Abstract
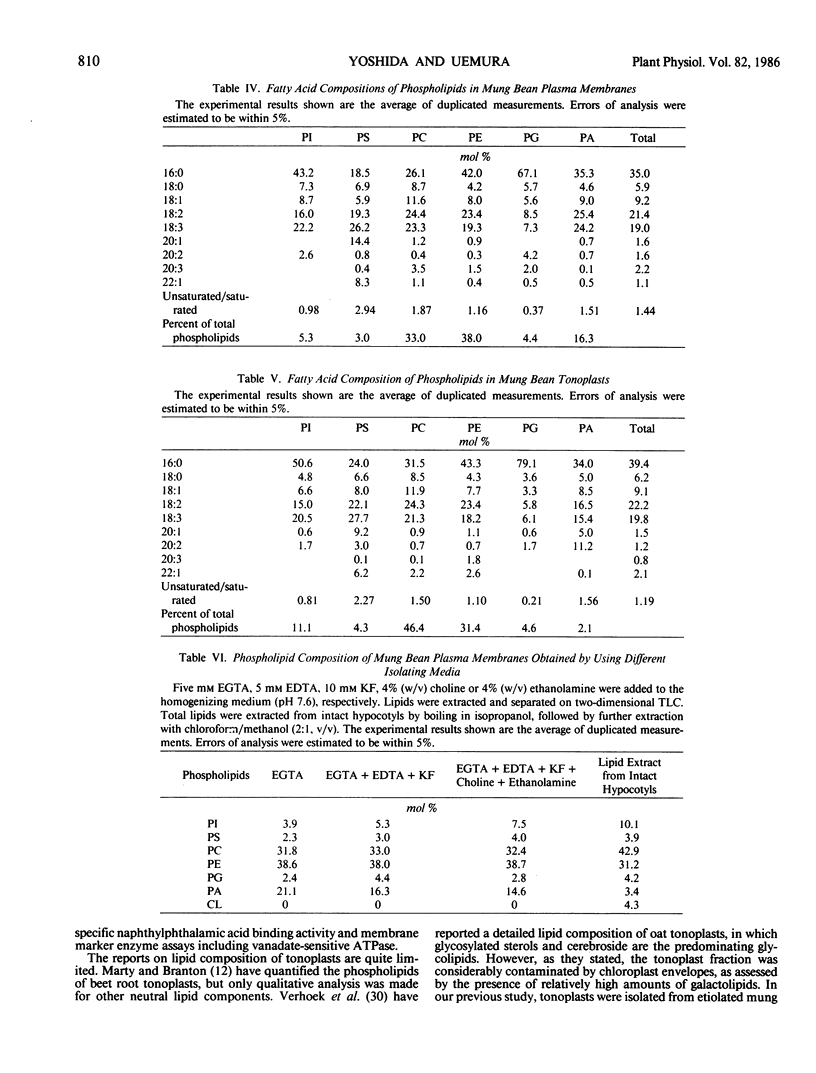
The lipid composition of plasma membranes and tonoplasts from etiolated mung bean hypocotyls was examined in detail. Phospholipids, sterols, and ceramide monohexoside(s) were the major lipid classes in both membranes. The content of phospholipids on a protein basis was higher in the tonoplast, but the content of total sterols was similar in both membranes. Accordingly, the sterol to phospholipid molar ratio in the plasma membrane was higher than that of the tonoplast. Phosphatidylethanolamine and phosphatidylcholine comprised the major phospholipids in both membranes. Phosphatidylinositol, phosphatidylserine, and phosphatidylglycerol were identified as minor phospholipid components. The content of phosphatidylinositol and phosphatidylglycerol was relatively high in the tonoplast, comprising 11 and 5% of the total phospholipids, respectively. Although special care was taken against the degradative action of phospholipase D and phosphatidic acid phosphatase during the isolation of these membranes, by adding EDTA, EGTA, KF, choline, and ethanolamine to the homogenizing medium, significant amounts of phosphatidic acid, about 15% of the total phospholipids, were detected in the plasma membrane. On the other hand, the content of phosphatidic acid in tonoplasts and other membrane fractions was very low. This fact may indicate that high levels of phosphatidic acid occur naturally in plasma membranes. Phosphatidylglycerol in both membranes and phosphatidylinositol in the tonoplast contained high levels of palmitic acid, which comprised more than 50% of the total fatty acids. Significant differences were observed in the sterol compositions of plasma membranes and tonoplasts. More than 90% of the sterols in the plasma membrane were unesterified, while the tonoplast was enriched in glycosylated sterols, especially acylated sterylglycosides. Ceramide monohexoside was found to be specifically located in these membranes, in particular, in the tonoplast, in which it comprised nearly 17% of the total lipids.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Galliard T. Techniques for overcoming problems of lipolytic enzymes and lipoxygenases in the preparation of plant organelles. Methods Enzymol. 1974;31:520–528. doi: 10.1016/0076-6879(74)31056-7. [DOI] [PubMed] [Google Scholar]
- Kogel K. H., Ehrlich-Rogozinski S., Reisener H. J., Sharon N. Surface galactolipids of wheat protoplasts as receptors for soybean agglutinin and their possible relevance to host-parasite interaction. Plant Physiol. 1984 Dec;76(4):924–928. doi: 10.1104/pp.76.4.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty F., Branton D. Analytical characterization of beetroot vacuole membrane. J Cell Biol. 1980 Oct;87(1):72–83. doi: 10.1083/jcb.87.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montezinos D., Brown M., Jr Surface architecture of the plant cell: biogenesis of the cell wall, with special emphasis on the role of the plasma membrane in cellulose biosynthesis. J Supramol Struct. 1976;5(3):277–290. doi: 10.1002/jss.400050303. [DOI] [PubMed] [Google Scholar]
- Murata N., Yamaya J. Temperature-dependent phase behavior of phosphatidylglycerols from chilling-sensitive and chilling-resistant plants. Plant Physiol. 1984 Apr;74(4):1016–1024. doi: 10.1104/pp.74.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarles R. H., Dawson R. M. The distribution of phospholipase D in developing and mature plants. Biochem J. 1969 May;112(5):787–794. doi: 10.1042/bj1120787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn P. J. A lipid-phase separation model of low-temperature damage to biological membranes. Cryobiology. 1985 Apr;22(2):128–146. doi: 10.1016/0011-2240(85)90167-1. [DOI] [PubMed] [Google Scholar]
- Quinn P. J., Williams W. P. Plant lipids and their role in membrane function. Prog Biophys Mol Biol. 1978;34(2):109–173. doi: 10.1016/0079-6107(79)90016-6. [DOI] [PubMed] [Google Scholar]
- Roughan P. G., Batt R. D. Quantitative analysis of sulfolipid (sulfoquinovosyl diglyceride) and galactolipids (monogalactosyl and digalactosyl diglycerides) in plant tissues. Anal Biochem. 1968 Jan;22(1):74–88. doi: 10.1016/0003-2697(68)90261-3. [DOI] [PubMed] [Google Scholar]
- Scherer G. F., Morré D. J. Action and Inhibition of Endogenous Phospholipases during Isolation of Plant Membranes. Plant Physiol. 1978 Dec;62(6):933–937. doi: 10.1104/pp.62.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel G. A., Hess W. M. Evidence for the presence of the toxin-binding protein on the plasma membrane of sugarcane cells. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1413–1417. doi: 10.1073/pnas.71.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis R. L., Berkowitz R. L. Characterization of Soybean Plasma Membrane during Development: FREE STEROL COMPOSITION AND CONCANAVALIN A BINDING STUDIES. Plant Physiol. 1980 May;65(5):871–879. doi: 10.1104/pp.65.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura M., Yoshida S. Involvement of Plasma Membrane Alterations in Cold Acclimation of Winter Rye Seedlings (Secale cereale L. cv Puma). Plant Physiol. 1984 Jul;75(3):818–826. doi: 10.1104/pp.75.3.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman C. E., Travis R. L. Phospholipid composition of a plasma membrane-enriched fraction from developing soybean roots. Plant Physiol. 1985 Oct;79(2):494–498. doi: 10.1104/pp.79.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S. Chemical and Biophysical Changes in the Plasma Membrane during Cold Acclimation of Mulberry Bark Cells (Morus bombycis Koidz. cv Goroji). Plant Physiol. 1984 Sep;76(1):257–265. doi: 10.1104/pp.76.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Kawata T., Uemura M., Niki T. Isolation and Characterization of Tonoplast from Chilling-Sensitive Etiolated Seedlings of Vigna radiata L. Plant Physiol. 1986 Jan;80(1):161–166. doi: 10.1104/pp.80.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Kawata T., Uemura M., Niki T. Properties of Plasma Membrane Isolated from Chilling-Sensitive Etiolated Seedlings of Vigna radiata L. Plant Physiol. 1986 Jan;80(1):152–160. doi: 10.1104/pp.80.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Uemura M. Protein and Lipid Compositions of Isolated Plasma Membranes from Orchard Grass (Dactylis glomerata L.) and Changes during Cold Acclimation. Plant Physiol. 1984 May;75(1):31–37. doi: 10.1104/pp.75.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]