Abstract
Von Hippel–Lindau (VHL) disease is an autosomal-dominant syndrome caused by mutations in the VHL gene, located on the short arm of chromosome 3. Patients with VHL are likely to manifest with a spectrum of multiple benign and malignant tumors involving various organ systems. We present a case of a 28-year-old female without a remarkable family history who presented with complaints of hematuria and abdominal discomfort. Initial laboratory investigations confirmed hematuria. Subsequent abdominal computed tomography scan revealed heterogeneous enhancing solid mass in bilateral kidneys, avidly enhancing mass in the right adrenal gland, bilateral simple renal cortical cysts, and a pancreatic cyst. With a provisional diagnosis of VHL disease, an MRI of the brain and spine was performed, which showed the presence of a cerebellar hemangioblastoma. Her catecholamine and vanillylmandelic acid levels were in the normal range not in line with pheochromocytoma. The patient then underwent bilateral partial renal nephrectomy and right adrenalectomy. Histopathologic examination reported clear renal cell carcinoma and pheochromocytoma of the right adrenal gland mass. Molecular genetic testing confirmed the presence of VHL disease.
Keywords: Cerebellar hemangioblastoma, Pheochromocytoma, Von-Hipple-Lindau disease
Introduction
Von Hippel-Lindau disease (VHL) is a rare autosomal dominant syndrome with a reported incidence of 1 in 36,000 live births. Caused by the mutation of the VHL suppressor gene in chromosome 3 [1], there is a predisposition to the formation of cysts and tumors in various organs [2]. The diagnostic criteria for VHL disease have been: (a) more than 1 central nervous system (CNS) hemangioblastoma, (b) 1 CNS hemangioblastoma and visceral manifestations of VHL disease, and (c) any manifestation and a known family history of VHL disease [3]. However, VHL gene mutation analysis has become a staple in establishing a definite diagnosis in cases where the criteria may not be met [4]. Since the lesions associated with the disease have significant morbidity and mortality; the need for detection, screening, and surveillance are crucial for optimal management. Here we report a case of VHL disease with CNS hemangioblastoma, unilateral pheochromocytoma, bilateral renal cell carcinoma, bilateral renal cortical cysts, and pancreatic cysts confirmed with histopathological evaluation and VHL gene mutation analysis.
Case report
The 28-year-old female presented to our institution with complaints of abdominal discomfort and blood-tinged urine for 1 month. Her vitals were normal and initial laboratory investigations confirmed the presence of hematuria. With the probability of renal pathology, the patient was then advised to undergo computed tomography (CT) intravenous urography for evaluation which showed:
-
-
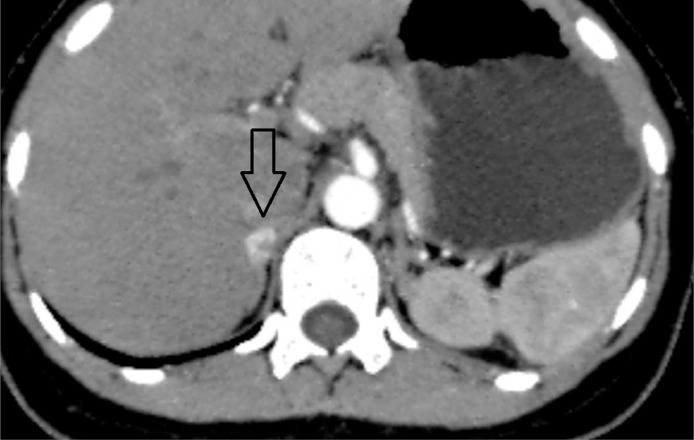
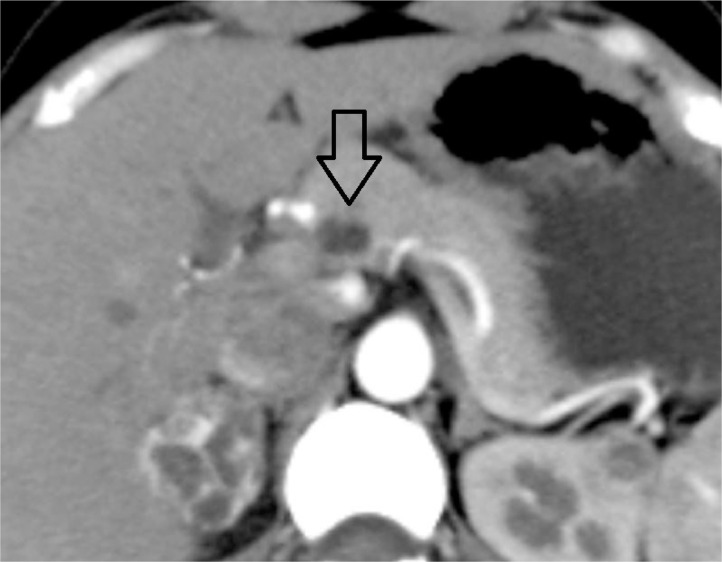
A well-defined elliptical hypodense lesion measuring approximately 13.4 × 11.7 × 7.3 mm in the body of the right adrenal gland with homogeneous avid enhancement on postcontrast study (Fig. 1).
-
-
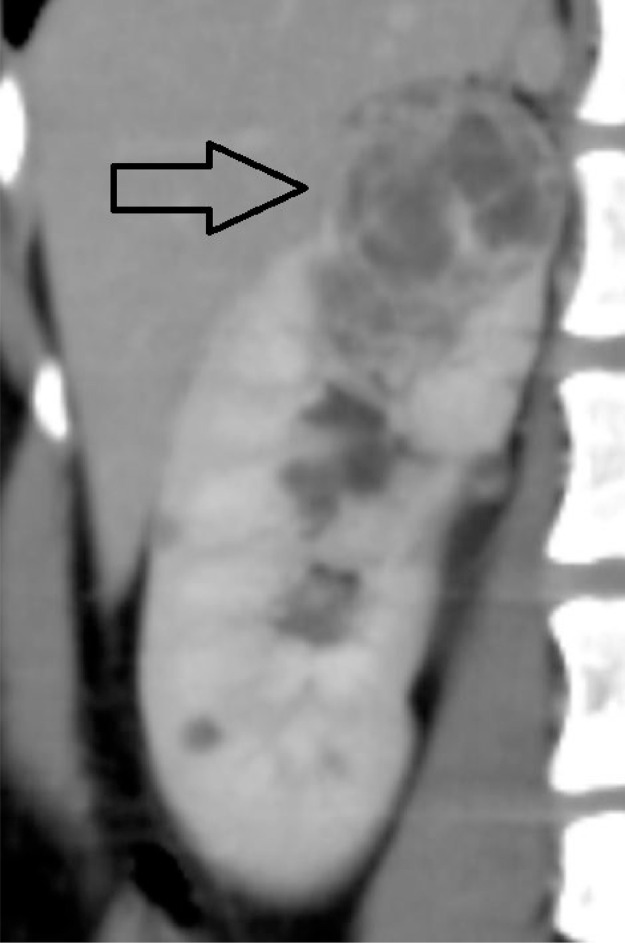
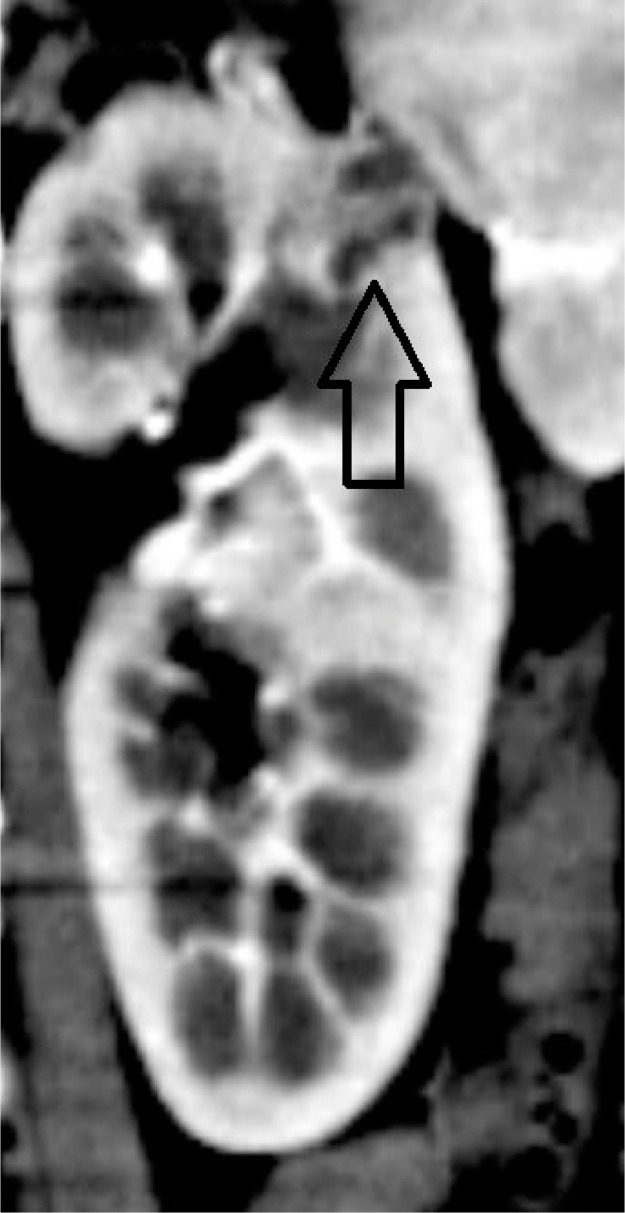
An irregular heterogeneous exophytic lesion (>50% exophytic) measuring approximately 4.2 × 3.3 × 3.6 cm with postcontrast enhancement of the septations noted in the upper pole of the right kidney (Fig. 2). A well-defined hypodense lesion (<50 % exophytic) measuring approximately 2.4 × 1.4 × 1.4 cm with the enhancement of the septations noted involving the upper pole of the left kidney (Fig. 3).
-
-
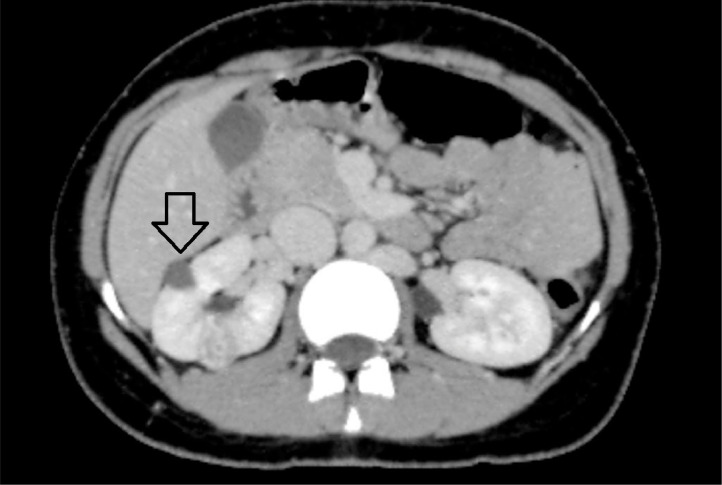
Few simple cortical cysts were noted in both kidneys (Fig. 4).
-
-
A well-defined hypodense lesion measuring approximately 10.5 × 8.4 mm was noted involving the body of the pancreas without postcontrast enhancement (Fig. 5).
Fig. 1.
Closed arrow pointing at the right adrenal lesion.
Fig. 2.
Closed arrow showing the lesion in the upper pole of the right kidney.
Fig. 3.
Closed arrow showing the lesion in the upper pole of the left kidney.
Fig. 4.
Open arrow showing right renal cortical cyst.
Fig. 5.
Open arrow showing pancreatic cyst in body of pancreas.
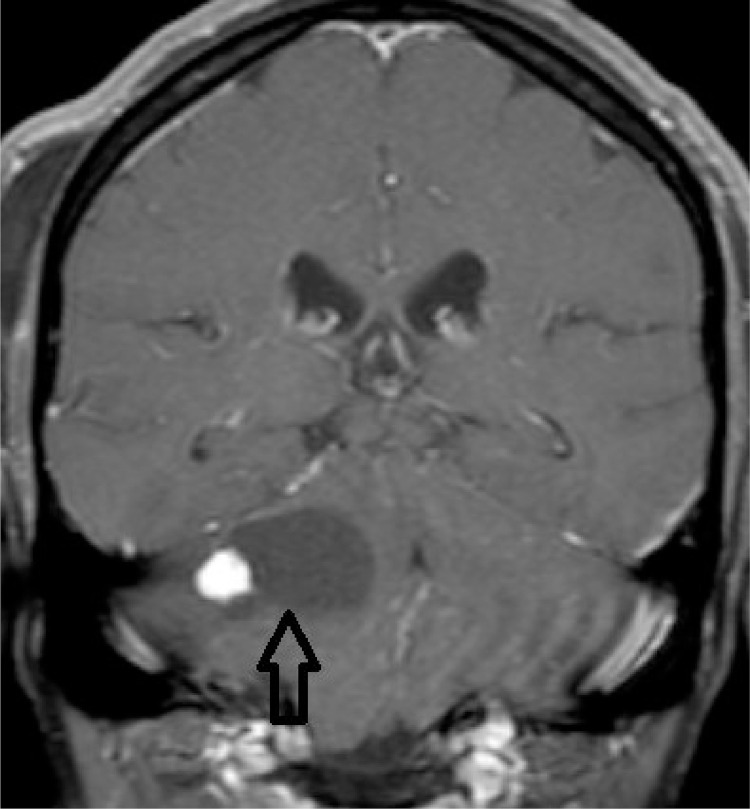
With the possibility of VHL disease, a contrast-enhanced MRI of the brain and spine was done to evaluate the presence of cerebellar hemangioblastoma. Contrast-enhanced MRI brain revealed a well-defined ∼ 3.3 × 3.3 cm cystic lesion with an eccentric mural nodule in the right brachium pontis. The mural nodule demonstrated avid enhancement in postcontrast images. The findings were suggestive of hemangioblastoma (Fig. 6). No lesion was seen in the spinal cord.
Fig. 6.
T1 Coronal postcontrast image of brain at the level of right brachium pontis. The image demonstrated a cystic lesion with enhancing eccentric mural nodule suggestive of hemangioblastoma.
With the possibility of pheochromocytoma in the right adrenal gland, 24-hour urinary metanephrine, normetanephrine, and vanillylmandelic acid (VMA) levels were evaluated which were found to be 94.57 µg/24 h, 343.77 µg/24 h and 4.06 mg/24 h respectively; all within the normal range.
The patient then underwent surgery for the removal of the right adrenal gland mass and partial nephrectomy of the bilateral kidney for removal of complex renal mass.
The histopathological evaluation of the postsurgical specimen yielded the following results:
-
-
Microscopic examination of the renal mass revealed features of clear cell renal cell carcinoma (WHO/ISUP grade I).
-
-
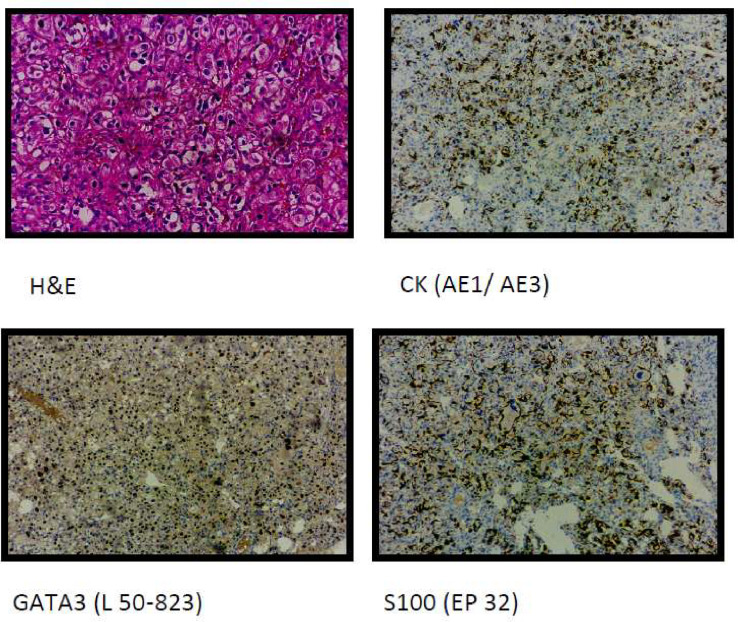
Microscopic examination of the paraffin block of the adrenal mass revealed a tumor surrounding native parenchyma comprising polygonal cells in nests having coarse chromatin and abundant cytoplasm. Immunohistochemical interpretation found the tumor cells to be positive for GATA3, CK, INHIBIN-1, and S100- features of pheochromocytoma (Fig. 7).
-
-
VHL gene mutation analysis found the patient to be Heterozygous for c.473T>C (p.L158P) confirming that the proband harbors a mutation in the VHL gene.
Fig. 7.
Histopathology of renal mass (upper 2 figures) suggestive of clear cell renal cell carcinoma. Histopathology of adrenal mass (lower 2 figures) suggestive of pheochromocytoma.
Since cerebellar hemangioblastoma was asymptomatic, the patient opted for a close follow-up. She was counseled for surgery if any symptoms developed.
Discussion
VHL is an autosomal dominant familial cancer syndrome with manifestations in multiple organ systems most frequently hemangioblastoma (retinal, cerebellar and spinal), renal cell carcinoma, pheochromocytoma and pancreatic tumors [5]. The presented case showed typical findings like clear cell renal cell carcinoma, cerebellar hemangioblastoma, pheochromocytoma, renal cortical cysts, and a pancreatic cyst; findings seen with VHL disease. Clear cell renal cell carcinoma represents around 75%-80% of cases of renal cell carcinoma originating from the epithelium of the proximal tubule. It is sporadic in over 95% of cases and in the remaining 5% of cases, most of them are associated with VHL disease [6]. The patient came to the hospital with symptoms of hematuria and abdominal discomfort which may be attributed to renal cell carcinoma confirmed on histopathological examination as clear cell renal cell carcinoma.
Pheochromocytoma is also a rare neoplasm with an incidence of 1-4/106 population/y arising from the chromaffin cells of the embryonic neural crest. The presentation of signs and symptoms depends on the location and amount of catecholamine secretion. The classic triad of presentation includes headaches, palpitations, and profuse sweating [7]. Several familial disorders are known to be associated with adrenal pheochromocytoma, which commonly includes VHL syndrome, multiple endocrine neoplasia type 2 (MEN2), and neurofibromatosis type 1 (NF1) [8]. Our patient did not have symptoms usually seen with pheochromocytoma like hypertension [9] or features of cardiac or neurologic dysfunction [10] as a result of increased catecholamine secretion. The levels of catecholamines and VMA were also found to be in the normal range. This may be because the neoplasm was detected in the “pre biochemical phase” [11] where the levels are normal and thus explaining the lack of symptoms. However, the diagnosis of pheochromocytoma in the right adrenal gland mass was confirmed on histopathological examination of the postsurgical specimen.
Hemangioblastoma mainly located in the cerebellar hemisphere is a relatively rare tumor accounting for 1.5%-2.5% of all brain tumors. It can occur as an isolated finding or present as part of a syndrome associated with other lesions such as in VHL disease. The clinical presentation is usually cerebellar signs like ataxia, dizziness, headache, and intracranial hypertension associated with mass effect [12]. However, our patient did not have any symptoms attributable to cerebellar hemangioblastoma. However, MRI of the brain showed its presence at the level of the right brachium pontis.
VHL disease can manifest in the pancreas as solid, cystic, or combined lesions including both solid and cystic components. Solid lesions are primarily neuroendocrine tumors and cystic lesions are commonly simple cysts or serous cystadenomas. Since renal cell carcinoma is associated with VHL disease, metastasis should be considered in the differential diagnosis of pancreatic lesions. Although cystic lesions usually encountered in VHL are mostly benign it is mandatory to differentiate them with neoplastic cysts like mucinous cystic tumors, intrauterine papillary mucinous tumors, and solid pseudopapillary neoplasms which can be premalignant or malignant. The pancreatic lesion observed in our patient was a well-defined hypodense lesion without solid component, septa, postcontrast enhancement, and ductal communication; thus consistent with a simple pancreatic cyst [13,14].
With the myriads of radiological and histopathological findings and fulfillment of criteria [4], a provisional diagnosis of VHL disease was made. Since no history of VHL disease or associated findings were present in the family; a definite diagnosis was required. The disease shows an autosomal dominant inheritance with high expression and penetrance; ∼80% of cases occur via an autosomal dominant pathway and ∼20% arising de novo [15]. Thus, it was imperative that a definite diagnosis was made; So VHL gene mutation gene analysis was done which confirmed the presence of a mutation in the VHL gene. Since the screening, surveillance, and genetic counseling are key aspects in the management of patients; general recommendations in regards to radiological investigations to screening and surveillance for organs without lesions include a baseline MRI brain and spine with contrast to be repeated every 2 years to screen for CNS lesions and MRI abdomen preferably to be done every 2 years to screen for abdominal lesions, with use of CT and ultrasonography (USG) to be decided on a case-to-case basis. However, in organs with a diagnosed malignancy, the imaging follow-up is personalized based on the lesion [16]. It is also important to know the possible manifestations of VHL disease in the lung which include pulmonary arterial hypertension, lung cancer, pulmonary fibrosis, and ARDS [17]. It is important to consider the possibility of metastasizing renal cell carcinoma, especially with multiple lung nodules [18]. The choice of imaging for screening and follow up however is dependent on multiple factors with CT and MRI both having advantages and disadvantages in different scenarios; however, CT is being considered in most scenarios [19].
Summary
VHL is an autosomal dominant multisystem familial cancer syndrome caused by the mutation of the VHL tumor suppressor gene on chromosome 3. Since the presence of VHL disease has grievous implications for the patient and also due to its autosomal dominant inheritance pattern, a definite diagnosis becomes paramount. Detection of the disease however requires knowledge of the constellation of clinical presentation, laboratory findings and radiological imaging findings associated with the VHL. A multidisciplinary approach for the management, screening, surveillance and genetic counseling are important to improve both life expectancy and quality of life.
Patient consent
Informed written consent was obtained from the patient for publication of the case including images.
Footnotes
Acknowledgments: No source of funding.
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Keutgen XM, Hammel P, Choyke PL, Libutti SK, Jonasch E, Kebebew E. Evaluation and management of pancreatic lesions in patients with von Hippel-Lindau disease. Nat Rev Clin Oncol. 2016;13(9):537–549. doi: 10.1038/nrclinonc.2016.37. [DOI] [PubMed] [Google Scholar]
- 2.Sharma A, Mukewar S, Vege SS. Clinical profile of pancreatic cystic lesions in von Hippel-Lindau disease: a series of 48 patients seen at a tertiary institution. Pancreas. 2017;46(7):948–952. doi: 10.1097/MPA.0000000000000875. [DOI] [PubMed] [Google Scholar]
- 3.Kanno H, Kuratsu JI, Nishikawa R, Mishima K, Natsume A, Wakabayashi T, et al. Clinical features of patients bearing central nervous system hemangioblastoma in von Hippel-Lindau disease. Acta Neurochir. 2013;155(1):1–7. doi: 10.1007/s00701-012-1514-y. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen SM, Rhodes L, Blanco I, Chung WK, Eng C, Maher E, et al. Von Hippel-Lindau disease: genetics and role of genetic counseling in a multiple neoplasia syndrome. J Clin Oncol. 2016;34(18):2172–2181. doi: 10.1200/JCO.2015.65.6140. [DOI] [PubMed] [Google Scholar]
- 5.Arao T, Okada Y, Tanikawa T, Inatomi H, Shuin T, Fujihira T, et al. A case of von Hippel-Lindau disease with bilateral pheochromocytoma, renal cell carcinoma, pelvic tumor, spinal hemangioblastoma and primary hyperparathyroidism. Endocr J. 2002;49(2):181–188. doi: 10.1507/endocrj.49.181. PMID: 12081237. [DOI] [PubMed] [Google Scholar]
- 6.Cairns P. Renal cell carcinoma. Cancer Biomark. 2010;9(1-6):461–473. doi: 10.3233/CBM-2011-0176. PMID: 22112490; PMCID: PMC3308682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antunes E, Lopes J, Silva I, Fernandes V. Pheochromocytoma: a case report. Cureus. 2022;14(11):e31409. doi: 10.7759/cureus.31409. PMID: 36523722; PMCID: PMC9744410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neumann HPH, Young WF, Jr, Eng C. Pheochromocytoma and Paraganglioma. N Engl J Med. 2019;381(6):552–565. doi: 10.1056/NEJMra1806651. PMID: 31390501. [DOI] [PubMed] [Google Scholar]
- 9.Zuber SM, Kantorovich V, Pacak K. Hypertension in pheochromocytoma: characteristics and treatment. Endocrinol Metab Clin North Am. 2011;40(2):295–311. doi: 10.1016/j.ecl.2011.02.002. viiPMID: 21565668; PMCID: PMC3094542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zelinka T, Petrák O, Turková H, Holaj R, Strauch B, Kršek M, et al. High incidence of cardiovascular complications in pheochromocytoma. Horm Metab Res. 2012;44(5):379–384. doi: 10.1055/s-0032-1306294. Epub 2012 Apr 19. PMID: 22517556. [DOI] [PubMed] [Google Scholar]
- 11.Young WF., Jr Endocrine hypertension: then and now. Endocr Pract. 2010;16(5):888–902. doi: 10.4158/EP10205.RA. PMID: 20713331. [DOI] [PubMed] [Google Scholar]
- 12.Lahkim M, Andour H, Laamrani FZ, Allaoui M, Saouab R, El Fenni J, et al. Cerebellar hemangioblastoma: case report with review of the literature. Radiol Case Rep. 2021;16(10):3109–3112. doi: 10.1016/j.radcr.2021.07.027. PMID: 34429813; PMCID: PMC8367806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamura K, Nishimori I, Ito T, Yamasaki I, Igarashi H, Shuin T. Diagnosis and management of pancreatic neuroendocrine tumor in von Hippel-Lindau disease. World J Gastroenterol. 2010;16(36):4515–4518. doi: 10.3748/wjg.v16.i36.4515. PMID: 20857520; PMCID: PMC2945481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilendarov A, Velkova K, Siracov N, Tchervenkov L, Georgiev A. Pathological unit for specific new mucinous benign pancreatic cysts. Highl Med Med Sci. 2021;15:157–163. [Google Scholar]
- 15.Ganeshan D, Menias CO, Pickhardt PJ, Sandrasegaran K, Lubner MG, Ramalingam P, et al. Tumors in von Hippel-Lindau Syndrome: from head to toe-comprehensive state-of-the-art review. Radiographics. 2018;38(3):849–866. doi: 10.1148/rg.2018170156. Epub 2018 Mar 30. Erratum in: Radiographics. 2018 May-Jun;38(3):982. PMID: 29601266. [DOI] [PubMed] [Google Scholar]
- 16.Bajaj S, Gandhi D, Nayar D, Serhal A. Von Hippel-Lindau Disease (VHL): characteristic lesions with classic imaging findings. J Kidney Cancer VHL. 2023;10(3):23–31. doi: 10.15586/jkcvhl.v10i3.293. PMID: 37555195; PMCID: PMC10404985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen T, Sun M, Zhou G. Von Hippel-Lindau protein and respiratory diseases. World J Respirol. 2013;3(3):48–56. [Google Scholar]
- 18.Lu L, Drew PA, Yachnis AT. Hemangioblastoma in the lung: metastatic or primary lesions? Case Rep Pathol. 2014;2014 doi: 10.1155/2014/468671. Epub 2014 Dec 14. PMID: 25574414; PMCID: PMC4276681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Georgiev A, Chervenkov L, Anastasova V, Kitova T. Comment on "Evaluation of pulmonary nodules by magnetic resonance imaging sequences: which sequence will replace computed tomography? Rev Assoc Med Bras (1992) 2023;69(4) doi: 10.1590/1806-9282.20221624. PMID: 37075370; PMCID: PMC10176654. [DOI] [PMC free article] [PubMed] [Google Scholar]