Highlights
-
•
Focal lesions like stroke and tumors cause highly-impacting cognitive impairment.
-
•
Pathophysiological mechanism (acute vs low-growing) affects differently the brain.
-
•
Focal lesions show a low-dimensional characterization of cognitive impairment.
-
•
Lesion location is correlated with cognitive deficits only in stroke, not in tumor.
Keywords: Stroke, Neuro-oncology, Neuropsychology, Cognition, Neural networks
Abstract
Introduction
Neuropsychological studies infer brain-behavior relationships from focal lesions like stroke and tumors. However, these pathologies impair brain function through different mechanisms even when they occur at the same brain’s location. The aim of this study was to compare the profile of cognitive impairment in patients with brain tumors vs. stroke and examine the correlation with lesion location in each pathology.
Methods
Patients with first time stroke (n = 77) or newly diagnosed brain tumors (n = 76) were assessed with a neuropsychological battery. Their lesions were mapped with MRI scans. Test scores were analyzed using principal component analysis (PCA) to measure their correlation, and logistic regression to examine differences between pathologies. Next, with ridge regression we examined whether lesion features (location, volume) were associated with behavioral performance.
Results
The PCA showed a similar cognitive impairment profile in tumors and strokes with three principal components (PCs) accounting for about half of the individual variance. PC1 loaded on language, verbal memory, and executive/working memory; PC2 loaded on general performance, visuo-spatial attention and memory, and executive functions; and, PC3 loaded on calculation, reading and visuo-spatial attention. The average lesion distribution was different, and lesion location was correlated with cognitive deficits only in stroke. Logistic regression found language and calculation more affected in stroke, and verbal memory and verbal fluency more affected in tumors.
Conclusions
A similar low dimensional set of behavioral impairments was found both in stroke and brain tumors, even though each pathology caused some specific deficits in different domains. The lesion distribution was different for stroke and tumors and correlated with behavioral impairment only in stroke.
1. Introduction
Research in neuropsychology has traditionally focused on the behavioral effects of focal brain injuries, especially stroke and brain tumors. Typically, the lesions caused by these different pathologies are combined in group analyses to examine the anatomical basis of behavioral deficits. However, it is unknown if tumors and strokes affect the same cognitive functions, even when the lesion is at the same location. The pathophysiology of tumors and strokes is different. Strokes cause an acute (minutes, hours) disruption of local neuronal activity through a loss of blood flow. Tumors in contrast grow slowly (weeks, months) and displace neural structures affecting function through different mechanisms (e.g., oedema, mechanical pressure, neuroinflammation). The relative slow growth may induce in theory an adaptation, and through the recruitment of neural plasticity mechanisms a different pattern of deficits (Klein et al., 2012).
There have been only a handful of studies that have directly compared the behavioral deficits induced by these two kinds of focal lesions. Anderson et al. (1990) found that tumors caused less severe and less specific deficits for the site of damage. Cipolotti et al. (2015) examined executive tasks in frontal lesions, and found no significant difference in performance among strokes, high grade gliomas, low grade gliomas and meningiomas. A recent study by van Grinsven et al. (2023) used a lesion-symptom mapping approach to examine the influence of the etiology (stroke, tumor) in the localization of verbal memory and verbal fluency. They found substantial differences in lesion volume and topography between the groups. Despite clear differences in lesion topography, the cognitive profile was quite similar at the group-level. However, different neuroanatomical correlates were found in the two pathologies.
Here we re-examine the issue of cognitive impairment in stroke vs. brain tumors by testing the hypothesis that these two kinds of focal lesions will produce a low dimensional pattern of correlated behavioral deficits analogously to what we recently reported in stroke (Corbetta et al., 2015, Bisogno et al., 2021). While traditionally teaching in neurology and neuropsychology emphasize the occurrence of many different cognitive syndromes, each with its own specific localization, more recent studies clearly show that cognitive deficits post-stroke are strongly correlated within and between functional domains across many patients. Accordingly, a small number of behavioral factors or components capture large fractions of inter-individual variability in cognitive performance. This low dimensionality has been shown with the National Institute of Health Stroke Scale (NIHSS; Lyden et al., 2004, Zandieh et al., 2012) a scale that measures cognition cursorily, but also with more in-depth experimental (Corbetta et al., 2015) or more clinical neurobehavioral assessments (Massa et al., 2015, Bisogno et al., 2021. Notably, these components accurately describe recovery of function and chronic impairment (Ramsey et al., 2017) representing robust behavioral phenotypes for large scale studies. The low dimensional organization of behavioral impairment corresponds to a low dimensional pattern of structural and functional connectivity abnormalities at the whole brain level (Corbetta et al., 2018, Thiebaut de Schotten et al., 2020, Salvalaggio et al., 2020).
Based on the above studies, we compared neuropsychological performance across multiple domains (language, memory, attention, executive function) in a prospective series of stroke and brain tumors asking the following questions. First, will the pattern of behavioral impairment be similar in stroke and tumors? To study the correlation among neuropsychological tests, we used principal component analysis (PCA) to find possible patterns of inter-test and inter-subject correlation. Second, is lesion location strongly predictive of behavioral deficits in tumor at the individual level, as previously found in stroke? (Corbetta et al., 2015, Salvalaggio et al., 2020, Karnath et al., 2004). To address the issue, we apply ridge regression to compare the degree of correlation between behavioral deficits and lesion location in stroke and brain tumors.
The discovery of similar vs. different patterns of cognitive impairment in stroke and tumors is theoretically and clinically relevant. Theoretically, it is important to ask whether stroke and tumors can be interchangeably used as examples of focal lesions, and whether the low dimensionality of behavioral impairment found in stroke generalizes to another pathology. Clinically, with improvements in acute stroke care and surgical treatment of brain tumors, cognitive deficits are increasingly recognized causes of long-term disability in stroke (Kruithof et al., 2016, Wassenius et al., 2020, Silva et al., 2021) and tumors (Hansen et al., 2021). A definition of behavioral phenotypes would be helpful to plan pharmacological and rehabilitation interventions.
2. Material and methods
2.1. Subjects
We enrolled patients with brain tumors (n = 76) and patients with first symptomatic ischemic or haemorrhagic stroke (n = 133) admitted to the Neurology and Neurosurgery Unit of Padua University Hospital from December 2017 to February 2019. For all patients the exclusion criteria were as follows: 1. Age under 18; 2. Prior history of neurological or psychiatric disorders; 3. Previous central nervous system surgeries; 4. Presence of other medical conditions that precluded active participation in research and/or might alter the interpretation of the behavioral/imaging studies. In the case of brain tumors, additional exclusion criteria were metastases and recurrences. In the case of stroke, additional exclusion criteria were more than two clinically silent lacunes, <15 mm in size on CT scan, and multifocal strokes. To make the two cohorts comparable from a neuropsychological perspective, we included only patients who were able to complete all tests of the neuropsychological assessment. All tumor patients were able to successfully complete the neuropsychological battery. A significant number of stroke patients failed to complete the battery for the following reasons: 19 patients for severe aphasia; 8 for hemiplegia of dominant upper limb that prevented the administration of paper-and-pencil tests; 11 for both severe aphasia and hemiplegia; 4 for insufficient knowledge of Italian language; 5 for refusing to complete the whole battery; 9 for early transfer to another rehabilitation hospital. The final numbers of patients in the two cohorts were as follows: tumors n = 76 and stroke n = 77. Therefore, our stroke cohort represents a sample of patients with milder deficits.
This is a retrospective and non-interventional study. This study received approval from the Ethics Committee of the Azienda Ospedale Università Padova (Protocol Number 70n/AO/20). We obtained a waiver for written informed consent since all data were collected retrospectively and anonymously.
2.2. Neuropsychological assessment
The neuropsychological assessment tested different cognitive domains. Specifically, we employed (1) the Oxford Cognitive Screen (OCS; Demeyere et al., 2015): a brief screening tool composed of tests of language, visual attention, spatial neglect, praxis abilities, visual and verbal memory, calculation, number reading and executive functions; (2) subtests of the Esame Neuropsicologico Breve 2 (Mondini et al., 2011): the Trail Making Test (TMT), forms A and B (selective attention and switching ability), Phonemic fluency, Prose memory immediate and delay recall, and Memory interference test; (3) Boston Naming Test (BNT, visual naming); (4) forward and backward Digit span; and, (5) forward and backward Corsi block-tapping test (short-term and working memory).
Subjects in the stroke cohort were tested within two weeks from their event; subjects in the tumor cohort were also tested within two weeks of their hospitalization for a first clinical manifestation (e.g., confusion, focal deficit, or seizures) and prior to surgery.
2.3. Imaging
For each patient, an MRI or CT scan was collected. The ITK-snap imaging software system (version 3.6.0; Yushkevich et al., 2006) was used to manually segment lesions. All lesions were segmented by two of the authors (SF segmented tumor lesions, AB segmented stroke lesions) and were checked by a board-certified neuro-radiologist (author MGA). For stroke, either the CT (n. 14) or the Fluid Attenuated Inversion Recovery (FLAIR) sequence (n. 63) were used and segmented lesions were mapped on the 2 mm version of the Montreal Neurological Institute 152 6th generation atlas (Grabner et al., 2006) using the Clinical Toolbox of the SPM software system (Rorden et al., 2012). For tumor lesions, 3D T1w, FLAIR, and T2w sequences were used to manually segment the tumor lesion core and the oedema region separately. Segmented lesions were mapped on the same atlas using the Advanced Normalization Toolbox (ANTs; Avants et al., 2011). The FMRIB Software Library (FSL; Jenkinson et al., 2012) was used to create a frequency map of individual lesions on the MNI152 atlas, thus producing an overlay map of all lesions for stroke, tumor core, and tumor core plus oedema.
2.4. Behavioral analysis
Firstly, we applied a dimensionality reduction using principal component analysis (PCA) to the behavioral scores (Turken and Dronkers, 2011). PCA is a technique that identifies hidden variables or factors that capture the correlation of behavioral scores across subjects. Since behavioral scores were expected to be correlated, an oblique rotation was used to maximize the segregation in different components as in previous work. A priori we set out to look at the first three components, based on prior stroke studies in which three components capture the majority of variance (Corbetta et al., 2015, Bisogno et al., 2021).
Secondly, a logistic regression model was run to test the discriminating power of neuropsychological tests in differentiating the two cognitive profiles. The model aim is to distinguish observations into two categories (Stock and Watson, 2015). In the model, these variables were controlled: age, gender, education, side of lesion (right vs. left).
2.5. Anatomical-clinical correlation
The second step involved modelling of the behavioral scores in relation to the anatomical lesions. To be consistent with our previous studies (Corbetta et al., 2015, Salvalaggio et al., 2020, Bisogno et al., 2021, Pini et al., 2021) we performed a ridge regression (RR) analysis (Hoerl and Kennard, 1970). This method is applied when the number of predictors is high as compared to the number of subjects. The aim of the lesion-behavior analysis was to use lesion information in terms of voxel-wise damage (location, volume) to explain behavioral deficits described in terms of behavioral PCs previously calculated. The predictor used was a binary matrix of lesioned voxels (for each subject and for each voxel, the entry of the matrix is set to 1 if the voxel is lesioned and 0 otherwise). The RR results furnished a map of weights linking lesion locations with behavioral deficits at the level of individual subjects. For example, a positive weight assigned to a voxel indicates that a lesion in such a voxel is likely related to the deficit appearance. It also provides an estimate of how that model accounts for the behavioral variability across subjects, in terms of variance explained (R2). Consistently with our previous papers (Corbetta et al., 2015, Salvalaggio et al., 2020, Favaretto et al., 2022) we did not use a nested validation loop. The main rationale for using a nested cross-validation loop would be to ensure generalization of results to a different data set, and it would be necessary to predict behavioral PCs from lesions in new subjects. However, our main goal here is to establish whether lesions can explain behavioral PCs within the current data set (without aiming to generalize results to other data sets). We first applied a spatial PCA, and we used as regressors only the first PCs, which explained at least 95% of the original variance.
Thus, for each of the behavioral PCs, we estimated the model weights vector as:
where is the predictors matrix ( is the number of subjects and is the number of selected spatial PCs), after z-scoring with respect to the whole matrix; is the transpose of , is the vector of the outcome variable to be predicted (i.e. the selected behavioral PC score, after z-scoring), is the identity matrix of dimension , and is the regularization parameter, optimized as follows.
For each of the three RR models, the regularization parameter was optimized by identifying a value within , with 200 logarithmic steps. For each of these 200 values of , each RR model was trained and tested using a leave-one-out cross validation loop (LOOCV). In each loop, the optimal () value was the one that minimized the prediction error over the training set.
Model accuracy was assessed through the coefficient of determination :
where represents the -th element of vector , and is its prediction.
The statistical significance of each model was estimated through a permutation test, with iterations. For each iteration, the behavioral scores were randomly permuted across subjects, and the LOOCV with optimization was used to fit the RR model to the randomized score. The p-value for the observed was defined as the probability of the of the randomized dataset to be larger than the observed (models with p-value < 0.05 were considered statistically significant).
To obtain the optimal set of RR model weights , the average weights across the loops at was considered. The distribution of weights obtained with the permutation test was used as null distribution to select the statistically significant weights. Only the that fall at the left or right ends (2.5%) of the tails of the null distribution were considered significant. These selected weights were back projected to the brain to display a map of the most predictive lesioned voxels. Finally, Gaussian smoothing (variance = 1) and scaling within was applied on the maps. Weights lower than 0.05 in absolute values were not shown.
RR models were employed to map lesion-behavioral deficits in both cohorts (brain tumor and stroke) separately. We repeated the RR estimation for the tumor patients’ cohort, considering the tumor core portion or the combination of both tumor core and oedema. The RR weights obtained for the tumor core as behavioral predictor were compared to the RR weights estimated when considering the core plus the oedema region. This analysis examined whether the oedema region is behaviorally relevant.
Statistics and Machine Learning Toolbox (https://it.mathworks.com/products/statistics.html) as implemented for Matlab R2018b was used for all statistical analysis.
3. Results
3.1. Demographic characteristics of the sample
The stroke sample (n = 77) included n = 8 haemorrhagic and n = 69 ischemic. The main risk factors identified in the medical history were hypertension (n = 53), smoke (n = 22), diabetes (n = 14), atrial fibrillation (n = 7). The mean NIHSS score was 2.08 (SD = 2.04; range = 0–9). Most stroke patients were mild in severity (80% of patients had NIHSS score < 4). 75% of patients showed at least one motor symptom (arm weakness, leg weakness, facial palsy, or dysarthria). The tumor cohort consisted of n = 16 meningiomas and n = 60 gliomas, of which 7 low grade and 53 high grade.
The stroke sample was slightly older than the oncological sample (t(1 5 0) = −2.44; p = 0.016), whereas the two groups were similar in terms of mean education (t(1 4 7) = −1.81; p = 0.07). Gender differences were similar with an overall majority of males affected (χ2(1) = 2.41; p = 0.12). Handedness did not differ among the two cohorts (χ2(1) = 2.38; p = 0.21) (Table 1).
Table 1.
Demographic characteristics for neuro-oncological and stroke cohorts.
|
Brain tumors N = 76 |
Stroke N = 77 |
Statistical differences | |
|---|---|---|---|
| Mean age (SD) | 59.6 (13.4) | 65.4 (13.9) | T = −2.44; p = 0.016 |
| Mean education (SD) | 10.4 (3.7) | 11.6 (4.4) | T = −1.81; p = 0.07 |
| Gender Male Female |
41 35 |
51 26 |
χ2 = 2.41; p = 0.12 |
| Handedness Right Left |
73 3 |
68 9 |
χ2 = 2.38; p = 0.21 |
| WHO 2016 Classification Meningioma II Oligodendroglioma II Astrocytoma III Gliosarcoma IV Astrocytoma IDH-WT IV Glioblastoma IDH-WT IV Glioblastoma IDH-M |
16 2 5 6 10 32 5 |
– |
Group differences were tested with a T-Test or a Pearson-Fisher χ2 test; significant difference was found in mean age only.
3.2. Principal components of behavioral impairment
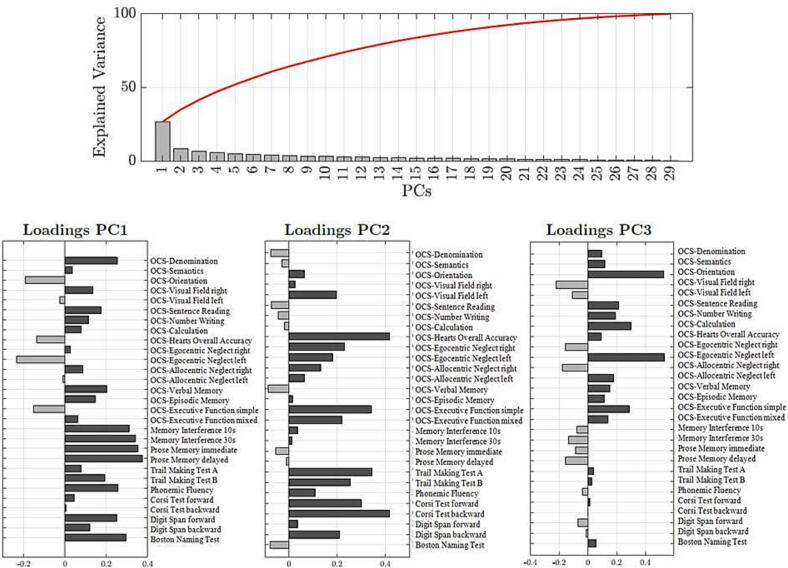
To directly compare the behavioral impairment in stroke and tumors, the PCA was run on the whole sample (n = 153) yielding three main factors that explained 41.5% of the variance (Fig. 1). We considered three PCs with oblique rotation as we assumed some correlation among tests. Positive loadings indicate lower performance, while negative loadings indicate higher performance. PC1 accounted for 25% of the variance, PC2 for 9% of the variance and PC3 for 7.5% of the variance. PC1 loaded on language, verbal memory, and executive/working memory (OCS-denomination, OCS-sentence reading, OCS-number writing, OCS-verbal memory, OCS-episodic memory, OCS-visual field right, Memory intereference 10 s, Memory interference 30 s, Prose memory immediate, Prose memory delayed, Phonemic fluency, Digit span forward and backward, Boston Naming Test, TMT form B); PC2 loaded on visuo-spatial attention, working memory, and executive functions (OCS-visual field left, OCS-hearts overall accuracy, OCS-egocentric and allocentric neglect, OCS-executive function, TMT form A and B, Corsi Test forward and backward, Digit span backward); PC3 loaded on calculation, reading and visuospatial attention (OCS-orientation, OCS-number writing, OCS-sentence reading, OCS-calculation, OCS-egocentric neglect left, OCS-executive function). A mixed measure ANOVA confirmed that the PC loadings were not significantly different in the tumor and stroke cohorts (F(2,296) = 1.47; p = 0.23).
Fig. 1.
Screening plot of explained variance in the total sample. The principal component analysis run on neuropsychological data revealed 29 components in the performance of the total population (n = 153). In the sample, the first three components explained the 41.5% of the total variance. The red line represents the sum of the percentages of the variance explained by the components. Below the loading of each test for the first three PCs are represented.
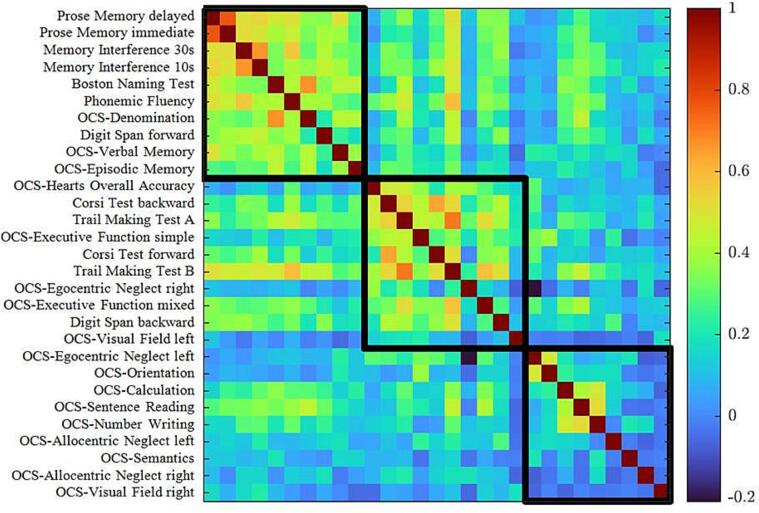
The strength of pairwise correlation between tests can be visualized through a correlation matrix (Fig. 2). A correlation is evident between language, verbal memory, executive/working memory (PC1) with r values ranging between r = 0.81 to r = 0.17; visuo-spatial attention correlated with executive functions (PC2) with r values ranging between r = 0.68 to r = −0.09; a third cluster included verbal functions (reading, writing, semantic knowledge), calculation, and visuo-spatial attention (PC3) with r values ranging between r = 0.53 to r = −0.16. In conclusion across domains and subjects, three factors accounted for a significant fraction of the variance, both in tumor and stroke.
Fig. 2.
Correlation matrix of behavioral subtests. The correlation between neuropsychological scores are represented for the total sample (brain tumor and stroke sample). The color bar represents Pearson r-values, the red color indicates strong positive correlation and the blue color indicates a weak or null correlation. Each square corresponds to the variables identified through the PCA (PC1, PC2, PC3).
To confirm these findings, we also ran the PCA separately in the two samples (Supplementary Fig. 1): in the two groups the first three components explained a similar percentage of the total variance (Stroke sample: 44.6%; Tumor sample: 48%). Moreover, the tests with the major loadings in each component were similar in the two groups: PC1 loaded on language, verbal memory, and executive/working memory (explained variance stroke sample: 28%; tumor sample: 27%) PC2 loaded on visuospatial attention and working memory, and executive functions (explained variance stroke sample: 9.2%; tumor sample: 12%); PC3 was the most different between the two groups, however it explained a low percentage of the total variance (explained variance stroke sample: 7.4%; tumor sample: 9%). We also repeated the analysis by considering only gliomas in the oncological cohort obtaining similar results (see Supplementary Fig. 2).
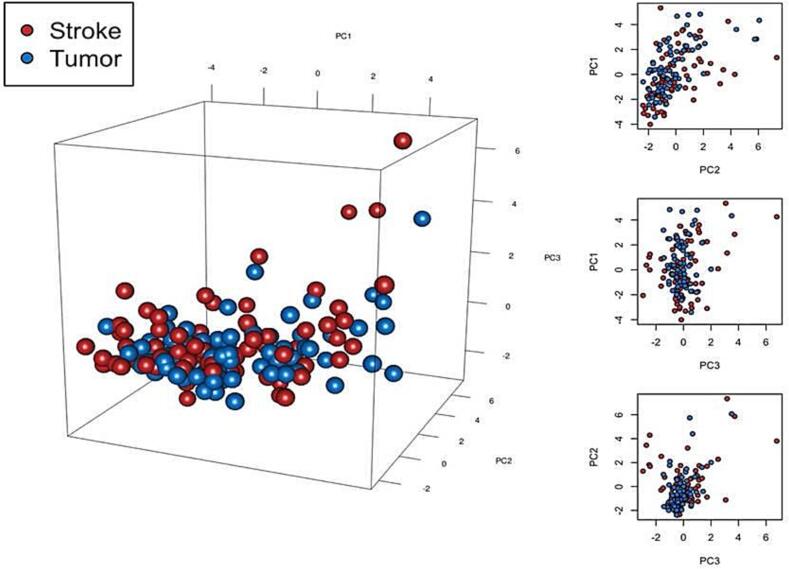
Finally, to test that the components found in stroke, as in previous studies (Corbetta et al., 2015, Bisogno et al., 2021), explain variance in the tumor data set, we first normalized both tumor data on stroke data and we applied the loadings coming from the PCA on stroke data. In this way, all patients could be projected into the same components space. Fig. 3 shows the distribution of scores along the three PC axes in stroke and tumor patients. It is apparent that the two populations cannot be separated, indicating that the cognitive profiles of stroke and tumor patients can be summarized in the same principal components space. Finally, we reconstructed the original tumor data from the first three PCs, by multiplying the matrix of individual scores on the first three PCs (Npatientsx3) with the rotated matrix of the loadings of the same PCs obtained from the stroke sample (Ntestsx3). Then, we correlated the data reconstructed in this way with the original data and obtained an R2 = 30.3%. Hence, the three PCs on the stroke dataset explain 30% of the variance in the tumor dataset.
Fig. 3.
Distribution of scores along the three PC axes in stroke and tumor patients. The individual scores of the first three components are represented in a three-dimensional space, for each subject of the two groups. Blue dots represent brain tumor patients, red dots represent stroke patients. On the right side, the same representation in two-dimensional space. The distribution of the two samples is similar.
3.3. Logistic regression
While the previous analyses show that a significant portion of individual cognitive performance variability can be summarized by a common cognitive structure, we were also interested in differences between groups. Table 2 shows scores for each test in the two groups (number of patients below normal cut-off, median and IQR). Supplementary Table 1 shows the median and IQR scores for each neuropsychological test for the different subgroups of the tumor sample (high grade glioma, low grade glioma, meningioma).
Table 2.
Descriptive incidences of cognitive performance for each test in the two groups.
|
Brain tumors |
Stroke |
|||
|---|---|---|---|---|
| N. of patients below normal cut-off | Median (IQR) | N. of patients below normal cut-off | Median (IQR) | |
| Oxford Cognitive Screen (OCS) | ||||
| Denomination | 12 | 4 (3.75–4) | 28 | 3 (3–4) |
| Semantics | 0 | 3 (3–3) | 1 | 3 (3–3) |
| Orientation | 0 | 4 (4-4) | 2 | 4 (4-4) |
| Visual Field-Right | 2 | 4 (4-4) | 2 | 4 (4-4) |
| Visual Field-Left | 5 | 2 (2-2) | 3 | 2 (2-2) |
| Sentence Reading | 15 | 15 (15–15) | 18 | 15 (15–15) |
| Number Writing | 4 | 3 (3-3) | 6 | 3 (3-3) |
| Calculation | 11 | 4 (4-4) | 20 | 4 (3-4) |
| Hearts Overall Accuracy | 27 | 46 (43.75–49) | 28 | 47 (43–49) |
| Egocentric Neglect-Right | 9 | 0 (0-1) | 9 | 0 (0-1) |
| Egocentric Neglect-Left | 7 | 0 (0-1) | 8 | 0 (0-1) |
| Allocentric Neglect-Right | 1 | 0 (0-0) | 4 | 0 (0-0) |
| Allocentric Neglect-Left | 1 | 0 (0-0) | 2 | 0 (0-0) |
| Verbal Memory | 24 | 3 (2-4) | 22 | 3 (3-4) |
| Episodic Memory | 16 | 4 (4-4) | 11 | 4 (4-4) |
| Executive Function-Simple | 8 | 12 (12-12) | 13 | 12 (11-12) |
| Executive Function-Mixed | 14 | 13 (11-13) | 23 | 13 (10-13) |
| Memory Interference-10 sec | 13 | 6 (4-8) | 13 | 7 (5-9) |
| Memory Interference-30 sec | 14 | 5 (3-7) | 13 | 6 (4-8) |
| Prose Memory-Immediate | 16 | 10 (7-14) | 15 | 12 (8-14) |
| Prose Memory-Delayed | 22 | 13 (9-17) | 14 | 14 (9-17) |
| Trail Making Test-A | 3 | 38 (18-51.25) | 10 | 44 (30-70) |
| Trail Making Test-B | 21 | 126 (91.75-204) | 22 | 140 (86-255) |
| Phonemic Fluency | 23 | 10.3 (7.93-13.7) | 17 | 11 (7.67-15) |
| Corsi Test forward | 4 | 5 (4-6) | 3 | 5 (4-6) |
| Corsi Test backward | 13 | 4 (4-5) | 3 | 4 (4-6) |
| Digit Span forward | 15 | 5 (4-6) | 5 | 5 (5-6) |
| Digit Span Backward | 13 | 4 (3-5) | 10 | 4 (3-5) |
| Boston Naming Test | 26 | 13 (12–14) | 33 | 13 (10–14) |
Descriptive incidences of cognitive performance for each test in the two groups (number of patients below normal cut-off, median).Five tests significantly discriminated a tumor from a stroke patient. High scores (more normal) in OCS-denomination and OCS-calculation were positively associated with tumors (bold font in the table); high scores (more normal) in OCS-episodic memory, Memory interference 10 s, and Phonemic fluency were positively associated with strokes (italic font in the table).
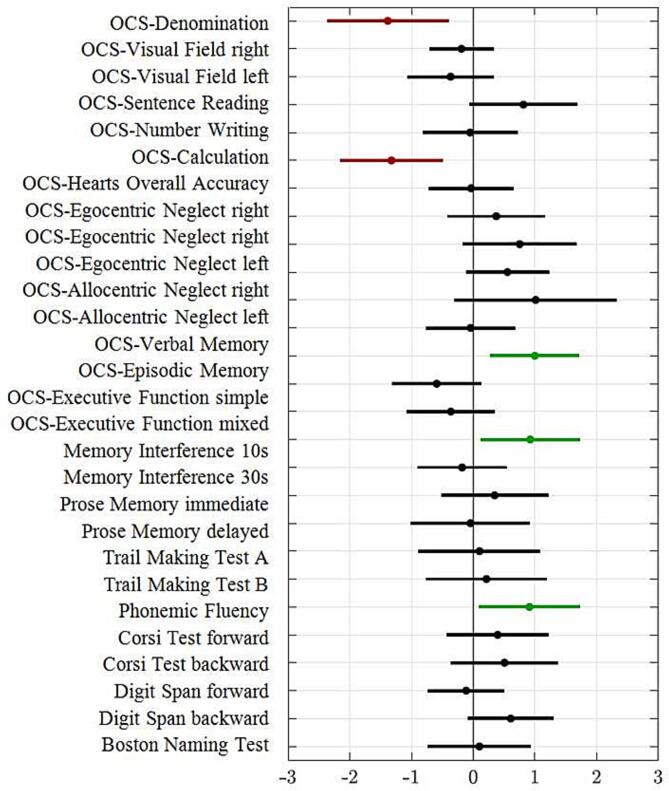
Differences in the cognitive profile were investigated by means of a logistic regression with age, education, gender, lesion side (right vs. left), and tests scores as predictors. Five tests significantly discriminated a tumor from a stroke patient. Higher scores (more normal) in OCS-denomination (z = −2.79; p = 0.005) and OCS-calculation (z = −3.17; p = 0.001) were positively associated with tumors; in contrast, high scores (more normal) in OCS-episodic memory (z = 2.75; p = 0.005), Memory interference 10 s (z = 2.28; p = 0.022), and Phonemic fluency (z = 2.21; p = 0.027) were positively associated with strokes (Fig. 4). We controlled all models for multicollinearity by means of the Variance Inflation Factors (VIF) which should be < 10 to suggest no potentially harmful collinearity. The AUC was 0.889. In other words, strokes showed more impairment in language denomination and calculation whereas tumors showed more impairment in episodic memory, verbal recall, and verbal executive function (fluency).
Fig. 4.
Results of logistic regression. The X-axis reported the coefficient of the logistic model (dots) with the confidence interval (lines) of each test (listed on the Y-axis). Scores greater than 0 indicate a higher score in the test in the stroke population, scores < 0 indicate a higher score in the test in the tumor population. Performance in five tests significantly discriminated a patient with a tumor or stroke: high scores in OCS-denomination and OCS-calculation were more probable in patients with tumor (red dots), while high scores in OCS-episodic memory, Memory interference 10 s and Phonemic fluency were more probable in patients with stroke (green dots).
3.4. Lesion anatomy
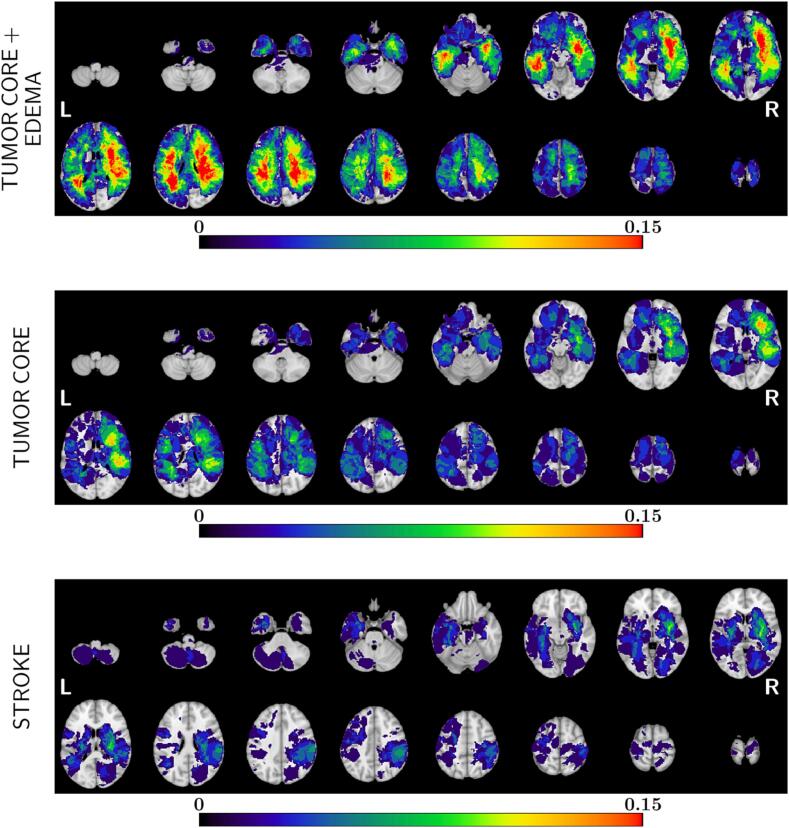
Lesions were segmented and normalized to the MNI152 atlas (Fig. 5). Stroke lesions were localized more commonly subcortically especially in the basal ganglia and central white matter in agreement with previous maps (Corbetta et al., 2015, Thiebaut de Schotten et al., 2020, Bisogno et al., 2021). Tumor lesions occurred prevalently in the frontal and temporal white–grey matter junction, with a more heterogeneous distribution (Supplementary Table 2 shows the top regions of damage in the two cohorts). In general, the overlap of individual lesions was low with <20% of patients with a lesion in the same location. The percentage of overlap increased in the tumor sample when considering the region of oedema.
Fig. 5.
Lesion frequency maps. The lesion frequency maps are represented for brain tumor population (tumor core and oedema on the top, tumor core only in the middle) and for stroke population (below). The red color indicates higher overlap between lesions. Tumor lesions occurred prevalently in frontal and temporal cortex, specifically at the white–grey matter junction. Stroke lesions were more common subcortically, especially in the basal ganglia and central white matter. The overlapping is generally low, indeed, <20% of patients had a lesion in the same location for both groups.
3.5. Ridge regression models of lesion-to-behavior
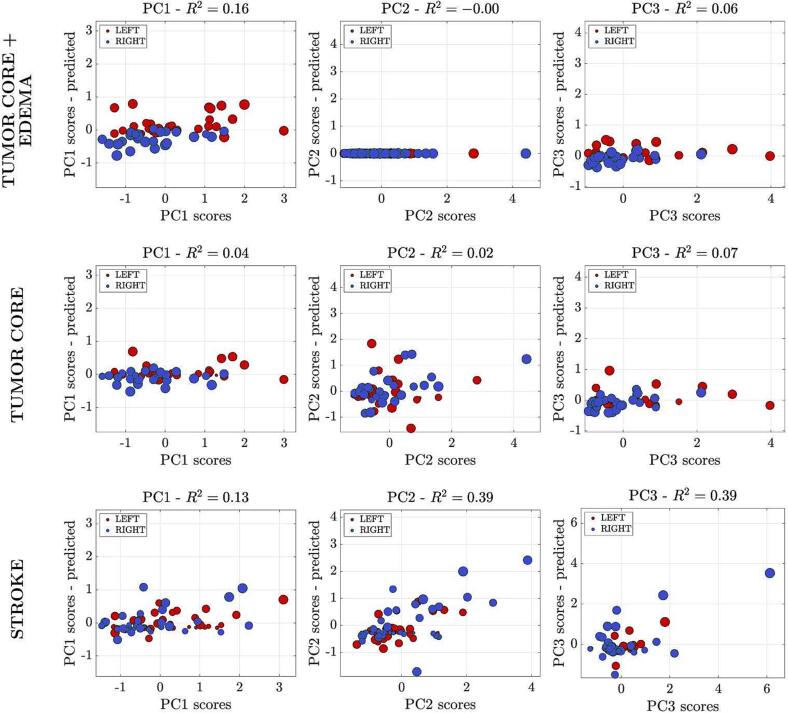
We used ridge regression to model the behavioral scores based on lesion information (location, volume). The analysis aims to use the lesion information to estimate behavioral scores at the individual level that are then compared with the empirically measured scores. This analysis was conducted on patients who had both neuroimaging and neuropsychological data (tumor: n = 66; stroke: n = 67). Fig. 6 shows on the Y-axis the predicted score based on the lesion, while on the X-axis the actual score. It is apparent that when considering the tumor core, the lesion location did not explain any of the PC scores (R2 < 10%). When considering the region of the tumor core plus oedema, the relationship was significant for PC1 (R2 = 0.16, p = 0.01). In the stroke population, the association was significant for PC1 (R2 = 0.13, p = 0.04), PC2 (R2 = 0.30, p < 0.001) scores, and not significant for PC3 (R2 = 0.39, p = 0.06). The analysis run by considering only gliomas was also not significant (Supplementary Fig. 3).
Fig. 6.
Ridge regression analysis. Scatter plots of predicted scores for each component are represented. Each dot represents a subject, and the color of each dot represents the side of the lesion (red = lesion in the left hemisphere; blue = lesion in the right hemisphere). The diameter of each colored circle is proportional to the lesion's volume. The model provides an accurate prediction of behavioral scores for PC1 and PC2 in stroke population (third line). The model is not significant in the neuro-oncological population when considering the tumor core only, without oedema. When considering the region of the tumor core plus oedema, the association was significant for PC1 (upper and middle lines).
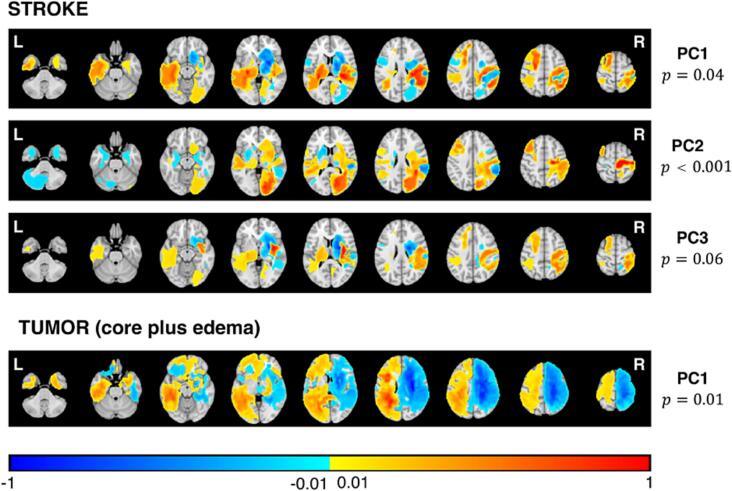
Fig. 7 shows the maps with the lesion locations significantly associated with the behavioral scores. PC1-RR stroke map involves left peri-sylvian cortex consistent with language impairments, and bilateral fronto-parietal and basal ganglia consistent with memory and executive deficits (both spatial and verbal). PC2-RR stroke map highlights posterior right hemisphere regions (occipito-parietal) consistent with left visuo-spatial and overall performance deficits. Bilateral basal ganglia and frontal lesions are consistent with executive deficits. PC3-RR map shows bilateral cortical and subcortical lesions. Interestingly, none of the tumor RR maps were significant except for a marginal PC1 that splits left and right hemisphere lesions with positive loadings associated with more severe deficits in the left peri-sylvian cortex.
Fig. 7.
Ridge regression maps. Ridge regression maps of PCs for significant correlation are represented. In the stroke population, significant correlation is found in PC1, PC2 and PC3 (lines above); in the tumor population, only PC1 reported a significant correlation when considering the core with the oedema (below). Warm colors represent positive correlation between anatomical voxels and high PC values (i.e. high level of impairment in the corresponding domains). Cold colors represent negative correlation between anatomical voxels and high PC values.
3.6. Cognitive performance of patients with similar lesion location (cluster analysis)
To compare the neuropsychological performance of stroke and tumor patients with similar lesion locations, we conducted an exploratory analysis using a spectral clustering algorithm to divide patients in four anatomical clusters (see Supplementary Analysis and Results for a detailed description of the method). The analysis was limited by the low numerosity of each cluster (cluster 1: tumor (T) = 7, stroke (S) = 10; cluster 2: T = 21, S = 28; cluster 3: T = 18, S = 20; cluster 4: T = 10, S = 9). Therefore, most details are presented in the Supplementary results (see also Supplementary Fig. 4, and Supplementary Table 3).
We note only that differences in cognitive performance found in the logistic regression analysis were localized to specific anatomical clusters. Lesions in cluster 1 localized to left prefrontal regions, and stroke patients, as shown in the logistic regression (Fig. 4), performed worse on the naming subtest of the OCS than tumor patients (F = 9.343, p < 0.01). Also in agreement with the logistic regression, tumor patients performed worse on memory tests than stroke patients in cluster 2 (left temporal) and cluster 3 (right frontal).
4. Discussion
Focal neurological disorders (stroke, tumors) have been the prime model for studying the localization and organization of sensory, motor, and cognitive functions in the brain. While sensory and motor deficits are accurately detected with a neurological examination, even in a structured scale like the NIHSS, the evaluation of cognitive deficits requires more in-depth neuropsychological testing. Cognitive deficits in focal brain disorders are clinically relevant as they represent the main cause of disability (Olesen et al., 2012). For instance, both brain tumor and stroke patients experience loss of memory and concentration that compromise their return to work (Treger et al., 2007, Randazzo and Peters, 2016, Ghanbari Ghoshchi et al., 2020).
This study compared the cognitive performance of patients with mild stroke vs. brain tumor lesions, specifically whether the low dimensional structure of cognitive deficits found in stroke also occurs in brain tumors. Secondly, stroke and brain tumors both occur preferentially in the white matter. Since lesion location is strongly associated with behavioral deficits in stroke (Corbetta et al., 2015, Salvalaggio et al., 2020, Karnath et al., 2004), we asked whether the same occurred for brain tumor lesions.
4.1. Methodological considerations
We focused on cognitive symptoms, and considered only data from patients who were able to complete the whole neuropsychological assessment. While every enrolled tumor patients completed the assessment, only about 60% of enrolled stroke patients were able to do so (n = 77 out of n = 133). As a result, our stroke sample was milder (mean NIHSS score on admission = 6.3 ± 4.1; score at the time of testing = 2.08 ± 2.05) as compared to other recently reported samples (Corbetta et al., 2015: mean NIHSS score on admission = 7.5; Bisogno et al., 2021: mean NIHSS score on admission = 7.1 ± 5.6; at the time of testing = 3.2 ± 2.9). Accordingly, the percentage of patients with motor deficits was lower (75% vs 90% in Bisogno et al., 2021). However, the stroke and tumor sample were similar in terms of mean education and gender, whereas the stroke population was slightly older. The tumor sample included both meningiomas (n = 16) and gliomas (n = 60), but the results did not change when only gliomas were analyzed.
The lesion topography (Fig. 5) was quite different: the core tumor lesions affected the grey-white matter junction, with a predominant frontal and temporal distribution (Mandal et al., 2020). When the oedema region was also considered the damage extended deeply and broadly in the white matter sparing only occipital and superior parietal cortex. Strokes damaged predominantly basal ganglia and central white matter, with only 10–15% involving cortex, as previously reported in larger cohorts (Corbetta et al., 2015, Thiebaut de Schotten et al., 2020, Bisogno et al., 2021, Kang et al., 2003, Wessels et al., 2006). The degree of overlap across lesions was low (<20%). Our maps resemble those reported recently by van Grinsven et al. (2023), who also showed a low lesion overlap per area (12.8 % for tumor, and 4.8 % per stroke) with only about 1/3 of the areas involved in both pathologies in more than 5% of patients.
4.2. A common low dimensional structure of cognitive impairment in brain tumors and stroke
The first notable result is that a low dimensional set of correlated cognitive deficits explained about half of the variability across subjects, both when all patients were considered jointly (Fig. 1, Fig. 2) or split by pathology (Supplementary Fig. 1). The variance explained by the first three components in the joint PCA (42%) was comparable to Bisogno et al.’s (2021) in which three components explained 50% of the variance in a larger and more severe stroke sample (n = 158). The first component loaded on language, verbal memory, and executive/working memory; the second component loaded on visuo-spatial attention (OCS-hearts overall accuracy, OCS-egocentric and allocentric neglect), working memory and executive functions (e.g., TMT A and B, Corsi Test, Digit span backwards); the third component loaded on deficits of reading, calculation, and visuo-spatial that were partly captured in components 1 and 2. The selection of three components was a priori based on previous work in stroke (Corbetta et al., 2015, Bisogno et al., 2021), and components 1 and 2 were similar to those reported after including motor deficits (Corbetta et al., 2015, Bisogno et al., 2021). We also directly tested how much variance of the cognitive scores in tumor patients could be predicted by the component structure derived from the stroke cohort. About 30% of the variability in tumor patients’ performance can be described by using the loadings derived from stroke; also the two populations are indistinguishable when plotted in the three component space (Fig. 3).
Recently, Sperber et al. (2023) have criticized the low dimensionality of behavioral deficits in stroke as an artifact of lesion anatomy. However, as recently discussed (Pini et al., 2023), anatomy alone does not explain the low dimensionality of cognitive deficits after focal injury. In fact, even after considering the variables indicated by Sperber et al. we showed that behavior is summarized by the lowest number of components as compared to anatomical models alone. Moreover, the current work shows a similar correlation for lesions of different etiologies that are localized on average to different anatomical sites. Finally, we found correlation among behavioral scores in tumors in which lesion location did not explain behavioral variance (Fig. 5). Overall, we conclude that the low dimensionality of behavioral deficits cannot be explained by anatomy alone.
Even though mild stroke and tumors yielded a similar pattern of correlated cognitive deficits, we also found some evidence of differentiation in their cognitive profile. Mild stroke patients were more affected in functions that require more localized processing such as language and calculation as shown in the logistic regression model (OCS-denomination; OCS-calculation, Fig. 4). The stronger impairment of stroke patients in OCS-denomination localized in left prefrontal regions (cluster 1, Suppl. Fig. 4). In contrast, tumor patients were more affected in memory performance (OCS-episodic memory, Memory interference, Phonemic fluency, Fig. 4) that localized in left temporal (cluster 2) and right frontal (cluster 3) (Suppl. Fig. 4).
A similar pattern has been recently reported in van Grinsven et al. (2023). Using lesion-symptom mapping they investigated performance for verbal memory and language in two cohorts of stroke and brain tumor patients. They found comparable group-level impairment, similarly to our PCA results, and a different lesion topography, as we did. When looking for anatomical locations related to behavioral performance, they reported a correlation between memory performance and areas surrounding the left hippocampus in brain tumors, but not in stroke sample. Moreover, several left hemisphere cortical regions (left insula, rolandic operculum, Heschl’s gyrus) were linked to verbal fluency impairment, but only when the lesion was a tumor. Therefore, although van Grinsven et al. (2023) emphasize differences across pathologies, the actual data are quite similar across studies with evidence of both similarity and mild differences. Despite the general low lesion-symptom correlation in brain tumors (see paragraph below for a discussion), we found some vulnerability to memory impairment in left temporal tumors as compared to strokes. The left temporal region is one of the most common sites in gliomas possibly because the hippocampus is one of the sites where neural stem cells are generated (Mandal et al., 2020). The left hippocampus was also found as an “eloquent” hotspot for verbal memory and critically linked to memory deficits in brain tumor patients (Campanella et al., 2018). Finally, when considering only the left hemisphere, patients suffering from tumors in the anterior temporal lobe were most frequently and severely impaired in verbal memory as compared to lesions in other areas (Behrens et al., 2021).
The description of cognitive phenotypes that capture large amount of variance, and that are specific for a certain pathology, could be clinically relevant because it allows to shift the focus of clinicians from the rare and interesting cases to the great majority of patients. This is especially true in the case of brain tumors, where symptoms are subtler. These phenotypes are robust and shall be used for large scale studies of treatment, genetics, or outcome. The importance of shifting from a modular to a network-wide view of cognitive impairment in brain tumors has been also advocated by Duffau and colleagues (Duffau, 2018, Herbet and Duffau, 2020, Duffau, 2021).
4.3. Low behavioral specificity of lesion location in tumors
The second important question concerned the correlation between the behavioral scores and the anatomical lesions. The lesion topography was different with tumors affecting predominantly the frontal and temporal white–gray matter junction while stroke damaging prevalently the basal ganglia and deep white matter (Fig. 5). This different topography is also consistent with van Grinsven et al. (2023).
The ridge regression detected a relationship between anatomical lesions and behavioral deficits at the individual subject level only in stroke. PC1 deficits loading on language and memory localized in left > right peri-sylvian, bilateral basal ganglia and frontal cortex. PC2 deficits loading on general performance and visuo-spatial localized to right occipito-parietal cortex. Notably, no significant correlation was obtained in brain tumors using the core lesion locations. A significant correlation for PC1 was obtained only when considering core-oedema lesions and localized to the left peri-sylvian cortex like in stroke.
The low behavioral specificity of lesion location in tumors shall be considered preliminary given the relatively low number of patients tested. Anderson et al. (1990) had previously noted that extensive tumors involving areas of different lobes do not lead to cognitive damage in contrast with strokes with same extension. Van Grinsven et al. (2023) found that lesion volumes were larger in the tumor group and correlated with cognitive performance. However, at the group level, this difference in volume did not result in more severe cognitive impairment in the tumor as compared to the stroke population.
Different research groups have pointed out that even large tumors in eloquent areas, or recurrences of brain tumors operated on, do not cause the expected cognitive deficits, and that this can be related to two mechanisms: plasticity and remapping of function that occurs as tumors grow; and those cognitive functions do not map onto specific regions but network of regions (Duffau, 2017, Nenning et al., 2020, Duffau, 2021). Studies on brain tumor prognosis have found contrasting values of lesion location and volume in terms of survival (Awad et al., 2017, Curtin et al., 2021). We have recently completed a study looking at variables accounting for post-surgical cognitive performance and found little value of pre-operative lesion location and volume. In contrast, we found pre-surgical neuropsychological scores to be highly predictive of post-surgical performance. These findings support the notion that cognitive functions are distributed and remapped in brain tumors, and that psychological evaluations more than anatomical lesions are a good read-out of current brain function (Zangrossi et al., 2022).
In contrast to the low predictive value of lesion core location, we did find some correlation for PC1 (language, memory) when the region of oedema was added to the lesion core location. The map highlighted the left peri-sylvian region that was one of the regions predictive in stroke. A link between oedema and cognitive function has been described in relation to radiotherapy treatment (Wang et al., 2021). Recently, we showed abnormal functional connectivity in cortical regions remote from the tumor core, but presumably connected to it by anatomical fibers that travel through the surrounding oedema region (Silvestri et al., 2022). In conclusion, the anatomical analysis provides preliminary evidence that tumor location is less correlated overall with cognitive impairment than stroke location.
5. Conclusions and limitations
This study shows that mild acute strokes and brain tumors cause a similar pattern of cognitive impairment across cognitive domains and subjects. We also show specific deficits that are stronger in one or the other pathology.
A common behavioral deficit correlation in mild stroke and tumors, notwithstanding their different lesion topography, provides evidence that lesion location alone is not enough to explain behavioral impairment. In stroke, we, and others (Thiebaut de Schotten et al., 2020) have argued that lesions in many locations cause a much lower dimensional pattern of structural disconnection and functional connectivity alteration (Siegel et al., 2016, Salvalaggio et al., 2020). Importantly, these network abnormalities are more associated with cognitive impairment than lesion location, which is instead strongly linked with sensory and motor functions (Siegel et al., 2016, Salvalaggio et al., 2020). We postulate that similar mechanisms are at work in tumors, as also supported by the surgical and neuropsychological observations of Duffau (2018).
The study is limited by the sample size that prevents any conclusion about differences in cognitive performance for lesions at the same location with different etiology. Another limitation is that we focused on milder strokes to make them more comparable to the performance of brain tumors. Therefore, our results cannot be generalized to the entire stroke population. Moreover, in our sample, stroke lesions incompletely covered the typical distribution as shown in prior studies (Corbetta et al., 2015, Thiebaut de Schotten et al., 2020, Bisogno et al., 2021). This limits conclusions about brain-behavior correlation in regions that were not damaged. However, the low-dimensional cognitive pattern described with the PCA is like previous work based on a larger sample and a wider distribution of lesions (Corbetta et al., 2015, Bisogno et al., 2021). Moreover, we did not perform a nested cross-validation to optimize the hyperparameter in the ridge regression, thus current ridge regression findings might be sample-specific and their generalization should be tested by a replication on an independent cohort.
Finally, our analysis was based just on lesion location and volume. Future studies may consider other structural or functional variables that may relate to performance and may differ between etiology: white matter integrity, functional networks, cortical atrophy. A natural extension of this study would be to examine behavioral predictions separately for tumor core and oedema region to assess the role of oedema in causing cognitive deficits, by comparing behavioral prediction using disconnection methods (Boes et al., 2015, Thiebaut de Schotten and Foulon, 2018, Thiebaut de Schotten et al., 2020, Pini et al., 2021). In clinical practice steroid treatment is empirically given to treat oedema, and patients’ neurological deficits do improve.
CRediT authorship contribution statement
Silvia Facchini: Conceptualization, Methodology, Writing – original draft. Chiara Favaretto: Formal analysis, Methodology, Software. Marco Castellaro: Formal analysis, Validation. Andrea Zangrossi: Formal analysis, Validation. Margherita Zannin: Investigation. Antonio Luigi Bisogno: Investigation. Valentina Baro: Investigation. Maria Giulia Anglani: Data curation, Supervision. Antonio Vallesi: Supervision. Claudio Baracchini: Supervision. Domenico D'Avella: Supervision. Alessandro Della Puppa: Supervision. Carlo Semenza: Supervision. Maurizio Corbetta: Conceptualization, Methodology, Writing – review & editing, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank our patients who participated in the study, and their family for support. Without their willingness to help for improving future clinical diagnosis and treatment this study would have not been possible.
Study funding
MC was supported by Fondazione Cassa di Risparmio di Padova e Rovigo (CARIPARO) Grant Agreement number 55403; Ministry of Health, Italy (RF-2008-12366899) Brain connectivity measured with high-density electroencephalography: a novel neurodiagnostic tool for stroke- NEUROCONN; BIAL foundation grant (Grant Agreement number 361/18); H2020 European School of Network Neuroscience (euSNN); H2020 Visionary Nature Based Actions For Heath, Wellbeing & Resilience in Cities (VARCITIES); Ministry of Health Italy (RF-2019-12369300): Eye-movement dynamics during free viewing as biomarker for assessment of visuospatial functions and for closed-loop rehabilitation in stroke (EYEMOVINSTROKE); European Union (ERC-2022-SYG NEMESIS Grant number 101071900). Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or the European Research Council Executive Agency. Neither the European Union nor the granting authority can be held responsible for them.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2023.103518.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Anderson S.W., Damasio H., Tranel D. Neuropsychological impairment associated with lesions caused by tumor or stroke. Arch. Neurol. 1990;47:397–405. doi: 10.1001/archneur.1990.00530040039017. [DOI] [PubMed] [Google Scholar]
- Avants B.B., Tustison N.J., Song G., Cook P.A., Klein A., Gee J.C. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54(3):2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad A.W., Karsy M., Sanai N., Spetzler R., Zhang Y., Xu Y., Mahan M.A. Impact of removed tumor volume and location on patient outcome in glioblastoma. J. Neurooncol. 2017;135(1):161–171. doi: 10.1007/s11060-017-2562-1. [DOI] [PubMed] [Google Scholar]
- Behrens M., Thakur N., Lortz I., Seifert V., Kell C.A., Forster M.-T. Neurocognitive deficits in patients suffering from glioma in speech-relevant areas of the left hemisphere. Clin. Neurol. Neurosurg. 2021;207:106816. doi: 10.1016/j.clineuro.2021.106816. [DOI] [PubMed] [Google Scholar]
- Bisogno A.L., Favaretto C., Zangrossi A., Monai E., Facchini S., De Pellegrin S., Pini L., Castellaro M., Basile A.M., Baracchini C., Corbetta M. A low-dimensional structure of neurological impairment in stroke. Brain Commun. 2021;3(2):1–13. doi: 10.1093/braincomms/fcab119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boes A.D., Prasad S., Liu H., Liu Q.i., Pascual-Leone A., Caviness V.S., Fox M.D. Network localization of neurological symptoms from focal brain lesions. Brain. 2015;138(10):3061–3075. doi: 10.1093/brain/awv228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella F., Del Missier F., Shallice T., Skrap M. Localizing memory functions in brain tumor patients: anatomical hotspots. World Neurosurg. 2018;120:e690–e709. doi: 10.1016/j.wneu.2018.08.145. [DOI] [PubMed] [Google Scholar]
- Cipolotti L., Healy C., Chan E., Bolsover F., Lecce F., White M., Spanò B., Shallice T., Bozzali M. The impact of different aetiologies on the cognitive performance of frontal patients. Neuropsychologia. 2015;68:21–30. doi: 10.1016/j.neuropsychologia.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Ramsey L., Callejas A., Baldassarre A., Hacker C.D., Siegel J.S., Astafiev S.V., Rengachary J., Zinn K., Lang C.E., Tabor Connor L., Fucetola R., Strube M., Carter A.R., Shulman G.L. Common behavioral clusters and subcortical anatomy in stroke. Neuron. 2015;85(5):927–941. doi: 10.1016/j.neuron.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Siegel J.S., Shulman G.L. On the low dimensionality of behavioral deficits and alterations of brain network connectivity after focal injury. Cortex. 2018;107:229–237. doi: 10.1016/j.cortex.2017.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin L., Whitmire P., White H., Bond K.M., Mrugale M.M., Hu L.S., Swanson K.R. Shape matters: morphological metrics of glioblastoma imaging abnormalities as biomarkers of prognosis. Sci. Rep. 2021;11(1):23202. doi: 10.1038/s41598-021-02495-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeyere N., Riddoch M.J., Slavkova E.D., Bickerton W.L., Humphreys G.W. The Oxford Cognitive Screen (OCS): Validation of a stroke-specific short cognitive screening tool. Psychol. Assess. 2015;27(3):883–894. doi: 10.1037/pas0000082. [DOI] [PubMed] [Google Scholar]
- Duffau H. A two-level model of interindividual anatomo-functional variability of the brain and its implications for neurosurgery. Cortex. 2017;86:303–313. doi: 10.1016/j.cortex.2015.12.009. [DOI] [PubMed] [Google Scholar]
- Duffau H. The error of Broca: From the traditional localizationist concept to a connectomal anatomy of human brain. J. Chem. Neuroanat. 2018;89:73–81. doi: 10.1016/j.jchemneu.2017.04.003. [DOI] [PubMed] [Google Scholar]
- Duffau H. The death of localizationism: The concepts of functional connectome and neuroplasticity deciphered by awake mapping, and their implications for best care of brain-damaged patients. Rev. Neurol. 2021;177(9):1093–1103. doi: 10.1016/j.neurol.2021.07.016. [DOI] [PubMed] [Google Scholar]
- Favaretto C., Allegra M., Deco G., Metcalf N.V., Griffis J.C., Shulman G.L., Brovelli A., Corbetta M. Subcortical-cortical dynamical states of the human brain and their breakdown in stroke. Nat. Commun. 2022;13(1):5069. doi: 10.1038/s41467-022-32304-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanbari Ghoshchi S., De Angelis S., Morone G., Panigazzi M., Persechino B., Tramontano M., Capodaglio E., Zoccolotti P., Paolucci S., Iosa M. Return to Work and Quality of Life after Stroke in Italy: A Study on the Efficacy of Technologically Assisted Neurorehabilitation. Int. J. Environ. Res. Public Health. 2020;17(14):5233. doi: 10.3390/ijerph17145233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabner, G., Janke, A.L., Budge, M.M., Smith, D., Pruessner, J., Collins, D.L., 2006. Symmetric Atlasing and Model Based Segmentation: An Application to the Hippocampus in Older Adults. In: Larsen R, Nielsen M, Sporring J (Eds.), Medical Image Computing and Computer-Assisted Intervention – MICCAI 2006. Lecture Notes in Computer Science, vol 4191. Springer, Berlin, Heidelberg. [DOI] [PubMed]
- Hansen A., Pedersen C.B., Minet L.R., Beier D., Jarden J.O., Søgaard K. Hemispheric tumor location and the impact on health-related quality of life, symptomatology, and functional performance outcomes in patients with glioma: an exploratory cross-sectional study. Disabil. Rehabil. 2021;43(10):1443–1449. doi: 10.1080/09638288.2019.1668486. [DOI] [PubMed] [Google Scholar]
- Herbet G., Duffau H. Revisiting the Functional Anatomy of the Human Brain: Toward a Meta-Networking Theory of Cerebral Functions. Physiol. Rev. 2020;100(3):1181–1228. doi: 10.1152/physrev.00033.2019. [DOI] [PubMed] [Google Scholar]
- Hoerl A.E., Kennard R.W. American Society for Quality Ridge Regression: Biased Estimation For Nonorthogonal Problems. Technometrics. 1970;12(1):55–67. [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. FSL. NeuroImage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Kang D.W., Chalela J.A., Ezzeddine M.A., Warach S. Association of ischemic lesion patterns on early diffusion-weighted imaging with TOAST stroke subtypes. Arch. Neurol. 2003;60:1730–1734. doi: 10.1001/archneur.60.12.1730. [DOI] [PubMed] [Google Scholar]
- Karnath H.O., Fruhmann Berger M., Küker W., Rorden C. The anatomy of spatial neglect based on voxelwise statistical analysis: a study of 140 patients. Cereb. Cortex. 2004;14(10):1164–1172. doi: 10.1093/cercor/bhh076. [DOI] [PubMed] [Google Scholar]
- Klein M., Duffau H., De Witt Hamer P.C. Cognition and resective surgery for diffuse infiltrative glioma: an overview. J. Neurooncol. 2012;108(2):309–318. doi: 10.1007/s11060-012-0811-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruithof W.J., Post M.W., van Mierlo M.L., van den Bos G.A., de Man-van Ginkel J.M., Visser-Meily J.M. Caregiver burden and emotional problems in partners of stroke patients at two months and one year post-stroke: Determinants and prediction. Patient Educ. Couns. 2016;99(10):1632–1640. doi: 10.1016/j.pec.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Lyden P., Claesson L., Havstad S., Ashwood T., Lu M. Factor analysis of the National Institutes of Health Stroke Scale in patients with large strokes. Arch. Neurol. 2004;61(11):1677–1680. doi: 10.1001/archneur.61.11.1677. [DOI] [PubMed] [Google Scholar]
- Mandal A.S., Romero-Garcia R., Hart M.G., Suckling J. Genetic, cellular, and connectomic characterization of the brain regions commonly plagued by glioma. Brain. 2020;143:3294–3307. doi: 10.1093/brain/awaa277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa M.S., Wang N., Bickerton W.L., Demeyere N., Riddoch M.J., Humphreys G.W. On the importance of cognitive profiling: A graphical modelling analysis of domain-specific and domain-general deficits after stroke. Cortex. 2015;71:190–204. doi: 10.1016/j.cortex.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Mondini, S., Mapelli, D,, Vestri, A., Arcara, G., Bisiacchi, P.S., 2011. Esame neuropsicologico breve II. Una batteria di test per lo screening neuropsicologico, Milano: Raffello Cortina Editore.
- Nenning K.H., Furtner J., Kiesel B., Schwartz E., Roetzer T., Fortelny N., Bock C., Grisold A., Marko M., Leutmezer F., Liu H., Golland P., Stoecklein S., Hainfellner J.A., Kasprian G., Prayer D., Marosi C., Widhalm G., Woehrer A., Langs G. Distributed changes of the functional connectome in patients with glioblastoma. Sci. Rep. 2020;10(1):18312. doi: 10.1038/s41598-020-74726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen J., Gustavsson A., Svensson M., Wittchen H.U., Jönsson B., CDBE2010 study group The economic cost of brain disorders in Europe: Economic cost of brain disorders in Europe. Eur. J. Neurol. 2012;19:155–162. doi: 10.1111/j.1468-1331.2011.03590.x. [DOI] [PubMed] [Google Scholar]
- Pini, L., Bisogno, A. L., Salvalaggio, A., Shulman, G.L., Corbetta, M., 2023. The correlation of behavioural deficits post-stroke: a trivial issue? Brain, Letter to the editor. [DOI] [PubMed]
- Pini, L., Salvalaggio, A., De Filippo De Grazia, M., Zorzi, M., Thiebaut de Schotten, M., Corbetta, M., 2021. A novel stroke lesion network mapping approach: improved accuracy yet still low deficit prediction. Brain Commun., 3(4), fcab259. [DOI] [PMC free article] [PubMed]
- Ramsey L.E., Siegel J.S., Lang C.E., Strube M., Shulman G.L., Corbetta M. Behavioral clusters and predictors of performance during recovery from stroke. Nat. Hum. Behav. 2017;1(3):0038. doi: 10.1038/s41562-016-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo D., Peters K.B. Psychosocial distress and its effects on the health-related quality of life of primary brain tumor patients. CNS Oncol. 2016;5(4):241–249. doi: 10.2217/cns-2016-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C., Bonilha L., Fridriksson J., Bender B., Karnath H.O. Age-specific CT and MRI templates for spatial normalization. Neuroimage. 2012;61(4):957–965. doi: 10.1016/j.neuroimage.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvalaggio, A., De Filippo De Grazia, M., Zorzi, M., Thiebaut de Schotten, M., Corbetta, M., 2020. Post-stroke deficit prediction from lesion and indirect structural and functional disconnection. Brain, 143(7), 2173–2188. [DOI] [PMC free article] [PubMed]
- Siegel J.S., Ramsey L.E., Snyder A.Z., Metcalf N.V., Chacko R.V., Weinberger K., Baldassarre A., Hacker C.D., Shulman G.L., Corbetta M. Disruptions of network connectivity predict impairment in multiple behavioral domains after stroke. PNAS. 2016;113(30) doi: 10.1073/pnas.1521083113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, C.R.R.D., Pimenta, C.J.L., Viana, L.R.C., Ferreira, G.R.S., Bezerra, T.A., Costa, T.F.D., et al., 2021. Specific health-related quality of life in Cerebrovascular accident survivors: associated factors. Rev. Bras. Enferm. 29, 75(3):e20210407. [DOI] [PubMed]
- Silvestri E., Moretto M., Facchini S., Castellaro M., Anglani M., Monai E., D’Avella D., Della Puppa A., Cecchin D., Bertoldo A., Corbetta M. Widespread cortical functional disconnection in gliomas: an individual network mapping approach. Brain Commun. 2022;4(2):fcac082. doi: 10.1093/braincomms/fcac082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperber, C., Gallucci, L., Umarova, R., 2023. The low dimensionality of post-stroke cognitive deficits: it’s the lesion anatomy! Brain, 146, 2443-2452. [DOI] [PubMed]
- Stock J.H., Watson M.W. Introduction to Econometrics. 3ª ed. Pearson; 2015. Regression with a Binary Dependent Variable; pp. 442–443. [Google Scholar]
- Thiebaut de Schotten M., Foulon C. The rise of a new associationist school for lesion-symptom mapping. Brain. 2018;141:2–4. doi: 10.1093/brain/awx332. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M., Foulon C., Nachev P. Brain disconnections link structural connectivity with function and behavior. Nat. Commun. 2020;11(1):5094. doi: 10.1038/s41467-020-18920-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treger I., Shames J., Giaquinto S., Ring H. Return to work in stroke patients. Disabil. Rehabi. 2007;29(17):1397–1403. doi: 10.1080/09638280701314923. [DOI] [PubMed] [Google Scholar]
- Turken A.U., Dronkers N.F. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Front. Syst. Neurosci. 2011;5:1. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Grinsven E.E., Smits A.R., van Kessel E., Raemaekers M.A.H., de Haan E.H.F., Huenges Wajer I.M.C., Ruijters V.J., Philippens M.E.P., Verhoeff J.J.C., Ramsey N.F., Robe P.A.J.T., Snijders T.J., van Zandvoort M.J.E. The impact of etiology in lesion-symptom mapping – A direct comparison between tumor and stroke. NeuroImage: Clinical. 2023;37:103305. doi: 10.1016/j.nicl.2022.103305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Chen D., Qiu J., Li S., Zheng X. The relationship between the degree of brain edema regression and changes in cognitive function in patients with recurrent glioma treated with bevacizumab and temozolomide. Quant. Imaging Med. Surg. 2021;11(11):4556–4568. doi: 10.21037/qims-20-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenius C., Claesson L., Blomstrand C., Jood K., Carlsson G. Integrating consequences of stroke into everyday life - Experiences from a long-term perspective. Scand. J. Occup. Ther. 2020;12:1–13. doi: 10.1080/11038128.2020.1857433. [DOI] [PubMed] [Google Scholar]
- Wessels T., Wessels C., Ellsiepen A., Reuter I., Trittmacher S., Stolz E., Jauss M. Contribution of diffusion-weighted imaging in determination of stroke etiology. AJNR Am. J. Neuroradiol. 2006;27:35–39. [PMC free article] [PubMed] [Google Scholar]
- Yushkevich P.A., Piven J., Hazlett H.C., Gimpel Smith R., Ho S., Gee J.C., Gerig G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Zandieh A., Kahaki Z.Z., Sadeghian H., Pourashraf M., Parviz S., Ghaffarpour M., Ghabaee M. The underlying factor structure of National Institutes of Health Stroke scale: an exploratory factor analysis. Int. J. Neurosci. 2012;122(3):140–144. doi: 10.3109/00207454.2011.633721. [DOI] [PubMed] [Google Scholar]
- Zangrossi A., Silvestri E., Bisio M., Bertoldo A., De Pellegrin S., Vallesi A., Della Puppa A., D'Avella D., Denaro L., Scienza R., Mondini S., Semenza C., Corbetta M. Predictors of cognitive outcome after brain tumor resection in glioma patients, personal communication. NeuroImage: Clinical. 2022;36 doi: 10.1016/j.nicl.2022.103219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.