyond episodic remembering: Elaborative retrieval of lifetime periods in young and older adults. Memory, 28(1), 83–93. doi: 10.1080/09658211.2019.1686152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis, D. R., Moscovitch, M., & McAndrews, M. P. (2007). Consequences of hippocampal damage across the autobiographical memory network in left temporal lobe epilepsy. Brain, 130, 2327–2342. doi: 10.1016/j.neuropsychologia.2005.01.001 [DOI] [PubMed] [Google Scholar]
- Addis, D. R., Sacchetti, D. C., Ally, B. A., Budson, A. E., & Schacter, D. L. (2009). Episodic simulation of future events is impaired in mild Alzheimer’s disease. Neuropsychologia, 47(12), 2660–2671. doi: 10.1016/j.neuropsychologia.2009.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis, D. R., Wong, A. T., & Schacter, D. L. (2008). Age-related changes in the episodic simulation of future events. Psychological Science (0956-7976), 19(1), 33–41. doi: 10.1111/j.1467-9280.2008.02043.x [DOI] [PubMed] [Google Scholar]
- Aizpurua, A., & Koutstaal, W. (2015). A matter of focus: Detailed memory in the intentional autobiographical recall of older and younger adults. Consciousness and Cognition, 33, 145–155. doi: 10.1016/j.concog.2014.12.006 [DOI] [PubMed] [Google Scholar]
- Albert, M. S., DeKosky, S. T., Dickson, D., Dubois, B., Feldman, H. H., Fox, N. C., Gamst, A., Holtzman, D. M., Jagust, W. J., Petersen, R. C., Snyder, P. J., Carrillo, M. C., Thies, B., & Phelps, C. H. (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7(3), 270–279. doi: 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer, T., Campbell, K. L., & Hasher, L. (2016). Cognitive control as a double-edged sword. Trends in Cognitive Sciences, 20(12), 905–915. doi: 10.1016/j.tics.2016.10.002 [DOI] [PubMed] [Google Scholar]
- Arbuckle, T. Y., & Gold, D. P. (1993). Aging, inhibition, and verbosity. Journal of Gerontology, 48(5), P225–P232. doi: 10.1093/geronj/48.5.p225 [DOI] [PubMed] [Google Scholar]
- Barnabe, A., Whitehead, V., Pilon, R., Arsenault-Lapierre, G., & Chertkow, H. (2012). Autobiographical memory in mild cognitive impairment and Alzheimer’s disease: A comparison between the Levine and Kopelman interview methodologies. Hippocampus, 22(9), 1809–1825. doi: 10.1002/hipo.22015 [DOI] [PubMed] [Google Scholar]
- Barnier, A. J., Priddis, A. C., Broekhuijse, J. M., Harris, C. B., Cox, R. E., Addis, D. R., Keil, P. G., & Congleton, A. R. (2014). Reaping what they sow: Benefits of remembering together in intimate couples. Journal of Applied Research in Memory and Cognition, 3(4), 261–265. doi: 10.1016/j.jarmac.2014.06.003 [DOI] [Google Scholar]
- Bastin, C., Feyers, D., Jedidi, H., Bahri, M. A., Degueldre, C., Lemaire, C., Collette, F., & Salmon, E. (2013). Episodic autobiographical memory in amnestic mild cognitive impairment: What are the neural correlates? Human Brain Mapping, 34(8), 1811–1825. doi: 10.1002/hbm.22032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza, R., & St Jacques, P. (2007). Functional neuroimaging of autobiographical memory. Trends in Cognitive Sciences, 11(5), 219–227. doi: 10.1016/j.tics.2007.02.005 [DOI] [PubMed] [Google Scholar]
- Coelho, S., Guerreiro, M., Chester, C., Silva, D., Maroco, J., Paglieri, F., & de Mendonça, A. (2019). Mental time travel in mild cognitive impairment. Journal of Clinical and Experimental Neuropsychology, 41(8), 845–855. doi: 10.1080/13803395.2019.1632269 [DOI] [PubMed] [Google Scholar]
- Devitt, A. L., Addis, D. R., & Schacter, D. L. (2017). Episodic and semantic content of memory and imagination: A multilevel analysis. Memory & Cognition, 45, 1078–1094. doi: 10.3758/s13421-017-0716-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond, N. B., Abdi, H., & Levine, B. (2020). Different patterns of recollection for matched real-world and laboratory-based episodes in younger and older adults. Cognition, 202, 104309. doi: 10.1016/j.cognition.2020.104309 [DOI] [PubMed] [Google Scholar]
- Diamond, N. B., Armson, M. J., & Levine, B. (2020). The truth is out there: Accuracy in recall of verifiable real-world events. Psychological Science, 31(12), 1544–1556. doi: 10.1177/0956797620954812 [DOI] [PubMed] [Google Scholar]
- Dickerson, B. C., Feczko, E., Augustinack, J. C., Pacheco, J., Morris, J. C., Fischl, B., & Buckner, R. L. (2009). Differential effects of aging and Alzheimer’s disease on medial temporal lobe cortical thickness and surface area. Neurobiology of Aging, 30(3), 432–440. doi: 10.1016/j.neurobiolaging.2007.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esopenko, C., & Levine, B. (2017). Autobiographical memory and structural brain changes in chronic phase TBI. Cortex, 89, 1–10. doi: 10.1016/j.cortex.2017.01.007 [DOI] [PubMed] [Google Scholar]
- Fenerci, C., Gurguryan, L., Spreng, R. N., & Sheldon, S. (2022). Comparing neural activity during autobiographical memory retrieval between younger and older adults: An ALE meta-analysis. Neurobiology of Aging, 119, 8–21. doi: 10.1016/j.neurobiolaging.2022.06.009 [DOI] [PubMed] [Google Scholar]
- van Genugten, R., & Schacter, D. L. (2022). Automated scoring of the Autobiographical Interview with natural language processing. PsyArXiv. doi: 10.31234/osf.io/nyurm [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa, A. (2004). Remembering our past: Functional neuroanatomy of recollection of recent and very remote personal events. Cerebral Cortex, 14(11), 1214–1225. doi: 10.1093/cercor/bhh082 [DOI] [PubMed] [Google Scholar]
- Gilboa, A., & Moscovitch, M. (2021). No consolidation without representation: Correspondence between neural and psychological representations in recent and remote memory. Neuron, 109(14), 2239–2255. doi: 10.1016/j.neuron.2021.04.025 [DOI] [PubMed] [Google Scholar]
- Grilli, M. D., & Sheldon, S. (2022). Autobiographical event memory and aging: Older adults get the gist. Trends in Cognitive Sciences, 26(12), 1079–1089. doi: 10.1016/j.tics.2022.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasher, L., & Zacks, R. T. (1988). Working memory, comprehension, and aging: A review and a new view. In Bower G. H. (Ed.), Psychology of learning and motivation (Vol. 22, pp. 193–225). Academic Press. doi: 10.1016/S0079-7421(08)60041-9 [DOI] [Google Scholar]
- Head, D., Rodrigue, K. M., Kennedy, K. M., & Raz, N. (2008). Neuroanatomical and cognitive mediators of age-related differences in episodic memory. Neuropsychology, 22(4), 491–507. doi: 10.1037/0894-4105.22.4.491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgetts, C. J., Postans, M., Warne, N., Varnava, A., Lawrence, A. D., & Graham, K. S. (2017). Distinct contributions of the fornix and inferior longitudinal fasciculus to episodic and semantic autobiographical memory. Cortex; A Journal Devoted to the Study of the Nervous System and Behavior, 94, 1–14. doi: 10.1016/j.cortex.2017.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, D. T., Knopman, D. S., Gunter, J. L., Graff-Radford, J., Vemuri, P., Boeve, B. F., Petersen, R. C., Weiner, M. W., & Jack, C. R.; Alzheimer’s Disease Neuroimaging Initiative (2016). Cascading network failure across the Alzheimer’s disease spectrum. Brain, 139(2), 547–562. doi: 10.1093/brain/awv338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killiany, R. J., Gomez-Isla, T., Moss, M., Kikinis, R., Sandor, T., Jolesz, F., Tanzi, R., Jones, K., Hyman, B. T., & Albert, M. S. (2000). Use of structural magnetic resonance imaging to predict who will get Alzheimer’s disease. Annals of Neurology, 47(4), 430–439. doi: [DOI] [PubMed] [Google Scholar]
- Kirwan, C. B., Bayley, P. J., Galván, V. V., & Squire, L. R. (2008). Detailed recollection of remote autobiographical memory after damage to the medial temporal lobe. Proceedings of the National Academy of Sciences of the United States of America, 105(7), 2676–2680. doi: 10.1073/pnas.0712155105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, B. (2004). Autobiographical memory and the self in time: Brain lesion effects, functional neuroanatomy, and lifespan development. Brain and Cognition, 55(1), 54–68. doi: 10.1016/S0278-2626(03)00280-X [DOI] [PubMed] [Google Scholar]
- Levine, B., Black, S. E., Cabeza, R., Sinden, M., Mcintosh, A. R., Toth, J. P., Tulving, E., & Stuss, D. T. (1998). Episodic memory and the self in a case of isolated retrograde amnesia. Brain, 121(10), 1951–1973. [DOI] [PubMed] [Google Scholar]
- Levine, B., Svoboda, E., Hay, J. F., Winocur, G., & Moscovitch, M. (2002). Aging and autobiographical memory: Dissociating episodic from semantic retrieval. Psychology and Aging, 17(4), 677–689. doi: 10.1037/0882-7974.17.4.677 [DOI] [PubMed] [Google Scholar]
- Maguire, E. A., & Frith, C. D. (2003). Aging affects the engagement of the hippocampus during autobiographical memory retrieval. Brain, 126(7), 1511–1523. doi: 10.1093/brain/awg157 [DOI] [PubMed] [Google Scholar]
- McIntyre, J. S., & Craik, F. I. M. (1987). Age differences in memory for item and source information. Canadian Journal of Psychology/Revue Canadienne de Psychologie, 41(2), 175–192. doi: 10.1037/h0084154 [DOI] [PubMed] [Google Scholar]
- McKinnon, M. C., Nica, E. I., Sengdy, P., Kovacevic, N., Moscovitch, M., Freedman, M., Miller, B. L., Black, S. E., & Levine, B. (2008). Autobiographical memory and patterns of brain atrophy in fronto-temporal lobar degeneration. Journal of Cognitive Neuroscience, 20(10), 1839–1853. doi: 10.1162/jocn.2008.20126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon, M. C., Palombo, D. J., Nazarov, A., Kumar, N., Khuu, W., & Levine, B. (2015). Threat of death and autobiographical memory: A study of passengers from Flight AT236. Clinical Psychological Science: A Journal of the Association for Psychological Science, 3(4), 487–502. doi: 10.1177/2167702614542280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melega, G., Lancelotte F., Johnen, A.-K., Hornberger, M., Levine, B., Renoult, L. (Submitted). Evoking episodic and semantic details with instructional manipulation: The semantic Autobiographical Interview. https://psyarxiv.com/6h23t/ [DOI] [PubMed]
- Meulenbroek, O., Rijpkema, M., Kessels, R. P. C., Rikkert, M. G. M. O., & Fernández, G. (2010). Autobiographical memory retrieval in patients with Alzheimer’s disease. Neuroimage, 53(1), 331–340. doi: 10.1016/j.neuroimage.2010.05.082 [DOI] [PubMed] [Google Scholar]
- Miller, T. D., Chong, T. T.-J., Aimola Davies, A. M., Johnson, M. R., Irani, S. R., Husain, M., Ng, T. W., Jacob, S., Maddison, P., Kennard, C., Gowland, P. A., & Rosenthal, C. R. (2020). Human hippocampal CA3 damage disrupts both recent and remote episodic memories. eLife, 9, e41836. doi: 10.7554/eLife.41836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miloyan, B., McFarlane, K., & Vásquez-Echeverría, A. (2019). The adapted Autobiographical Interview: A systematic review and proposal for conduct and reporting. Behavioural Brain Research, 370, 111881. doi: 10.1016/j.bbr.2019.03.050 [DOI] [PubMed] [Google Scholar]
- Palombo, D. J., Bacopulos, A., Amaral, R. S. C., Olsen, R. K., Todd, R. M., Anderson, A. K., & Levine, B. (2018). Episodic autobiographical memory is associated with variation in the size of hippocampal subregions. Hippocampus, 28(2), 69–75. doi: 10.1002/hipo.22818 [DOI] [PubMed] [Google Scholar]
- Petersen, R. C., & Negash, S. (2008). Mild cognitive impairment: An overview. CNS Spectrums, 13(1), 45–53. doi: 10.1017/s1092852900016151 [DOI] [PubMed] [Google Scholar]
- Ranganath, C., & Ritchey, M. (2012). Two cortical systems for memory-guided behaviour. Nature Reviews Neuroscience, 13(10), Article 10. doi: 10.1038/nrn3338 [DOI] [PubMed] [Google Scholar]
- Renoult, L., Armson, M. J., Diamond, N. B., Fan, C. L., Jeyakumar, N., Levesque, L., Oliva, L., McKinnon, M., Papadopoulos, A., Selarka, D., St Jacques, P. L., & Levine, B. (2020). Classification of general and personal semantic details in the Autobiographical Interview. Neuropsychologia, 144, 107501. doi: 10.1016/j.neuropsychologia.2020.107501 [DOI] [PubMed] [Google Scholar]
- Rosenbaum, R. S., Moscovitch, M., Foster, J., Schnyer, D. M., Gao, F., Kovacevic, N., Verfaellie, M., Black, S., & Levine, B. (2008). Patterns of autobiographical memory loss in medial–temporal lobe amnesic patients. Journal of Cognitive Neuroscience, 20(8), 1490–1506. doi: 10.1162/jocn.2008.20105 [DOI] [PubMed] [Google Scholar]
- Setton, R., Mwilambwe-Tshilobo, L., Sheldon, S., Turner, G. R., & Spreng, R. N. (2022). Hippocampus and temporal pole functional connectivity is associated with age and individual differences in autobiographical memory. Proceedings of the National Academy of Sciences of the United States of America, 119(41), e2203039119. doi: 10.1073/pnas.2203039119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strikwerda-Brown, C., Mothakunnel, A., Hodges, J. R., Piguet, O., & Irish, M. (2019). External details revisited—A new taxonomy for coding ‘non-episodic’ content during autobiographical memory retrieval. Journal of Neuropsychology, 13(3), 371–397. doi: 10.1111/jnp.12160 [DOI] [PubMed] [Google Scholar]
- Svoboda, E., McKinnon, M. C., & Levine, B. (2006). The functional neuroanatomy of autobiographical memory: A meta-analysis. Neuropsychologia, 44(12), 2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral, P. P., Madore, K. P., Kalinowski, S. E., & Schacter, D. L. (2020). Modulation of hippocampal brain networks produces changes in episodic simulation and divergent thinking. Proceedings of the National Academy of Sciences of the United States of America, 117(23), 12729–12740. doi: 10.1073/pnas.2003535117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral, P. P., Madore, K. P., & Schacter, D. L. (2017). A role for the left angular gyrus in episodic simulation and memory. The Journal of Neuroscience, 37(34), 8142–8149. doi: 10.1523/jneurosci.1319-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vásquez Echeverría, A. (2015). Replication of “age-related changes in the episodic simulation of future events” in estimating the reproducibility of psychological science. Science, 349(6251), aac4716.26315443 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used to conduct this meta-analysis are available on request. This study was not preregistered.