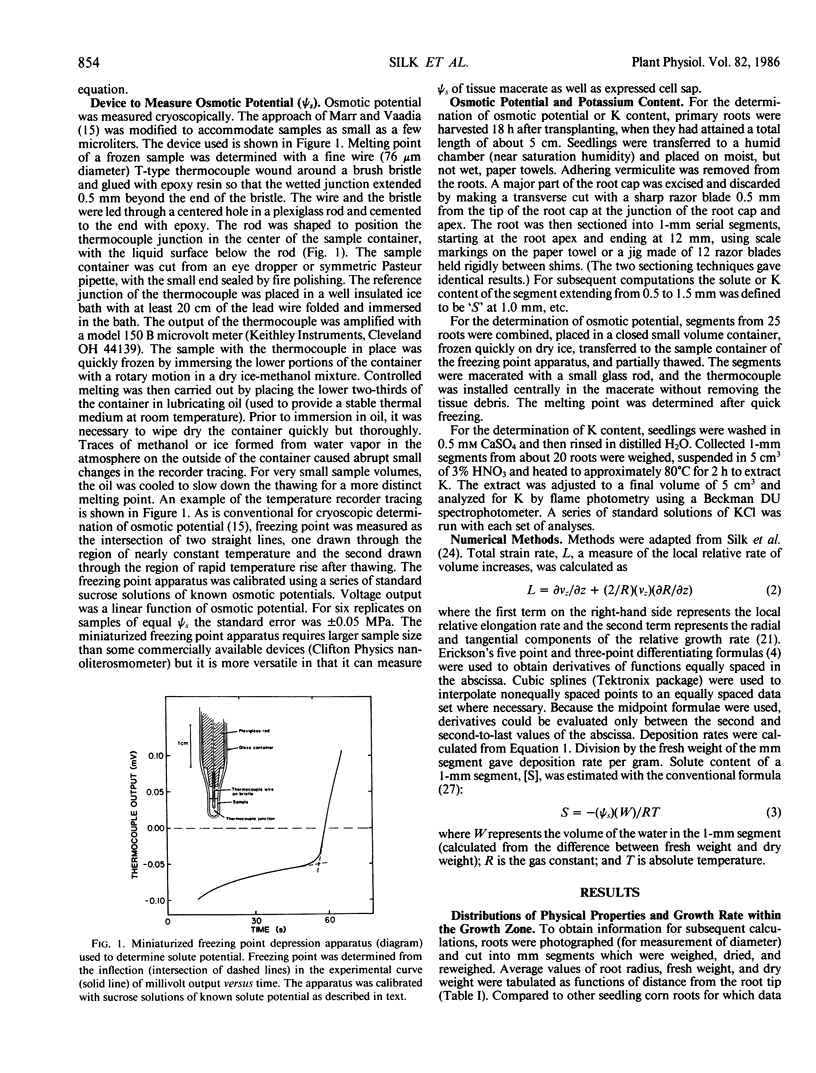
Abstract
Densities of osmoticum and potassium were measured as a function of distance from the tip of the primary root of Zea mays L. (cv WF9 × mo17). Millimeter segments were excised and analyzed for osmotic potential by a miniaturized freezing point depression technique, and for potassium by flame spectrophotometry. Local deposition rates were estimated from the continuity equation with values for density and growth velocity. Osmotic potential was uniform, −0.73 ± 0.05 megapascals, throughout the growth zone of well-watered roots. Osmoticum deposition rate was 260 μosmoles per gram fresh weight per hour. Potassium density fell from 117 micromoles per gram in the first mm region to 48 micromoles per gram at the base of the growth zone. Potassium deposition rates had a maximum of 29 micromoles per gram per hour at 3.5 millimeters from the tip and were positive (i.e. potassium was being added to the tissue) until 8 millimeters from the tip. The results are discussed in terms of ion relations of the growing zone and growth physics.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck W. A. PRODUCTION OF SOLUTES IN GROWING EPIDERMAL CELLS. Plant Physiol. 1941 Jul;16(3):637–642. doi: 10.1104/pp.16.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D. J. Cell wall yield properties of growing tissue : evaluation by in vivo stress relaxation. Plant Physiol. 1985 Jun;78(2):347–356. doi: 10.1104/pp.78.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman G. L., LeWinter M. M. Pericardial adaptations during chronic cardiac dilation in dogs. Circ Res. 1984 Mar;54(3):294–300. doi: 10.1161/01.res.54.3.294. [DOI] [PubMed] [Google Scholar]
- Giaquinta R. T. Phloem loading of sucrose: involvement of membrane ATPase and proton transport. Plant Physiol. 1979 Apr;63(4):744–748. doi: 10.1104/pp.63.4.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J. B., Kahn J. S. The Kinetics of Potassium Accumulation by Corn Roots as a Function of Cell Maturity. Plant Physiol. 1957 Sep;32(5):497–498. doi: 10.1104/pp.32.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart J. A. An analysis of irreversible plant cell elongation. J Theor Biol. 1965 Mar;8(2):264–275. doi: 10.1016/0022-5193(65)90077-9. [DOI] [PubMed] [Google Scholar]
- Marr A. G., Vaadia Y. Rapid cryoscopic technique for measuring osmotic properties of drop size samples. Plant Physiol. 1961 Sep;36(5):677–680. doi: 10.1104/pp.36.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk W. K., Erickson R. O. Local biosynthesis rates of cytoplasmic constituents in growing tissue. J Theor Biol. 1980 Apr 21;83(4):701–703. doi: 10.1016/0022-5193(80)90197-6. [DOI] [PubMed] [Google Scholar]
- Silk W. K., Walker R. C., Labavitch J. Uronide Deposition Rates in the Primary Root of Zea mays. Plant Physiol. 1984 Mar;74(3):721–726. doi: 10.1104/pp.74.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward F. C., Prevot P., Harrison J. A. ABSORPTION AND ACCUMULATION OF RUBIDIUM BROMIDE BY BARLEY PLANTS. LOCALIZATION IN THE ROOT OF CATION ACCUMULATION AND OF TRANSFER TO THE SHOOT. Plant Physiol. 1942 Jul;17(3):411–421. doi: 10.1104/pp.17.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii K., Laties G. G. Dual mechanisms of ion uptake in relation to vacuolation in corn roots. Plant Physiol. 1966 May;41(5):863–870. doi: 10.1104/pp.41.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]


