Abstract
Enamel, being the hardest and the highest mineralized tissue of the human body, contains nearly 96% inorganic components and 4% organic compounds and water. Dentin contains 65% inorganic components and 35% organic and water content. The translucency and white appearance of enamel are attributed to Hydroxyapatite (HA), which constitutes the major part of the inorganic component of dental hard tissue. With the advent of nanotechnology, the application of Nanohydroxyapatite (nHA) has piqued interest in dentistry due to its excellent mechanical, physical, and chemical properties. Compared to HA, nHA is found to have superior properties such as increased solubility, high surface energy and better biocompatibility. This is due to the morphological and structural similarity of nanosized hydroxyapatite particles to tooth hydroxyapatite crystals. These nanoparticles have been incorporated into various dental formulations for different applications to ensure comprehensive oral healthcare. To prevent dental caries, several nHA based dentifrices, mouth rinsing solutions and remineralizing pastes have been developed. nHA-based materials, such as nanocomposites, nano impression materials, and nanoceramics, have proven to be very effective in restoring tooth deformities (decay, fracture, and tooth loss). The nHA coating on the surface of the dental implant helps it bind to the bone by forming a biomimetic coating. A recent innovative strategy involves using nHA to reduce dentinal hypersensitivity and to reconstruct periodontal bone defects. The purpose of the present review is to discuss the different applications of nHA in dentistry, especially in preventive and restorative dentistry, dental implantology, bleaching and dentine hypersensitivity management.
Keywords: Nanohydroxyapatite, Dental caries, Remineralization, Hydroxyapatite, Nanoparticles, Dental Implantology, Bleaching
1. Introduction
Hydroxyapatite (HA) has been one of the most studied biomaterials in medicine and dentistry due to its biocompatibility and role as the primary constituent of the body's hard tissues, namely bone and teeth. HA, an effective source of calcium and phosphate, hence it is useful for remineralizing the demineralized enamel regions in case of incipient carious lesions (Pepla, et al., 2014). Owing to its porous structure, HA exhibits poor mechanical properties. When compared with normal HA, the nHA has some exceptional properties like superior solubility, increased surface energy and better biocompatibility. It also has a larger reaction surface due to its small size and has excellent bioactivity property when compared to larger sized crystals (Amaechi et al., 2019). nHA has gained interest in prevention of caries by remineralizing the affected enamel and dentin due to carious attack. In early carious lesion, the dental hard tissue loses mineral ions by bacterial acid attack, but leaves the collagen matrix intact. nHA remineralizes the organic scaffold in the carious attack by directly replacing lost minerals or as a carrier for lost mineral ions. To achieve this remineralizing effect, nHA is incorporated in dentifrices. When synthetic nHA is applied onto the tooth surface, the nanoparticles penetrate the tooth porosities, forming a protecting coating on the tooth surface (Souza et al., 2015). The nHA is considered has a favorable scaffolding material for bone regeneration since it resembles bone in chemical and crystal structure. A study reports that the Gel-nHA scaffold has odontogenic activity which is favorable for endodontic regeneration (Shahi, et al 2022). Even though the diverse application of nanotechnology is vastly researched, there is still little knowledge about its cytotoxicity and safety. Few studies reports that nano-hydroxyapatite can infiltrate systemic regions through oral epithelium and cause cytotoxicity (Tay et al., 2014, Komiyama et al., 2019). In dentistry, the utilization of various materials for oral usage necessitates the use of low or non-toxic agents, which is important for both patients and staff. Moreover, prior to clinical application, screening tests should evaluate any potential toxicity (Shahi, S et al., 2019).(See Table 1).
Table 1.
Recent Evidences on the Nanohydroxyapatite Application in the Field of Dental Implantology, Dentin Hypersensitivity Management, Bleaching, and Dental Caries Prevention and Restoration of Teeth.
| Authors, Year of Publication | Type of Study | Aim of the study | Conclusion |
|---|---|---|---|
| Yadav and Meena, 2022 [70] | In -vitro | Formed a resin-based micro-nano particulate-filled restorative composite and analyzed the consequence of variable nHA filler concentration on their thermomechanical and thermogravimetric properties. | Thermal stability was better with Aluminium oxide-nHA filled dental composite t than Titanium dioxide–nHA filled dental composite |
| Soares et al., 2022 [78] | In -vitro | Studied the activity of human dental pulp cells on polycaprolactone/nHA nanofibrous scaffolds, for dentin regeneration in vital pulp therapy | Mineralized matrix formed after 21 days was 9 times higher for PCL + 2%nHA formulation as compared with the control |
| Baskar et al., 2022 [79] | In -vitro | Assessed odontogenic differentiation capabilities of porous bio-mineralizable composite scaffolds with eggshell derived nHA and Carboxymethyl Chitosan (CMC) on dental pulp stem cells. | DPSCs on 1:5 nHA-CMC scaffolds showed enhanced cell viability nd proliferation along with increased expression of DSPP as well as VEGF |
| Ardani et al., 2022[80] | In -silico | Evaluated the binding molecular docking of Polyether Ether Ketone (PEEK) incorporated with nHA as a biomaterial for orthodontic mini-implant fabrication | PEEK with nHA displayed a striking binding affinity with ALP and IGF-1 osteogenic markers and hence has increased potential for osseointegration. |
| Netalkar et al., 2022[81] | In -vitro | Evaluated the effect of nHA incorporation on fluoride releasing ability, penetration, and adaptation of pit and fissure sealant. | The nHA incorporated sealant showed more samples with no bubbles or debris. The fluoride releasing ability was higher in nHA incorporated sealant group. |
| Tamburaci and Tihminlioglu 2021[82] | In -vitro | Fabricated a bilayer nanocomposite membrane with microporous sublayer composed of chitosan and silicon doped nanohydroxyapatite particles (Si-nHap) and chitosan/polyethylene oxidenanofiber upper layer. | Novel bilayer nanocomposite membranes can be utilised as a material for guided bone regeneration in dentistry especially in periodontal applications. |
| Niu et al., 2021[83] | In- vitro | A novel polyamide-6/chitosan@nano-hydroxyapatite/polyamide-6 (PA6/CS@n-HA/PA6) bilayered tissue guided membranes was formed. | PA6/CS@n-HA/PA6 bilayered scaffolds exhibited safety, good bioactivity, biocompatibility and osteo-conductivity for bone regeneration |
| Fang et al., 2022 [84] | In -vitro | A scaffold using gelatin, nano-hydroxyapatite, metformin(GHMS) was made and its effectiveness in bone regeneration was studied in a rat alveolar bone defect model. | GHMS showed superior bone regeneration compared to extraction-only, Sinbone and Bio-Oss Collagen groups. |
| Amaechi et al., 2022 [85] | RCT | Investigated whether combined usage of nHA dental lotion (Apagard Deep Care) after tooth-brushing with nanoHAP toothpaste (Apagard M−plus) enhanced the remineralization process. | The application of a dental lotion containing 5% nHA after brushing resulted in superior remineralization compared to a placebo lotion. |
| Yoshida et al., 2021 [86] | In -vitro | Studied the effectiveness of dental adhesive material Super-bond (SB), which included 10%, 30%, and 50% nHA (naHAp/SB) on odontoblastic differentiation of dental pulp stem cells) and the formation of reparative dentin was also investigated. | 30% nHA/SB promoted maximum reparative dentin formation and hence can be used as an apt direct pulp capping agent. |
| Gamal et al., 2022 [87] | Animal Study | Compared histologically the bone regenerative ability of combination of hyaluronic acid (HLA) with nanohydroxyapatite (HANP)and using HANP alone in treating bony defects in rabbit calveria. | Both the groups showed bone formation but with the HLA + HANP group the bone formation was better as HLA accelerated the initiation of new bone production when coupled with HANP. |
| Sharifi et al., 2022 [88] | In-vitro study | Formed and analyzed the properties of a novel root repair material with nHA, Portland cement, and bismuth oxide. | There was a significant increase in cell viability in the novel root repair material containing hydroxyapatite nanoparticles after 3 and 7 days |
| Bozoglu et al., 2022 [89] | In-vitro | Osteogenic potential of PEEK implants coated with boron doped nHA on periodontal ligament cells | Enhanced periodontal ligament cells adhesion and proliferation on the surface of PEEK implants coated with boron doped nHA compared with untreated PEEK implants |
| Ji, D. and Lu, D 2022 [90] | In-vitro and Animal study | Effect of nHA composite polyamide 66 at the interface between bone tissue and titanium implants on repairing type II diabetics bone defects | Enhanced bone repair nHA composite polyamide 66 material showed enhance bone repair between bone and implant. The material had good biocompatibility and less cytotoxicity |
| Karthika, 2022 [91] | In vitro | Fe (III) and Cu (II) incorporated hydroxyapatite coatings (Fe/Cu-HAP) on titanium implant was developed and assessed for biocompatibility and antibacterial activity | Fe/Cu-HAP coating on titanium implant showed uniform deposition with improved biocompatibility and bioactivity. The coating had improved antibacterial activity against Staphylococcus aureus and Escherichia coli |
| Hajinaebi et al., 2022 [92] | In-vitro | Ciprofloxacin (CIP) was loaded onto a nHA-coated Ti-6Al-4 V implant. | Implant coated with CIP-nHA showed crack-free homogenous coating on the surface with sustained release of CIP. The antibacterial activity of S. aureus and E. coli was higher on the implants coated with CIP-nHA |
| Moharam et al., 2022 [30] | Clinical study | Effect of 2.5% Arginine and nHA application on the post-bleaching hypersensitivity and color change. | The use of desensitising agents had no effect on the bleaching outcome, but there was a statistically significant colour change. |
| Alsen et al., 2022 [93] | Clinical study | Comparison of nHA with fluoride for the treatment of dentin hypersensitivity following ultrasonic scaling: | Both fluoride and nHA had similar effect |
| Vitiello et al., 2022 [94] | In-vitro | Remineralization potential of CPP-ACP mousse nHA gel, 5% SF varnish, ACP functionalized with fluoride and carbonate-coated with citrate toothpaste and | CPP-ACP mousse nHA gel showed better surface remineralization compared other remineralizing agents |
| Elembaby et al., 2022 [95] | In-vitro | Effect of nHA incorporation into resin infiltrant on the mineral content, surface tomography, and resin tag penetration of demineralized enamel. | High-quality resin tags in demineralized enamel with enhanced mineral density, resin penetration and smooth surfaces |
| Wahba et al., 2022 [96] | In-vitro | Comparison of fluoride varnish, fluoride mouthwash, Self- Assembling Peptide (P11-4), CPP-ACP, and nHA in prevention and arrest of Primary Tooth Enamel Lesions | Fluoride varnish, fluoride mouthwash showed caries-preventive effects whereas other agents did not show any effect |
| Sebastian et al., 2022 [97] | In-vitro | Comparison of CPP-ACP, nHA and Calcium Sucrose Phosphate (CSP) on artificial enamel lesion | CSP showed highest surface microhardness followed by nHA and CPP-ACP |
| Erdilek et al., 2022 [98] | In-vitro | Comparison of remineralization potential of fluoride gel, sodium fluoride toothpaste, and homemade nHA paste on artificial early enamel caries. | Homemade nHA paste had a enhanced remineralization potential of early enamel caries lesions |
| El-Gar et al., 2022 [99] | In-vitro | Assessment of biocompatibility and antibiofilm activity of suspension of nHA of large nanoparticle size (NHA-LPS) and nHA -small particle size (NHA-SPS) | NHA-LPS suspension showed enhanced bacterial adhesion and biofilm thickness in compared to NHA-SPS. |
| Atef et al., 2022 [1 0 0] | In-vitro | Effectiveness of diode laser, fluoride varnish and nHA on the enamel microhardness and microstructural alterations of the primary teeth enamel was assessed | All the remineralizing agents were equally effective in increasing microhardness and maintaining enamel microstructure integrity. |
| Eliwa et al., 2022 [1 0 1] | In-vitro | Comparison of remineralization potential of nano-seashell, nano-pearl, and nHA pastes with fluoride-based toothpaste. | Enamel surface microhardness was highest in fluoride-based toothpaste, nano pearl paste, nano-seashell paste and nHA.Fluorescence was decreased to greatest in nano-seashell, fluoride-based toothpaste, nHA pastes and least in nano pearls. |
1.1. Synthesis of Nanohydroxyapatite
To synthesize nanohydroxyapatite, various methods such as co-precipitation, wet precipitation, hydrothermal, mechanochemical, hydrolysis, solid state and sol-gel method can be used. Among these, wet chemical precipitation method is the most commonly used method as it is highly reproducible, simple as well as economical. A key advantage of this method of synthesis is that the only byproduct is water. nHA obtained by wet precipitation is non-stoichiometric whereas that obtained from solid state method is stoichiometric nHA (Abidi and Murtaza, 2014). Microwave hydrothermal method with ultra-sonic atomization precipitation can be used for big scale and rapid synthesizing of nHA powder. The resulting nano-powder exhibits homogenous size distribution and excellent dispersibility [Cai et al., 2019]. However, using an electrospining technique, nHA coated Nanofibrous scaffolds are created, which are a promising material for bone tissue engineering, wound dressing applications, healing bone defects, and healing bone defects. The electrospining method of coating nHA on various polymers such as cellulose, poly(L-lactide), collagen, and chitosan improves the bioactivity of polymeric nanofibers (Fauziyah et al., 2021; Sato et al., 2021; Ao et al., 2017; Seyedjafari et al., 2010; Stocco et al., 2021; Ribeiro et al., 2014).
2. Applications of Nanohydroxyapatite in dentistry
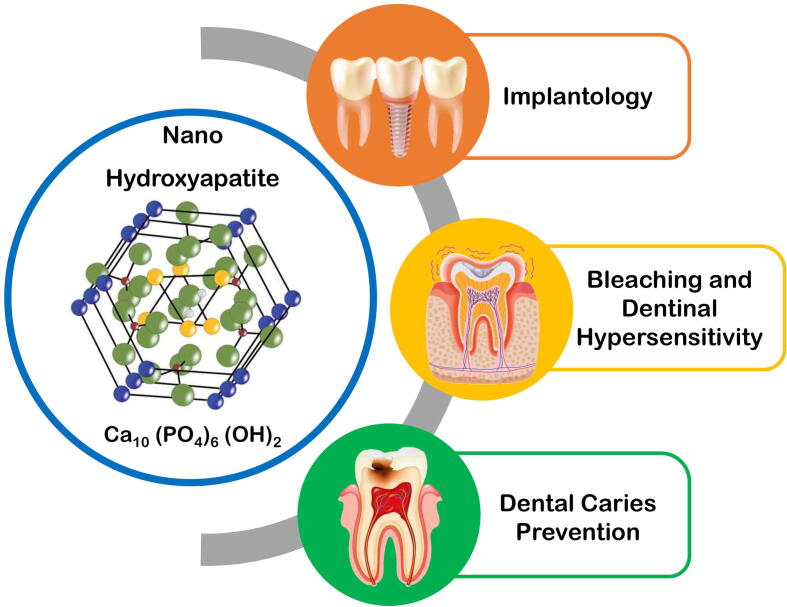
The nHA is a ground-breaking material with numerous applications in dentistry. It is used in a diverse array of dental applications, including implantology, dentin hypersensitivity management, bleaching, and for the prevention and restoration of dental caries teeth which is discussed in the below section (Fig. 1) (Bordea et al., 2020).
Fig. 1.
Various Dental Applications of Nanohydroxyapatite.
2.1. Implantology
Nanohydroxyapatite is most often utilized to coat stainless steel implants and titanium implants to improve bone bonding and new bone genesis, resulting in improved bone-to-implant contact. In-vivo study has reported that nHA crystals deposited onto dental implants have a biological effect and revealed significant presence of osteoblast, osteoclast, and proinflammatory markers (Breding et al., 2014). Oliveira et al. investigated the response of a nHA coating implant using gene expression analysis, indicating its benefits in enhancing bone formation in diabetic rats (de Oliveira et al., 2021). Nanohydroxyapatite-silicate based cement could enhance the primary stability of dental implants in case of circumferential bony defects around implants (Khorshidi et al., 2017). The nHA combined with Zinc Oxide nanoparticles (nZnO) found to have antibacterial action on titanium discs of three coatings (nZnO; nZnO + nHA; nHA) and untreated titanium (Abdulkareem et al., 2015). Memarzadeh et al. investigated the efficiency of nZnO as a coating material, as well as a composite coating material composed of 75% nZnO and 25% nHA. He demonstrated that these materials have strong antibacterial action when exposed to Staphylococcus aureus (Memarzadeh et al., 2015). Jimbo et al. compared nanoscale HA-coated implant with uncoated implant and found that both implants have almost same bone to implant contact. The nano-indentation showcased that the quality of tissues was significantly improved around the hydroxyapatite coated implants (Jimbo et al., 2012). The performance, mechanical, and biological properties of Ti-6Al-4 V dental implants were improved by coating them with nHA using a modified mechanical coating technique (Ahmadzadeh et al., 2020). In an in-vitro study, a collagen, bone morphogenic protein-2, and nHA composite solution coated onto dental implants powerfully expressed osteo-nectin, a marker of osteoblastic differentiation, and induced mesenchymal stem cell differentiation and osteoblastic differentiation. Whereas, placement of this coated dental implants on rabbit tibias showed greater bone formation after 4 weeks (Pang et al., 2021).
2.2. Bleaching and dentinal hypersensitivity
Desensitizing gels containing remineralizing agents like fluoride, casein phosphopeptide amorphous calcium phosphate (CPP-ACP), and nHA are used to reduce hypersensitivity after bleaching, which occurs in 70% of bleached patients (Santoset al., 2015). Because of nHA surface remineralization property, which adds an apatite coating to the tooth's surface, nHA is preferred over desensitizing agents in the treatment of hypersensitivity. The nHA has a high affinity for demineralized surfaces and binds to them. The nHA multiply and aggregate to form microclusters, resulting in an even layer of apatite that can fully overlap prismatic and interprismatic enamel. Thus, nHA restores the altered enamel morphology by preserving the crystallinity of the enamel. Browning et al. tested the efficacy of a nHA paste in alleviating bleaching-related tooth sensitivity. In 42 subjects, a nHA paste and a placebo (zero-HA) paste were assigned at random. The researchers looked into three characteristics of tooth sensitivity: the percentage of participants, the number of days, and the intensity level. The colour change was evaluated. Tooth sensitivity was reported by only 29 percent in the nHA group whereas the non-nano hydroxyapatite group showed 51 percent sensitivity. The study concluded that paste containing nHA can effectively lessen the duration of tooth sensitivity in people who use teeth whiteners (Browning et al., 2012). A study by Shetty et al. showed that desensitization was significantly boosted by hydroxyapatite at 25% concentration in a liquid slurry and at 100% concentration in a dry sol form. (Shetty et al., 2010). nHA paste can heal minute enamel flaws, preventing the sensory reaction. The existence of enamel flaws has been demonstrated to promote bleaching-related tooth sensitivity. These flaws have been repaired using a nHA paste. According to Ferraz et al., the inclusion of varied concentrations of nHA in 35% hydrogen peroxide had no effect on the bleaching of enamel and deep dentin, nor did it affect bond strength of the enamel post bleaching (Ferraz et al., 2018). Another study found that bleached human enamel treated with nHA derived from chicken eggshell increased enamel microhardness after bleaching significantly (Kunam et al., 2019). The application of bleaching agent containing 6% and 12% hydrogen peroxide loaded with nHA preserved the interprismatic enamel structure without altering the enamel morphology and chemical composition (Orilisi et al., 2021). Monterubbianesi et al demonstrated that 6% hydrogen peroxide bleaching gel enriched with nHA safe and effective at home bleaching system since it has a potential to maintain the enamel microstructure, without changing the color (Monterubbianesi et al., 2021). In a comparative study showed that 2.5% Arginine and nHA are equally effective for the management of post-bleaching dentin sensitivity (Moharam et al., 2022). Studies report that there is enhanced clinical performance and dentine hypersensitivity relief using nHA containing agents (de Melo Alencar et al., 2019, Oubenyahya, 2021). Clinically toothpaste containing either 10% or 15% nHA (10 or 15%) solely or supplemented with potassium nitrate was as effective as calcium sodium phosphosilicate for relief of dentine hypersensitivity symptoms when applied at least twice daily. The study also found that toothpaste containing 15% nHA was more effective than toothpaste containing 10% nHA at reducing dentin sensitivity (Amaechi et al., 2021). 20% nHA cream is equally effective to 20% pure silica containing dental cream in relieving the dentine hypersensitivity symptoms (Amaechi et al., 2018).Dentifrices containing nHA have showed complete closure of dentine tubules and intertubular dentine coverage with significant mineral deposition on the dentin surface (Bologa, et al., 2020).
2.3. Nano-hydroxyapatite in dental caries prevention
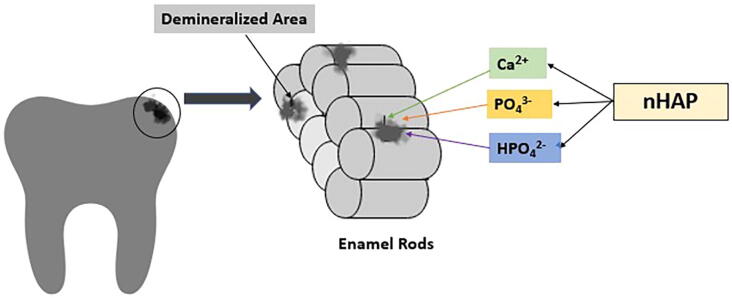
Nano-hydroxyapatite has been used in toothpastes for caries prevention since 1980 mainly in Japan, and in the year 1993, it was approved as an anti-caries agent by the Japanese Government (Kani et al., 1989). nHA prevents dental caries mainly by exhibiting strong binding affinity with proteins and plaque and bacteria fragments, thus there is an increase in the surface area for the proteins which binds to it. It acts as filler and repairs the enamel surface depression which is enhanced due to its small size. According to few researchers, the mechanism of action of nHA is that it promotes remineralization by depositing apatite nano-particles in enamel defects. Another mechanism of action is that nHA acts as a calcium phosphate reservoir, keeping a state of super-saturation with regard to enamel minerals, enhancing remineralization and inhibiting demineralization. (Huang et al., 2011, Philip, 2019).The nHA has the ability to reprecipitate the minerals on the carious lesion surface which is aided by its surface bio-activity combined with its chemical as well as physical resemblance to tooth enamel. The nHA was found to induce constant enamel caries remineralization by producing a homogeneous apatite layer on the demineralized enamel surfaces by chemically bonding to natural apatite crystals. The hardness along with the modulus of elasticity of the restored enamel is same as the natural enamel. Incipient enamel lesion has highly porous enamel surface hence allows greater penetration of nHA. These particles will then act as a template and continue to attract calcium and phosphate ions thus leading to promotion of crystal growth and integrity (Fig. 2).Research by Huang et al revealed that a 10% nHA suspension is the most optimum concentration for remineralizing incipient carious lesions. There was a strident increase between the remineralization effect at 5% and 10% concentrations. For the concentrations above 10%, not much increase in remineralization was seen. This might be because the nHA deposited on the outer enamel layer will block the porosities on the enamel surface and limit further diffusion into the carious lesions in the short period of remineralization. The calcium concentration in nHA solutions was considerably higher than the micro hydroxyapatite solutions. The increased calcium concentration promotes the saturation of oral fluids with hydroxyapatite, thus facilitating the apatite minerals deposition in carious regions and leading to remineralization. The mineralization effect was maximum at a pH of 4.0 whereas pH 7.0 showed least mineralization (Huang et al., 2011). Along with remineralization, the nHA rinses have also proven to be effective in increasing tooth microhardness. nHA may also have a synergetic role in remineralization with fluoride. nHA in a 0.05% Sodium fluoride mouth rinse is found to be helpful to remineralize early carious lesion (Haghgoo et al., 2014). Tschoppe et al. in 2011 developed a compound called zinc carbonate nHA and demonstrated that it has similar remineralizing capacity on enamel and dentin when compared with fluoride (Tschoppe et al., 2011).
Fig. 2.
Mechanism of Action of Nanohydroxyapatite (nHAP) on Initial Enamel Caries.
Katarzyna Grocholewicz et al. showed that the combination of nHA and ozone therapy is more effective than when applied individually for remineralization of approximal initial carious lesions (Grocholewicz, et al., 2020). Daas in 2018 compared the remineralization potential of nHA paste and fluoride varnish and concluded that there is no significant difference between the two (Daas et al., 2018). Juntavee et al. devised an intriguing strategy by studying the effect of nHA gel and Clinpro (CP) on the remineralization ability of enamel and cementum on the cavo-surface area, and it was discovered that nHA was highly capable in the remineralization of enamel and cementum. (Juntavee et al., 2021). The use of 10% nHA dentifriceon the demineralized enamel surface after post orthodontic debonding showed enhanced remineralization assessed using extracted maxillary premolar (Verma et al, 2021). In primary teeth, GC Tooth Mousse® and nHA toothpaste had the same remineralizing effect as 1000 ppm fluoridated toothpaste, as evaluated by surface microhardness on artificial caries (Kasemkhun et al., 2021). The highest remineralizing potential was found in nHA-containing dentifrice, followed by bioactive glass, CPP-ACP (casein phosphopeptide-amorphous calcium phosphate), and fluoride. There was a noteworthy difference in surface microhardness and the formation of hydroxyapatite crystals after treating the demineralized teeth samples in all groups (Geeta et al., 2020). An in-vitro study to assess root dentin demineralization using high fluoride (F−) concentration (5,000 µg F/g) and nHA showed that both dentifrices reduced dentine demineralization (Leal et al., 2020). A comparative study of the remineralization potential of nHA, NovaMin, and amine fluoride dentifrices on artificial enamel caries found that all are efficient in remineralizing artificial carious lesions. However nHA dentifrices that nHA dentifrice produced better effects with significantly decreased lesion depth compared to amine fluoride and NovaMin dentifrices (Manchery et al., 2019).Another study compared the effects of various dentifrices, such as bioactive glass Novamin, nHA, functionalized tricalcium phosphate, and grape seed extract, on the initial stage of demineralization through remineralization. The bioactive glass Novamin recovered the most surface microhardness, followed by functionalized tricalcium phosphate, nHA, and grape seed extract (Joshi et al., 2019). In-situ study showed that dental lotion with 5% nHA was effective in remineralizing initial caries and inhibition of enamel demineralization when applied twice daily for 6 months in patients undergoing fixed orthodontic treatment (Amaechi B.T et al., 2021). Immediate application of Iranian nHA toothpaste and fluoride toothpaste after debonding the fixed orthodontic treatment for 6 months consistently showed reduced lesion extent and increased amount of remineralization on early enamel lesions. (Badiee et al., 2020). Wierichs et al. suggested that both fluoride-free dentifrices, one containing nHA did not hamper demineralization (Wierichs et al., 2020). In a clinical study, initial carious lesions detected using ICDAS caries diagnostic criteria subjected to three different remineralizing agents such as Tricalcium phosphate paste (TCP), Fluoride varnish, and nHA. At week five, the DIAGNOdent scores of incipient carious lesions were recorded to assess the remineralizing effect. The results of the study showed that all the 3 remineralizing agents had a momentous remineralizing effect on incipient caries on both occlusal and smooth surfaces. The nHA gel showed highest significant effect on initial caries as compared to both fluoride varnish and TCP paste (Alhamed et al., 2020). Compared to in-vitro study, there is insufficient clinical evidence for nHA's efficacy in reducing dental caries formation and remineralizing early carious lesions. Apart from remineralization properties, the nHA can also inhibit bacterial colonization of Streptococcus mutans due to excellent absorptive properties of the nHA on salivary proteins (Lu et al., 2007). The effect of nHA on the glucosyltransferase genes expression showed enhanced the transcription of gtfB, gtfC and gtfD which encodes enzymes responsible for insoluble glucans production (Park et al., 2019).Two different nHA-based toothpastes containing substituted with metal ions namely Zinc-carbonate substituted nHA (α toothpaste) and Fluoride, Magnesium, Strontium-carbonate substituted nHA (β toothpaste) assessed on early colonization and biofilm formation. The effect of α toothpaste was high on early colonization and biofilm formation compared to β toothpaste (Ionescu et al., 2020).
2.4. Nanohydroxyapatite in GIC
Hydroxyapatite being the most stable derivative of calcium phosphate saltswere incorporated into GIC to improve its properties like, compressive strength, flexural strength, microhardness, reduction in cytotoxicity, increases fluoride ion release and enhance its antibacterial properties. Sincehydroxyapatite exhibits increased crystallinity, nHA is incorporated into cement and has an increased compressive strength ranging between 107 and 113.6 MPa. (Barandehfard F et al., 2016). A study by Moheet et al. showed that there was an increase of 53.34% in the flexural strength with the addition of 10% nHA to GIC. This change in the flexural strength is because of porosity of HA. The release of fluoride ions is enhanced because of the bigger surface area of nHA particles contributing to an increase in acid - base reactivity (Moheet IA et al., 2018). Kheur et al. studied the effects of integrating nHA in glass ionomer luting agents and evaluated the shear bond strength to the tooth as well as the flexural strength. This was compared with the conventional GICs, resin modified GIC, and also with adhesive resin. While adhesive resin showcased maximum flexural strength as well as showed highest shear bond strengths, the authors inferred that experimental nHA modified glass ionomer cement had better bonding of the polyacid’s carboxyl groups with the calcium present in the natural tooth to synthetic hydroxyapatite (Kheur et al., 2020). Alatawi et al. conducted a similar experiment, by incorporating various concentrations of nHA into GIC. The results showed that adding nHA increased the release of fluoride ion by the conventional GIC. Furthermore, when an 8% concentration of nHA was used, the bacterial inhibition against Streptococcus mutans was quite significant (Alatawi RAS et al., 2019). Noorani et al. compared the cytotoxicity of nHA-silica incorporated into GIC to that of conventional GIC and resin-modified GIC on human Dental Pulp Stem Cells (DPSCs). In terms of cytotoxicity, the experimental nHA-silica-GIC was comparable to the conventional GIC but superior to the resin-modified GIC (Noorani et al., 2017). A more complex hybrid product containing GIC—nHA–mucosal defensive agent–antibiotic emulsion was tested by Pagano et al. for its mechanical, thermal and biological properties. The study showed that the complex hybrid product improved the mechanical properties, especially in oral cavity because of its wet environment and with the powder formulated antibiotic. The addition of nHA which has antibiotic and mucosal defensive properties, significantly decreased the overall toxicity of the cement (Pagano et al., 2019).
2.5. Nanohydroxyapatite in dental composite
Meena et al investigated on adding 5–20 wt% nHA and Mineral Trioxide Aggregate (MTA) on various properties of dental composite. The nHA filler was seen to improve the thermal stability of dental composite than MTA filler (Meena et al., 2017). Another study evaluated the effect of micro/nHA rod addition in composite resins on its strength and remineralization. Hybrid nano-rods and micro-rod HA filled composites displayed great potential application for teeth restoration (Wu et al., 2019). Lung et al. found that composite resin matrix when reinforced with 30 wt% silanized-nHA improved the physical and mechanical properties of a Bis-GMA based composite resin (Lung et al., 2016). Yadav et al. aimed to study the efficacy of 3-methacryloxypropyl trimethoxy silane and 3-aminopropyl triethoxysilane treated nHAP filler in developing high-strength, thermally-stable photocurable dental composite with enhanced thermo-mechanical properties. This functionalized nHA based dental composite material was considered as a substitute for restorative dentistry (Yadav and Gangwar, 2021). The alumina nHA and titanium oxide nHA hybrid form of dental composite restorative material was assessed for thermo-mechanical and thermogravimetric properties. The study results showed that alumina nHA filled dental composite was more significant than titanium oxide dental composite material (Yadav and Meena, 2022).
2.6. Application of Nanohydroxyapatite in orthodontics
The use of nHA under orthodontic bands reduced microleakage but had no effect on tooth colour changes following orthodontic debonding (Malekpour et al., 2022, Enan and Hammad, 2013). During active orthodontic treatment, a biomimetic nHA solution reduced hard tissue demineralization. Bond strength values for brackets and attachments were reduced following remineralizing treatment. Composite attachments had higher adhesion values than brackets. When compared to demineralized enamel, remineralized enamel with biomimetic nHA had higher microhardness values (Scribante et al 2020).
2.7. Nanohydroxyapatite in Periodontal Tissue Regeneration
Recently nHA is considered as a viable alternative to autogenous bone grafts in periodontal tissue and alveolar tissue regeneration because it has several advantages such as biocompatibility, minimal morbidity as well as absence of toxicity. If nHA is coupled with some other active particles or biologic mediators, it can boost periodontal tissue regeneration even more when used alone. nHA has been studied to be helpful in periodontitis in supporting alveolar bone regeneration. An animal study by Tanongpitchayes et al. on preservation of post-extraction sockets of dogs with clinical periodontitis by development of a nHA-based Hydrogel found that it could enhance regeneration of extraction socket in dogs with periodontitis. Hence, it can be used as a bone substitute for post-extraction socket preservation, which would further help in planning for future dental implant placement (Tanongpitchayes et al., 2021). A recent article concluded that the nHA graft displayed promising results clinically in periodontal regeneration and hence can be used for treating the intrabony defects (Wang et al., 2020). The Nano-Hydroxyapatite-Modified Collagen material (nHAC) and Fibroblast Growth Factor (bFGF) composite scaffold material was developed to regenerate and repair periodontal tissue. This scaffold material had a dense and porous 3D structure that increased bFGF loading and locked the periodontal ligament cells, promoting cell growth and attachment (Wang et al., 2020). The osteogenic differenting capability of Periodontal Ligament Stem Cells (PDLSCs) derived from ovariectomized rats was demonstrated by the collage-based composite scaffold composed of nHA and poly(L-lactide). PDLSCs adhered, expanded, and proliferated on the collagen-based composite scaffold, producing a large amount of mineral matrix (Xu et al., 2016). The 1% nHA/chitosan scaffold demonstrated a favourable environment for the growth of Human Periodontal Ligament Cells (HPLCs) as well as improved cytocompatibility. Furthermore, the alkaline phosphatase and type I collagen levels produced by the HPLCs on the 1% nHA/chitosan scaffold were significantly higher than those produced by the pure chitosan scaffold (Zhang et al., 2007). The nHA deposited on polymer blends of Poly (D,L-lactic acid)/poly(D, L-lactic- co-glycolic acid) by sonication method could potentially improve bone cell adhesion, structural stability, detrimental pH variations, and wetting properties. The developed composite membrane exhibited extended membrane degradation time with a potential to enhance the process of periodontal tissue regeneration disease (Higuchi et al., 2019). To regenerate periodontal bone tissues in diabetic patients with impaired immune systems, a conductive alginate/gelatin scaffold composed of polydopamine-mediated graphene oxide and hydroxyapatite nanoparticle was developed. This scaffold exhibited cell affinity with good conductivity which stimulated Ca2+ ion channels on the cell membrane and promoted influx of Ca2+ ion. The scaffold aided in secretion of osteogenesis-related cytokines by suppressing M1 macrophage polarization and M2 macrophages activation (Li et al., 2022). Besides above mentioned applications nHA can also be utilized to repair bone abnormalities caused by trauma or surgery. nHA can be utilized with success in tissue engineering and regeneration of bone/cementum when conjucted with stem cells and growth factors and placed on a scaffold. It can be used in oral surgical operations such as repair of cleft lip and palate and periodontal therapies (Al-Ahmady et al., 2018).
2.8. Cytotoxicity of nanohydroxyapatite
Despite the fact that nanotechnology is already part of our daily lives, there has been much debate on the safety of nanomaterials. According to reports, nano-silica has different biological features from micro-level material in terms of skin penetration and nuclear entrance, and it has a number of negative biological impacts both locally and systemically, including DNA fragmentation. A study by Tay et al has shown that n-HAP particles are taken up and cause cytotoxicity in monolayer grown human oral epithelial (TR146)cells (Tay et al., 2014). Komiyama et al conducted a study on the permeability of n-HA on oral epithelium, showed that Nano-hydroxyapatite may encounter resistance when trying to enter the oral epithelium due to stratum corneum. Furthermore, given the lifespan of oral epithelial cells is 5-7 days, the surface cells of the non-keratinized mucosa where nanoparticles are absorbed are likely to degenerate within that time. Their findings imply that it is unlikely for nano-hydroxyapatite to infiltrate systemic regions through oral epithelium (Komiyama et al., 2019).
3. Conclusion
Nanohydroxyapatite is a promising revolutionary material in the prevention of early carious lesion mainly due to greater source of free calcium. The development of Nanofiber loaded with nHA as a composite material is explored minimally. 3D printed nHA with varied polymeric composition still need to be further explored for dentin-pulp regeneration. Apart from remineralization various other applications of nanohydroxyapatite in dentistry is also extensively researched upon. Its use in regeneration has opened new vistas in the field of implantology [El-Gar et al., 2022]. However, long term studies are required to establish safety of continuous use nHA in daily life.
CRediT authorship contribution statement
C. Pushpalatha: Conceptualization, Resources, Data curation, Writing – original draft, Supervision. V.S. Gayathri: Conceptualization, Resources, Data curation, Writing – original draft. S.V. Sowmya: Writing – original draft, Writing – review & editing, Visualization. Dominic Augustine: Writing – original draft, Writing – review & editing, Visualization. Ahmed Alamoudi: Writing – review & editing, Visualization. Bassam Zidane: Writing – review & editing, Visualization. Nassreen Hassan Mohammad Albar: Writing – review & editing, Visualization. Shilpa Bhandi: Writing – review & editing, Visualization, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
References
- Abdulkareem E.H., Memarzadeh K., Allaker R.P., Huang J., Pratten J., Spratt D. Anti-biofilm activity of zinc oxide and hydroxyapatite nanoparticles as dental implant coating materials. J. Dent. 2015 Dec;43(12):1462–1469. doi: 10.1016/j.jdent.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Abidi S.S.A., Murtaza Q. Synthesis and characterization of nano-hydroxyapatite powder using wet chemical precipitation reaction. J. Mater. Sci. Technol. 2014 Apr;30(4):307–310. [Google Scholar]
- Ahmadzadeh H., Isfahani T., Kharazi A.Z. Modified mechanical coating technique for the preparation of Nanohydroxyapatite coated Ti–6Al–4V dental implants. Prot. Met. Phys. Chem. 2020;56(4):766–771. [Google Scholar]
- Al-Ahmady HH, Abd Elazeem AF, Bellah Ahmed NE moataz, Shawkat WM, Elmasry M, Abdelrahman MA, et al. Combining autologous bone marrow mononuclear cells seeded on collagen sponge with Nano Hydroxyapatite, and platelet-rich fibrin: Reporting a novel strategy for alveolar cleft bone regeneration. Journal of Cranio-Maxillofacial Surgery. 2018 Sep;46(9):1593–600.8. [DOI] [PubMed]
- Alatawi R.A.S., Elsayed N.H., Mohamed W.S. Influence of hydroxyapatite nanoparticles on the properties of glass ionomer cement. J. Mater. Res. Technol. 2019 Jan;8(1):344–349. [Google Scholar]
- Alhamed M., Almalki F., Alselami A., Alotaibi T., Elkwatehy W. Effect of different remineralizing agents on the initial carious lesions–A comparative study. Saudi Dent. J. 2020;32(8):390–395. doi: 10.1016/j.sdentj.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaechi B.T., Alshareif D.O., Azees P.A.A., Shehata M.A., Lima P.P., Abdollahi A., Kalkhorani P.S., Evans V., Bagheri A., Okoye L.O. Anti-caries evaluation of a nano-hydroxyapatite dental lotion for use after toothbrushing: An in situ study. J. Dent. 2021;115 doi: 10.1016/j.jdent.2021.103863. [DOI] [PubMed] [Google Scholar]
- Amaechi, B.T., AbdulAzees, P.A., Alshareif, D.O., Shehata, M.A., Lima, P.P.d.C.S., Abdollahi, A., et al., 2019 Dec, Comparative efficacy of a hydroxyapatite and a fluoride toothpaste for prevention and remineralization of dental caries in children, BDJ Open, 5 (1) 18. [DOI] [PMC free article] [PubMed]
- Amaechi B.T., Lemke K.C., Saha S., Gelfond J. Clinical efficacy in relieving dentin hypersensitivity of nanohydroxyapatite-containing cream: A randomized controlled trial. Open Dent. J. 2018;12:572. doi: 10.2174/1874210601812010572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaechi B.T., Lemke K.C., Saha S., Luong M.N., Gelfond J. Clinical efficacy of nanohydroxyapatite-containing toothpaste at relieving dentin hypersensitivity: An 8 weeks randomized control trial. BDJ open. 2021;7(1):1–8. doi: 10.1038/s41405-021-00080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ao C., Niu Y., Zhang X., He X., Zhang W., Lu C. Fabrication and characterization of electrospun cellulose/nano-hydroxyapatite nanofibers for bone tissue engineering. Int. J. Biol. Macromol. 2017;97:568–573. doi: 10.1016/j.ijbiomac.2016.12.091. [DOI] [PubMed] [Google Scholar]
- Ardani I.G., Nugraha A.P., Suryani M.N., Pamungkas R.H., Vitamamy D.G., Susanto R.A., Sarno R., Fajar A., Kharisma V.D., Putera A. Molecular docking of polyether ether ketone and nano-hydroxyapatite as biomaterial candidates for orthodontic mini-implant fabrication. J. Pharm. Pharmacogn. Res. 2022;10(4):676–686. [Google Scholar]
- Atef R., Zaky A.A., Waly N., El Rouby D., Ezzeldin N. Effect of diode laser and remineralizing agents on microstructure and surface microhardness of therapeutic gamma-irradiated primary teeth enamel. open access macedonian. J. Med. Sci. 2022;10(D):243–250. [Google Scholar]
- Badiee M., Jafari N., Fatemi S., Ameli N., Kasraei S., Ebadifar A. Comparison of the effects of toothpastes containing nanohydroxyapatite and fluoride on white spot lesions in orthodontic patients: A randomized clinical trial. Dent. Res. J. (Isfahan) 2020;17:354–359. [PMC free article] [PubMed] [Google Scholar]
- Barandehfard F., Kianpour Rad M., Hosseinnia A., Khoshroo K., Tahriri M., Jazayeri H.E., et al. The addition of synthesized hydroxyapatite and fluorapatite nanoparticles to a glass-ionomer cement for dental restoration and its effects on mechanical properties. Ceram. Int. 2016 Nov;42(15):17866–17875. [Google Scholar]
- Baskar K., Saravana Karthikeyan B., Gurucharan I., Mahalaxmi S., Rajkumar G., Dhivya V., Kishen A. Eggshell derived nano-hydroxyapatite incorporated carboxymethyl chitosan scaffold for dentine regeneration: A laboratory investigation. Int. Endodont. J. 2022;55(1):89–102. doi: 10.1111/iej.13644. [DOI] [PubMed] [Google Scholar]
- Bologa E., Stoleriu S., Iovan G., Ghiorghe C.A., Nica I., Andrian S., Amza O.E. Effects of dentifrices containing nanohydroxyapatite on dentinal tubule occlusion—A scanning electron microscopy and edx study. Appl. Sci. 2020;10(18):6513. [Google Scholar]
- Bordea I.R., Candrea S., Alexescu G.T., Bran S., Băciuț M., Băciuț G., Lucaciu O., Dinu C.M., Todea D.A. Nano-hydroxyapatite use in dentistry: A systematic review. Drug Metab. Rev. 2020;52(2):319–332. doi: 10.1080/03602532.2020.1758713. [DOI] [PubMed] [Google Scholar]
- Bozoğlu Ü.Ç., Kiremitçi A., Yurtsever M.Ç., Gümüşderelioğlu M. Peek dental implants coated with boron-doped nano-hydroxyapatites: Investigation of in-vitro osteogenic activity. J. Trace Elem. Med. Biol. 2022:127026. doi: 10.1016/j.jtemb.2022.127026. [DOI] [PubMed] [Google Scholar]
- Breding, K., Jimbo, R., Hayashi, M., Xue, Y., Mustafa, K. and Andersson, M., 2014. The effect of hydroxyapatite nanocrystals on osseointegration of titanium implants: an in vivo rabbit study. International journal of dentistry, 2014. [DOI] [PMC free article] [PubMed]
- Browning W.D., Cho S.D., Deschepper E.J. Effect of a nano-hydroxyapatite paste on bleaching-related tooth sensitivity. J. Esthet. Restor. Dent. 2012;24(4):268–276. doi: 10.1111/j.1708-8240.2011.00437.x. [DOI] [PubMed] [Google Scholar]
- Cai Z., Wang X., Zhang Z., Han Y., Luo J., Huang M., et al. Large-scale and fast synthesis of nano-hydroxyapatite powder by a microwave-hydrothermal method. RSC Adv. 2019;9(24):13623–13630. doi: 10.1039/c9ra00091g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daas I., Badr S., Osman E. Comparison between fluoride and nano-hydroxyapatite in remineralizing initial enamel lesion: an in vitro study. J. Contemp. Dent. Pract. 2018;19(3):306–312. [PubMed] [Google Scholar]
- de Melo Alencar C., de Paula B.L.F., Ortiz M.I.G., Magno M.B., Silva C.M., Maia L.C. Clinical efficacy of nano-hydroxyapatite in dentin hypersensitivity: A systematic review and meta-analysis. J. Dent. 2019;82:11–21. doi: 10.1016/j.jdent.2018.12.014. [DOI] [PubMed] [Google Scholar]
- de Oliveira, P.G.F.P., de Melo Soares, M.S., Silveira e Souza, A.M.M., Taba Jr, M., Palioto, D.B., Messora, M.R., Ghiraldini, B., Nunes, F.A.D.S. and de Souza, S.L.S., 2021. Influence of nano‐hydroxyapatite coating implants on gene expression of osteogenic markers and micro‐CT parameters. An in vivo study in diabetic rats. J. Biomed. Mater. Res. Part A, 109(5), pp.682-694. [DOI] [PubMed]
- Elembaby A., AlHumaid J., El Tantawi M., Akhtar S. The impact of nano-hydroxyapatite resin infiltrant on enamel remineralization: An in vitro study. Int. J. Periodont. Restor. Dent. 2022;42(2) doi: 10.11607/prd.5599. [DOI] [PubMed] [Google Scholar]
- El-Gar Y.H.A., Etman W.M., Genaid T.M., Al-Madboly L.A. Potent antibacterial and antibiofilm activities of a synthetic remineralizing preparation of nano-hydroxyapatite against cariogenic streptococcus mutans using an ex-vivo animal model. Front. Dent. Med. 2022;3:738326. doi: 10.3389/fdmed. [DOI] [Google Scholar]
- Eliwa M., Aidaros N., Kamh R. A comparative evaluation of remineralization potential of nano-seashell, nano-pearl, and nano-hydroxyapatite pastes versus fluoride-based toothpaste on non-cavitated initial enamel lesion: An in vitro study. Egypt. Dent. J. 2022;68(1) [Google Scholar]
- Enan E.T., Hammad S.M. Microleakage under orthodontic bands cemented with nano-hydroxyapatite-modified glass ionomer: An in vivo study. Angle Orthod. 2013 Nov;83(6):981–986. doi: 10.2319/022013-147.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdilek A.D., Burke S., Şahin M., Efes A., Efes B.G. Effects of homemade nano-hydroxyapatite and olive oil paste on remineralization of early caries lesions. Trop. Health Med. Res. 2022;4(1):1–9. [Google Scholar]
- Fang C.H., Sun C.K., Lin Y.W., Hung M.C., Lin H.Y., Li C.H., et al. Metformin-incorporated gelatin/nano-hydroxyapatite scaffolds promotes bone regeneration in critical size rat alveolar bone defect model. IJMS. 2022 Jan 5;23(1):558. doi: 10.3390/ijms23010558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauziyah, M., Salsabila, T., Setyawan, H. and Widiyastuti, W., 2021. Synthesis of Chitosan/Hydroxyapatite Nanofibers as A Wound Dressing via Electrospinning Method. In Journal of Physics: Conference Series (Vol. 1726, No. 1, p. 012019). IOP Publishing.
- Ferraz L.N., Júnior W.F.V., Ambrosano G.M.B., Giorgi M.C.C., Aguiar F.H.B., Lima D.A.N.L. Effect of different concentrations of nanohydroxyapatite on tooth bleaching effectiveness and enamel bond strength. Braz. Dent. Sci. 2018;21(1):17–25. [Google Scholar]
- M. Gamal SM, Bilal MM, El Kholey SE. Evaluation of the effectiveness of hyaluronic acid plus nanohydroxyapatite versus nanohydroxyapatite alone on bone regeneration in rabbits. Tanta Dent J 2022;19:77-83
- Geeta R.D., Vallabhaneni S., Fatima K. Comparative evaluation of remineralization potential of nanohydroxyapatite crystals, bioactive glass, casein phosphopeptide-amorphous calcium phosphate, and fluoride on initial enamel lesion (scanning electron microscope analysis)–An in vitro study. J. Conserv. Dent.: JCD. 2020;23(3):275. doi: 10.4103/JCD.JCD_62_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grocholewicz K., Matkowska-Cichocka G., Makowiecki P., Droździk A., Ey-Chmielewska H., Dziewulska A., Tomasik M., Trybek G., Janiszewska-Olszowska J. Effect of nano-hydroxyapatite and ozone on approximal initial caries: A randomized clinical trial. Sci. Rep. 2020;10(1):1–8. doi: 10.1038/s41598-020-67885-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghgoo R., Rezvani M.B., Salehi Z.M. Comparison of nano-hydroxyapatite and sodium fluoride mouthrinse for remineralization of incipient carious lesions. J. Dent. (Tehran) 2014 Jul;11(4):406–410. [PMC free article] [PubMed] [Google Scholar]
- Hajinaebi M., Ganjali M., Nasab N.A. Antibacterial activity and drug release of ciprofloxacin loaded PVA-nHAp nanocomposite coating on Ti-6Al-4 V. J. Inorg. Organomet. Polym. Mater. 2022:1–12. [Google Scholar]
- Higuchi J., Fortunato G., Woźniak B., Chodara A., Domaschke S., Męczyńska-Wielgosz S., Kruszewski M., Dommann A., Łojkowski W. Polymer membranes sonocoated and electrosprayed with nano-hydroxyapatite for periodontal tissues regeneration. Nanomaterials. 2019;9(11):1625.6. doi: 10.3390/nano9111625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.B., Gao S.S., Cheng L., Yu H.Y. Remineralization potential of nano-hydroxyapatite on initial enamel lesions: an in vitro study. Caries Res. 2011;45:460–468. doi: 10.1159/000331207. [DOI] [PubMed] [Google Scholar]
- Ionescu, A.C., Cazzaniga, G., Ottobelli, M., Garcia-Godoy, F. and Brambilla, E., 2020. Substituted Nano-Hydroxyapatite toothpastes reduce biofilm formation on enamel and resin-based composite surfaces. Journal of Functional Biomaterials, 11(2), p.36. [DOI] [PMC free article] [PubMed]
- Ji, D. and Lu, D., 2022. Efficiency of Nanohydroxyapatite on Repairing Type II Diabetes Dental Implant-Bone Defect. BioMed Research International, 2022. [DOI] [PMC free article] [PubMed] [Retracted]
- Jimbo R., Coelho P.G., Bryington M., Baldassarri M., Tovar N., Currie F., et al. Nano hydroxyapatite-coated implants improve bone nanomechanical properties. J. Dent. Res. 2012 Dec;91(12):1172–1177. doi: 10.1177/0022034512463240. [DOI] [PubMed] [Google Scholar]
- Joshi C., Gohil U., Parekh V., Joshi S. Comparative evaluation of the remineralizing potential of commercially available agents on artificially demineralized human enamel: An In vitro study. Contemp. Clin. Dent. 2019;10(4):605. doi: 10.4103/ccd.ccd_679_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juntavee, A., Juntavee, N. and Sinagpulo, A.N., 2021. Nano-hydroxyapatite gel and its effects on remineralization of artificial carious lesions. International Journal of Dentistry, 2021. [DOI] [PMC free article] [PubMed]
- Kani T., Kani M., Isozaki A., Shintani H., Ohashi T., Tokumoto T. Effect to apatite-containing dentifrices on dental caries in school children. J. Dent. Health. 1989;39(1):104–109. [Google Scholar]
- Karthika A. Biocompatible iron and copper incorporated nanohydroxyapatite coating for biomedical implant applications. Mater. Today: Proc. 2022;51:1754–1759. [Google Scholar]
- Kasemkhun P., Rirattanapong P. The efficacy of non-fluoridated toothpastes on artificial enamel caries in primary teeth: An in vitro study. J. Int. Soc. Prevent. Community Dent. 2021;11(4):397. doi: 10.4103/jispcd.JISPCD_64_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheur M., Kantharia N., Iakha T., Kheur S., Husain N.A.H., Özcan M. Evaluation of mechanical and adhesion properties of glass ionomer cement incorporating nano-sized hydroxyapatite particles. Odontology. 2020 Jan;108(1):66–73. doi: 10.1007/s10266-019-00427-5. [DOI] [PubMed] [Google Scholar]
- Khorshidi H., Raoofi S., Najafi M., Kalantari M.H., KhorshidiMalahmadi J., Derafshi R. Nanohydroxyapatite silicate-based cement improves the primary stability of dental implants: An in vitro study. Adv. Mater. Sci. Eng. 2017;2017:1–5. [Google Scholar]
- Komiyama S., Miyasaka R., Kikukawa K., Hayman R. Can nano-hydroxyapatite permeate the oral mucosa? A histological study using three-dimensional tissue models. PLoS One. 2019;14(4):e0215681. doi: 10.1371/journal.pone.0215681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunam D., Sampath V., Manimaran S., Sekar M. Effect of indigenously developed nano-hydroxyapatite crystals from chicken egg shell on the surface hardness of bleached human enamel: An In Vitro study. Contemp. Clin. Dent. 2019;10(3):489. doi: 10.4103/ccd.ccd_810_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal A.M.C., Dos Santos M.V.B., da Silva Filho E.C., de Carvalho A.L.M., Tabchoury C.P.M., Vale G.C. Development of an experimental dentifrice with hydroxyapatite nanoparticles and high fluoride concentration to manage root dentin demineralization. Int. J. Nanomed. 2020;15:7469. doi: 10.2147/IJN.S264754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., Yang, L., Hou, Y., Zhang, Z., Chen, M., Wang, M., Liu, J., Wang, J., Zhao, Z., Xie, C. and Lu, X., 2022. Polydopamine-mediated graphene oxide and nanohydroxyapatite-incorporated conductive scaffold with an immunomodulatory ability accelerates periodontal bone regeneration in diabetes. Bioactive materials, 18, pp.213-227.7. [DOI] [PMC free article] [PubMed]
- Lu K.L., Meng X.C., Zhang J.X., Li X.Y., Zhou M.L. Inhibitory effect of synthetic nanohydroxyapatite on dental caries. KEM. 2007 Apr;336–338:1538–1541. [Google Scholar]
- Lung C.Y.K., Sarfraz Z., Habib A., Khan A.S., Matinlinna J.P. Effect of silanization of hydroxyapatite fillers on physical and mechanical properties of a bis-GMA based resin composite. J. Mech. Behav. Biomed. Mater. 2016 Feb;54:283–294. doi: 10.1016/j.jmbbm.2015.09.033. [DOI] [PubMed] [Google Scholar]
- Malekpour B., Ajami S., Salehi P., Hamedani S. Use of nano-hydroxyapatite serum and different finishing/polishing techniques to reduce enamel staining of debonding after orthodontic treatment: A randomized clinical trial. J. Orofac. Orthoped./Fortschritte der Kieferorthopädie. 2022;83(3):205–214. doi: 10.1007/s00056-021-00365-4. [DOI] [PubMed] [Google Scholar]
- Manchery N., John J., Nagappan N., Subbiah G.K., Premnath P. Remineralization potential of dentifrice containing nanohydroxyapatite on artificial carious lesions of enamel: A comparative in vitro study. Dent. Res. J. 2019;16(5):310. [PMC free article] [PubMed] [Google Scholar]
- Meena, A., Mali, H.S., Patnaik, A., Kumar, S.R., 2017 Jun 27. Comparative investigation of physical, mechanical and thermomechanical characterization of dental composite filled with nanohydroxyapatite and mineral trioxide aggregate. e-Polymers.17 (4) 311–9.
- Memarzadeh K., Sharili A.S., Huang J., Rawlinson S.C.F., Allaker R.P. Nanoparticulate zinc oxide as a coating material for orthopedic and dental implants: Nanoparticulate ZnO as a Coating Material. J. Biomed. Mater. Res. 2015 Mar;103(3):981–989. doi: 10.1002/jbm.a.35241. [DOI] [PubMed] [Google Scholar]
- Moharam L.M., Khadr S., Abdou A., Nagi S.M. Effect of Arginine and nano-hydroxyapatite application on the hypersensitivity and color change of bleached enamel: A randomized controlled clinical trial. J. Clin. Exp. Dent. 2022;14(6):e499. doi: 10.4317/jced.59423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moheet I.A., Luddin N., Ab Rahman I., Masudi S.M., Kannan T.P., Abd Ghani N.R.N. Evaluation of mechanical properties and bond strength of nano-hydroxyapatite-silica added glass ionomer cement. Ceram. Int. 2018 Jun;44(8):9899–9906. [Google Scholar]
- Monterubbianesi R., Tosco V., Bellezze T., Giuliani G., Özcan M., Putignano A., Orsini G. A comparative evaluation of Nanohydroxyapatite-enriched hydrogen peroxide home bleaching system on color, hardness and microstructure of dental enamel. Materials. 2021 Jan;14(11):3072. doi: 10.3390/ma14113072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netalkar PP, Sr M, Ym K, Natarajan S, Gadipelly T, Bhat P D, et al. Effect of nano‐hydroxyapatite incorporation on fluoride‐releasing ability, penetration, and adaptation of a pit and fissure sealant. Int J Paed Dentistry. 2022 May;32(3):344–51. [DOI] [PubMed]
- Niu X., Wang L., Xu M., Qin M., Zhao L., Wei Y., et al. Electrospun polyamide-6/chitosan nanofibers reinforced nano-hydroxyapatite/polyamide-6 composite bilayered membranes for guided bone regeneration. Carbohydr. Polym. 2021 May;260:117769. doi: 10.1016/j.carbpol.2021.117769. [DOI] [PubMed] [Google Scholar]
- Noorani T.Y., Luddin N., Rahman I.A., Masudi S.M. In vitro cytotoxicity evaluation of novel nano-hydroxyapatite-silica incorporated glass ionomer cement. J. Clin. Diagn. Res. 2017;11(4):ZC105. doi: 10.7860/JCDR/2017/24753.9739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orilisi G., Tosco V., Monterubbianesi R., Notarstefano V., Özcan M., Putignano A., Orsini G. ATR-FTIR, EDS and SEM evaluations of enamel structure after treatment with hydrogen peroxide bleaching agents loaded with nano-hydroxyapatite particles. PeerJ. 2021;9:e10606. doi: 10.7717/peerj.10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oubenyahya H. Nano hydroxyapatite toothpaste as a treatment for dentine hypersensitivity: A systematic review. Saudi J. Oral Sci. 2021 [Google Scholar]
- Pagano S., Chieruzzi M., Balloni S., Lombardo G., Torre L., Bodo M., et al. Biological, thermal and mechanical characterization of modified glass ionomer cements: The role of nanohydroxyapatite, ciprofloxacin and zinc l-carnosine. Mater. Sci. Eng. C. 2019 Jan;94:76–85. doi: 10.1016/j.msec.2018.09.018. [DOI] [PubMed] [Google Scholar]
- Pang K., Seo Y.K., Lee J.H. Effects of the combination of bone morphogenetic protein-2 and nano-hydroxyapatite on the osseointegration of dental implants. J. Korean Assoc. Oral Maxillofac. Surg. 2021;47(6):454–464. doi: 10.5125/jkaoms.2021.47.6.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M., Sutherland J.B., Rafii F. Effects of nano-hydroxyapatite on the formation of biofilms by Streptococcus mutans in two different media. Arch. Oral Biol. 2019;107 doi: 10.1016/j.archoralbio.2019.104484. [DOI] [PubMed] [Google Scholar]
- Pepla E., Besharat L.K., Palaia G., Tenore G., Migliau G. Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: a review of literature. Ann. Stomatol. (Roma) 2014 Jul;5(3):108–114. [PMC free article] [PubMed] [Google Scholar]
- Philip N. State of the art enamel remineralization systems: the next frontier in caries management. Caries Res. 2019;53(3):284–295. doi: 10.1159/000493031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro N., Sousa S.R., Van Blitterswijk C.A., Moroni L., Monteiro F.J. A biocomposite of collagen nanofibers and nanohydroxyapatite for bone regeneration. Biofabrication. 2014;6(3):035015. doi: 10.1088/1758-5082/6/3/035015. [DOI] [PubMed] [Google Scholar]
- Santos L.F.T.F., Torres C.R.G., Caneppele T.M.F., Magalhães A.C., Borges A.B. Effect of home-bleaching gels modified by calcium and/or fluoride and the application of nano-hydroxyapatite paste on in vitro enamel erosion susceptibility. Acta Odontol. Scand. 2015;74:1–6. doi: 10.3109/00016357.2015.1053150. [DOI] [PubMed] [Google Scholar]
- Sato T.P., Rodrigues B.V., Mello D.C., Münchow E.A., Ribeiro J.S., Machado J.P.B., Vasconcellos L.M., Lobo A.O., Bottino M.C., Borges A.L. The role of nanohydroxyapatite on the morphological, physical, and biological properties of chitosan nanofibers. Clin. Oral Invest. 2021;25(5):3095–3103. doi: 10.1007/s00784-020-03633-6. [DOI] [PubMed] [Google Scholar]
- Scribante, A., Dermenaki Farahani, M.R., Marino, G., Matera, C., Rodriguez y Baena, R., Lanteri, V., Butera, A., 2020. Biomimetic effect of nano-hydroxyapatite in demineralized enamel before orthodontic bonding of brackets and attachments: visual, adhesion strength, and hardness in in vitro tests. BioMed Research International, 2020. [DOI] [PMC free article] [PubMed]
- Sebastian R., Paul S.T., Azher U., Reddy D. Comparison of remineralization potential of casein phosphopeptide: Amorphous calcium phosphate, nano-hydroxyapatite and calcium sucrose phosphate on artificial enamel lesions: An in vitro study. Int. J. Clin. Pediatr. Dent. 2022;15(1):69. doi: 10.5005/jp-journals-10005-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyedjafari E., Soleimani M., Ghaemi N., Shabani I. Nanohydroxyapatite-coated electrospun poly (l-lactide) nanofibers enhance osteogenic differentiation of stem cells and induce ectopic bone formation. Biomacromolecules. 2010;11(11):3118–3125. doi: 10.1021/bm1009238. [DOI] [PubMed] [Google Scholar]
- Shahi S., Özcan M., Maleki Dizaj S., Sharifi S., Al-Haj Husain N., Eftekhari A., Ahmadian E. A review on potential toxicity of dental material and screening their biocompatibility. Toxicol. Mech. Methods. 2019;29(5):368–377. doi: 10.1080/15376516.2019.1566424. [DOI] [PubMed] [Google Scholar]
- Shahi S., Dehghani F., Abdolahinia E.D., Sharifi S., Ahmadian E., Gajdács M., Kárpáti K., Dizaj S.M., Eftekhari A., Kavetskyy T. Effect of gelatinous spongy scaffold containing nano-hydroxyapatite on the induction of odontogenic activity of dental pulp stem cells. J. King Saud Univ.-Sci. 2022;34(8) [Google Scholar]
- Sharifi S., Maleki-Dizaj S., Shahi S., Mahdilouy M. Synthesis, characterization, and evaluation of nano-hydroxyapatite based experimental calcium silicate cement as a root repair material. J. Oral Res. 2022;11(1):1–13. [Google Scholar]
- Shetty S., Kohad R., Yeltiwar R. Hydroxyapatite as an in-office agent for tooth hypersensitivity a clinical and scanning electron microscope study. J. J. Periodontal. 2010;81:1781–1789. doi: 10.1902/jop.2010.100172. [DOI] [PubMed] [Google Scholar]
- Soares, Igor Paulino Mendes, Caroline Anselmi, Fernanda Ali Kitagawa, Rafael Antonio de Oliveira Ribeiro, Maria Luísa Leite, Carlos Alberto de Souza Costa, and Josimeri Hebling. “Nano-hydroxyapatite-incorporated polycaprolactone nanofibrous scaffold as a dentin tissue engineering-based strategy for vital pulp therapy.” Dental Materials 38, no. 6 (2022): 960-977. [DOI] [PubMed]
- Souza B.M., Comar L.P., Vertuan M., Fernandes Neto C., Buzalaf M.A.R., Magalhães A.C. Effect of an experimental paste with hydroxyapatite nanoparticles and fluoride on dental demineralisation and remineralisation in situ. Caries Res. 2015;49(5):499–507. doi: 10.1159/000438466. [DOI] [PubMed] [Google Scholar]
- Stocco T.D., Rodrigues P.J.G., de Almeida Filho M.A., Lobo A.O. Nanohydroxyapatite electrodeposition onto electrospun nanofibers: Technique overview and tissue engineering applications. Bioengineering. 2021;8(11):151. doi: 10.3390/bioengineering8110151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamburaci S., Tihminlioglu F. Development of Si doped nano hydroxyapatite reinforced bilayer chitosan nanocomposite barrier membranes for guided bone regeneration. Mater. Sci. Eng.: C. 2021 Sep;128:112298. doi: 10.1016/j.msec.2021.112298. [DOI] [PubMed] [Google Scholar]
- Tanongpitchayes K, Randorn C, Lamkhao S, Chokethawai K, Rujijanagul G, Na Lampang K, et al. Effectiveness of a Nanohydroxyapatite-Based Hydrogel on Alveolar Bone Regeneration in Post-Extraction Sockets of Dogs with Naturally Occurring Periodontitis. Veterinary Sciences. 2021 Dec 26;9(1):7.2. [DOI] [PMC free article] [PubMed]
- Tay C.Y., Fang W., Setyawati M.I., Chia S.L., Tan K.S., Hong C.H., et al. Nano-hydroxyapatite and nano-titanium dioxide exhibit different subcellular distribution and apoptotic profile in human oral epithelium. ACS Appl. Mater. Interfaces. 2014;6:6248–62569. doi: 10.1021/am501266a. [DOI] [PubMed] [Google Scholar]
- Tay C.Y., Fang W., Setyawati M.I., Chia S.L., Tan K.S., Hong C.H.L., Leong D.T. Nano-hydroxyapatite and nano-titanium dioxide exhibit different subcellular distribution and apoptotic profile in human oral epithelium. ACS Appl. Mater. Interfaces. 2014;6(9):6248–6256. doi: 10.1021/am501266a. [DOI] [PubMed] [Google Scholar]
- Tschoppe P., Zandim D.L., Martus P., Kielbassa A.M. Enamel and dentine remineralization by nano-hydroxyapatite toothpastes. J. Dent. 2011 Jun;39(6):430–437. doi: 10.1016/j.jdent.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Verma P., Muthuswamy Pandian S. Bionic effects of nano hydroxyapatite dentifrice on demineralised surface of enamel post orthodontic debonding: in-vivo split mouth study. Prog. Orthod. 2021;22(1):1–8. doi: 10.1186/s40510-021-00381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello F., Tosco V., Monterubbianesi R., Orilisi G., Gatto M.L., Sparabombe S., Memé L., Mengucci P., Putignano A., Orsini G. Remineralization efficacy of four remineralizing agents on artificial enamel lesions: SEM-EDS investigation. Materials. 2022;15(13):4398. doi: 10.3390/ma15134398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahba N., Schwendicke F., Kamel M.A., Allam G., Kabil N., Elhennawy K. Preventing and arresting primary tooth enamel lesions using self-assembling peptide P11–4 in vitro. J. Int. Soc. Prevent. Commun. Dent. 2022;12(1):58. doi: 10.4103/jispcd.JISPCD_257_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Wu Y., Yao Z., Wang C. Study of a new nano-hydroxyapatite/basic fibroblast growth factor composite promoting periodontal tissue regeneration. Mater. Express. 2020;10(11):1802–1807.3. [Google Scholar]
- Wierichs R.J., Musiol J., Erdwey D., Esteves-Oliveira M., Apel C., Meyer-Lückel H. Re-and demineralization characteristics of dentin depending on fluoride application and baseline characteristics in situ. J. Dent. 2020;94 doi: 10.1016/j.jdent.2020.103305. [DOI] [PubMed] [Google Scholar]
- Wu Y., Chang C., Chang K., Lin D., Ko C., Wu H., et al. Effect of micro-/nano-hybrid hydroxyapatite rod reinforcement in composite resins on strength through thermal cycling. Polym. Compos. 2019 Sep;40(9):3703–3710. [Google Scholar]
- Xu, W.H., Feng, L., Liu, Y., Cai, D.Q., Wen, N. and Zheng, W.J., 2016. Estrogen enhances the bone regeneration potential of periodontal ligament stem cells derived from osteoporotic rats and seeded on nano-hydroxyapatite/collagen/poly (L-lactide). International journal of molecular medicine, 37(6), pp.1475-1486.4. [DOI] [PMC free article] [PubMed]
- Yadav S., Gangwar S. An investigation of experimental dental restorative composites filled with nano-hydroxyapatite treated with different silanes. Silicon. 2021 Apr;13(4):1127–1137. [Google Scholar]
- Yadav R., Meena A. Comparative study of thermo-mechanical and thermogravimetric characterization of hybrid dental restorative composite materials. Proc. Inst. Mech. Eng., Part L: J. Mater.: Des. Appl. 2022;236(5):1122–1129. [Google Scholar]
- Yoshida S., Sugii H., Itoyama T., Kadowaki M., Hasegawa D., Tomokiyo A., et al. Development of a novel direct dental pulp-capping material using 4-META/MMA-TBB resin with nano hydroxyapatite. Mater. Sci. Eng.: C. 2021 Nov;130:112426. doi: 10.1016/j.msec.2021.112426. [DOI] [PubMed] [Google Scholar]
- Zhang, Y.F., Cheng, X.R., Chen, Y., Shi, B., Chen, X.H., Xu, D.X. and Ke, J., 2007. Three-dimensional nanohydroxyapatite/chitosan scaffolds as potential tissue engineered periodontal tissue. Journal of biomaterials applications, 21(4), pp.333-349.5. [DOI] [PubMed]