Abstract
Androgenetic alopecia (AGA) is the most common nonscarring alopecia and is characterised by distinct gradual patterned hair loss. AGA is mediated by genetic predisposition and excessive follicular sensitivity to androgens, mainly in males, leading to the progressive conversion of scalp terminal hair into vellus hair. Although highly prevalent, it is not fatal but may have a severe psychosocial impact, especially on females and younger males. Significant advances have been made in understanding AGA's epidemiology and pathophysiology, but only 2 drugs remain approved by the FDA - finasteride and minoxidil. Prolonged use of these drugs, is a prerequisite for enhanced treatment response. However, this leads to poor medication adherence and adverse effects from extended use eg, the “postfinasteride syndrome” which persists beyond stopping the drug. Hence, there is a need for research on more effective alternative treatments for AGA, with fewer side effects.
This paper reviewed recent advances in AGA pathophysiology and its treatment options. The recently characterized structure of type 2, 5-alpha reductase holds significance in comprehending present and prospective treatments of AGA.
Key words: AGA, androgenetic alopecia, cyproterone, female pattern baldness, finasteride, flutamide hair follicle transplantation, hair loss, male pattern baldness, minoxidil, nonscarring alopecia, spironolactone
Capsule Summary.
-
•
Despite extensive research on the epidemiology and pathophysiology of AGA, only 2 FDA-approved drugs currently exist.
-
•
A comprehensive review of interventions in AGA, focuses on the recently published structure of type 2, 5-alpha reductase for developing more effective drugs.
Background
Androgenetic alopecia (AGA) is a common patterned hair loss affecting men and women.1 It is influenced by genetics and sensitivity to androgens.2 Prevalence differs by gender and race, with >50% of older men and 15% of postmenopausal women affected. However, hair thinning can start as early as puberty.3 AGA's impact on appearance and psychosocial experiences can affect the quality of life (QOL).4 Despite numerous models exploring AGA pathogenesis, there are only 2 FDA approved treatments (finasteride and minoxidil).5
Balding has been observed throughout history, with Aristotle noting its absence in women and eunuchs.6,7 Darwin believed baldness was a secondary sexual characteristic. He reasoned that while hair is a common feature in most adult humans, males tend to experience hair loss more frequently than females.8 Osborn discovered that baldness follows a dominant inheritance pattern.9 Hamilton and Orentreich linked genetic predisposition, androgens and age to baldness.2,10
AGA progresses differently in males and females, with different classification scales.11 While the cause may be similar in both genders, the evidence for androgens is stronger in males and there are variations in hair loss patterns.12
This review discusses AGA's history, causes, diagnosis, management, and recent advancements in research and clinical approaches.
AGA etiology
Androgens and androgen receptors
Androgens are important hormones for the growth and development of the human body. They function through intracellular signalling pathways, with testosterone being the main and most active androgen in males.13 Testosterone is converted by type 2, 5-alpha reductase (SRD5A2) into dihydrotestosterone (DHT), leading to AGA. Excessive DHT shrinks hair follicles, replacing terminal hairs, with vellus hairs (Fig 1).14
Fig 1.
Androgen-mediated effect on the hair growth cycle leading to AGA: Excessive activation of the AR results in the miniaturisation of the follicles, shortening the anagen phase of the hair cycle. The hair shafts become thinner and shorter and may not penetrate the epidermis.
Androgen receptors (ARs) in hair follicles bind to DHT, changing protein shape, and initiating a signalling cascade (Fig 2).15 Occipital hairs are less sensitive due to AR methylation, protecting them from miniaturization and loss. AR has a strong affinity for DHT compared to testosterone, explaining their binding strength.16
Fig 2.
A schematic illustration of the cellular mechanism of androgen, DHT, and AR action in mediating androgenetic alopecia. AR, Androgen receptors; DHT, dihydrotestosterone; SRD5A2, 5 alpha-reductase type 2.
The role of genetics
AGA susceptibility is primarily influenced by hereditary factors, contributing to around 80% of the predisposition to baldness.17 AGA follows a polygenic model, characterized by varying expression levels, which accounts for the diverse range of clinical phenotypes and initial variations observed in individuals affected by this condition.18
Modifications of the androgen receptor (AR) genes on the Xq12 chromosome lead to AGA,19 through increased AR gene activity in hair follicles triggered by dihydrotestosterone (DHT) binding. The mechanism of AR-mediated hair loss remains unknown.20 However, gene polymorphisms in the AR gene consistently correlate with AGA susceptibility in men21 and are implicated in FPHL.22 Variation of the AR gene accounts for about 40% of the heritability of AGA in men,23 and variation in the DNA responsible for hair loss is close to the AR locus in regions responsible for the regulatory effect of AR, including the Ectodysplasin A2 Receptor (EDA2R).24 Studies have provided partial insight into the intricate heritability of AGA, revealing a reciprocal interplay between genetics and androgens, as evidenced by the development of alopecia in individuals from families with bald adult males upon androgenic therapy.10,20
The role of steroid 5-alpha reductase (SRD5A) enzyme
The SRD5A enzyme is a full membrane-embedded protein with 5 members: SRD5A type 1 to type 3, the glycoprotein synaptic 2 (GSPN2) and GSPN2- like.25 SRD5A1 predominates in the liver, skin, and scalp, and metabolises neurosteroids. SRD5A2 is expressed in hair follicles, the prostate, and the genitourinary tract, and has high specificity for steroids containing the Δ4,3-keto configuration, like testosterone. SRD5A2 was characterised based on its ability to convert Δ4,3-keto steroids into metabolites with distinct predetermined roles26 and is the one associated with AGA. SRD5A3 functions in protein N-linked glycosylation and is expressed in malignant human prostate tissues, prostate cancer, and breast cancer.27
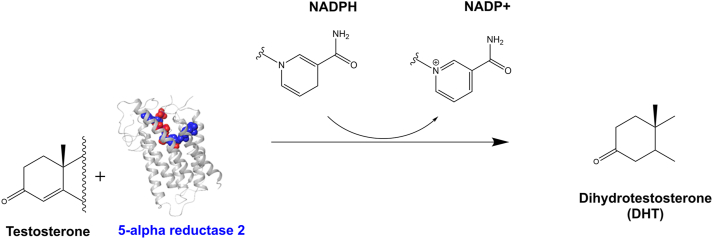
SRD5A mediates 3 metabolic pathways; bile acid biosynthesis, androgen, and oestrogen metabolism. In androgen metabolism, SRD5A reduces Δ4,5 bond in substrates using NADPH, for example, SRD5A1 converts testosterone to DHT causing BPH and SRD5A2 is involved in AGA. These 2 iso-enzymes reduce the Δ4 group (double bond) of C-19 and C-21 steroids into 5α-stereoisomers – DHT (Fig 3).28
Fig 3.
Dihydrotestosterone (DHT) synthesis by SRD5A2 in AGA. The SRD5A2 synthesizes DHT by converting testosterone in the presence of NADPH to DHT.
AGA management
Diagnosis
Male and female pattern hair loss have similarities but also significant differences in their presentation and treatment. The pattern of hair loss and the response to treatment vary between genders. Advanced ADA is a clinical diagnosis. However, assessing patients for early AGA involves invasive and noninvasive methods such as scalp dermoscopy, hair/scalp examination, pull tests, trichograms, and scalp biopsies.29
Histologically, AGA is characterized by follicular miniaturization, perifollicular inflammation, fibrosis in the pilosebaceous unit angiofibrotic tracts, follicular stelae and signs of follicle destruction or hair shaft damage.30 Evaluation is critical to exclude other scalp conditions, like seborrheic dermatitis, which can aggravate AGA and result in smooth and shiny skin.31
The management of AGA involves FDA-approved and nonapproved treatments, which are reported to effectively slow down the progression of hair loss. It's important to note that discontinuing treatment may result in a rapid progression of hair loss.
Treatment
Despite the widespread occurrence of AGA, effectively managing and treating the condition remains challenging, leading many individuals to seek medical help. Various options, including lifestyle adjustments, hair care practices, and medications are available, but only 2 drugs, oral finasteride (for men) and topical minoxidil (for both men and women), are FDA-approved for AGA treatment. Off-label use of topical minoxidil for AGA exists, but its effectiveness and safety require further investigation to assess potential benefits and risks.32
Finasteride
Finasteride – a competitive and specific SRD5A2 inhibitor – is an FDA-approved drug used to treat adult men with mild to moderate AGA at an oral dosage of 1 mg/day, thereby decreasing serum, prostate, and scalp DHT by 60% to 70%.33 Finasteride preferentially inhibitions SRD5A2 by forming a stable complex with the enzyme in the presence of NADPH (Fig 4). SRD5A2 inhibitors prevent the hydride transfer from NADPH to testosterone responsible for converting testosterone to DHT. The SRD5A2 and finasteride complex have a half-life (t1/2) of approximately 31 days, and DHT takes about 14 days to increase after the discontinuation of finasteride. Hair regrowth stops within 12 months of stopping systemic finasteride.34 A systematic review of randomized controlled trials (RCTs) recommended a combination therapy of minoxidil 2% and systemic finasteride 1 mg, citing its superiority to monotherapies.35
Fig 4.
Mechanism of inhibition by finasteride - covalent adduct between NADPH and finasteride. E57TM2 facilitates the hydride transfer to the Δ1,2 bond of finasteride to the covalent bond in the red circle. The covalent bond prevents a further hydride transfer from NADPH to testosterone and prevents testosterone synthesis to DHT.
(Adapted from Xiao et al25).
Topical finasteride, not FDA-approved but found to be effective, is recommended for females to avoid hormonal side effects.36 It reduces hair loss, improves hair growth and lowers DHT levels.37 While finasteride has shown positive effects, there have been reported drawbacks, leading to a decline in its prescription.38 Studies suggest higher doses may be more effective for female pattern hair loss,39 but caution is required due to the risk of teratogenicity in premenopausal women.40
Finasteride side effects may result from interactions with proteins beyond SRD5A2 inhibition. Recent research discovered that finasteride can bind and inhibit phenylethanolamine N-methyltransferase, an enzyme that controls epinephrine production. This interaction might contribute to systemic side effects like sexual and psychological symptoms.41
Minoxidil
Topical minoxidil is the main treatment for AGA and is also used off-label for other forms of hair loss. It was repurposed as a hair loss treatment when hypertensive patients on oral treatment experienced increased hair growth as a side effect.42 Topical minoxidil is thought to dilate scalp blood vessels, promoting hair growth by improving nutrient delivery to hair follicles.43 It is available in different forms, such as solutions, foam, and shampoo, with the 5% solution being more effective than the 2% solution.44
Recent studies have shown that low-dose oral minoxidil (2.5-5 mg/day for male and 0.25-1.25 mg daily for female AGA) can be safe and effective but should be used with caution in individuals at risk for cardiovascular events.45, 46, 47
Laser therapy
In 1967, a study showed that low-level light/laser therapy (LLLT) using a ruby laser promoted hair growth in mice. LLLT devices were later FDA-cleared in 2007 for men and 2011 for women as a potential treatment for hair loss. LLLT stimulates hair growth by influencing the hair cycle using specific wavelengths between 650 nm and 1200 nm,48 with LLLT red or near-infrared light ranging between 600 and 950 nm and fluences between 2 and 10 Joules per square centimetres (J/cm2), over 15-20 minutes, 3 times a week for 6 months.49
Recent reviews of randomized trials have found that LLLT can increase hair diameter or density compared to sham devices, with minor side effects like dry skin and scalp irritation reported. These findings support the use of LLLT as a treatment option for AGA. However, challenges exist in standardizing treatment parameters, study designs, and assessing long-term outcomes in LLLT studies.50,51
Hair transplantation
Hair follicle transplantation is a surgical procedure that involves removing and transplanting hair follicles from non-androgen sensitive areas to areas affected by AGA. The transplanted follicles do not miniaturize, grow in groups of 1 to 4 hairs and are harvested as units. In 2009, the FDA-approved a robotic hair restoration device to assist surgeons. Later in 2011, the ARTAS system was also approved to harvest curly hair in black men.52 ARTAS robotic hair transplant is a minimally invasive hair restoration system that uses artificial intelligence technology to restore hair faster and more precisely than traditional hair restoration methods.
Non–FDA-approved AGA treatments
Off-label medications and hormonal therapies
Dutasteride is an effective off-label drug for AGA and inhibits SRD5A2,53 and was more effective than finasteride in a meta-analysis of 24 weeks of treatment with comparable side effects.54 In Japan and South Korea, oral dutasteride (0.5 mg/d) has been approved for male AGA.55
Other treatment options for AGA include oral cyproterone and spironolactone, sometimes used off-label in females.56 Cyproterone blocks the androgen receptor, while spironolactone slows androgen production by blocking androgen receptors in target tissues and decreasing testosterone production in the adrenal gland. Flutamide, a nonsteroidal antiandrogen that binds to the AR and blocks the action of testosterone, is not commonly used in the treatment of AGA in either males or females due to adverse side effects, such as liver toxicity.57,58
Phytomedicine
Phytomedicine, the use of plant-based products for medicinal purposes, is popular for treating AGA and can be used as a complementary or alternative treatment. Plants like Serenoa repens, Panax ginseng, Curcuma aeruginosa, Cucurbita pepo and Trifolium pratense59; palm extract (tocotrienol/tocopherol complex), horsetail and ashwagandha have been reported to treat AGA. Although high quality evidence from controled studies are needed these plants are reported to inhibit 5-alpha-reductase, lower cortisol levels, reduce inflammation, promote homeostasis, and maintain collagen stores.60
Injectables
Platelet-rich plasma (PRP) refers to a naturally derived mixture of platelets in a concentrated plasma solution, typically containing over 1,000,000 platelets per microliter or 2-7 times the concentration found in regular blood.61 The application of PRP has been shown to stimulate hair growth, enhance cell survival, and extend the active growth phase (anagen) of the hair cycle.62 It enhances grafting and improves follicular unit survival,63 resulting in hair density and thickness, according to recent meta-analyses of 30 articles with 687 patients using various injection methods.64 However, the efficacy of PRP compared to other treatments for androgenetic alopecia (AGA) remains uncertain due to a lack of standardized protocols, long-term follow-up outcomes, and limited clinical evidence.65
Exosomes
Mesenchymal stem cell-derived exosomes hold great promise in the field of hair restoration, as they contain cytokines and growth factors that promote hair growth.66,67
Research suggests that exosomes derived from dermal papilla cells can accelerate hair follicle growth, delay regression, and reduce hair loss and inflammation in preclinical models.68
Although no clinical trials have been completed yet, anecdotal evidence and case reports suggest promising results69 and initial studies indicate increased hair thickness and density after exosome therapy in individuals with pattern baldness.70
A Korean pilot study showed that exosome therapy improved hair thickness (increased from 57.5 to 64.0 mm, P ≤ .001) and hair density (increased from 105.4 to 122.7 counts/cm2, P ≤ .001) after 12 weeks.71,72 However, further research is needed to assess exosomes' biodistribution, pharmacokinetic profile, and safety.73
Adjuvant therapy
Microneedling, a minimally invasive cosmetic procedure, stimulates the release of growth factors and stem cells, promoting collagen formation and improving topical treatment absorption,74 leading to improved hair density and thickness.75 Case studies demonstrate its effectiveness when combined with therapies like PRP and minoxidil for patients unresponsive to conventional treatments, resulting in improved hair density and thickness.76 Reported side effects include pain, bruising, and folliculitis.77
Camouflage techniques
Camouflage techniques can be helpful in disguising hair loss and boosting self-confidence.78 These methods include temporary solutions like wigs, hair-thickening fibres, and pigmented powders79 as well as semi-permanent options such as scalp micro-pigmentation, which creates the appearance of closely shaved hair follicles through tattooing.80
Future prospects
The discovery of SRD5A2's structure (Fig 5) in 2020 has opened new possibilities for developing more effective AGA treatments. Understanding its function and interactions allows researchers to identify drug targets and design drugs specifically targeting the protein. This breakthrough has the potential to enhance drug efficacy and safety. It also has implications for treating other diseases. The discovery represents a milestone in AGA treatment and promises a brighter future for patients.
Fig 5.
The structure of human SRD5A2. (A), Spheres represent NADP-DHF adduct. L1-6 are the 6 loops connecting the 7 transmembranes (TM), and the TM portion has 254 amino acid residues. (B), The active site inside the 7 TM channels surrounded by L1, L3, and L5, with 2 separate pockets for NADP and DHF (shown in red circle).
(Adapted from Xiao et al25).
New treatments
New insights into AGA's pathogenesis have led to new research prospects. Promising treatments are being developed, including clascoterone, an FDA-approved topical androgen receptor inhibitor initially used for acne. Recent studies indicate clascoterone's potential effectiveness in treating AGA.81 Patients treated with clascoterone 7.5% twice daily for 6 months experienced reduced hair loss and improvement compared to the placebo group and their baseline.82,83
Other upcoming treatments for AGA include Janus kinase inhibitors, which block the immune system response causing hair loss, and stem cell therapies that stimulate hair growth.
Conclusion
Androgenetic alopecia (AGA) poses significant challenges for individuals, particularly among younger males, females, and those seeking treatment. Although limited treatment options currently exist, ongoing research and expanding knowledge about its underlying mechanisms and potential interventions offer hope for improving outcomes for those affected by this condition. As we continue to learn more about AGA and the structure of SRD5A2, we can work toward developing better targeted approaches to managing and treating this common and distressing condition.
Conflicts of interest
None disclosed.
Acknowledgments
Hair and Skin Research Laboratory, University of Cape Town, South Africa.
Footnotes
Funding sources: South African National Research Foundation (NRF) SARChI Chair, Post-doctoral Fellow- PSTD2204041931; Thuthuka Funding Instrument (NRF Rating Track, grant number: TTK170413227114).
IRB approval status: Not applicable.
References
- 1.Paik J.H., Yoon J.B., Sim W.Y., Kim B.S., Kim N.I. The prevalence and types of androgenetic alopecia in Korean men and women. Br J Dermatol. 2001;145:95–99. doi: 10.1046/j.1365-2133.2001.04289.x. [DOI] [PubMed] [Google Scholar]
- 2.Dawber R. Aetiology and pathophysiology of hair loss. Dermatologica. 1987;175:23–28. doi: 10.1159/000248896. [DOI] [PubMed] [Google Scholar]
- 3.Rinaldi F., Marzani B., Pinto D., Sorbellini E. Randomized controlled trial on a PRP-like cosmetic, biomimetic peptides based, for the treatment of alopecia areata. J Dermatol Treat. 2019;30(6):588–593. doi: 10.1080/09546634.2018.1544405. [DOI] [PubMed] [Google Scholar]
- 4.Kabir Y., Goh C. Androgenetic alopecia: update on epidemiology, pathophysiology, and treatment. J Egypt Women's Dermatologic Soc. 2013;10:107–116. [Google Scholar]
- 5.Ntshingila S., Khumalo N., Engel M., Arowolo A. An appraisal of laboratory models of androgenetic alopecia: a systematic review. Skin Health Dis. 2021;1(2):e15. doi: 10.1002/ski2.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trüeb R.M., Lee W.-S. Springer; 2014. Male Aalopecia. [Google Scholar]
- 7.Zirkle C. In: Studies and Essays in the History of Service and Learning in Honour of George Sarton. Montagn M.F.A., editor. Schuman; 1946. The discovery of sex-influenced, sex-limited, and sex-linked heredity; pp. 169–194. [Google Scholar]
- 8.Darwin C.R. Variation of Plants and Animals Under Domestication. John Murray; 1868. 1st issue. Volume 2. [Google Scholar]
- 9.Osborn D. Inheritance of baldness: various patterns due to heredity and sometimes present at birth—a sex-limited character—dominant in man—women not bald unless they inherit tendency from both parents. J Hered. 1916;7:347–355. [Google Scholar]
- 10.Hamilton J.B. Male hormone stimulation is prerequisite and an incitant in common baldness. Am J Anat. 1942;71:451–480. [Google Scholar]
- 11.Hamilton J.B. Patterned loss of hair in man: types and incidence. Ann N Y Acad Sci. 1951;53:708–728. doi: 10.1111/j.1749-6632.1951.tb31971.x. [DOI] [PubMed] [Google Scholar]
- 12.Ludwig E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977;97:247–254. doi: 10.1111/j.1365-2133.1977.tb15179.x. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman K.D. Androgens and alopecia. Mol Cell Endocrinol. 2002;198:89–95. doi: 10.1016/S0303-7207(02)00372-6. [DOI] [PubMed] [Google Scholar]
- 14.Guarrera M., Rebora A. Kenogen in female androgenetic alopecia. Dermatology. 2005;210:18–20. doi: 10.1159/000081477. [DOI] [PubMed] [Google Scholar]
- 15.Kim A.R., Kim S.N., Jung I.K., Kim H.H., Park Y.H., Park W.S. The inhibitory effect of Scutellaria baicalensis extract and its active compound, baicalin, on the translocation of the androgen receptor with implications for preventing androgenetic alopecia. Planta Med. 2014;80:153–158. doi: 10.1055/s-0033-1360300. [DOI] [PubMed] [Google Scholar]
- 16.Messner E.A., Steele T.M., Tsamouri M.M., Hejazi N., Gao A.C., Mudryj M., et al. The androgen receptor in prostate cancer: effect of structure, ligands and spliced variants on therapy. Biomedicines. 2020;8:422. doi: 10.3390/biomedicines8100422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katzer T., Leite Junior A., Beck R., da Silva C. Physiopathology and current treatments of androgenetic alopecia: going beyond androgens and anti-androgens. Dermatol Ther. 2019;32 doi: 10.1111/dth.13059. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Jacobo L., Villarreal-Villarreal C., Ortiz-López R., Ocampo-Candiani J., Rojas-Martínez A. Genetic and molecular aspects of androgenetic alopecia. Indian J Dermatol Venereol Leprol. 2018;84:263–268. doi: 10.4103/ijdvl.IJDVL_262_17. [DOI] [PubMed] [Google Scholar]
- 19.Tan M.H., Li J., Xu H.E., Melcher K., Yong E.L. Androgen receptor: structure, role in prostate cancer and drug discovery. Acta Pharmacol Sin. 2015;36:3–23. doi: 10.1038/aps.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asadi S. The role of mutations on gene AR, in androgenetic alopecia syndrome. Int J Mol Biol Open Access. 2020;5:46–49. [Google Scholar]
- 21.Zhuo F., Xu W., Wang L., Wu Y., Xu Z.L., Zhao J.Y. Androgen receptor gene polymorphisms and risk for androgenetic alopecia: a meta-analysis. Clin Exp Dermatol. 2012;37:104–111. doi: 10.1111/j.1365-2230.2011.04186.x. [DOI] [PubMed] [Google Scholar]
- 22.Yip L., Zaloumis S., Irwin D., et al. Gene-wide association study between the aromatase gene (CYP19A1) and female pattern hair loss. Br J Dermatol. 2009;161:289–294. doi: 10.1111/j.1365-2133.2009.09186.x. [DOI] [PubMed] [Google Scholar]
- 23.Ellis J.A., Stebbing M., Harrap S.B. Polymorphism of the androgen receptor gene is associated with male pattern baldness. J Invest Dermatol. 2001;116:452–455. doi: 10.1046/j.1523-1747.2001.01261.x. [DOI] [PubMed] [Google Scholar]
- 24.Chew E.G., Tan J.H.J., Bahta A.W., et al. Differential expression between human dermal papilla cells from balding and non-balding scalps reveals new candidate genes for androgenetic alopecia. J Invest Dermatol. 2016;136:1559–1567. doi: 10.1016/j.jid.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 25.Xiao Q., Wang L., Supekar S., et al. Structure of human steroid 5α-reductase 2 with the anti-androgen drug finasteride. Nat Commun. 2020;11:1–11. doi: 10.1038/s41467-020-19249-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imperato-McGinley J., Guerrero L., Gautier T., Peterson R.E.J.S. Steroid 5α-reductase deficiency in man: an inherited form of male pseudohermaphroditism. Science. 1974;186:1213–1215. doi: 10.1126/science.186.4170.1213. [DOI] [PubMed] [Google Scholar]
- 27.Srivilai J., Minale G., Scholfield C.N., Ingkaninan K. Discovery of natural steroid 5 alpha-reductase inhibitors. Assay Drug Dev Technol. 2019;17:44–57. doi: 10.1089/adt.2018.870. [DOI] [PubMed] [Google Scholar]
- 28.Azzouni F., Godoy A., Li Y., Mohler J. The 5 alpha-reductase isozyme family: a review of basic biology and their role in human diseases. Adv Urol. 2012;2012 doi: 10.1155/2012/530121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips T.G., Slomiany W.P., Allison R. Hair loss: common causes and treatment. Am Fam Physician. 2017;96:371–378. [PubMed] [Google Scholar]
- 30.Valdebran M., Mo J., Elston D.M., Doan L. Pattern hair loss: assessment of inflammation and fibrosis on histologic sections. J Am Acad Dermatol. 2020;82:757–758. doi: 10.1016/j.jaad.2019.09.013. [DOI] [PubMed] [Google Scholar]
- 31.Alves R. Androgenetic alopecia: a review and emerging treatments. Clin Res Dermatol Open Access. 2017;4:1–13. [Google Scholar]
- 32.Nestor M.S., Ablon G., Gade A., Han H., Fischer D.L. Treatment options for androgenetic alopecia: efficacy, side effects, compliance, financial considerations, and ethics. J Cosmet Dermatol. 2021;20:3759–3781. doi: 10.1111/jocd.14537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez P., Serrano-Falcon C., Torres J.M., Serrano S., Ortega E. 5α-Reductase isozymes and aromatase mRNA levels in plucked hair from young women with female pattern hair loss. Arch Dermatol Res. 2018;310:77–83. doi: 10.1007/s00403-017-1798-0. [DOI] [PubMed] [Google Scholar]
- 34.Lee S.W., Juhasz M., Mobasher P., Ekelem C., Mesinkovska N.A. A systematic review of topical finasteride in the treatment of androgenetic alopecia in men and women. J Drugs Dermatol. 2018;17:457. [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Y., Chen C., Qu Q., et al. The effectiveness of combination therapies for androgenetic alopecia: a systematic review and meta-analysis. Dermatol Ther. 2020;33 doi: 10.1111/dth.13741. [DOI] [PubMed] [Google Scholar]
- 36.Dhariwala M.Y., Ravikumar P. An overview of herbal alternatives in androgenetic alopecia. J Cosmet Dermatol. 2019;18:966–975. doi: 10.1111/jocd.12930. [DOI] [PubMed] [Google Scholar]
- 37.Gupta A.K., Talukder M. Topical finasteride for male and female pattern hair loss: is it a safe and effective alternative? J Cosmet Dermatol. 2022;21:1841–1848. doi: 10.1111/jocd.14895. [DOI] [PubMed] [Google Scholar]
- 38.Goren A., Naccarato T. Minoxidil in the treatment of androgenetic alopecia. Dermatol Ther. 2018;31 doi: 10.1111/dth.12686. [DOI] [PubMed] [Google Scholar]
- 39.Iamsumang W., Leerunyakul K., Suchonwanit P. Finasteride and its potential for the treatment of female pattern hair loss: evidence to date. Drug Des Devel Ther. 2020;14:951–959. doi: 10.2147/dddt.S240615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oliveira-Soares R., Andre M.C., Peres-Correia M. Adverse effects with finasteride 5 mg/day for patterned hair loss in premenopausal women. Int J Trichology. 2018;10:48–50. doi: 10.4103/ijt.ijt_73_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giatti S., Di Domizio A., Diviccaro S., et al. Three-dimensional proteome-wide scale screening for the 5-alpha reductase inhibitor finasteride: identification of a novel off-target. J Med Chem. 2021;64:4553–4566. doi: 10.1021/acs.jmedchem.0c02039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bryan J. How minoxidil was transformed from an antihypertensive to hair-loss drug. Pharm J. 2011;287:137. [Google Scholar]
- 43.Choi N., Shin S., Song S.U., Sung J.-H. Minoxidil promotes hair growth through stimulation of growth factor release from adipose-derived stem cells. Int J Mol Sci. 2018;19:691. doi: 10.3390/ijms19030691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pekmezci E., Turkoglu M., Gokalp H., Kutlubay Z. Minoxidil downregulates interleukin-1 alpha gene expression in HaCaT cells. Int J Trichology. 2018;10:108–112. doi: 10.4103/ijt.ijt_18_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lueangarun S., Panchaprateep R., Tempark T., Noppakun N. Efficacy and safety of oral minoxidil 5 mg daily during 24-week treatment in male androgenetic alopecia. J Am Acad Dermatol. 2015;72:638. [Google Scholar]
- 46.Adil A., Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:136–141.e5. doi: 10.1016/j.jaad.2017.02.054. [DOI] [PubMed] [Google Scholar]
- 47.Gupta A.K., Mays R.R., Dotzert M.S., Versteeg S.G., Shear N.H., Piguet V. Efficacy of non-surgical treatments for androgenetic alopecia: a systematic review and network meta-analysis. J Eur Acad Dermatol Venereol. 2018;32:2112–2125. doi: 10.1111/jdv.15081. [DOI] [PubMed] [Google Scholar]
- 48.Lueangarun S., Visutjindaporn P., Parcharoen Y., Jamparuang P., Tempark T. Systematic review and meta-analysis of randomized controlled trials of United States Food and drug Administration-approved, home-use, low-level light/laser therapy devices for pattern hair loss: device design and technology. J Clin Aesthet Dermatol. 2021;14:E64–E75. [PMC free article] [PubMed] [Google Scholar]
- 49.Pillai J.K., Mysore V. Role of low-level light therapy (LLLT) in androgenetic alopecia. J Cutan Aesthet Surg. 2021;14:385–391. doi: 10.4103/jcas.Jcas_218_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Egger A., Resnik S.R., Aickara D., et al. Examining the safety and efficacy of low-level laser therapy for male and female pattern hair loss: a review of the literature. Skin Appendage Disord. 2020;6:259–267. doi: 10.1159/000509001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Afifi L., Maranda E.L., Zarei M., et al. Low-level laser therapy as a treatment for androgenetic alopecia. Laser Surg Med. 2017;49:27–39. doi: 10.1002/lsm.22512. [DOI] [PubMed] [Google Scholar]
- 52.Revene A. Hair Transplant: Procedures, Complications and Tips. https://myhealthguide.org/hair-transplant/
- 53.Arif T., Dorjay K., Adil M., Sami M. Dutasteride in androgenetic alopecia: an update. Curr Clin Pharmacol. 2017;12:31–35. doi: 10.2174/1574884712666170310111125. [DOI] [PubMed] [Google Scholar]
- 54.Zhou Z., Song S., Gao Z., Wu J., Ma J., Cui Y. The efficacy and safety of dutasteride compared with finasteride in treating men with androgenetic alopecia: a systematic review and meta-analysis. Clin Interv Aging. 2019;14:399. doi: 10.2147/CIA.S192435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gupta A.K., Talukder M., Williams G. Comparison of oral minoxidil, finasteride, and dutasteride for treating androgenetic alopecia. J Dermatol Treat. 2022;33:2946–2962. doi: 10.1080/09546634.2022.2109567. [DOI] [PubMed] [Google Scholar]
- 56.Levy L.L., Emer J.J. Female pattern alopecia: current perspectives. Int J Womens Health. 2013;5:541–556. doi: 10.2147/ijwh.S49337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson D.B., Sonthalia S. StatPearls [Internet] StatPearls Publishing; 2021. [Google Scholar]
- 58.Shaw J.C. Antiandrogen therapy in dermatology. Int J Dermatol. 1996;35:770–778. doi: 10.1111/j.1365-4362.1996.tb02970.x. [DOI] [PubMed] [Google Scholar]
- 59.Zgonc Škulj A., Poljšak N., Kočevar Glavač N., Kreft S. Herbal preparations for the treatment of hair loss. Arch Dermatol Res. 2020;312:395–406. doi: 10.1007/s00403-019-02003-x. [DOI] [PubMed] [Google Scholar]
- 60.Farris P.K., Rogers N., McMichael A., Kogan S. A novel multi-targeting approach to treating hair loss, using standardized nutraceuticals. J Drugs Dermatol. 2017;16:s141–s148. [PubMed] [Google Scholar]
- 61.Stevens J., Khetarpal S. Platelet-rich plasma for androgenetic alopecia: a review of the literature and proposed treatment protocol. Int J Womens Dermatol. 2019;5:46–51. doi: 10.1016/j.ijwd.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gupta A.K., Cole J., Deutsch D.P., et al. Platelet-rich plasma as a treatment for androgenetic alopecia. Dermatol Surg. 2019;45:1262–1273. doi: 10.1097/DSS.0000000000001894. [DOI] [PubMed] [Google Scholar]
- 63.Gentile P., Garcovich S., Bielli A., Scioli M.G., Orlandi A., Cervelli V. The effect of platelet-rich plasma in hair regrowth: a randomized placebo-controlled trial. Stem Cells Transl Med. 2015;4:1317–1323. doi: 10.5966/sctm.2015-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Evans A.G., Mwangi J.M., Pope R.W., et al. Platelet-rich plasma as a therapy for androgenic alopecia: a systematic review and meta-analysis. J Dermatol Treat. 2022;33:498–511. doi: 10.1080/09546634.2020.1770171. [DOI] [PubMed] [Google Scholar]
- 65.Giordano S., Romeo M., di Summa P., Salval A., Lankinen P. A meta-analysis on evidence of platelet-rich plasma for androgenetic alopecia. Int J Trichology. 2018;10:1–10. doi: 10.4103/ijt.ijt_74_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Panda B., Sharma Y., Gupta S., Mohanty S. Mesenchymal stem cell-derived exosomes as an emerging paradigm for regenerative therapy and Nano-Medicine: a comprehensive review. Life. 2021;11:784. doi: 10.3390/life11080784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chahla J., Cinque M.E., Piuzzi N.S., et al. A call for standardization in platelet-rich plasma preparation protocols and composition reporting: a systematic review of the clinical orthopaedic literature. J Bone Joint Surg Am. 2017;99:1769–1779. doi: 10.2106/JBJS.16.01374. [DOI] [PubMed] [Google Scholar]
- 68.Kost Y., Muskat A., Mhaimeed N., Nazarian R.S., Kobets K. Exosome therapy in hair regeneration: a literature review of the evidence, challenges, and future opportunities. J Cosmet Dermatol. 2022;21:3226–3231. doi: 10.1111/jocd.15008. [DOI] [PubMed] [Google Scholar]
- 69.Krane N.A., Christofides E.A., Halaas Y. Advances in hair restoration. Curr Otorhinolaryngol Rep. 2021;9:436–441. [Google Scholar]
- 70.Gupta A.K., Renaud H.J., Halaas Y., Rapaport J.A. Exosomes: a new effective non-surgical therapy for androgenetic alopecia? Skinmed. 2020;18:96–100. [PubMed] [Google Scholar]
- 71.Huh C.-H., Kwon S.H. Exosome for hair regeneration: from bench to bedside. J Am Acad Dermatol. 2019;81:AB62. Mosby-Elsevier. [Google Scholar]
- 72.Yuan A.-R., Bian Q., Gao J.-Q. Current advances in stem cell-based therapies for hair regeneration. Eur J Pharmacol. 2020;881 doi: 10.1016/j.ejphar.2020.173197. [DOI] [PubMed] [Google Scholar]
- 73.Food, U. & Administration, D. (Food and Drug Administration, 2019).
- 74.Ocampo-Garza S.S., Fabbrocini G., Ocampo-Candiani J., Cinelli E., Villani A. Micro needling: a novel therapeutic approach for androgenetic alopecia, A review of literature. Dermatol Ther. 2020;33 doi: 10.1111/dth.14267. [DOI] [PubMed] [Google Scholar]
- 75.Dhurat R., Sukesh M., Avhad G., Dandale A., Pal A., Pund P. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6. doi: 10.4103/0974-7753.114700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Faghihi G., Nabavinejad S., Mokhtari F., Fatemi Naeini F., Iraji F. Microneedling in androgenetic alopecia; comparing two different depths of microneedles. J Cosmet Dermatol. 2021;20:1241–1247. doi: 10.1111/jocd.13714. [DOI] [PubMed] [Google Scholar]
- 77.Neerja P. A study on the efficacy of microneedling with minoxidil solution versus microneedling with hair multivitamin solution for the treatment of androgenetic alopecia. Int J Dermatol Clin Res. 2020;6:10–12. [Google Scholar]
- 78.Daruwalla S.B., Dhurat R.S., Hamid S.A.T. All that a dermatotrichologist needs to know about hair camouflage: a comprehensive review. Int J Trichology. 2022;14:77–83. doi: 10.4103/ijt.ijt_6_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saed S., Ibrahim O., Bergfeld W.F. Hair camouflage: a comprehensive review. Int J Womens Dermatol. 2017;3:S75–S80. doi: 10.1016/j.ijwd.2017.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dhurat R.S., Shanshanwal S.J.S., Dandale A.L. Standardization of SMP procedure and its impact on outcome. J Cutan Aesthet Surg. 2017;10:145–149. doi: 10.4103/jcas.Jcas_116_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hebert A., Thiboutot D., Stein Gold L., et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial Acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621–630. doi: 10.1001/jamadermatol.2020.0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rosette C., Rosette N., Mazzetti A., Moro L., Gerloni M. Cortexolone 17α-propionate (clascoterone) is an androgen receptor Antagonist in dermal papilla cells in vitro. J Drugs Dermatol. 2019;18:197–201. [PubMed] [Google Scholar]
- 83.Sun H.Y., Sebaratnam D.F. Clascoterone as a novel treatment for androgenetic alopecia. Clin Exp Dermatol. 2020;45:913–914. doi: 10.1111/ced.14292. [DOI] [PubMed] [Google Scholar]