Take Home Message
Standardised reporting of repeat magnetic resonance imaging scans in active surveillance of prostate cancer, using the Prostate Cancer Radiological Estimation of Change in Sequential Evaluation recommendations, is feasible and offers a prognostic value.
Keywords: Active surveillance, Follow-up, Imaging, Magnetic resonance imaging, Prostate Cancer Radiological Estimation of Change in Sequential Evaluation, Prostate cancer
Abstract
Background
The Prostate Cancer Radiological Estimation of Change in Sequential Evaluation (PRECISE) score has been developed to standardise prostate magnetic resonance imaging (MRI) reporting in men on active surveillance (AS) for prostate cancer (PCa).
Objective
To evaluate the feasibility of PRECISE scoring and assess its diagnostic accuracy.
Design, setting, and participants
All PCa patients on AS with a baseline MRI and at least one follow-up MRI scan between January 2008 and September 2022 at a single tertiary referral centre were included in a database. The follow-up protocol of the Prostate Cancer International Active Surveillance (PRIAS) study was used. All scans were retrospectively re-reported by a dedicated uroradiologist and appointed a Prostate Imaging Reporting and Data System (version 2.1) and PRECISE score.
Outcome measurements and statistical analysis
Clinically significant progression was defined by histopathological upgrading (on biopsy or radical prostatectomy) to grade group ≥3 and/or evolution to T3 stage. A survival analysis was performed to assess differential progression-free survival (PFS) according to the PRECISE score.
Results and limitations
A total of 188 patients were included for an analysis with a total of 358 repeat MRI scans and 144 repeat biopsies. The median follow-up was 46 mo (interquartile range 21–74). Radiological progression (PRECISE 4–5) had sensitivity, specificity, negative predictive value, and positive predictive value of, respectively, 78%, 70%, 90%, and 49% for clinically significant progression. Four-year PFS was 91% for PRECISE 1–3 versus 66% for PRECISE 4–5 (p < 0.001). In total, 137 patients underwent a confirmation MRI scan within 18 mo after diagnosis. Four-year PFS in this group was 81% for PRECISE 1–3 versus 43% for PRECISE 4–5 (p < 0.001). Limitations include retrospective design and no strict adherence to AS protocol.
Conclusions
Implementation of PRECISE scoring for PCa patients on AS is feasible and offers a prognostic value. Patients with PRECISE score 4–5 on confirmation MRI within 18 mo after diagnosis have a three-fold higher risk of clinically significant progression after 4 yr.
Patient summary
Patients with low-risk prostate cancer can be followed up carefully. In this study, we evaluate the standardised reporting of repeat magnetic resonance imaging scans (using the Prostate Cancer Radiological Estimation of Change in Sequential Evaluation [PRECISE] recommendations). PRECISE scoring is feasible and helps identify patients in need of further treatment.
1. Introduction
Active surveillance (AS) is a recommended management strategy for low-risk and selected favourable intermediate-risk prostate cancer (PCa) according to current guidelines [1]. This treatment strategy reduces overtreatment and treatment-related side effects, with proven long-term oncological safety [2], [3].
In addition to the usual clinical (digital rectal examination), biochemical (prostate-specific antigen [PSA]), and pathological (prostate biopsy) parameters, magnetic resonance imaging (MRI) is increasingly used for AS of PCa. Baseline MRI is considered mandatory for diagnosis and proper risk classification of PCa [4]. Standardised MRI reporting (using the Prostate Imaging Reporting and Data System [PI-RADS] score [5]) and MRI-guided targeted biopsy have clearly been shown to improve patient selection for AS and reduce the risk of reclassification [4]. The place of serial MRI scans in the surveillance phase is more controversial, and the optimal MRI intervals and triggers remain unclear due to a scarcity of data and a lack of standardised reporting. Furthermore, comparison and pooling of data are complicated by the large heterogeneity in inclusion criteria and follow-up protocols for AS.
A standardised approach to prostate MRI reporting for AS was proposed in the Prostate Cancer Radiological Estimation of Change in Sequential Evaluation (PRECISE) recommendations [6]. These are based on a consensus statement by an international panel of experts in the field of MRI and AS. The probability of significant radiological progression is determined using a 1–5 Likert scale (PRECISE score): PRECISE 1–2 indicates radiological regression, PRECISE 3 indicates stability, and PRECISE 4–5 implies progression.
We report the application of the PRECISE recommendations in a real-life cohort. Our objective is to evaluate the feasibility of PRECISE scoring and to estimate its accuracy and predictive value for disease progression.
2. Patients and methods
All PCa patients on AS with a baseline MRI and at least one follow-up MRI scan between January 2008 and September 2022 at a single tertiary referral centre were retrospectively included in a database. The AS inclusion criteria were PSA ≤20, clinical stage <cT3, and biopsy-confirmed PCa with grade group (GG) 1 or GG 2 without invasive cribriform and intraductal carcinoma. No maximum cancer core length or number of positive cores was set. At our centre, we use the follow-up protocol of the Prostate Cancer International Active Surveillance (PRIAS) study [7], [8]. A time path for standard follow-up as indicated by the PRIAS protocol is shown in Table 1. Adverse PSA kinetics will also lead to early repeat MRI ± targeted biopsies. A detailed overview of the updated PRIAS protocol is available online (www.prias-project.org). In this real-life setting, deviation from the protocol could occur at the treating physician’s discretion or patient’s preference. The study was approved by the hospital's ethics committee (EC2011/495 with amendment on November 18, 2015).
Table 1.
Timetable for follow-up of patients on active surveillance (PRIAS protocol)
| Year | 1 |
2 |
3 |
4 |
5 |
6 |
7 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Month | 0a | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 30 | 36 | 42 | 48 | 54 | 60 | 66 | 72 | 78 | 84 |
| PSA | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × |
| MRI + targeted biopsy | × | ×b | × | × | × | ||||||||||||||
| Systematic biopsy | × | × | × | × | |||||||||||||||
MRI = magnetic resonance imaging; PRIAS = Prostate Cancer International Active Surveillance; PSA = prostate-specific antigen.
Time of diagnosis.
MRI after 3 mo with targeted biopsies only if no MRI is used before diagnosis. Repeat MRI + biopsies after 1, 4, 7, and 10 yr and subsequently every 5 yr.
Patient and tumour characteristics and outcome were recorded. All MRI scans were retrospectively re-reported by a dedicated expert uroradiologist, who was blinded to the original MRI report and the patient’s outcome. Suspicious lesions were scored according to PI-RADS (version 2.1) guidelines at each time point. For each follow-up scan, the overall PRECISE score for the likelihood of radiological progression was compared with the last most recent scan and was determined on a global visual basis. A large majority of MRI scans were performed at our institution on a 3 Tesla scanner (Siemens Magnetom Prisma, Erlangen, Germany) without an endorectal coil and were biparametric, consisting of 3 mm axial T2-weighted images, 3 mm axial T1-weighted images, and 3 mm axial diffusion-weighted images (b values 50, 400, and 800, respectively, and calculated b2000). Before the scan, a rectal clysma was applied and intravenous butylscopalamine was administered. A limited number of patients were scanned at another institution with consequently slightly different scan protocols. Follow-up scans performed at other centres were also included and re-reported.
As earlier suggested by Giganti et al. [9], we applied the following specific interpretation of the PRECISE criteria:
-
1.
“PRECISE 3” (ie, stability): either a scan with a stable lesion over time or a persistent negative scan.
-
2.
“PRECISE 4” (ie, progression): either a new focal lesion (scored as PI-RADS ≥3) in a previous negative scan or a lesion with more suspicious MRI features (volume or conspicuity) since the last scan.
The primary outcome of “clinically significant progression” was defined by histopathological upgrading to GG ≥3 (on biopsy or definitive pathology in case of radical prostatectomy) and/or evolution to clinical, radiological, or pathological stage T3. The secondary endpoint of “any progression” also included those patients with upgrading to GG 2.
Radiological progression on MRI is defined by PRECISE score 4–5, and radiological stability/involution is defined by PRECISE score 1–3. In patients with multiple follow-up scans, the highest PRECISE score was considered most representative on a patient level. Furthermore, all repeat MRI scans were also evaluated separately on a scan level. In this per-scan analysis, each scan was compared with the previous one and appointed a PRECISE score. When no pathological data or subsequent imaging was available, scans were considered to be “progression free” when they had at least 2 yr of follow-up with stable PSA (PSA doubling time ≥3 yr). MRI scans for which no 2 yr of follow-up was available were excluded from the analysis.
A subgroup analysis was performed for those patients who underwent a first follow-up MRI scan within 18 mo after diagnosis (ie, the confirmation MRI after 1 yr, with a margin of 6 mo).
In a subgroup analysis of PRECISE score 3, we made a distinction between PRECISE 3n (stable MRI findings, but no visible lesion) and PRECISE 3v (stable MRI findings, with visible lesion unchanged).
Continuous variables are presented as median with interquartile range (IQR), and categorical variables as frequency and percentage. Sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) of the PRECISE score for PCa progression were calculated from 2 × 2 contingency tables. Univariate Cox proportional hazard regression was performed to identify predictors of clinically significant progression. Most significant predictors were used for a multivariate Cox regression model. Progression-free survival (PFS) was assessed using Kaplan-Meier curves, and the log-rank test was used to assess differences between curves. A statistical analysis was performed using SPSS (version 28; IBM Corporation, Armonk, NY, USA). All tests were two sided, with a level of statistical significance set at p < 0.05.
3. Results
A total of 188 patients were included for the analysis, with a total of 358 repeat MRI scans (one to six repeat MRI scans per patient) and 144 repeat biopsies (zero to four repeat biopsies per patient). Image quality was adequate to appoint a PRECISE score to each follow-up scan.
Table 2 shows the baseline characteristics of our study population. The median follow-up was 46 mo (IQR 21–74). The median time to first follow-up scan was 13 mo (IQR 11–25).
Table 2.
Baseline characteristics of study populationa
| Overall (n = 188) | |
|---|---|
| Age (yr) | 65 (59–71) |
| PSA (ng/ml) | 6.0 (4.9–8.3) |
| Prostate volume (ml) | 46.0 (36.5–61.0) |
| PSA density (ng/ml/ml) | 0.14 (0.10–0.19) |
| cT, n (%) | |
| T1a | 18 (9.6) |
| T1b | 5 (2.7) |
| T1c | 162 (86.2) |
| T2a | 3 (1.6) |
| GG, n (%) | |
| 1 | 171 (91.0) |
| 2 | 17 (9.0) |
| PI-RADS score, n (%) | |
| 1–2 | 76 (40.8) |
| 3 | 49 (26.1) |
| 4 | 57 (30.3) |
| 5 | 6 (3.2) |
cT = clinical T stage; GG = Gleason grade group; PI-RADS = Prostate Imaging Reporting and Data System; PSA = prostate-specific antigen.
Data are shown as medians and interquartile range for continuous variables, and as counts and percentages for categorical variables.
Eighty-four (44.7%) patients had any progression, whereas 50 (26.6%) had clinically significant progression.
Ninety-three (49.5%) patients stayed on AS, 86 (45.7%) switched to active treatment, seven (3.7%) switched to watchful waiting, and two (1.2%) died of another cause. No patient died of PCa. Of the 86 patients who switched to active treatment, 66 (76.7%) underwent a radical prostatectomy, 19 (22.1%) received radiotherapy, and one (1%) received high-intensity focused ultrasound.
The reason for treatment included biopsy-confirmed tumour upgrading in 49 (57.0%) patients. Nineteen (22.1%) patients with radiological progression directly switched to active treatment without a confirmatory biopsy. Other reasons for treatment were “rising PSA only” in six (7.0%) patients, symptomatic outflow obstruction in three (3.5%) patients and “patient preference” in nine (10.5%) patients.
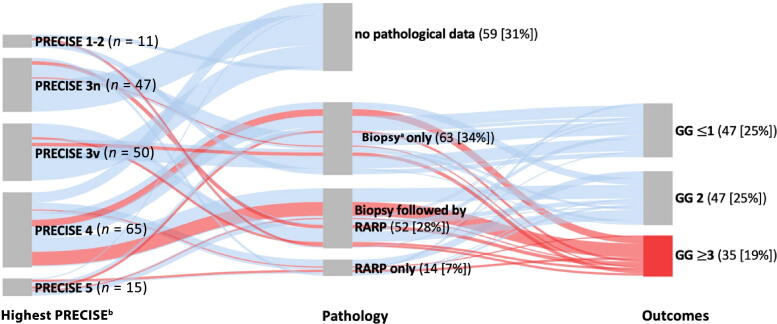
Figure 1 gives an overview of the histopathological follow-up data within our cohort stratified by the highest PRECISE score. Definitive pathology after radical prostatectomy serves as a true control for PRECISE scoring. When no radical prostatectomy was performed, we looked at the control biopsy after establishing the highest PRECISE score. Not all MRI scans had a histopathological control.
Fig. 1.
Sankey diagram showing histopathological follow-up data stratified by the highest PRECISE score. One patient with highest PRECISE score 5 directly switched to radiotherapy without control biopsy. GG = Gleason grade group; PRECISE = Prostate Cancer Radiological Estimation of Change in Sequential Evaluation (3n, nonvisible lesion; 3v, stable visible lesion); RARP = robot-assisted radical prostatectomy; [%] = percentage of the whole population (n = 188). a Control biopsy after establishing the highest PRECISE score. b Highest PRECISE score for each patient.
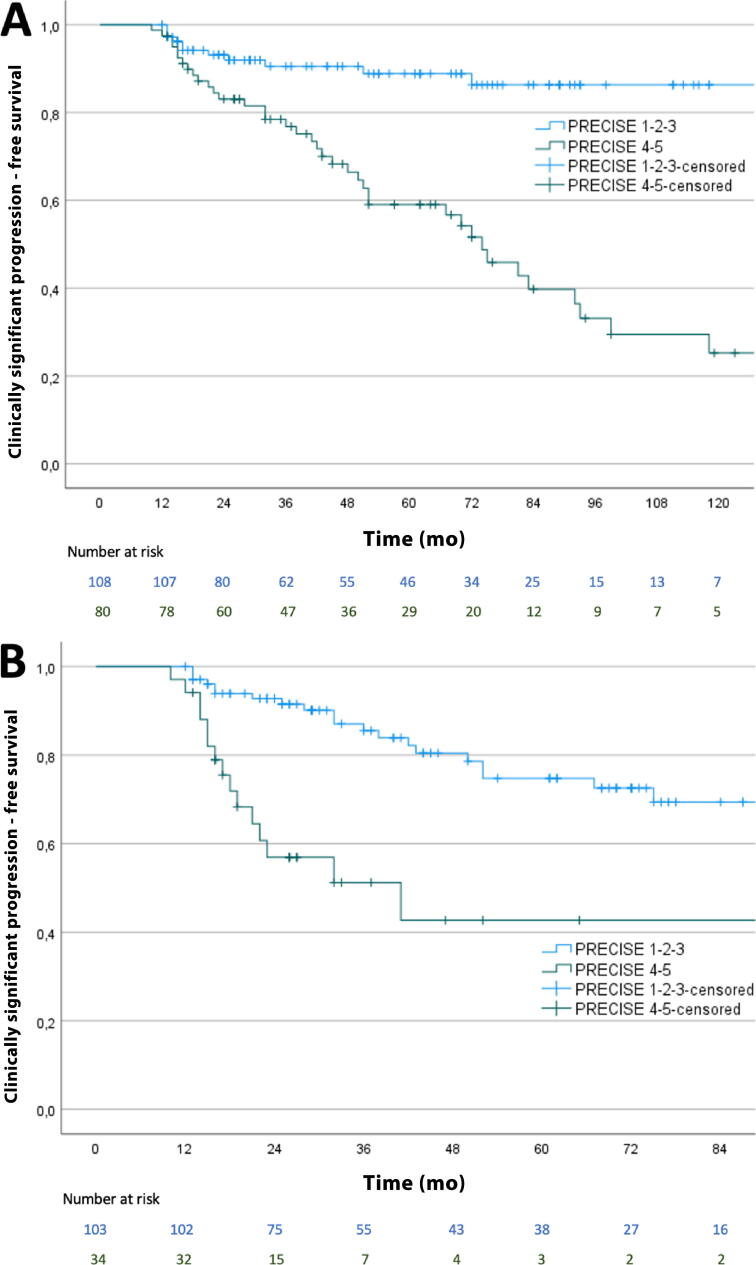
On a patient level, radiological progression (PRECISE 4–5, 80/188) had sensitivity of 78%, specificity of 70%, NPV of 90%, and PPV of 49% for clinically significant progression. Four-year PFS was 91% (95% confidence interval [CI] 84.6–96.3%) for PRECISE 1–3 versus 66% (95% CI 55.0–77.8%) for PRECISE 4–5 (p < 0.001; Fig. 2A). At 1, 2, and 5 yr after diagnosis, PFS was 100%, 93%, and 89% for PRECISE 1–3 versus 98%, 83%, and 59% for PRECISE 4–5, respectively.
Fig. 2.
Kaplan-Meier curves showing the rate of clinically significant progression stratified by (A) the highest PRECISE score in the overall population and (B) the PRECISE score within 18 mo after diagnosis (ie, the confirmation MRI). MRI = magnetic resonance imaging; PRECISE = Prostate Cancer Radiological Estimation of Change in Sequential Evaluation.
Radiological progression had sensitivity of 65%, specificity of 76%, NPV of 73%, and PPV of 69% for any progression. According to this outcome, 4-yr PFS was 77% (95% CI 68.3–85.5%) for PRECISE 1–3 versus 57% (95% CI 45.0–68.2%) for PRECISE 4–5 (p < 0.001). At 1, 2, and 5 yr after diagnosis, PFS was 100%, 86%, and 75% for PRECISE 1–3 versus 98%, 77%, and 49% for PRECISE 4–5, respectively.
Results from the Cox proportional hazard analysis are shown in Table 3, Table 4. The PRECISE score remains an independent predictor of clinically significant progression.
Table 3.
Univariate Cox regression analysis for identifying predictors of clinically significant progression
| Predictors | Univariate analysis |
|
|---|---|---|
| HR (95% CI) | p value | |
| Age at diagnosis (yr) | 1.04 (0.99–1.08) | 0.063 |
| Baseline PSA density (ng/ml/ml) | 3.40 (0.63–18.37) | 0.155 |
| Baseline GG | ||
| GG 1 | Reference | – |
| GG 2 | 0.76 (0.24–2.45) | 0.648 |
| Baseline PI-RADS | ||
| PI-RADS 1–2 | Reference | – |
| PI-RADS 3–5 | 1.86 (1.03–3.36) | 0.041 |
| Highest PRECISEa | ||
| PRECISE 1–3 | Reference | – |
| PRECISE 4–5 | 5.09 (2.61–9.95) | <0.001 |
| No. of repeat MRI scans | ||
| 1 | Reference | – |
| 2 | 0.53 (0.28–1.01) | 0.054 |
| ≥3 | 0.181 (0.08–0.43) | <0.001 |
| No. of repeat biopsies | ||
| 0 | Reference | – |
| 1 | 12.48 (3.84–40.49) | <0.001 |
| ≥2 | 7.98 (2.11–30.11) | 0.002 |
CI = confidence interval; GG = Gleason grade group; HR = hazard ratio; MRI = magnetic resonance imaging; PI-RADS = Prostate Imaging Reporting and Data System; PRECISE = Prostate Cancer Radiological Estimation of Change in Sequential Evaluation; PSA = prostate-specific antigen.
Highest PRECISE score for each patient.
Table 4.
Multivariate Cox regression model predicting clinically significant progression
| Predictors | Multivariate analysis |
|
|---|---|---|
| HR (95% CI) | p value | |
| Baseline PI-RADS | ||
| PI-RADS 1–2 | Reference | – |
| PI-RADS 3–5 | 1.09 (0.58–2.08) | 0.784 |
| No. of repeat MRI scans | ||
| 1 | Reference | – |
| 2 | 0.44 (0.21–0.90) | 0.024 |
| ≥3 | 0.12 (0.05–0.33) | <0.001 |
| No. of repeat biopsies | ||
| 0 | Reference | – |
| 1 | 6.03 (1.83–19.92) | 0.003 |
| ≥2 | 6.03 (1.46–24.90) | 0.013 |
| Highest PRECISEa | ||
| PRECISE 1–3 | Reference | – |
| PRECISE 4–5 | 5.20 (2.57–10.52) | <0.001 |
CI = confidence interval; HR = hazard ratio; MRI = magnetic resonance imaging; PI-RADS = Prostate Imaging Reporting and Data System; PRECISE = Prostate Cancer Radiological Estimation of Change in Sequential Evaluation.
Highest PRECISE score for each patient.
In total, 137 patients underwent a confirmation MRI scan within 18 mo after diagnosis (median 12 mo; IQR 11–14). Radiological progression on this first follow-up MRI scan (PRECISE 4–5, 34/137) had sensitivity of 42%, specificity of 82%, NPV of 79%, and PPV of 47% for clinically significant progression. Four-year PFS in this group was 81% (95% CI 71.3–89.7%) for PRECISE 1–3 versus 43% (95% CI 24.6–60.8%) for PRECISE 4–5 (p < 0.001; Fig. 2B).
Evaluating any progression in this subgroup, radiological progression had sensitivity of 37%, specificity of 85%, NPV of 70%, and PPV of 57%. Four-year PFS was 68% (95% CI 58.2–78.6%) for PRECISE 1–3 versus 25% (95% CI 6.67–42.7%) for PRECISE 4–5 (p < 0.001).
Of the patients, 52% (97/188) showed radiological stability during follow-up (PRECISE 3). When comparing PRECISE 3n with PRECISE 3v, 19% (nine/47) versus 34% (17/50) of patients had any progression (p = 0.060) and 9% (four/47) versus 10% (five/50) of patients had clinically significant progression (p = 0.597).
In total, 358 repeat MRI scans were performed. Forty-three MRI scans did not have follow-up histopathological data, a subsequent MRI scan, or at least 2 yr of clinical follow-up, and thus were excluded from the analysis. The remaining 315 MRI scans were evaluated. On a scan level, radiological progression (PRECISE 4–5, 97/315) had sensitivity of 76%, specificity of 78%, NPV of 94%, and PPV of 39% for clinically significant progression.
Supplementary Figure 1 shows a case classified as PRECISE 4 (radiological progression) and Supplementary Figure 2 shows a case classified as PRECISE 2 (radiological regression). Furthermore, a case with PRECISE 3v (stable visible lesion) is illustrated in Supplementary Figure 3 and a case with PRECISE 3n (stable nonvisible lesion) is illustrated in Supplementary Figure 4.
4. Discussion
In this study, we showed that the implementation of PRECISE scoring in a clinical setting is feasible and offers a prognostic value. Our expert radiologist could allocate a PRECISE score to each repeat MRI scan. All scans were re-reported by one single radiologist. Therefore, interobserver variability could not be assessed. Recent work by Giganti et al. [9], however, shows good interobserver reproducibility of PRECISE scoring. The same author also demonstrated that a dedicated teaching course on PRECISE scoring significantly improves diagnostic accuracy [10]. Furthermore, there is a trend toward improved diagnostic accuracy of PRECISE scoring in predicting clinical progression compared with other radiological definitions [11].
In our study, radiological progression (PRECISE 4–5) on a patient level had sensitivity of 78%, specificity of 70%, NPV of 90%, and PPV of 49% for clinically significant progression. On a scan level, results were quite similar (respectively, 76%, 78%, 94%, and 39%). These results are in line with previous reports. So far, five different study groups have used PRECISE scoring in their AS cohorts [12], [13], [14], [15], [16]. Despite differences regarding inclusion criteria, AS protocols, and definition of progression, all studies report a high NPV (ranging from 76% to 100%) and moderate PPV (ranging from 32% to 66%). A meta-analysis by Rajwa et al. [11] shows a pooled NPV of 88% (95% CI 81.1–94.4%) and PPV of 51% (95% CI 44.0–87.5%).
There is growing interest in MRI-based surveillance, whereby routine prostate biopsy can be avoided in the absence of radiological progression [4]. After all, prostate biopsy forms a barrier to patient adherence and tolerability. We know that compliance rates with a protocol biopsy can be as low as 20–30%, both in routine practice and in formal studies such as PRIAS [8], [16]. In our cohort, 38% of patients did not get a control biopsy after establishing the highest PRECISE score (Fig. 1). Mostly those patients with radiological stability or regression tended to be noncompliant with protocol biopsy. With an NPV of 90% in our study, however, MRI as a stand-alone tool is not accurate enough to omit repeat biopsy during AS. Furthermore, the moderate PPV of 39% suggests that a confirmatory biopsy can be helpful in identifying patients with true pathological progression. When we look at the reason for treatment in our own cohort, 22.1% (19/86) of patients directly switched to active treatment without a confirmatory biopsy. While nine patients had a PRECISE score of 5, indicating definitive radiological progression (mostly appearance of extracapsular extension), the other patients might have benefited from confirmatory biopsies. Furthermore, we observed that only 7% (6/86) of the treated patients showed a rising PSA value and did not get a repeat scan or biopsy.
PRECISE scoring had better NPV and sensitivity for clinically significant progression (90% and 78%, respectively) than for any progression (73% and 65%, respectively). These findings suggest that PRECISE has a better value in detecting progression to higher-grade tumours (GG ≥3). The higher sensitivity of MRI to detect more high-grade, clinically relevant cancer is already well described in a diagnostic setting [17].
Patients with PRECISE score 4–5 on confirmation MRI within 18 mo after diagnosis have a three-fold higher risk of clinically significant progression after 4 yr (57% vs 19%). These findings show that a first follow-up MRI scan around 1 yr after diagnosis is very useful in identifying early progressors, which accounted for 12% of patients in our cohort. Furthermore, in the group with radiological stability/involution (PRECISE 1–3), we see very little progression in the first 2.5 yr after diagnosis (10%), after which there is a somewhat steeper decline in the survival curve (Fig. 2B), suggesting the need for timely repeat MRI.
In patients with radiological stability/involution (PRECISE 1–3), only 9% had clinically significant progression after 4 yr. On the contrary, patients with radiological progression (PRECISE 4–5) had a 34% likelihood of clinically significant progression within 4 yr (p < 0.001).
In our cohort, 45.7% of the patients switched to active treatment over a median follow-up period of 46 mo. While 44.7% of patients had any progression, a smaller percentage of 26.6% met the primary endpoint of clinically significant progression. In order to estimate the diagnostic accuracy and prognostic value of PRECISE scoring, we need to define progression. Of course, definitive pathology after radical prostatectomy could serve as a true control. In the absence of definitive pathology, however, the most reliable way to ascertain true progression is a topic of debate and an important reason for heterogeneity in the published data.
There are other reasons to switch to treatment than tumour upgrading or stage progression. The decision to switch to active treatment is therefore not a good definition of progression. Furthermore, a lot of patients with tumour upgrading from GG 1 to GG 2 are still eligible to stay on AS. Only when upgrading to GG ≥3, active treatment is recommended for all patients [1]. Furthermore, stage progression to T3 disease, irrespective of whether it was clinical, radiological, or pathological, would also always lead to active treatment. We thus proposed a composite primary endpoint whereby “clinically significant progression” is defined by histopathological upgrading to GG ≥3 and/or evolution to T3 stage. As progression to GG 2 is often a trigger for active treatment (especially in the presence of >10% of Gleason pattern 4, cribriform, or intraductal growth), the definition of “any progression” was also included.
We highlight the importance of quality control and use of the same MRI device during follow-up to optimise diagnostic accuracy. Recent data, for instance, revealed that only 60% of all MRI scans in the PRECISION trial had at least good quality [18]. Furthermore, the use of different MRI devices in the follow-up of patients on AS makes reliable PRECISE scoring more difficult (other noise, resolution, apparent diffusion coefficient values, and others), although no data on this topic are available.
We suggest standard reporting of the PI-RADS as well as the PRECISE score in protocolling repeat MRI scans. While clinicians already have good experience with the PI-RADS recommendations, PRECISE offers more subtle information on the evolution of the lesions in time. For instance, PI-RADS 4 to PI-RADS 4 could mean PRECISE 3 (stable lesion) as well as PRECISE 4 (radiological progression of a small lesion). It is also important to note that PI-RADS 3 and PRECISE 3 are very similar in name and may cause confusion in daily practice as these have completely different meanings. PI-RADS 3 is generally regarded as “probably suspicious” (indeterminate lesion, usually considered as positive MRI), while PRECISE 3 should rather be interpreted as “not suspicious” (no progression).
It is well known that patients with no visible tumour on MRI, both at baseline and during follow-up, have the best outcomes [11]. It could therefore be useful to subdivide PRECISE 3 into “PRECISE 3n” (stable nonvisible lesion) and “PRECISE 3v” (stable visible lesion). A subgroup analysis in our cohort, however, fails to show any statistically significant difference, possibly due to a lack of power. This should be evaluated in a larger patient cohort.
In our experience, PRECISE scoring has already added value in clinical practice. Future research will further determine the place of MRI in AS, in particular the ideal interval for repeat MRI, and its safety in replacing routine biopsies and the exact triggers for performing early prostate biopsy. Furthermore, it contributes to standardised MRI reporting, enabling more reliable data collection and synthesis, and will therefore help develop future guideline recommendations.
We acknowledge that this is a small single-centre retrospective analysis. In our real-life cohort, adherence to the PRIAS protocol was not strict, specifically when it comes to compliance with routine repeat prostate biopsies. Different scanner types and biopsy techniques were used. Furthermore, MRI quality has improved over time, and the early scans might be less accurate than the most recent ones. This huge diversity poses an important risk for all kinds of biases. For instance, there is a significant risk of verification bias from patients with PRECISE 1–3 who did not get a repeat biopsy or radical prostatectomy. The lower the PRECISE score, the less chance of repeat biopsy and finding pathological upgrading. There is also a risk of under-reporting of pathological upgrade in patients with PRECISE 4–5 who were treated with radiotherapy without a repeat biopsy. Nevertheless, it was our objective to evaluate the feasibility of PRECISE scoring in clinical practice, with all related advantages and disadvantages. Our work is an early step towards its validation and promotes structured data collection.
Larger cohorts are needed to perform a multivariable analysis with other clinical parameters to rule out multicollinearity and to assess the independent predictive value of PRECISE scoring. Prospective studies generating high-level evidence have yet to be performed.
5. Conclusions
Implementation of PRECISE scoring for PCa patients on AS is feasible and offers a prognostic value. Patients with PRECISE score 4–5 on confirmation MRI within 18 mo after diagnosis have a three-fold higher risk of clinically significant progression after 4 yr.
Author contributions: Jan Aerts had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Aerts, Van Praet, De Visschere.
Acquisition of data: Aerts, Hendrickx, Berquin, Lumen, Verbeke, Villeirs, Van Praet, De Visschere.
Analysis and interpretation of data: Aerts, Van Praet, De Visschere.
Drafting of the manuscript: Aerts, Van Praet, De Visschere.
Critical revision of the manuscript for important intellectual content: Hendrickx, Berquin, Lumen, Verbeke, Villeirs, Van Praet, De Visschere.
Statistical analysis: Aerts, Van Praet.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Van Praet, De Visschere.
Other: None.
Financial disclosures: Jan Aerts certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Associate Editor: Roderick van den Bergh
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.euros.2023.08.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Mottet N., van den Bergh R.C.N., Briers E., et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer—2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79:243–262. doi: 10.1016/j.eururo.2020.09.042. [DOI] [PubMed] [Google Scholar]
- 2.Klotz L., Loblaw A., Sugar L., et al. Active Surveillance Magnetic Resonance Imaging Study (ASIST): results of a randomized multicenter prospective trial. Eur Urol. 2019;75:300–309. doi: 10.1016/j.eururo.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 3.Welty C.J., Cowan J.E., Nguyen H., et al. Extended followup and risk factors for disease reclassification in a large active surveillance cohort for localized prostate cancer. J Urol. 2015;193:807–811. doi: 10.1016/j.juro.2014.09.094. [DOI] [PubMed] [Google Scholar]
- 4.Ploussard G., Rouvière O., Rouprêt M., van den Bergh R., Renard-Penna R. The current role of MRI for guiding active surveillance in prostate cancer. Nat Rev Urol. 2022;19:357–365. doi: 10.1038/s41585-022-00587-0. [DOI] [PubMed] [Google Scholar]
- 5.Turkbey B., Rosenkrantz A.B., Haider M.A., et al. Prostate Imaging Reporting and Data System version 2.1: 2019 update of Prostate Imaging Reporting and Data System version 2. Eur Urol. 2019;2019:340–351. doi: 10.1016/j.eururo.2019.02.033. [DOI] [PubMed] [Google Scholar]
- 6.Moore C.M., Giganti F., Albertsen P., et al. Reporting magnetic resonance imaging in men on active surveillance for prostate cancer: the PRECISE recommendations—a report of a European school of Oncology Task Force. Eur Urol. 2017;71:648–655. doi: 10.1016/j.eururo.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Bul M., Zhu X., Valdagni R., et al. Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol. 2013;63:597–603. doi: 10.1016/j.eururo.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Bokhorst L.P., Valdagni R., Rannikko A., et al. A decade of active surveillance in the PRIAS study: an update and evaluation of the criteria used to recommend a switch to active treatment. Eur Urol. 2016;70:954–960. doi: 10.1016/j.eururo.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Giganti F., Pecoraro M., Stavrinides V., et al. Interobserver reproducibility of the PRECISE scoring system for prostate MRI on active surveillance: results from a two-centre pilot study. Eur Radiol. 2020;30:2082–2090. doi: 10.1007/s00330-019-06557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giganti F., Aupin L., Thoumin C., et al. Promoting the use of the PRECISE score for prostate MRI during active surveillance: results from the ESOR Nicholas Gourtsoyiannis teaching fellowship. Insights Imaging. 2022;13:1–13. doi: 10.1186/s13244-022-01252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajwa P., Pradere B., Quhal F., et al. Reliability of serial prostate magnetic resonance imaging to detect prostate cancer progression during active surveillance: a systematic review and meta-analysis. Eur Urol. 2021;80:549–563. doi: 10.1016/j.eururo.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 12.O’Connor L.P., Wang A.Z., Yerram N.K., et al. Changes in magnetic resonance imaging using the prostate cancer radiologic estimation of change in sequential evaluation criteria to detect prostate cancer progression for men on active surveillance. Eur Urol Oncol. 2020;4:227–234. doi: 10.1016/j.euo.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caglic I., Sushentsev N., Gnanapragasam V.J., et al. MRI-derived PRECISE scores for predicting pathologically-confirmed radiological progression in prostate cancer patients on active surveillance. Eur Radiol. 2021;31:2696–2705. doi: 10.1007/s00330-020-07336-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ullrich T., Arsov C., Quentin M., et al. Multiparametric magnetic resonance imaging can exclude prostate cancer progression in patients on active surveillance: a retrospective cohort study. Eur Radiol. 2020;30:6042–6051. doi: 10.1007/s00330-020-06997-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dieffenbacher S., Nyarangi-Dix J., Giganti F., et al. Standardized magnetic resonance imaging reporting using the prostate cancer radiological estimation of change in sequential evaluation criteria and magnetic resonance imaging/transrectal ultrasound fusion with transperineal saturation biopsy to select men. Eur Urol Focus. 2019;7:102–110. doi: 10.1016/j.euf.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Giganti F., Stabile A., Stavrinides V., et al. Natural history of prostate cancer on active surveillance: stratification by MRI using the PRECISE recommendations in a UK cohort. Eur Radiol. 2021;31:1644–1655. doi: 10.1007/s00330-020-07256-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moldovan P.C., Van den Broeck T., Sylvester R., et al. What is the negative predictive value of multiparametric magnetic resonance imaging in excluding prostate cancer at biopsy? A systematic review and meta-analysis from the European Association of Urology Prostate Cancer Guidelines Panel. Eur Urol. 2017;72:250–266. doi: 10.1016/j.eururo.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 18.Giganti F., Allen C., Emberton M., Moore C.M., Kasivisvanathan V. Prostate Imaging Quality (PI-QUAL): a new quality control scoring system for multiparametric magnetic resonance imaging of the prostate from the PRECISION trial. Eur Urol Oncol. 2020;3:615–619. doi: 10.1016/j.euo.2020.06.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.