Abstract
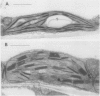
Morphometric, electrophoretic, and immunological procedures were used to probe the structural and physiological differences between triazine-resistant (R) and susceptible (S) isolines of canola (Brassica napus L.). The R biotype exhibited increased grana stacking and decreased amounts of starch compared to the S biotype. Likewise, characters associated with an increase in grana stacking (lower chlorophyll a/b ratio, increased chlorophyll a/b light-harvesting complex, and relatively lower amounts of the P700 chlorophyll a protein and chloroplast coupling factor) were all observed in the R isoline of canola. Proteins which occur with approximately equal frequency in grana and stroma lamellae (plastocyanin, cutochrome f) or present only in the stroma (ribulose 1,5-bisphosphate carboxylase/oxygenase) were not quantitatively different in the two biotypes. Gross anatomical parameters (volume of epidermis, palisade mesophyll, spongy mesophyll, and air space) were similar in the two isolines. Thus, the triazine-resistance mutation does not confer a shade-type anatomy despite the chloroplast changes that are characteristic of shade biotypes or shade adaptions. These data indicate that the differences in chloroplast structure noted previously in comparisons of nonisonuclear R and S weed biotypes reflect differences in the triazineresistance factor rather than characters unrelated to triazine resistance.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burke J. J., Wilson R. F., Swafford J. R. Characterization of Chloroplasts Isolated from Triazine-Susceptible and Triazine-Resistant Biotypes of Brassica campestris L. Plant Physiol. 1982 Jul;70(1):24–29. doi: 10.1104/pp.70.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg J., McIntosh L. Molecular Basis of Herbicide Resistance in Amaranthus hybridus. Science. 1983 Dec 23;222(4630):1346–1349. doi: 10.1126/science.222.4630.1346. [DOI] [PubMed] [Google Scholar]
- Kyle D. J., Staehelin L. A., Arntzen C. J. Lateral mobility of the light-harvesting complex in chloroplast membranes controls excitation energy distribution in higher plants. Arch Biochem Biophys. 1983 Apr 15;222(2):527–541. doi: 10.1016/0003-9861(83)90551-9. [DOI] [PubMed] [Google Scholar]
- Mattoo A. K., St John J. B., Wergin W. P. Adaptive reorganization of protein and lipid components in chloroplast membranes as associated with herbicide binding. J Cell Biochem. 1984;24(2):163–175. doi: 10.1002/jcb.240240207. [DOI] [PubMed] [Google Scholar]
- Pillai P., John J. B. Lipid composition of chloroplast membranes from weed biotypes differentially sensitive to triazine herbicides. Plant Physiol. 1981 Sep;68(3):585–587. doi: 10.1104/pp.68.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinback K. E., McIntosh L., Bogorad L., Arntzen C. J. Identification of the triazine receptor protein as a chloroplast gene product. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7463–7467. doi: 10.1073/pnas.78.12.7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierling E., Alberte R. S. P(700) Chlorophyll a-Protein : Purification, Characterization, and Antibody Preparation. Plant Physiol. 1983 Jul;72(3):625–633. doi: 10.1104/pp.72.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]