Abstract
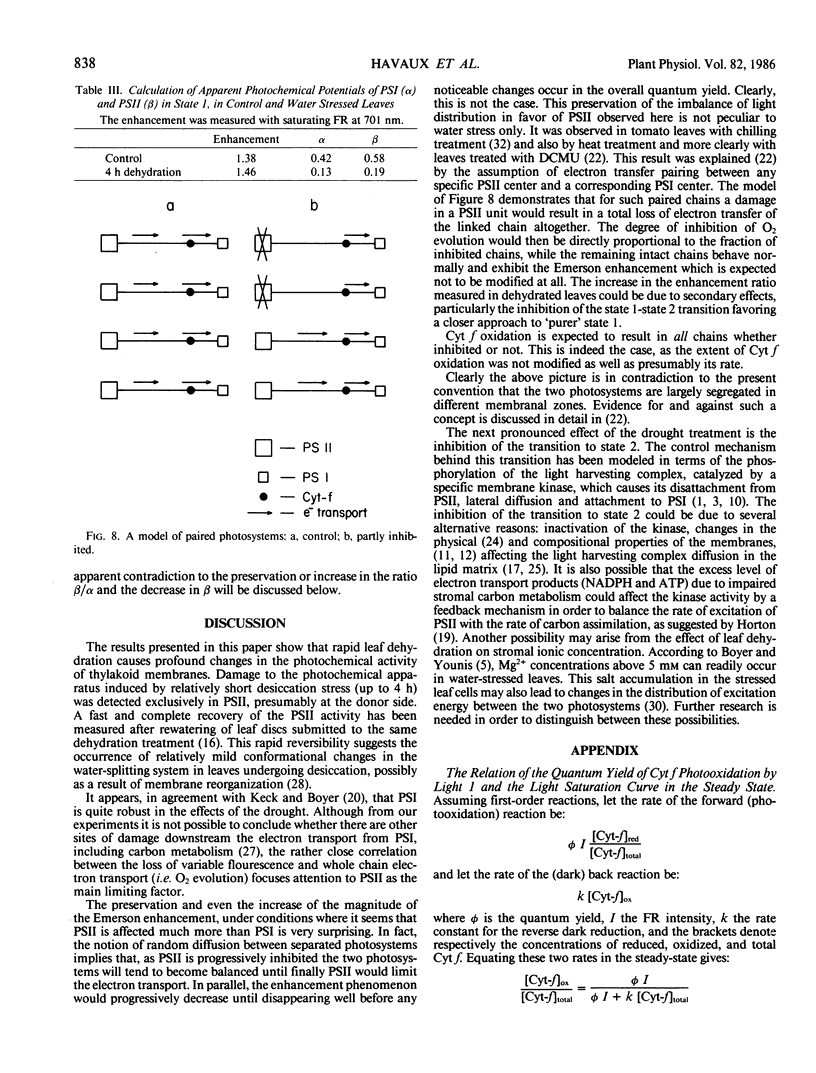
The effect of rapid dehydration of detached tobacco leaves (Nicotiana tabacum L.) on the photochemical apparatus of photosynthesis was studied in vivo by a combination of methods: photoacoustics, chlorophyll a fluorescence, and cytochrome f difference spectroscopy. It was shown that the inhibition of gross O2 evolution was mainly caused by inactivation of PSII: (a) The saturation curve of cytochrome-f photooxidation by farred (>710 nanometers) light was resistant to the stress, leading to the conclusion that photosystem I (PSI) was largely unaffected by the stress. (b) The extent of the chlorophyll a variable fluorescence arising from photosystem II (PSII) decreased with the progression of the stress, but was largely unaffected when the leaf was preincubated with electron donors to PSII, such as hydroxylamine. It is concluded that the drought damage to PSII occurred on the photooxidative side. Despite the extensive inhibition of PSII and the relative preservation of PSI, the apparent PSII/PSI activity balance was somewhat larger in stressed leaves than in the control, as indicated by photoacoustic measurements of Emerson enhancement. These measurements were performed continuously under conditions which favor transitions to either state 1 or 2, showing that the transition to state 2 was considerably inhibited. Simultaneous measurements of chlorophyll fluorescence induction at 680 and 730 mm at room temperature were also used to probe changes in energy distribution between PSII and PSI and indicated that the transition from a dark adapted state to state 2 was also affected in water-stressed leaves. The saturation curve of the far-red light effect in Emerson enhancement was not changed by the stress, giving another independent evidence for the drought resistance of PSI activity. This apparent preservation of the imbalance in photochemical activities in favor of PSII, despite the fact that PSII is strongly inhibited, and PSI is not, supports a previous suggestion that the electron transfer between the two photosystems is not random but that a large extent of PSII and PSI units are specifically linked.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J., Steinback K. E., Arntzen C. J. Chloroplast phosphoproteins: regulation of excitation energy transfer by phosphorylation of thylakoid membrane polypeptides. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5253–5257. doi: 10.1073/pnas.77.9.5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. G., Andersen K. S., Smillie R. M. Photoreduction and Oxidation of Cytochrome f in Bundle Sheath Cells of Maize. Plant Physiol. 1972 Apr;49(4):467–470. doi: 10.1104/pp.49.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury M., Baker N. R. Analysis of the slow phases of the in vivo chlorophyll fluorescence induction curve. Changes in the redox state of photosystem II electron acceptors and fluorescence emission from photosystems I and II. Biochim Biophys Acta. 1981 May 13;635(3):542–551. doi: 10.1016/0005-2728(81)90113-4. [DOI] [PubMed] [Google Scholar]
- Canaani O., Barber J., Malkin S. Evidence that phosphorylation and dephosphorylation regulate the distribution of excitation energy between the two photosystems of photosynthesis in vivo: Photoacoustic and fluorimetric study of an intact leaf. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1614–1618. doi: 10.1073/pnas.81.6.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth P. Protein phosphorylation-induced State I-State II transitions are dependent on thylakoid membrane microviscosity. Arch Biochem Biophys. 1983 Oct 1;226(1):145–154. doi: 10.1016/0003-9861(83)90279-5. [DOI] [PubMed] [Google Scholar]
- Keck R. W., Boyer J. S. Chloroplast Response to Low Leaf Water Potentials: III. Differing Inhibition of Electron Transport and Photophosphorylation. Plant Physiol. 1974 Mar;53(3):474–479. doi: 10.1104/pp.53.3.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty P., Boyer J. S. Chloroplast Response to Low Leaf Water Potentials: IV. Quantum Yield Is Reduced. Plant Physiol. 1976 May;57(5):704–709. doi: 10.1104/pp.57.5.704. [DOI] [PMC free article] [PubMed] [Google Scholar]


