This editorial refers to ‘Prediction of coronary artery disease using urinary proteomics’, by D. Wei et al., https://doi.org/10.1093/eurjpc/zwad087.
Proteomics is a rapidly evolving field of molecular biology that studies the structure, function, and interactions of proteins within a cell, tissue, or organism. It involves the analysis of a set of proteins expressed by an organism or a particular cell type under specific conditions. The main goal of proteomics is to understand the complex network of protein interactions and their regulation in health and disease.1
Cardiovascular disease is a leading cause of mortality worldwide,2 and there is a growing interest in using proteomics to predict cardiovascular risk. Proteomics can be used to identify proteins that are differentially expressed or modified in patients with cardiovascular disease compared to healthy individuals. By analyzing the patterns of protein expression or modification, researchers can identify potential biomarkers of disease and develop new diagnostic and therapeutic strategies.3
For example, proteomics can be used to identify proteins that are associated with atherosclerosis. Several studies have shown that changes in the levels of specific proteins, such as lipoprotein particles, apolipoproteins, and complement proteins, are associated with an increased risk of cardiovascular disease. Proteomics can also be used to study the effects of lifestyle interventions, such as diet and exercise, on cardiovascular risk. By analyzing changes in the expression or modification of specific proteins, researchers can identify molecular mechanisms underlying the protective effects of these interventions.4,5
In summary, proteomics is a powerful tool for studying the complex molecular mechanisms underlying cardiovascular disease and for identifying potential biomarkers and therapeutic targets. In this issue of the European Journal of Preventive Cardiology, Dongmei Wei and collaborators6 assess the association of biomarkers derived from urine proteomics with development of coronary artery disease (CAD) in a retrospective analysis of longitudinal data.
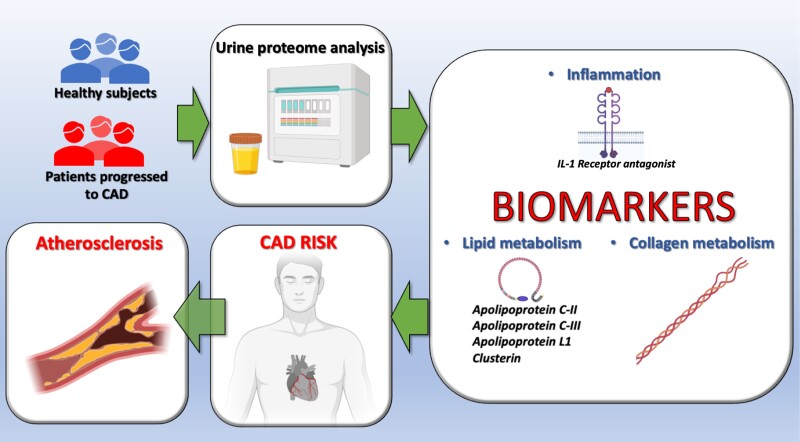
A derivation cohort of 72 individuals free of CAD matched by age, sex, hypertension, renal function, and total cholesterol were evaluated. Urine biomarkers were compared in those who developed or not CAD after 8 years (36 with and 36 without CAD, respectively). The validation cohort comprised 893 individuals (115 with CAD) from the Flemish Study on Environment, Genes and Health Outcomes study7 as well 156 individuals (80 with CAD) from the Human Urinary Proteome Database,8 followed up to 8 years. Intriguingly, 160 urinary peptides related to collagen turnover, lipid metabolism, and inflammation were selected. New classifiers were compared with 2 sets of urine proteomic classifiers that had been previously tested (CAD238 and ACSP75).9,10 Biomarkers were selected using robust statistical methods and machine learning. In the validation cohort, biomarkers were tested by multivariate Cox models.
The new proteomic classifier was positively associated with CAD, independent of the Framingham risk score (adjusted HR: 1.44, 95% CI: 1.01–2.95) or the SCORE2 risk score, recommended by the European Society of Cardiology11 (adjusted HR:1.67, 95% CI: 1.14–2.45). Overall, the new classifier provided a strong discrimination for CAD that was superior to the two previous classifiers, improving both risk discrimination and reclassification (Figure 1).
Figure 1.
The peptides constituting the proteomic classifier used to predict coronary artery disease (CAD) are involved in diverse pathways associated with atherosclerosis, including inflammation, lipid metabolism, and collagen turnover.
These findings are in agreement with a previous report demonstrating that higher plasma concentrations of 15 inflammatory proteins were associated with higher risks of myocardial infarction after adjustment for medications and established cardiovascular risk factors; among these 15 biomarkers, five (IL-6, IL-18R1, CXCL1, CD6, and CDCP1) were independently associated with myocardial infarction.12 Equally important, a proteomic approach had identified the pathways involved in cardiovascular disease attributable to physical inactivity,13 leading to the understanding of a number of proteins and enzymes involved in atherosclerotic processes in sedentary individuals, emphasizing that physical activity is linked to protective pathways including anti-oxidation and anti-inflammation.14
Unfortunately, Wei et al. did not investigate the association between the urinary proteomic classifier and the severity or subtypes of CAD. Further studies, ideally prospective and with a large population, are warranted to evaluate whether the new urinary proteomic classifier can identify plaque volume and composition measured by ultrasound or computerized tomography. The peptides constituting the proteomic classifier were involved in diverse pathways associated with atherosclerosis, including inflammation, lipid metabolism, and collagen turnover (Figure 1); however, the exact molecular mechanisms linking these markers to CAD need to be determined in dedicated studies.
Contributor Information
Pasquale Mone, Department of Medicine, Division of Cardiology, Wilf Family Cardiovascular Research Institute, Einstein Institute for Neuroimmunology and Inflammation (INI), Albert Einstein College of Medicine, 1300 Morris Park Avenue, 10461 New York City, NY 10461, USA; University of Campania ‘Luigi Vanvitelli’, Naples, Italy.
Tullio Tesorio, ‘Montevergine’ Clinic, Mercogliano (Avellino), Italy.
Antonio De Donato, University of Campania ‘Luigi Vanvitelli’, Naples, Italy.
Angelo Cioppa, ‘Montevergine’ Clinic, Mercogliano (Avellino), Italy.
Stanislovas S Jankauskas, Department of Medicine, Division of Cardiology, Wilf Family Cardiovascular Research Institute, Einstein Institute for Neuroimmunology and Inflammation (INI), Albert Einstein College of Medicine, 1300 Morris Park Avenue, 10461 New York City, NY 10461, USA; University of Campania ‘Luigi Vanvitelli’, Naples, Italy.
Luigi Salemme, ‘Montevergine’ Clinic, Mercogliano (Avellino), Italy.
Gaetano Santulli, Department of Medicine, Division of Cardiology, Wilf Family Cardiovascular Research Institute, Einstein Institute for Neuroimmunology and Inflammation (INI), Albert Einstein College of Medicine, 1300 Morris Park Avenue, 10461 New York City, NY 10461, USA; Department of Molecular Pharmacology, Einstein-Mount Sinai Diabetes Research Center (ES-DRC), Fleischer Institute for Diabetes and Metabolism (FIDAM), Einstein Institute for Aging Research, Albert Einstein College of Medicine, 1300 Morris Park Avenue, 10461 New York City, NY 10461, USA.
Funding
The Santulli’s Lab is currently supported in part by the National Institutes of Health (NIH): National Heart, Lung, and Blood Institute (NHLBI: R01-HL164772, R01-HL159062, R01-HL146691, T32-HL144456), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK: R01-DK123259, R01-DK033823), National Center for Advancing Translational Sciences (NCATS: UL1-TR002556-06, UM1-TR004400) to G.S., by the Diabetes Action Research and Education Foundation (to G.S.), and by the Monique Weill-Caulier and Irma T. Hirschl Trusts (to G.S.). S.S.J. is supported in part by a postdoctoral fellowship of the American Heart Association (AHA-21POST836407).
References
- 1. Sidoli S, Cheng L, Jensen ON. Proteomics in chromatin biology and epigenetics: elucidation of post-translational modifications of histone proteins by mass spectrometry. J Proteomics 2012;75:3419–3433. [DOI] [PubMed] [Google Scholar]
- 2. D'Ascenzi F, Sciaccaluga C, Cameli M, Cecere A, Ciccone MM, Di Francesco S, et al. When should cardiovascular prevention begin? The importance of antenatal, perinatal and primordial prevention. Eur J Prev Cardiol 2021;28:361–369. [DOI] [PubMed] [Google Scholar]
- 3. Fu Q, Van Eyk JE. Proteomics and heart disease: identifying biomarkers of clinical utility. Expert Rev Proteomics 2006;3:237–249. [DOI] [PubMed] [Google Scholar]
- 4. Ferrannini E, Manca ML, Ferrannini G, Andreotti F, Andreini D, Latini R, et al. Differential proteomics of cardiovascular risk and coronary artery disease in humans. Front Cardiovasc Med 2022;8:790289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wallentin L, Eriksson N, Olszowka M, Grammer TB, Hagstrom E, Held C, et al. Plasma proteins associated with cardiovascular death in patients with chronic coronary heart disease: A retrospective study. PLoS Med 2021;18:e1003513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wei D, Melgarejo JD, Van Aelst L, Vanassche T, Verhamme P, Janssens S, et al. Prediction of coronary artery disease using urinary proteomics. Eur J Prev Cardiol 2023;30:1537–1546. [DOI] [PubMed] [Google Scholar]
- 7. Zhang H, Thijs L, Kuznetsova T, Fagard RH, Li X, Staessen JA. Progression to hypertension in the non-hypertensive participants in the flemish study on environment, genes and health outcomes. J Hypertens 2006;24:1719–1727. [DOI] [PubMed] [Google Scholar]
- 8. Zhao M, Li M, Yang Y, Guo Z, Sun Y, Shao C, et al. A comprehensive analysis and annotation of human normal urinary proteome. Sci Rep 2017;7:3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Delles C, Schiffer E, von Zur Muhlen C, Peter K, Rossing P, Parving HH, et al. Urinary proteomic diagnosis of coronary artery disease: identification and clinical validation in 623 individuals. J Hypertens 2010;28:2316–2322. [DOI] [PubMed] [Google Scholar]
- 10. Htun NM, Magliano DJ, Zhang ZY, Lyons J, Petit T, Nkuipou-Kenfack E, et al. Prediction of acute coronary syndromes by urinary proteome analysis. PLoS One 2017;12:e0172036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Matsushita K, Kaptoge S, Hageman SHJ, Sang Y, Ballew SH, Grams ME, et al. Including measures of chronic kidney disease to improve cardiovascular risk prediction by SCORE2 and SCORE2-OP. Eur J Prev Cardiol 2023;30:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Valdes-Marquez E, Clarke R, Hill M, Watkins H, Hopewell JC, Consortium P. Proteomic profiling identifies novel independent relationships between inflammatory proteins and myocardial infarction. Eur J Prev Cardiol 2023. [DOI] [PubMed] [Google Scholar]
- 13. Kruger R. Proteomics insights on how physical inactivity can influence cardiovascular health. Eur J Prev Cardiol 2019;26:1862–1864. [DOI] [PubMed] [Google Scholar]
- 14. Gambardella J, Morelli MB, Wang XJ, Santulli G. Pathophysiological mechanisms underlying the beneficial effects of physical activity in hypertension. J Clin Hypertens (Greenwich) 2020;22:291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]