Abstract
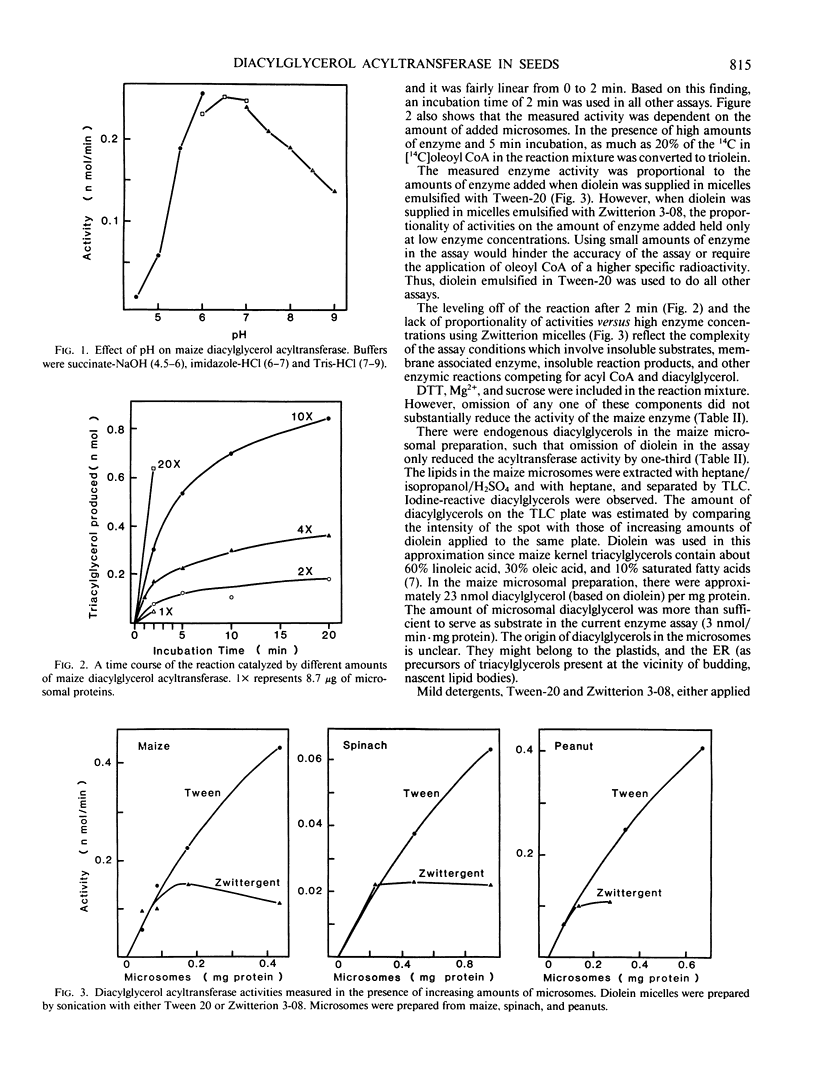
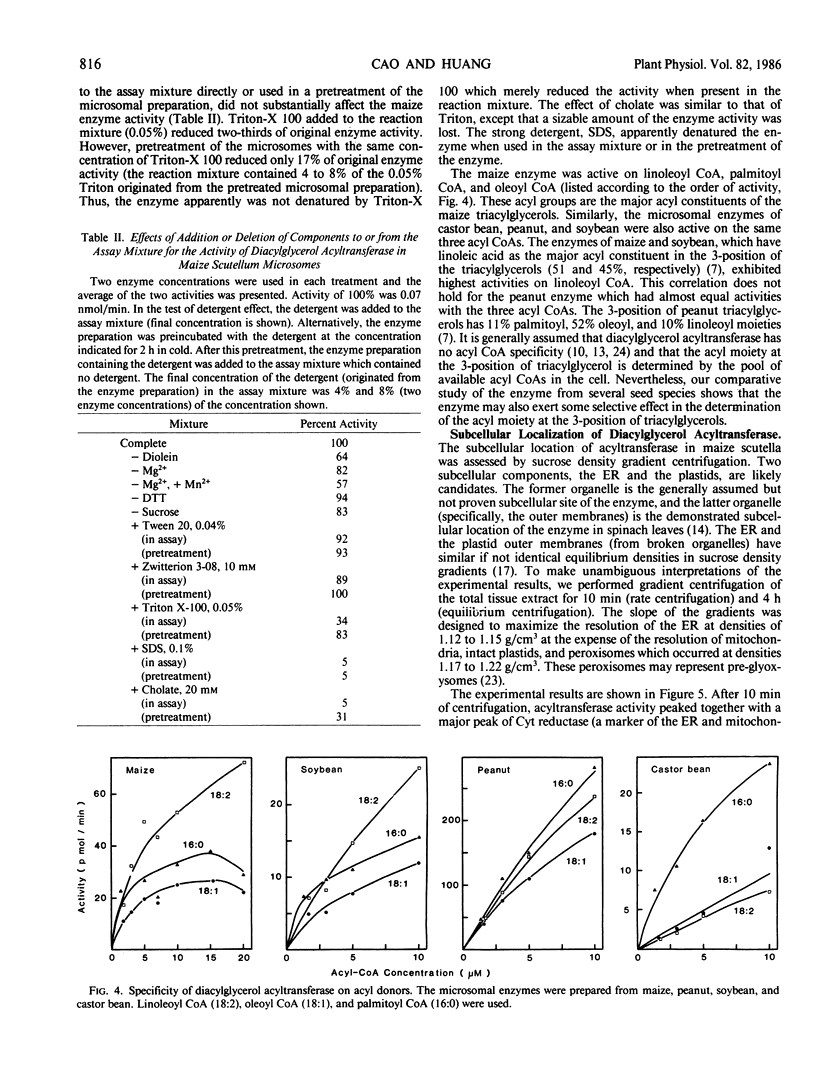
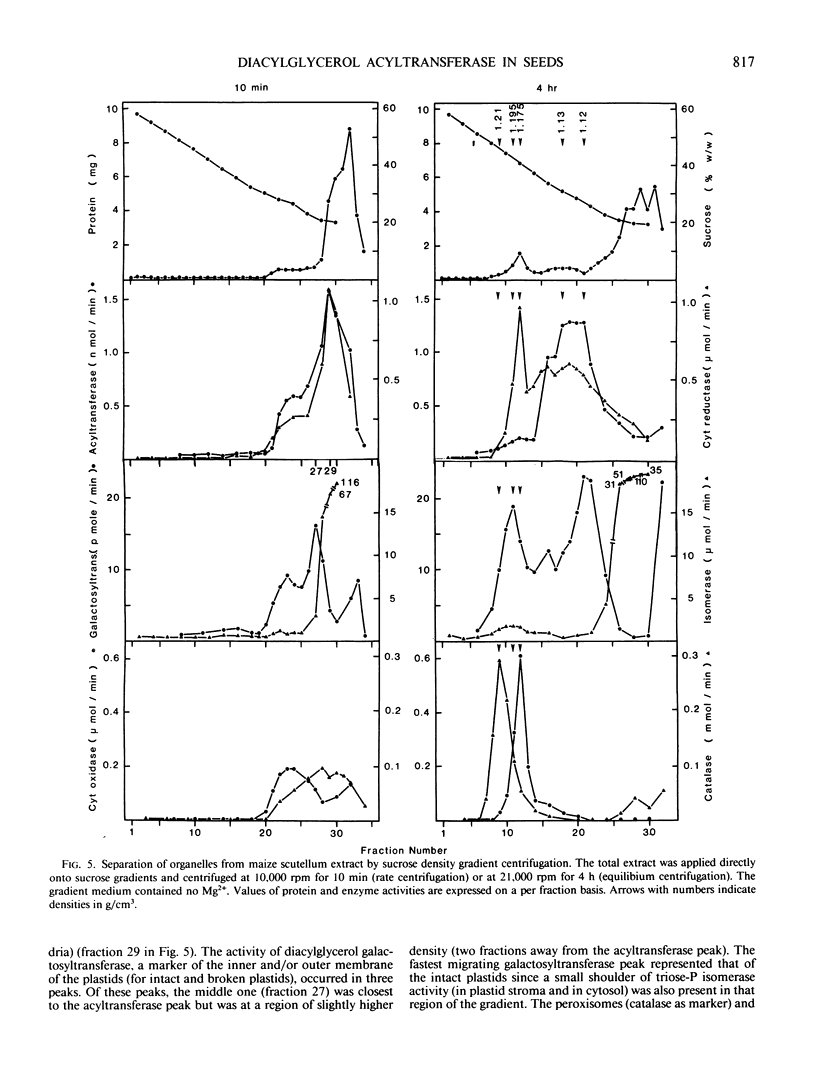
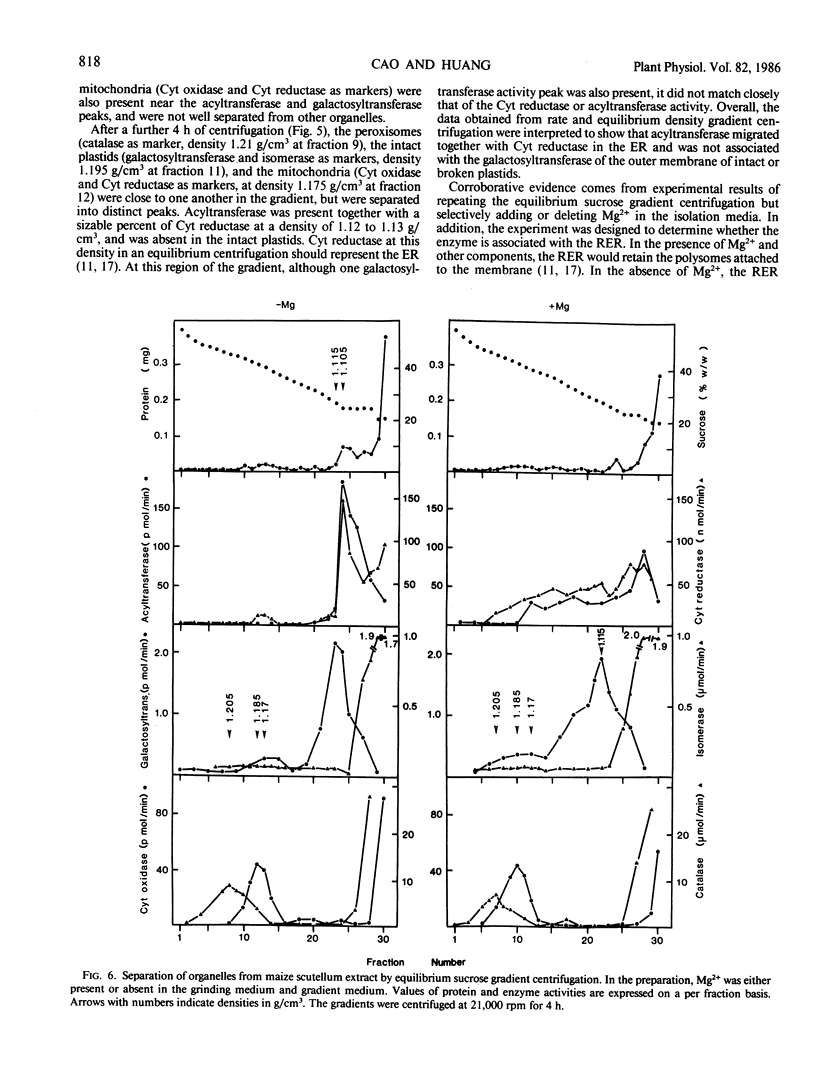
Diacylglycerol acyltransferase (EC 2.3.1.20) activity was detected in the microsomal fractions of maturing maize scutellum, soybean cotyledon, peanut cotyledon, and castor bean endosperm. The activity detected was high enough to account for the in vivo rate of triacylglycerol synthesis. The activity of the maize enzyme was characterized using diolein micelles prepared by sonication in Tween 20 as the substrate. The activity was highest at pH values of 6 to 7. The activity was proportional to the amount of enzyme added, and the reaction rate was linear for about 2 minutes. The enzyme was not inactivated by Tween 20, Zwitterion 3-08, Triton-X 100, and cholate, but was inactivated completely by sodium dodecyl sulfate. The enzyme was active on linoleoyl coenzyme A (CoA), palmitoyl CoA, and oleoyl CoA, although the activity was highest on linoleoyl CoA. Endogenous diacylglycerol was present in the microsomes, and the enzyme activity was only partially dependent on the addition of external diolein. Subcellular fractionation of the total scutellum extract in sucrose density gradients was performed. By comparing the migration of the enzyme between rate and equilibrium centrifugation, and between equilibrium centrifugation in the presence and absence of magnesium ions in the preparative media, the enzyme was shown to be associated with the rough endoplasmic reticulum. Some of the above findings on the maize enzyme were extended to the enzymes from castor bean, soybean, and peanuts.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Huang A. H. Enzymes of glycerol metabolism in the storage tissues of Fatty seedlings. Plant Physiol. 1975 Mar;55(3):555–558. doi: 10.1104/pp.55.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Moore T. S., Beevers H. Endoplasmic reticulum as the site of lecithin formation in castor bean endosperm. J Cell Biol. 1973 Jun;57(3):659–667. doi: 10.1083/jcb.57.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu R., Wang S. M., Lin Y. H., Vance V. B., Huang A. H. Characteristics and biosynthesis of membrane proteins of lipid bodies in the scutella of maize (Zea mays L.). Biochem J. 1986 Apr 1;235(1):57–65. doi: 10.1042/bj2350057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine W. E., Mancha M., Stumpf P. K. Fat metabolism in higher plants. Differential incorporation of acyl-coenzymes A and acyl-acyl carrier proteins into plant microsomal lipids. Arch Biochem Biophys. 1976 Apr;173(2):472–479. doi: 10.1016/0003-9861(76)90284-8. [DOI] [PubMed] [Google Scholar]
- Yamashita S., Hosaka K., Miki Y., Numa S. Glycerolipid acyltransferases from rat liver: 1-acylglycerophosphate acyltransferase, 1-acylglycerophosphorylcholine acyltransferase, and diacylglycerol acyltransferase. Methods Enzymol. 1981;71(Pt 100):528–536. doi: 10.1016/0076-6879(81)71063-2. [DOI] [PubMed] [Google Scholar]


