Abstract
Objectives
The goal of this systematic review was to analyze, in randomized controlled clinical trials (RCTs), regenerative techniques used to treat peri-implantitis (PI).
Methods
Three databases (PubMed/Medline, EMBASE, and On-Line Knowledge Library) were accessed, applying the PICO strategy (Population [P], Intervention [I], Comparison [C], and Outcomes [O]), with the following focused questions: (i) “In patients who received regenerative treatments for peri-implantitis (P), is the regenerative surgical treatment (I) clinically effective and predictable compared to non-regenerative (C) to treat PI (O)?”; and (ii) “In patients who received regenerative treatments for peri-implantitis (P), the regenerative approach (I), compared to non-regenerative (C), significantly increase the prognosis and implant survival rate in the mid- and long-term (O)?” The inclusion criteria were RCTs published in English between 2012 and 2022, with at least a one-year follow-up, which applied regenerative techniques to treat peri-implantitis. Cochrane’s collaboration tool for assessing the risk of bias was used.
Main results
Nine articles were included with 404 patients (225 females and 179 males; mean age of 60.44 years). One study evaluated patients after 48 months and another after 88 months. The techniques and devices used were: (i) implantoplasty with Er:YAG laser, (ii) blood concentrate (growth factors), and (iii) EMD, with no statistically significant outcome. Two studies considered the use of titanium granules with a significant increase in radiographic bone identification, whereas regenerative techniques with bone graft (autogenous, alloplastic, and xenograft) were the majority chosen, associated or not, with a collagen membrane. Xenograft had better results radiographically when compared to the autogenous bone graft and presented better results for bone level. There was an overall decrease in bleeding on probing, independent of the control or test group, and a reduction in pocket depth in the groups analyzed. Titanium granules, EMD, Er:YAG laser, and CGF had non-significant results; better results were observed when using bone grafts. The RoB showed a low risk in four studies (44.44%), three with moderate (33.33%), and two with high risk (22.23%).
Conclusion
Surgical regenerative treatment was a predictable option in the management of PI and in improving the clinical parameters of peri-implant tissues in the short term, mainly when using porous titanium granules, alloplastic bone grafts, and xenografts.
Keywords: Peri-implantitis, Regeneration, Surgery, Treatment, Systematic review
1. Introduction
Osseointegration understanding became dental implants a feasible and effective choice for oral rehabilitation (Fernandes et al., 2021a). Even though it is a complex process, it depends on the successful result of bone-implant contact (BIC) (Branemark et al., 2001). The clinical assessment involves the mechanical anchorage of the implant into the bone, permitting regular oral functions, which are obtained after several weeks (Puleo and Nanci, 1999). Even with a high success rate (Borges et al., 2020), clinicians face a significant increase in peri-implant diseases. In fact, implant failure may cause supporting bone loss, and around 25% of the patients rehabilitated with implants may develop peri-implant diseases (Wang et al., 2017).
Peri-implantitis (PI) is a pathological condition associated with bacterial colonization. It is an emphatic and crescent problem characterized by the inflammatory process around the implant, commonly associated with suppuration, involving soft and hard tissue loss (Sanz and Chapple, 2012, Poli et al., 2017). It is characterized by the loss of supporting bone and soft tissue inflammation (Berglundh et al., 2019), reaching a pocket depth (PD) of ≥ 6 mm, bleeding upon probing (BoP), or suppuration and radiographic bone loss (RBL) ≥ 3 mm (Berglundh et al., 2018). Then, the main goal of peri-implantitis treatment is to eliminate inflammation, preserve the structure around the implant, and bring a healthy status to the tissues (de Waal et al., 2013, Fernandes, 2021b). Moreover, some authors reported that PI is between 2.7% and 47.1%, and the success rate in high-risk groups (patients with a family or personal history of periodontitis and smokers) can be around 70% (Zitzmann et al., 2008).
In this sense, the correct diagnosis and selection of an accurate therapy for PI treatment should aim to reduce/eliminate local inflammation and bone loss. The steps for PI therapy should include (i) the control of the infection, (ii) debridement, corrective surgeries, or regenerative surgical procedures when necessary, and (iii) supportive therapy (Murray et al., 2013, Renvert and Polyzois, 2018). In an umbrella review, Martins et al. (2022) concluded that surgical therapy had a better outcome than non-surgical therapies for PI treatment. Surgical regenerative therapy is mainly based on the principles of bone regeneration or guided bone regeneration (GBR), which must be considered if there is a vertical infrabony defect with two or three walls compromised (Mishler & Shiau, 2014). The regenerative technique used bone substitutes/grafts associated with resorbable or non-resorbable membrane (barrier) to promote bone regeneration (Khoury and Buchmann, 2001, Lang and Lindhe, 2015). It can be used for autologous bone, allograft, xenograft, or synthetic bone materials, or even a mixture of them (Fernandes et al., 2009, Aghazadeh et al., 2012, Fernandes et al., 2012 Wang et al., 2021, Monteiro et al., 2022) always covered by membranes. However, several other adjunctive techniques have been suggested with the proposal to regenerate the tissues around the implants, such as methods for detoxifying implant surfaces, implantoplasty, and antimicrobial prescriptions (Martins et al., 2022).
One umbrella review developed by Martins et al. (2022) concluded that surgical approaches are the best option to treat PI. Thus, several regenerative surgical techniques have been identified and referred to in the literature, aiming to favor the reconstruction of bone defects (Larsson et al., 2016). Regenerative procedures can be characterized using only resorbable or non-resorbable membranes or GBR (Hermann & Buser, 1996), with xenografts, allografts, alloplastic materials, or autografts (Roccuzzo et al., 2016, Baskaran et al., 2022), enamel matrix derivative (EMD, Emdogain®) (Isehed et al., 2016, Dias et al., 2022), or titanium granules (Jepsen et al., 2016, Andersen et al., 2017). In addition, it is essential to mention that the regenerative surgical treatment of PI has a high level of complexity and depends on several factors such as the patient’s systemic health status, oral hygiene habits, setting of the defects, decontamination procedure, implant surface characteristics, surgical technique used, postoperative maintenance, and several other factors that may influence clinical outcome and results.
Nevertheless, depending on the morphology of the defect, the use of a membrane may or not be indicated (Schwarz et al., 2018), and the regenerative surgery result may not be predictable. It can be questionable (Schwarz et al., 2009). Furthermore, factors such as complete decontamination of the implant surface, materials in contact with the patient’s saliva, and complete bacteria removal increase the level of difficulties of the treatment. Otherwise, in most studies, surgical augmentative peri-implant therapy has shown improved outcomes, clinically and radiographically, with follow-up between six months and ten years of follow-up (Khoury et al., 2019).
Within this scenario, the goal of this review was to verify the current literature about regenerative techniques used to treat PI, to understand better the regenerative approach and outcomes obtained, detailing techniques and materials applied to improve the knowledge and practice.
2. Materials and methods
2.1. Protocol and focus question
This systematic review followed the PRISMA guidelines (Page et al., 2021). The focused questions were based on the PICO (Population [P], Intervention [I], Comparison [C], and Outcomes [O]) strategy (Table 1): (i) “In patients who received regenerative treatments for peri-implantitis (P), is the regenerative surgical treatment (I) clinically effective and predictable compared to non-regenerative (C) to treat PI (O)?”; and (ii) “In patients who received regenerative treatments for peri-implantitis (P), the regenerative approach (I), compared to non-regenerative (C), significantly increase the prognosis and implant survival rate in the mid- and long-term (O)?”
Table 1.
Eligibility criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| − 10 years (2012–2022) and at least 6 months of follow-up | - Studies involving peri-implantitis treatment but without approach regenerative techniques |
| - Clinical studies (Randomized Controlled Trial [RCT]) | - Patient with peri-implantitis and other systemic conditions |
| - Language: English | - Reviews (systematic, narrative, scoping), letters to the editor, pre-clinical studies, in silica and in vitro studies |
| - Articles that applied the surgical regenerative treatment for peri-implantitis | - Lack of information about the surgical therapy used and materials applied |
| - Full text available | - Same cohort patients (keeping the last follow-up) |
2.2. Search strategy
Electronic research was performed on February 23rd, 2022, and carried out on PubMed/Medline, EMBASE, and On-Line Knowledge Library (B-On). It was applied specific keywords associated with the Boolean connectors (“AND” and “OR”) to combine them, achieving a more significant number of articles: “randomized clinical trial” AND (regenerative OR “regenerative technique”) AND (“surgical treatment” OR “surgical therapy”) AND (periimplantitis OR peri-implantitis OR “periimplant disease” OR “peri-implant disease”) AND (“bone loss” OR “bone defect”) AND (“bleeding on probing”) AND (“guided bone regeneration” OR GRB OR “bone regeneration” OR “bone reconstruction” OR “bone graft” OR “bone grafting” OR “bone substitute”) AND (membrane OR “collagen membrane” OR “non-absorbable membrane” OR “non-resorbable membrane” OR “resorbable membrane”). A manual search was conducted to verify cross-references.
2.3. Eligibility criteria
The inclusion criteria were: (i) 10 years (2012–2022) and at least a one-year follow-up; (ii) clinical studies, considering only Randomized Controlled Trials [RCT]; (iii) language restriction (English); and (iv) articles that applied the surgical regenerative treatment for peri-implantitis with full text available. The exclusion criteria were: (i) studies involving peri- implantitis treatment but without approach regenerative techniques; (ii) patients with peri-implantitis and other systemic conditions; (iii) reviews (systematic, narrative, scoping), letter to the editor, consensus, pre-clinical studies, in silica and in vitro studies, case series or case report; (iv) article with a lack of information about the surgical therapy used and materials applied; and (v) same cohort patients results reported (only the longest follow-up was included) (Table 2).
Table 2.
Description of the PICO strategy and focus questions.
| Population (P) | Patients with dental implants affected by peri-implantitis |
|---|---|
| Intervention (I) | Regenerative surgical approach in dental implants with peri-implantitis |
| Comparison (C) | Non-surgical treatment or resective surgical treatment of dental implants affected by peri-implantitis |
| Outcome (O) | Survival rate of dental implants after regenerative surgical treatment |
| Focus questions | (i) Is the regenerative surgical treatment clinically effective to treat peri-implantitis? And (ii) will this approach significantly increase the implant survival rate? |
2.4. Selection of articles and data extraction
Two independent reviewers (ASB and FCC) performed the appraisal, and a third reviewer (GVOF) was consulted in case of initial disagreement. The reviewers discussed the results based on the inclusion/exclusion criteria, observing first the title and abstract and after reading the full text. Duplicate articles were removed. The following information was extracted from the articles: (i) author, (ii) type study, (iii) follow-up, (iv) sample size and sample characterization, (v) clinical characteristics and details (defect existent and bone gain, PD, keratinized tissue, BoP, plaque index), (vi) treatment done, adverse effects observed, and results found.
2.5. Risk of bias
All included studies were qualitatively assessed. Two independent investigators (GVOF and JCHF) performed the biases assessment. It used the Cochrane Collaboration’s tool for assessing the risk of bias in randomized trials (Higgins et al., 2011), focusing on (i) Random sequence generation, (ii) Allocation concealment, (iii) Blinding of participants and personnel, (iv) Blinding of outcome assessment, (v) Incomplete outcome data, (vi) Selective reporting, and (vii) Other sources of bias (such as funding or conflict of interest), where each group is classified as “low risk” (green), “unclear” (yellow), or “high risk” (red). In the case of divergences, a third researcher was consulted (FCC). If the study had green for all options evaluated, it was classified as low RoB; if it had one red or two yellows, it was classified as a moderate level for RoB; more than one red or two yellows, high RoB.
3. Results
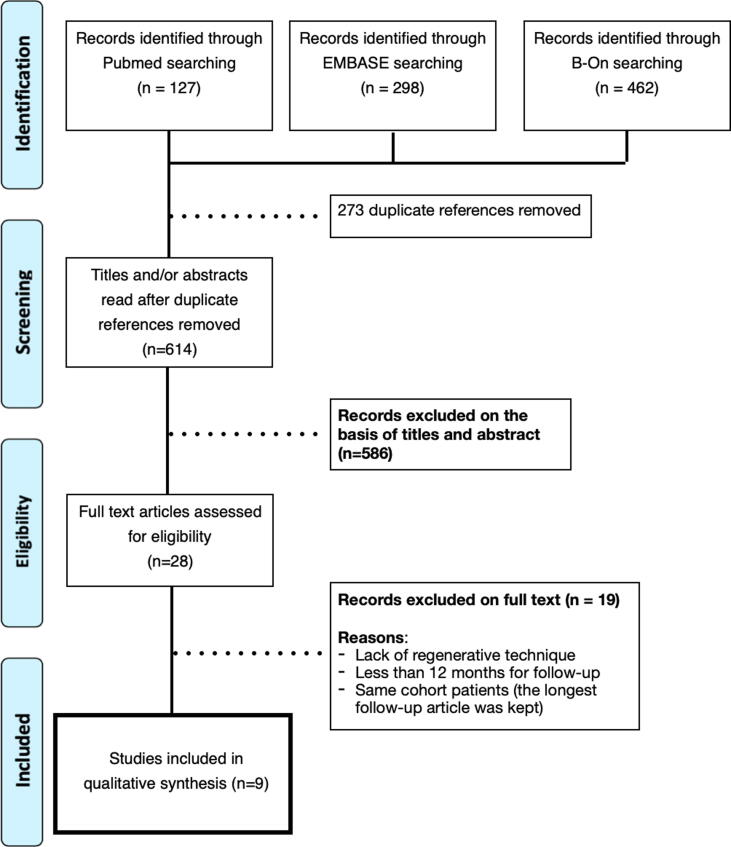
Initially, 887 articles were identified (462 through B-On, 298 in the EMBASE, and 127 in the PubMed/Medline databases). Duplicate articles (n = 273) were removed, resting 614 articles. In the first analysis by title and abstract, 586 were excluded (k = 0.95). After, 28 studies were full-text reading (k = 0.98). 19 out of 28 were removed due to lack of use of the regenerative technique, <12-month follow-up, and same cohort group (the article with the most extended follow-up was kept), which resulted in nine articles that were finally included (Fig. 1).
Fig. 1.
PRISMA flowchart for the screening and records.
3.1. Demographic data and Follow-up
A total of 404 patients were treated in the nine included studies (225 females and 179 males, respectively, 55.69% and 44.31%), with a mean age of 60.44 (±9.47) years. There were 54 dropouts with the following justifications: loss of follow-up, technical problems related to removing the suprastructures, the patient died, implant removed before follow-up (failure), psychological illness, and the patient received another crown. Only two studies (Aghazadeh et al., 2012, Renvert et al., 2018) did not report dropouts. Smokers were considered in all studies except Schwarz et al. (2013), who excluded smokers. Seven out of 9 studies included had 12-month follow-ups; Schwarz et al. (2013) evaluated patients after 48 months; Andersen et al. (2017) assessed the patients after 88 months.
3.2. Techniques and materials/devices used to treat PI
Only one study included implantoplasty associated with Er:YAG laser (Schwarz et al., 2013). Another study used blood concentrate (CGF, concentrated growth factors) (Isler et al., 2018), and Isehed et al. (2016) used EMD in the test group. These three materials/devices did not significantly improve the results. Two studies considered to approach the use of titanium granules (Jepsen et al., 2016, Andersen et al., 2017), with a significant increase in radiographic bone identification. The regenerative technique using bone graft was majority chosen for the more substantial part of the studies, which involved autogenous bone grafts (Aghazadeh et al., 2012), alloplastic bone grafts (Tapia et al., 2019), and xenograft or bovine bone grafts (Aghazadeh et al., 2012, Schwarz et al., 2013, Isler et al., 2018, Renvert et al., 2018, Renvert et al., 2021), associated or not with the collagen membrane. Xenograft had better results radiographically when compared to the autogenous bone graft.
3.3. Detoxification method
Different methodologies were applied to promote implant surface detoxification, but the most common was the use of 3% hydrogen peroxide (H2O2) for 1 min (Aghazadeh et al., 2012, Jepsen et al., 2016, Renvert et al., 2018, Tapia et al., 2019, Renvert et al., 2021). Titanium curettes and rotary titanium brushes were the second most used (Isehed et al., 2016, Jepsen et al., 2016, Isler et al., 2018, Tapia et al., 2019, Renvert et al., 2021). Ultrasonic scalers were also considered instead of plastic scalers or special implant tips (Isehed et al., 2016, Tapia et al., 2019). Other options were 24% EDTA (Andersen et al., 2017) and implantoplasty with Er:YAG laser (Schwarz et al., 2013). Titanium brushing before regenerative treatment significantly reduced PD (Tapia et al., 2019); otherwise, implantoplasty and laser therapy did not substantially improve the results (Schwarz et al., 2013).
3.4. Submersion of the implant and antimicrobial medication postoperative
Three articles considered complete closure of the flap (submerging the implants) after surgery and, thus, temporarily removing the crown (Andersen et al., 2017, Isler et al., 2018, Renvert et al., 2018). Renvert et al.’s (2021) study considered submerging the implants in cases of the screw-retained prosthesis and non-submerging them in cases of cemented crowns. Other five studies did not submerge the implants (Aghazadeh et al., 2012, Schwarz et al., 2013, Isehed et al., 2016, Jepsen et al., 2016, Tapia et al., 2019).
The postoperative antimicrobial therapy varied according to the study. Therefore, all therapies used chlorhexidine except Jepsen et al., 2016, Andersen et al., 2017. Azithromycin was considered in three studies (Aghazadeh et al., 2012, Renvert et al., 2018, Renvert et al., 2021), with the posology of 500 mg and/or 250 mg. All other studies administrated the association of amoxicillin 500 mg with metronidazole 500 mg or 400 mg (3x a day for one week) (Jepsen et al., 2016, Andersen et al., 2017, Isler et al., 2018, Tapia et al., 2019). Tapia et al. (2019) reported using clindamycin 300 mg in the case of allergy to amoxicillin.
3.5. Clinical parameters (Table 3)
3.5.1. BoP
There was an overall improvement (decrease) for bleeding on probing, independent of the group. Aghazadeh et al. (2012) had initial and final values of 87.5% and 48.4% in the control group and 79.4% and 26.7%, respectively, in the test group, with a significant BoP decrease. Andersen et al. (2017) reported a percentual reduction of 22% and 17% in the control and test groups. Isehed et al. (2016) described non-significative values between groups through medians, and the percentual found for the control and test groups (EMD) were, respectively, 51.11% and 37.5%. Jepsen et al. (2016) reported 45.4% (control) and 56.1% (test group) of the BoP reduction. Schwarz et al.’s (2013) study had a significative BoP improvement of 85.2% (CPS group) and 71.7% (ERL group), whereas, Renvert et al. (2018) had an improvement of 35% and 47.6% for the control and test groups, respectively. In another study by Renvert et al. (2021), the control group had a 64.28% improvement compared to 69.23% in the test group. Tapia et al. (2019) had a reduction of 54% and 80% (control and test group, respectively), and Isler et al. (2018) reported an improvement of 61.31% and 61.54%, respectively, in the CM and CGF group. (See Table 3)
Table 3.
Data extraction of the included studies.
| Author/year (Study design) | n / groups / mean age (SD) | Groups | Dropped out | Smoker | Follow-up (months) | PD | BoP | Bone Level | Detoxification method | Submerge or not | Antimicrobial | Comments/Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aghazadeh et al., 2012 (Single-blind randomised clinical trial) |
45 AB group: 22 70.1 y.o. (±6.2) 14 female, 8 male. BDX group: 23 67 y.o. (±7.5) 13 female, 10 male. |
AB group: autogenous bone + resorbable bovine collagen membrane BDX group: bovine xenograft + resorbable bovine collagen membrane |
None | AB group: 23.2 (±13.4) packs/year BDX group: 18.8 (±11) packs/year |
12 | AB Group: - baseline: 6 (±1.3) mm - final: 3.8 mm BDX group: - baseline: 6.2 (±1.4) mm - final: 3.3 mm |
AB Group: - baseline: 87.5% (±20.1) - final: 48.4% BDX group: - baseline: 79.4% (±28.9) - final: 26.7% |
AB group: - baseline: 5.9 (±1.8) mm - final: 5.8 (0.3) mm BDX group: - baseline: 5.2 (±1.8) mm - final: 4.2 (±0.3) mm |
3% Hydrogen peroxide for 1 min | Non-submerged | Azithromycin + 0.1% Chlorhexidine | BDX provided more evidence of radiographic bone fill than AB. The overall success of the treatment within both groups was limited |
| Andersen et al., 2017 (RCT) |
33 (final: 12) PTG group: 16 (final: 6)67 (±12.9), 45–79 y.o. 3 female, 3 male. Test group: 17 (final: 6)67.2 (±11.8), 53–85 y.o. 4 female, 2 male. |
Test group: open flap mechanical and chemical debridement with titanium curettes and EDTA gel + Porous titanium granules (PTG) Control: open flap debridement alone |
5 died; 10 subjects lost the follow-up; 3 lost implants; 2 excluded due to technical complications; 1 received a new cemented single crown | Porous titanium granules (PTG): 2/6 (33.3%) Control group: 3/6 (50%) |
88 | PTG group:- baseline 6.5 (±1.9) mm- final 4.3 (±2.4) mm Control group:- baseline: 6.5 (±2.3) mm- final: 3.5 (±1.2) mm |
PTG group: - baseline: 92% - final: 75% Control group: - baseline: 100% - final: 78% |
PTG group:- baseline: 5.4 (±1.5) mm- final: 4.6 (±3.0) mm Control group:- baseline: 4.3 (±1.3) mm- final: 4.5 (±1.8) mm |
24% EDTA | Submerged | Amoxicillin 500 mg + metronidazole | PTG significantly better radiographic peri-implant defect fill compared with controls; therefore, the long-term follow-up of surgical treatment of peri-implant osseous defects showed unpredictable results |
| Isehed et al., 2016 (RCT) |
29 EMD group: 15 61 − 819 female, 6 male.no -EMD group: 14 67 − 83 9 female, 5 male. |
Control group: debridement + saline solution Test group: debridement + saline solution + EMD (0.3 mL Emdogain) |
EMD group: 1 discontinued to follow-up and 2 lost due to infection no-EMD group: 1 implant loss |
EMD group: 26.7% no-EMD group: 42.9% |
12 | Median difference control: 3.0 mm (1.9 to 7.0 mm) test: 2.8 mm (0.4 to 3.9 mm) |
Control: median- baseline: 22.5 (1–79) - final: 11.0 Test: median- baseline: 16 (8–44) - final: 10 |
Median change control: −0.1 mm (-0.7 to 1.2 mm) test: 0.9 mm (0.0 to 1.3 mm) |
Debridement (removal of granulation tissue with UltraSons and Ti curettes) and saline solution | Non-submerged | 10 mL chlorhexidine (2 mg/ml) - twice daily, for 6 weeks | EMD did not improve PD and BoP after 12 months |
| Jepsen et al., 2016 (Multicenter, Multinational RCT) |
63 TG group: 33 65 y.o. (±10) 17 female, 16 male. Control: 30 59.1 y.o. (±12.2) 19 female, 11 male. |
Control group: Open flap debridement alone Test group: Open flap debridement + Tigran titanium granules (TG) |
4 patients in the control group were excluded (at the time of analysis - missing data) | Control: 18% TG group: 20% |
12 | TG group: - baseline: 6.3 (±1.3) mm - final: 3.5 (±1.5) mm Control group: - baseline: 6.3 (±1.6) mm - final: 3.5 (±1.1) mm |
TG group: - baseline: 89.4% (±20.7) - final: 33.3% (31.7) Control group: - baseline: 85.8% (±23.9) - final: 40.4% (±37.1) |
TG group: - baseline: 4.64 (±1.95) mm - final: 1.03 (±1.4) mm Control group: - baseline: 3.98 (±2.1) mm - final: 2.88 (±1.86) mm |
Rotary titanium brush + 3% hydrogen peroxide | Non-submerged | Amoxicillin 500 mg + metronidazole 400 mg | TG significantly increased radiographic bone defect identification in comparison to open flap debridement |
| Schwarz et al., 2013 (RCT) |
32 (21 female and 11 male) 60.8 y.o. (±10.9) CPS group: 16 ERL group: 16 |
CPS group: Plastic curettes + implantoplasty + saline solution + Bovine-derived natural bone + native collagen membrane Grupo ERL: implantoplasty + Er:YAG laser + Bovine-derived natural bone + native collagen membrane |
4 from the CPS group 7 from the ERL group (missing recall sessions or severe signs of reinfection) |
Smoker was excluded | 48 | CPS group: - baseline: 5.5 (±1.7) mm - final: 4.3 (±1.2) mm ERL group: - baseline: 5.1 (±1.5) mm - final: 3.8 (±1.1) mm |
CPS group: - baseline: 100% - final: 14.8% (±16.4) ERL group: - baseline: 95.2% (12.6) - final: 23.5% (23.4) |
CPS group:- baseline: 6.7 (±1.8) mm- final: 5.2 (±1.9) mm ERL group:- baseline: 7.3 (±1.9) mm- final: 6.1 (±1.1) mm |
CPS group = implantoplasty + saline solution ERL group = implantoplasty + Er:YAG laser |
Non-submerged | 0.2% Chlorhexidine digluconate | Different methods of surface decontamination did not show significant differences in the treatment of peri-implantitis |
| Renvert et al., 2018 (RCT) |
41 Test group: 21 67.5 y.o. 13 female, 8 male. Control group: 20 70 y.o. 9 female, 11 male. |
Control group: Surgical debridement Test group: Surgical debridement + xenograft |
None | 25% in the control group 23.81% in the test group |
12 | Control group: - baseline: 6.0 (±1.7) mm - final: 3.9 (±2.7) mm Test group: - baseline: 6.6 (±1.8) mm - final: 2.6 (±1.5) mm |
Control group: - baseline: 100% - final: 65% Test group: - baseline: 100% - final: 52.4% |
Control group: - baseline: 3.7 (±2.0) mm - final: 3.1 (±1.2) mm Test group: - baseline: 3.6 (±1.0) mm - final: 2.9 (±1.2) mm |
3% Hydrogen peroxide + saline solution | Submerged | Zitromax® (Sandoz AS, Copenhagen, Denmark) 500 mg day 1 and 250 mg days 2–4) + chlorhexidine 0.2% (twice daily) | Successful treatment outcome using a bone substitute was more predictable when a composite therapeutic endpoint was considered. |
| Renvert et al., 2021 (Multicenter, RCT) |
71 Control group: 34 62.9 y.o. (±13.0) 17 female, 17 male. Test group: 37 62.2 y.o. (±10.2) 20 female, 17 male. |
Control group: surgical debridement Test group: surgical debridement + adjunct use of DBBM (deproteinized bovine bone mineral) and NBCM (native bilayer collagen membrane) |
Control group: 2 patients due to implant failure Test group: 3 patients (2 due to implant failure and 1 lost the follow-up) |
Control group: 9 patients Test group: 8 smokers |
12 | Control group: (mean) - baseline: 6.8 (±1.3) mm - final: 4.5 (±1.5) mm Test group: (mean) - baseline: 6.7 (±1.5) mm - final: 4.8 (±1.5) mm |
Control group: (mean) - baseline: 1.4 (±1.0) - final: 0.5 (±0.6) Test group: (mean) - baseline: 1.3 (±0.9) - final: 0.4 (±0.6) |
Control group: (mean) - baseline: 4.9 (±1.8) mm - final: 3.6 (±2.3) mm Test group: (mean) - baseline: 4.4 (±1.8) mm - final: 2.1 (±1.6) mm |
Titanium curettes and a rotary titanium brush + 3% hydrogen peroxide for 1 min + saline solution (2 × 20 mL) | Submerged in cases of screw-retained prosthesis; non-submerged for cemented | Azitromycin: 500 mg on day 1 and 250 mg for 4 days + chlorhexidine (0.2%), twice daily, for 3 weeks | DBBM and NBCM resulted in significantly more radiographic defect filled (RDF) than debridement alone. Therefore, no difference was found in any clinical parameters |
| Tapia et al., 2019 (RCT) |
30 Test group: 15 65.53 y.o. (±10.3) 11 female, 4 male. Control group: 15 55.47 y.o. (±11.75) 9 female, 6 male. |
Control group: Surgical mechanical debridement + alloplastic bone graft + collagen membrane Test group: Surgical mechanical debridement (with titanium brush) + alloplastic bone graft + collagen membrane |
Control group: 2 lost the follow-up and 1 implant was removed | 4 (26.7%) in the control group 6 (40%) in the test group |
12 | Control group: - baseline: 6.17 (±0.98) mm - final: 3.87 (±0.81) mm Test group: - baseline: 6.16 (±1.27) mm - final: 3.31 (±0.72) mm |
Control group: - baseline: 100% - final: 46% (±52) Test group: - baseline: 100% - final: 20% (±41) |
Control group:- baseline: 3.41 (±0.78)- final: 0.89 (±1.36) Test group:- baseline: 3.71 (±0.77)- final: 0.75 (±0.86) |
Control group: Mechanical debridement with plastic ultrasonic scalers + 3% H2O2 Test group: Mechanical debridement with plastic ultrasonic scalers + 3% H2O2 + titanium brush (with an oscillating low speed of 900 rpm) |
Non-submerged | 0.12% Chlorexidine (twice daily for 2 weeks) + 500 mg amoxicillin and 500 mg metronidazole (3x a day for 7 days). In the case of allergy to amoxicillin, clindamycin (300 mg every 6 hr) | Use of a titanium brush before regenerative treatment resulted in statistically significant benefits in terms of PD reduction after 12 months |
| Isler et al., 2018 (RCT) |
60 CM group: 26 56.15 y.o. (±9.23) 15 female, 11 male. CGF group: 26 57.96 y.o. (±9.07) 10 female, 16 male. |
Collagen membrane (CM) group: Surgical mechanical debridement + xenograft (BioOss spongiosa granules, particle size 0.25–1 mm) + Biogide Concentrated growth factor (CGF) membrane group: Surgical mechanical debridement + xenograft (BioOss spongiosa granules, particle size 0.25–1 mm) + 2 membranes of CGF |
3 patients were excluded (technical problems related to removing the suprastructures) 5 patients (three in the test group and two in the control group) - lost of the follow-up |
CM group: 9 (34.6%) CGF group: 6 (23.1%) |
12 | CM group: (mean) - baseline: 5.41 (±1.16) mm - final: 2.70 (±0.80) mm CGF group: (mean) - baseline: 5.92 (±1.26) mm - final: 3.71 (±1.09) mm |
CM group: (mean) - baseline: 97.12% (±8.15) - final: 29.81% (±30.02) CGF group: (mean) - baseline: 97.12% (±10.79) - final: 35.58% (±30.14) |
CM group: (mean) - baseline: 5.47 (±1.31) mm - final: 2.92 (±1.0) mm CGF group: (mean) - baseline: 5.95 (±1.18) mm - final: 3.98 (±1.22) mm |
Titanium curettes + saline-soaked cotton gauzes | Submerged | Amoxicillin 500 mg + metronidazole 500 mg (3x a day for 1 week) + chlorhexidine 0.12% twice a day for 2 weeks | Both regenerative approaches yielded significant clinical and radiographic improvements. Therefore, collagen membrane + bone substitute showed better results |
AB = autogenous bone; BDX = bovine-derived xenograft; CPS = plastic curette + cotton pellets + sterile saline; EDTA = ethylenediaminetetraacetic acid; ERL = Er:YAG laser; PTG = Porous Titanium Granules; SD = standard deviation; TG = titanium granules.
3.5.2. Bone level
For this parameter, Aghazadeh et al. (2012) had 0.1 mm of improvement in the autogenous group, whereas the xenograft group had 1.0 mm, resulting in a better outcome than the gold standard and also demonstrating more radiographic evidence of bone filling (RBL, p < 0.001). For the studies that used titanium granules (Andersen et al., 2017), the authors found significantly better filling of radiographic peri-implant defects in the test group, with a baseline and final results for the test group of 5.4 (±1.5) mm and 4.6 (±3.0) mm, respectively; and for the control group, a baseline value of 4.3 (±1.3) mm and the final value of 4.5 (±1.8) mm. They had low values compared to Jepsen et al. (2016), who reported baseline and final values, respectively, of 3.98 (±2.1) mm and 2.88 (±1.86) mm (control group) and 4.64 (±1.95) mm and 1.03 (±1.4) mm (test group). Therefore, Jepsen et al. had a 12-month follow-up, whereas Andersen et al. (2017) had an 88-month follow-up.
Schwarz et al. (2013) was the only study that evaluated the effect of implantoplasty associated with xenograft and collagen membrane and Er:YAG laser. Negatively, the result for the test group (laser) was not significant and did not have improved outcomes compared to plastic curettes and saline solution (CPS group), respectively, at baseline: 7.3 (±1.9) mm and after 48 months 6.1 (±1.1) mm, and 6.7 (±1.8) mm and 5.2 (±1.9) mm.
In the only study (Isler et al., 2018) that used a biological blood material, CGF, there were improvements in bone levels (2.55 mm) for the collagen group and 1.98 mm for the CGF group. Another study used EMD (Isehed et al., 2016) and presented median results of −0.1 mm (-0.7 to 1.2 mm) in the control group and 0.9 mm (0.0 to 1.3 mm) in the EMD group. It was suggested that EMD has potential application in the management of peri-implantitis, noting that its adjunctive use was associated with an increase in marginal bone level and an increased prevalence of aerobic bacteria after 12 months of healing.
These results were similar to two other studies by Renvert et al. (2018, 2021), which used xenograft. There was an improvement for the control and test group, respectively, of 0.6 mm and 0.7 mm (Renvert et al., 2018) and 1.3 mm and 2.3 mm (Renvert et al., 2021), with better results found in the xenograft group (in the most recent article). Using alloplastic materials, Tapia et al. (2019) found similar improvement in the control and test groups, respectively, with mean differences of 2.52 mm and 2.96 mm.
3.5.3. Pocket depth
The PD had a similar reduction between groups (control and test)/period. Therefore, Renvert et al. (2018) showed the best results, with a reduction of 2.1 mm and 4.0 mm, respectively, in the control and test group. In another study by Renvert et al. (2021), the decline was not significant, as in the previous research, mainly in the test group, presenting 2.3 mm and 1.9 mm in the control and test groups.
For the blood concentrate (CGF), the PD findings were not significant, with lower reduction compared to the collagen membrane group, 2.21 mm (CGF group) and 2.71 mm (collagen membrane group). Also, there was no significant PD improvement in the study by Schwarz et al. (2013) after applying the laser, with initial and final values of 5.5 mm and 4.3 mm (control group) and 5.1 mm and 3.8 mm (test group), respectively. A similar result was found by Isehed et al. (2016), but using EMD instead of a laser, and reporting a non-significative result between the groups, with a median difference of 3.0 mm (1.9 to 7.0 mm) in control and 2.8 mm (0.4 to 3.9 mm) in the test group.
Analyzing and comparing autograft and xenograft (Aghazadeh et al., 2012), PD was slightly better in the xenograft group, which had a reduction of 2.9 mm. In contrast, the control group had 2.2 mm for PD reduction. For the studies that used titanium granules (Jepsen et al., 2016, Andersen et al., 2017), there was similar, and non-significative PD reduction (difference) between the groups analyzed, showing a non-effective result for this material. The authors (Andersen et al., 2017) reported 6.5 mm (±2.3) and 3.5 mm (±1.2) (baseline and final result) in the control group, and in the test group, 6.5 mm (±1.9) and 4.3 mm (±2.4) (baseline and final). In the Jepsen et al. (2016) study, they presented 6.3 mm (±1.6) and 3.5 mm (±1.1) in the control group (baseline and final results); while 6.3 mm (±1.3) and 3.5 mm (±1.5) (baseline and final results) was presented in the test group.
Finally, a significant improvement was found in the test group using a titanium brush in the study by Tapia et al. (2019), who reported a PD reduction of 2.3 mm in the control group (alloplastic graft and collagen membrane) and 2.85 mm in the test group (alloplastic graft and collagen membrane associated with a titanium brush).
3.6. Risk of bias
The RoB resulted in four studies with low risk (Aghazadeh et al., 2012, Schwarz et al., 2013, Tapia et al., 2019, Renvert et al., 2021); three with moderate (middle-level) risk (Jepsen et al., 2016, Isler et al., 2018, Renvert et al., 2018); and two with high risk (Isehed et al., 2016, Andersen et al., 2017), as demonstrated in Fig. 2.
Fig. 2.
RoB for the studies included.
4. Discussion
In the present systematic investigation, our goal was to evaluate regenerative procedures used to treat PI, including only RCTs. A total of 404 patients (225 females and 179 males), with a mean age of 60.44 years, diagnosed with PI were enrolled and received different regenerative surgical approaches. Nevertheless, even though regenerative surgical treatments may result in a healthier peri-implant clinical condition, most of the included studies (Aghazadeh et al., 2012, Isehed et al., 2016, Jepsen et al., 2016, Andersen et al., 2017, Isler et al., 2018, Renvert et al., 2018, Tapia et al., 2019, Renvert et al., 2021) had a postoperative follow-up of 12 months, which can be considered a short period. Only one study had a 48-month follow-up (Schwarz et al., 2013). This fact raises questions about mid- and long-term success when surgically treating PI.
Also, a significant part of the studies included smokers, except for Schwarz et al. (2013); therefore, no relevant data was explicitly provided for this group, even though it is known that smokers have inferior results for tissue healing (Naji et al., 2020). Moreover, the included studies reported several brands of dental implants with different designs, surface treatments, and abutments, combined or not to bone substitutes and barriers. Consequently, comparing different cases of peri-implant regenerative techniques, they were not precisely feasible, and there were many reasons for marginal bone loss. In addition, there were diversified etiologies, such as inadequate occlusal contact, infection, or mechanical problems.
Furthermore, a central role in the resistance to inflammatory processes has been given to soft tissue thickness. In this sense, it is difficult to directly compare different studies due to a lack of information or standardization. Also, it is noted that including patients with risk factors, such as smokers and those with systemic diseases and a history of periodontitis, may contribute to the differences found in the results. Moreover, the authors did not find any significant effect for a specific type of decontamination method (Khoury et al., 2019, Roccuzzo et al., 2021), which was similarly found in our systematic study.
According to Daugela et al. (2016), a greater success level of PI treatment was demonstrated when bone graft material was added, reducing the pocket depth (Aljohani et al., 2019). However, consensus data (Hallstrom et al., 2012) about the surgical treatment of PI supported that the regenerative approaches had a better clinical and radiographic performance. This fact agrees with the results found in a recent umbrella review (Martins et al., 2022). Still, there is no solid evidence supporting the use of a single material or product.
Our results agree with those findings, and the best PD reduction result was obtained in the study by Renvert et al. (2018), decreasing 4.0 mm in the test group (surgical debridement with bone substitute [xenograft]). In another study by Renvert et al. (2021), the results for PD were not as effective as in the previous publication, with the best result in the xenograft group (1.9 mm). Aghazadeh et al. (2012) also inserted xenograft material and found 2.9 mm of PD reduction; similar results were presented by Jepsen et al. (2016) using titanium granules (PD decreased of 2.8 mm). Therefore, when comparing control and test treatments, most studies did not find statistically significant results for PD.
The common finding for peri-implant disease (mucositis and peri-implantitis) is the positive presence of BoP. Even though BoP is an important clinical parameter, a systematic study (Khoshkam et al., 2013) showed that the studies included in the review did not accurately inform the BoP presence. Therefore, in our systematic review, all RCTs included had improved outcomes and reduced bleeding, supporting that all detoxification methods had effective results. Schwarz et al. (2013) found the most significant BoP reduction, with an improvement of 85.2% in the CPS group and 71.7% in the laser group (ERL); nevertheless, no significant result was found when using the laser. It can be explained because of the period studied, 48 months, against the 12-month follow-up of the other studies, which had around 40–60% bleeding reduction results.
The last parameter studied was bone level. Andersen et al. (2017) found a difference of −0.2 mm in the control group and 0.8 mm in the test group (porous titanium granules). These data reflect the long-term assessment, which can be contrasted to the short-term results of Jepsen et al. (2016), who found differences in the control and test groups of 1.1 mm and 3.61 mm.
Renvert et al.’s (2018) study did not cite the impact of xenograft used on bone level, but there was no statistical significance between groups. Differently from Renvert et al.’s (2021) study, the authors reported a difference in the bone level for the control and test groups of 1.3 mm and 2.3 mm. Tapia et al. (2019), using alloplastic biomaterial with or without previous titanium brushes, had a mean improvement of 2.52 mm and 2.96 mm, respectively, for the control and test groups. Also, Er:YAG laser had no significant results compared to plastic curettes, with differences between initial and final values of 1.2 mm and 1.5 mm, respectively, after 48 months.
Aljohani et al. (2019) showed the highest bone defect fill was achieved with porous titanium granule (PTG) application. Sequentially, it was followed by bovine xenograft and autogenous bone graft. Similarly, our results also have this perspective, with the best improvement for bone level found in PTG, sequentially followed by alloplastic bone graft, xenograft, CGF, EMD, and lastly, by the autogenous bone graft. This fact permitted to confirm that the “gold standard” biomaterial is not the best choice to treat PI, with significant differences shown in the Aghazadeh et al.’s (2012) study (p < 0.001).
4.1. Study limitations
The articles included in this systematic had different implant systems (brands), with different macro and microtopography, designs, and surfaces, associated with many biomaterials and membranes, making direct comparisons difficult. Also, varied types of cases and approaches cannot permit accurate to compare surgical procedures and different methods to treat and decontaminate the implant surface, and the inclusion of smokers might be reasons to explain the variability found in the results. Moreover, the lack of information caused difficulty in comparing studies.
The periods for patient observation reported ranged from 12 months (in 7 studies), 48 months (Schwarz et al., 2013), and 88 months (Andersen et al., 2017), with a diversified period of reexamination. Re-evaluation in the long term is required for a more robust assessment; only two studies provided long-term results, bringing more reliability to the data obtained.
Also, different etiologies (infection, inadequate occlusal contact, and mechanical problems) can be responsible for peri-implant bone loss. In addition, there is variability in the patient’s gingival thickness, which may cause variations in the resistance threshold to inflammation. Another limitation is the RoB found for the articles; most studies had a moderate or high risk of bias (5 articles), leading to reduced reliability of the data collected and raising questions about the viability and effectiveness of peri-implantitis surgical treatments.
5. Conclusion
Within the limitations of this study, it was possible to conclude that the regenerative surgical approach is a predictable choice in the management of PI and in improving the clinical parameters of peri-implant tissues in short-term, mainly when using porous titanium granules, alloplastic bone grafts, and xenograft biomaterials. Therefore, all data must be carefully interpreted due to the different materials used, the period of evaluation, and the number of articles included. More RCTs need to be carried out, with a long follow-up period (at least 24 months), standardization, and systematization of their protocols for regenerative surgical treatment of PI.
Ethical statement
Institutional ethical approval was not obtained for the study as the protocol only involved a systematic review of literature.
CRediT authorship contribution statement
Filipe Castro: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Writing – original draft, Visualization, Supervision, Project administration. Ahmed Sami Bouzidi: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing – original draft, Visualization, Project administration. Juliana Campos Hasse Fernandes: Validation, Formal analysis, Investigation, Resources, Visualization. Marco C. Bottino: Writing – original draft, Visualization. Gustavo Vicentis Oliveira Fernandes: Validation, Formal analysis, Investigation, Resources, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University. Production and hosting by Elsevier
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sdentj.2023.05.022.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Aghazadeh A., Rutger Persson G., Renvert S. A single-centre randomized controlled clinical trial on the adjunct treatment of intra-bony defects with autogenous bone or a xenograft: results after 12 months. J. Clin. Periodontol. 2012;39:666–673. doi: 10.1111/j.1600-051X.2012.01880.x. [DOI] [PubMed] [Google Scholar]
- Aljohani M., Yong S.L., Rahmah A.B. The effect of surgical regenerative treatment for peri-implantitis: a systematic review. Saudi Dent. J. 2019;32:109–119. doi: 10.1016/j.sdentj.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen H., Aass A.M., Wohlfahrt J.C. Porous titanium granules in the treatment of peri-implant osseous defects - a 7-year follow-up study. Int. J. Imp. Dent. 2017;3:50–56. doi: 10.1186/s40729-017-0106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskaran P., Prakash P., Appukuttan D., Mugri M.H., Sayed M., Subramanian S., Al Wadei M.H.D., Ahmed Z.H., Dewan H., Porwal A., et al. Clinical and radiological outcomes for guided implant placement in sites preserved with bioactive glass bone graft after tooth extraction: a controlled clinical trial. Biomimetics. 2022;7:43. doi: 10.3390/biomimetics7020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglundh T., Armitage G., Araujo M.G., Avila-Ortiz G., Blanco J., Camargo P.M., Chen S., Cochran D., Derks J., Figuero E., Hämmerle C.H.F., Heitz-Mayfield L.J.A., Huynh-Ba G., Iacono V., Koo K.-T., Lambert F., McCauley L., Quirynen M., Renvert S., Salvi G.E., Schwarz F., Tarnow D., Tomasi C., Wang H.-L., Zitzmann N. Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J. Clin. Periodontol. 2018;45(Suppl 20):S286–S291. doi: 10.1111/jcpe.12957. [DOI] [PubMed] [Google Scholar]
- Berglundh T., Jepsen S., Stadlinger B., Terheyden H. Peri-implantitis and its prevention. Clin. Oral Impl. Res. 2019;30:150–155. doi: 10.1111/clr.13401. [DOI] [PubMed] [Google Scholar]
- Borges H., Correia A.R.M., Castilho R.M., Fernandes G.V.O. Zirconia implants and marginal bone loss: a systematic review and meta-analysis of clinical studies. Int. J. Oral Maxillofac. Implants. 2020;35:707–720. doi: 10.11607/jomi.8097. [DOI] [PubMed] [Google Scholar]
- Branemark R., Branemark P., Rydevik B., Myers R.R. Osseointegration in skeletal reconstruction and rehabilitation: a review. J. Rehabil. Res. Dev. 2001;38:175–181. [PubMed] [Google Scholar]
- Daugela P., Cicciù M., Saulacic N. Surgical regenerative treatments for peri-implantitis: meta-analysis of recent findings in a systematic literature review. J. Oral Maxillofac. Res. 2016;7:e15. doi: 10.5037/jomr.2016.7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Y.C.M., Raghoebar G.M., Slater J.J.R.H., Meijer H.J.A., Winkel E.G., van Winkelhoff A.J. Implant decontamination during surgical peri-implantitis treatment: a randomized, double-blind, placebo-controlled trial. J. Clin. Periodontol. 2013;40:186–195. doi: 10.1111/jcpe.12034. [DOI] [PubMed] [Google Scholar]
- Dias A.T., Menezes C.C., Kahn S., Fischer R.G., Figueredo C.M.S., Fernandes G.V.O. Gingival recession treatment with enamel matrix derivative associated with coronally advanced flap and subepithelial connective tissue graft: a split-mouth randomized controlled clinical trial with molecular evaluation. Clin. Oral Investig. 2022;26(2):1453–1463. doi: 10.1007/s00784-021-04119-9. [DOI] [PubMed] [Google Scholar]
- Fernandes G.V.O. Editorial. J. Dent. Oral Sci. 2021;3(2):1. [Google Scholar]
- Fernandes G.V.O. Peri-Implantitis matter: possibilities of treatment but without a strong predictability for solution. Env. Dental J. 2021;3(2) [Google Scholar]
- Fernandes G.V.O., Calasans-Maia M., Mitri F.F., Bernardo V.G., Rossi A., Almeida G.D.S., Granjeiro J.M. Histomorphometric analysis of bone repair in critical size defect in rats calvaria treated with hydroxyapatite and zinc-containing hydroxyapatite 5% Key Eng. Mat. 2009;396–398:15–18. [Google Scholar]
- Fernandes G.V.O., Alves G.G., Linhares A.B.R., Silva M.H.P., Granjeiro J.M. Evaluation of cytocompatibility of bioglass-niobium granules with human primary osteoblasts: a multiparametric approach. Key Eng. Mat. 2012;493–494:37–42. [Google Scholar]
- Hallstrom H., Persson G.R., Lindgren S., Olofsson M., Renvert S. Systemic antibiotics and debridement of peri-implant mucositis. A randomized clinical trial. J. Clin. Periodontol. 2012;39:574–581. doi: 10.1111/j.1600-051X.2012.01884.x. [DOI] [PubMed] [Google Scholar]
- Hermann J.S., Buser D. Guided bone regeneration for dental implants. Curr. Opin. Periodontol. 1996;3:168–177. [PubMed] [Google Scholar]
- Higgins J.P.T., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savović J., Schulz K.F., Weeks L., Sterne J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isehed C., Holmlund A., Renvert S., Svenson B., Johansson I., Lundberg P. Effectiveness of enamel matrix derivative on the clinical and microbiological outcomes following surgical regenerative treatment of peri-implantitis. A randomized controlled trial. J. Clin. Periodontol. 2016;43:863–873. doi: 10.1111/jcpe.12583. [DOI] [PubMed] [Google Scholar]
- Isler S.C., Soysal F., Ceyhanlı T., Bakırarar B., Unsal B. Regenerative surgical treatment of peri-implantitis using either a collagen membrane or concentrated growth factor: a 12-month randomized clinical trial. Clin. Implant Dent. Relat. Res. 2018;20:703–712. doi: 10.1111/cid.12661. [DOI] [PubMed] [Google Scholar]
- Jepsen K., Jepsen S., Laine M.L., Moin D.A., Pilloni A., Zeza B., Sanz M., Ortiz-Vigon A., Roos-Jansåker A.M., Renvert S. Reconstruction of peri-implant osseous defects: a multicenter randomized trial. J. Dental Res. 2016;95:58–66. doi: 10.1177/0022034515610056. [DOI] [PubMed] [Google Scholar]
- Khoshkam V., Chan H.L., Lin G.H., MacEachern M.P., Monje A., Suarez F., Giannobile W.V., Wang H.L. Reconstructive procedures for treating peri-implantitis: a systematic review. J. Dent. Res. 2013;92(Suppl 12):131S–1318S. doi: 10.1177/0022034513509279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury F., Buchmann R. Surgical therapy of peri-implant disease: a 3-year follow-up study of cases treated with 3 different techniques of bone regeneration. J. Periodontol. 2001;72:1498–1508. doi: 10.1902/jop.2001.72.11.1498. [DOI] [PubMed] [Google Scholar]
- Khoury F., Keeve P.L., Ramanauskaite A., Schwarz F., Koo K.-T., Sculean A., Romanos G. Surgical treatment of peri-implantitis - Consensus report of working group 4. Int. Dent. J. 2019;69(Suppl 2):18–22. doi: 10.1111/idj.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang N.P., Lindhe J. sixth ed. Wiley-Blackwell; 2015. Clinical Periodontology and Implant Dentistry. [Google Scholar]
- Larsson L., Decker A.M., Nibali L., Pilipchuk S.P., Berglundh T., Giannobile W.V. Regenerative medicine for periodontal and peri-implant diseases. J Dental Res. 2016;95:255–266. doi: 10.1177/0022034515618887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins B.G.S., Fernandes J.C.H., Martins A.G., Castilho R.M., Fernandes G.V.O. Surgical and nonsurgical treatment protocols for peri-implantitis: an overview of systematic reviews. Int. J. Oral Maxillofac. Implants. 2022;37:660–676. doi: 10.11607/jomi.9659. [DOI] [PubMed] [Google Scholar]
- Mishler O.P., Shiau H.J. Management of peri-implant disease: a current appraisal. J. Evidence Based Dent. Pract. 2014;14:53–59. doi: 10.1016/j.jebdp.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Monteiro R.J.S.V., Moura-Netto C., Veiga N.J., Amaral S.A., Fernandes G.V.O. Periodontal regeneration after third molar extraction causing attachment loss in distal and furcation sites of the second molar: a case report with 12 months follow-up. J. Clin. Rev. Case Reports. 2022;7:128–132. [Google Scholar]
- Murray C.M., Knight E.T., Russell A.A., Tawse-Smith A., Leichter J.W. Peri-implant disease: current understanding and future directions. N. Z. Dent. J. 2013;109:55–62. [PubMed] [Google Scholar]
- Naji A., Edman K., Holmlund A. Influence of smoking on periodontal healing one year after active treatment. J. Clin. Periodontol. 2020;47:343–350. doi: 10.1111/jcpe.13228. [DOI] [PubMed] [Google Scholar]
- Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., Chou R., Glanville J., Grimshaw J.M., Hróbjartsson A., Lalu M.M., Li T., Loder E.W., Mayo-Wilson E., McDonald S., McGuinness L.A., Stewart L.A., Thomas J., Tricco A.C., Welch V.A., Whiting P., Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli P.P., Cicciu M., Beretta M., Maiorana C. Peri-implant mucositis and peri-implantitis: a current understanding of their diagnosis, clinical implications, and a report of treatment using a combined therapy approach. J. Oral Implantol. 2017;43:45–50. doi: 10.1563/aaid-joi-D-16-00082. [DOI] [PubMed] [Google Scholar]
- Puleo D., Nanci A. Understanding and controlling the bone-implant interface. Biomaterials. 1999;20:2311–2321. doi: 10.1016/s0142-9612(99)00160-x. [DOI] [PubMed] [Google Scholar]
- Renvert, S., Polyzois, I., 2018. Treatment of pathologic peri-implant pockets. Periodontol. 2000. 76, 180-190. [DOI] [PubMed]
- Renvert S., Roos-Jansåker A.-M., Persson G.R. Surgical treatment of peri-implantitis lesions with or without the use of a bone substitute - a randomized clinical trial. J. Clin. Periodontol. 2018;45:1266–1274. doi: 10.1111/jcpe.12986. [DOI] [PubMed] [Google Scholar]
- Renvert S., Giovannoli J.-L., Roos-Jansåker A.-M., Rinke S. Surgical treatment of peri-implantitis with or without a deproteinized bovine bone mineral and a native bilayer collagen membrane: A randomized clinical trial. J. Clin. Periodontol. 2021;48:1312–1321. doi: 10.1111/jcpe.13513. [DOI] [PubMed] [Google Scholar]
- Roccuzzo M., Gaudioso L., Lungo M., Dalmasso P. Surgical therapy of single peri-implantitis intrabony defects, by means of deproteinized bovine bone mineral with 10% collagen. J. Clin. Periodontol. 2016;43:311–318. doi: 10.1111/jcpe.12516. [DOI] [PubMed] [Google Scholar]
- Roccuzzo A., Stahli A., Monje A., Sculean A., Salvi G.E. Peri-implantitis: a clinical update on prevalence and surgical treatment outcomes. J. Clin. Med. 2021;10:1107. doi: 10.3390/jcm10051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz M., Chapple I.L. Working Group 4 of the VIII European Workshop on Periodontology. Clinical research on peri-implant diseases: consensus report of Working Group 4. J. Clin. Periodontol. 2012;39(Suppl12):202–206. doi: 10.1111/j.1600-051X.2011.01837.x. [DOI] [PubMed] [Google Scholar]
- Schwarz F., Sahm N., Bieling K., Becker J. Surgical regenerative treatment of peri-implantitis lesions using a nanocrystalline hydroxyapatite or a natural bone mineral in combination with a collagen membrane: a four-year clinical follow-up report. J. Clin. Periodontol. 2009;36:807–814. doi: 10.1111/j.1600-051X.2009.01443.x. [DOI] [PubMed] [Google Scholar]
- Schwarz F., Hegewald A., John G., Sahm N., Becker J. Four-year follow-up of combined surgical therapy of advanced peri-implantitis evaluating two methods of surface decontamination. J. Clin. Periodontol. 2013;40:962–967. doi: 10.1111/jcpe.12143. [DOI] [PubMed] [Google Scholar]
- Schwarz F., Derks J., Monje A., Wang H.-L. Peri-implantitis. J. Clin. Periodontol. 2018;45:S246–S266. doi: 10.1111/jcpe.12954. [DOI] [PubMed] [Google Scholar]
- Tapia B., Valles C., Herrera D., Sanz M., Nart J. The adjunctive effect of a titanium brush in implant surface decontamination at peri-implantitis surgical regenerative interventions: a randomized controlled clinical trial. J. Clin. Periodontol. 2019;46:586–596. doi: 10.1111/jcpe.13095. [DOI] [PubMed] [Google Scholar]
- Wang C.-W., Ashnagar S., Di Gianfilippo R., Arnett M., Kinney J., Wang H.-L. Laser-assisted regenerative surgical therapy for peri-implantitis: a randomized controlled clinical trial. J. Periodontol. 2021;92:378–388. doi: 10.1002/JPER.20-0040. [DOI] [PubMed] [Google Scholar]
- Wang W.C.W., Lagoudis M., Yeh C.-W., Paranhos K.S. Management of peri-implantitis - A contemporary synopsis. Singapore Dental J. 2017;38:8–16. doi: 10.1016/j.sdj.2017.10.001. [DOI] [PubMed] [Google Scholar]
- Zitzmann N.U., Berglundh T. Definition and prevalence of peri-implant diseases. J. Clin. Periodontol. 2008;35:286–291. doi: 10.1111/j.1600-051X.2008.01274.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.