Abstract
Background:
Malaria is the most important tropical and parasitic disease in the world. Endophagy of many malaria vectors advocates that impeding their entry into houses and preventing their contact with the occupants from infective bites could protect them against malaria.
Methods:
The study was carried out in Jaisalmer District, India and three villages were selected as test villages and three as control. Cross-sectional malaria prevalence surveys and mosquito collections were conducted in all the study villages. Insecticide-treated nets (ITNs) were tied below the beds for personal protection against the mosquito bite. Door and window curtains along with partition curtains were treated with insecticide for baring the entry of mosquito vectors.
Results:
Plasmodium vivax and P. falciparum were reported from the study villages. Higher malaria cases were detected in the control villages than the insecticide-treated bed net-distributed villages. The percentages of reduction of mosquito density in the houses of the ITNs distributed villages were significantly higher than the control villages. The insecticide activity was decreased slowly, and the knockdown time (KD50) values were found to be increased with the duration of usage of net. The KD50 of Anopheles subpictus s.l. was found to be more than the An. stephensi. Rooms where the ITNs were found to be significantly lower per man-hour density of mosquitoes.
Conclusion:
The use of alternative forms of ITNs shows a potential for preventing malaria and are making a significant contribution to the mosquito control. Effective use of ITNs could be helpful in the malaria elimination in India by 2030.
Keywords: Anopheles, Desert, Insecticide, Insecticide-treated nets (ITNs), Malaria
Introduction
Malaria is a major public health problem, causing 247 million cases and 0.619 million deaths throughout the world in 2021 (1). Eighty-seven countries and territories are endemic for malaria, and India contributes the majority of the eight million reported cases in the South-East Asia Region (2). Most of the malaria cases and deaths in India are reported from Chhattisgarh, Jharkhand, Madhya Pradesh, North Eastern States, Orissa, and Rajasthan (3).
The desert region of the Western Part of India was free from malaria and cases were reported after the introduction of the canal system for irrigation. The malaria epidemics occurred in this region from the mid-1980s onwards. The construction and introduction of three major canal systems namely, the Gang canal (1927), the Bhakra Sirhind feeder canal (1954), and the Indira Gandhi canal (1957) have a bearing on malaria epidemics in the region. These canals together irrigate about 14000 km2. Furthermore, large areas on the course of the canals, most notably the Indira Gandhi with its 9000 km of channels, are perennially inundated by seepage water from the irrigation channels. These inputs have raised the water label, altered the texture, water holding capacity, and salinity of the soil, and have affected crop patterns (4). The ambient temperature and humidity in the irrigated area have for some years changed in a way that suits mosquitoes.
There are six efficient vectors of malaria in India, of which three are common in central India, namely Anopheles stephensi, An. culicifacies s.l. and An. fluviatilis s.l. (5, 6). Malaria epidemics in this desert could not have occurred until either the existing desert species An. stephensi had drastically modified its behavior so as to be able to breed also in the rain-water collections after the monsoon. Anopheles stephensi is prevalent throughout the year but most abundant during months of rainfall (June–August) which coincides with the transmission period. In urban areas, it is generally endophilic and breeds in domestic containers, building construction sites, overhead tanks, underground cement tanks, and evaporator coolers (7, 8). In rural areas, An. stephensi is predominantly a zoophilic species and rests outdoors in cattle sheds, barracks and poorly constructed houses, and breeds in freshwater ponds, stream beds, seepage canals, wells, etc. Peak biting activity is recorded between 22:00 to 24:00 hours but varies seasonally in different localities (9, 10). It is an invasive species and enters new towns and settlements. Understanding the factors associated with the reduction of indoor mosquito bites and disease transmission in different settings is crucial for vector control and elimination.
The aim of the study was to evaluate the effectiveness of the insecticide in alternate forms like the use of insecticide-treated nets (ITNs) beneath the bed and insecticide-treated door and window curtains. The outcome of the study will provide information on the effectiveness of the usage of the alternate forms of the ITNs. This will be an additional control strategy against the vector-borne diseases.
Materials and Methods
Study area
The study site constituted six villages (Ujjala, Bilia, That, Kelwa, Nathusar, Devalpura) of the Pokaran Community Health Centre (CHC) of the Jaisalmer district. Jaisalmer (26.913°N 70.915°E) is located 575 kilometers (357 miles) west of the state capital Jaipur. The CHC has been selected because of the higher annual parasite index (API) (Table 1). All the study villages are located in rural areas. The housing pattern in the study sites is mixed of permanent, semi-permanent, and temporary in the ratio of 50, 2, and 48% respectively. Three villages were selected as control villages and three villages as study villages where treated nets were supplied. The study sites were selected by purposeful sampling. The control and study villages were selected based on population, mosquito density, slide positivity rate, annual parasite density, and geographical locations. The population and API of the three villages are shown in Table 1. The climate was dry with an average annual rainfall of about 93.5 mm and temperature varying between 5 °C to 49.2 °C. Study area was covered with many sand dunes and herbs. The area was habited with Acacia nilotica trees, and forests, which provide sufficient shelter for the resting and breeding habitat for mosquitoes. The villages have containers for storing drinking water for camels and cattle.
Table 1.
Number of house holds in the study villages, annual parasite index (API), and the mosquitoes species collected from the villages with density of Anopheles spp. Malaria cases in the form of the slide positivity rate (SPR), Plasmodium falciparum % (Pf%), P. vivax % (Pv%) reported from the study villages in Rajasthan State of India (2013–2015)
| Case/Control Village | Names of the villages | Population | Mosquito species | Per hour density of Anopheles | SPR | API | Pf% | Pv% | Mixed cases |
|---|---|---|---|---|---|---|---|---|---|
| Study villages | Nathusar | 2060 | An. subpictus s.l | 6 | 53.3% | 6.8 | 25% | 87.5 | 12.5% |
| An. stephensi | |||||||||
| Cx. quinquefasciatus | |||||||||
| That | 1189 | An. subpictus s.l. | 5 | 40% | 32 | 75% | 75% | 50% | |
| An. culicifacies s.l. | |||||||||
| Cx. quinquefasciatus | |||||||||
| Kelawa | 390 | An. subpictus s.l. | 6 | 28.6% | 17.9 | 50% | 100% | 50% | |
| An. stephensi | |||||||||
| Cx. quinquefasciatus | |||||||||
| Control Villages | Ujjala | 2250 | An. subpictus s.l. | 5 | 0% | 9.8 | 0 | 0 | 0 |
| An. stephensi | |||||||||
| Cx. quinquefasciatus | |||||||||
| Billia | 1152 | An. subpictus s.l. | 8 | 12% | 24.3 | 100% | 0 | 0 | |
| An. culicifacies s.l. | |||||||||
| Devalpura | 586 | An. culicifacies s.l. | 6 | 25% | 1.7 | 100% | 0 | 0 | |
| An. annularis |
Awareness Program
Meetings were fixed (July–August 2013) in each village before the initiation of the project. The villagers were informed about the introduction of the disease in their locality due to irrigation and changes in the water storage habitat. All the adult persons (> 18 years of age) present in the houses were invited to attend the awareness meeting. The villagers were also informed earlier regarding the problems and diseases caused by the vectors. They were informed about the interventions for the vector-borne diseases. They were also informed about the usefulness of the various methods for mosquito repellent and insecticide activity. They were told about the usefulness of the ITNs, how it is better than the conventional net, and how to use them. Before the start of the trial, community group meetings were organized in the study villages, and inhabitants were educated on proper and regular use of nets and the importance of the study.
Cross-sectional malaria prevalence surveys
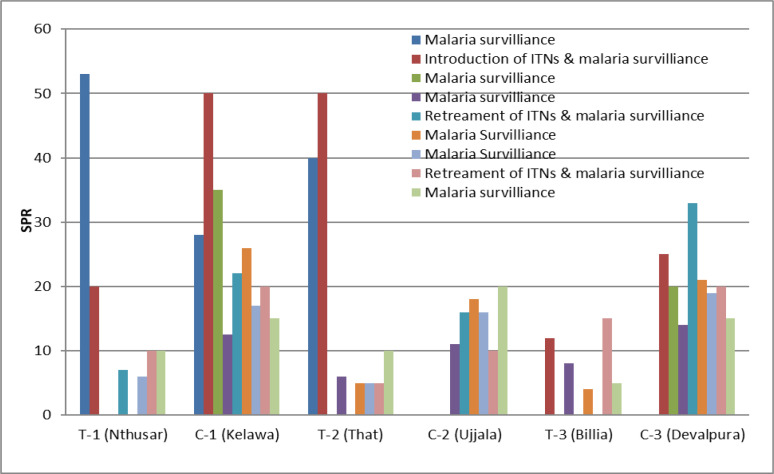
Cross-sectional malaria prevalence surveys were conducted in all the study villages during the pre-intervention, intervention, and post intervention phases (Fig. 4). The schedule for malaria prevalence surveys was announced 1 week in advance. 35% of the houses in each village were selected randomly and all the occupants of these houses were included in the survey. A blood smear was prepared from the finger-prick of everyone, irrespective of any clinical symptoms. Persons found positive for malaria parasites during cross-sectional surveys were treated with antimalarial drugs. The surveillance was conducted in the study villages from 2013 to 2015.
Fig. 4.
The Slide Positivity Rate (SPR) in the deltamethrin treated nets distributed villages (T-1, T-2, T-3) and control villages (C-1, C-2, C-3) during the pre-intervention, intervention and post-intervention period
Indoor mosquito collection
The mosquito collection was done in the six villages of the study site. The baseline survey was conducted in all six villages for the collection of entomological data. Baseline surveys were made in the post-monsoon period (September 2013 to January 2014). Indoor resting mosquitoes were collected in the selected houses for 15 minutes in each dwelling with the help of suction tubes using flashlights. The collections were made in the dawn from 0600–0700 hours. Mosquitoes were identified morphologically using standard keys used for the identification of medically important mosquito species Christophers (11), Barraud (12), Belkin (13), Reuben et al. (14) and Rattanarithikul et al. (15). The mosquitoes were brought to the laboratory in cloth cages, identified and kept under observations for 24 h under optimal conditions. The mean density of indoor-resting mosquitoes was calculated as person-man hour density (pmh).
Treating and distribution of nets
The technical staff of the NIIRNCD, Jodhpur (formerly Desert Medicine Research Centre, Jodhpur) treated the nets in the presence of the paramedical staff (state medical and health department), Anganwadi (Integrated Child Development Scheme) personnel and village level volunteers. The bed nets, cloth curtains, cloth partitions, and bed tops present in the households were treated with the insecticide by a target dose of 1 g of deltamethrin/m2 of netting. The Anganwadi personnel and village-level volunteers were trained for the net treatment.
Insecticide specifications
The insecticide used for the study was deltamethrin 2.5% suspension concentrate a synthetic pyrethroid. The product has been brought from Bayer India limited with the brand name K-Othrine flow.
Specification of net and distribution
The net material is polyester with a density of 34 g/m2 and 60 holes/cm2. Nets were procured from the local market and were weaved in a raschel design. The nets were treated with deltamethrin at 2% wt: wt, corresponding to 1 g/m2, and were made of white polyethylene of 100 denier strength with 156 mesh size. The requirement of the nets was ascertained through a sleeping pattern survey in the trial villages. Before the start of the trial, community group meetings were organized in the study villages, and inhabitants were educated on the proper and regular use of nets and the importance of the study. The distribution of nets as per the sleeping pattern survey was carried out in September 2013, and the number of treated nets distributed to each household was recorded in the register. The technical and supervisory staff of the Institute in consultation with the community constituted a village committee consisting of panchayat (governing body) members and other opinion leaders to monitor the proper use and maintenance of mosquito nets.
Sizes of the net and requirement of insecticide
The sizes of the nets were estimated, which were to be placed beneath the bed. Insecticides to be used for the door curtain and window curtains were also estimated.
Proper utilization of the nets
The nets were distributed in the study villages through the village-level volunteers. A total of 150 nets were treated using 2.5% Suspension Concentrate (SC) of deltamethrin with a target dose of 1g of deltamethrin/m2 and distributed without cost to all households. All safety measures were taken into considerations during net impregnation. Nets were provided to the villagers taking into account the number of beds that must be covered, and everyone should sleep on the beds with treated bed nets. All the wall-drobe, door and window curtains were also impregnated during the study. About 95% of the target population received the nets and did their door and window curtains impregnation. The nets for the absentees were covered in the second visit and distributed later. Proper use of the nets was demonstrated to the villagers and they were also informed that the ITNs were supplied to them for control of malaria.
Re-treatment of nets
Technical staff under the supervision of a scientist carried out the first re-treatment of the insecticide nets at the Community Centre and Common places of the village. The re-treatment was done six months after the distribution (September–March 2013). Deltamethrin 2.5% Suspension Concentrate (SC) was used at a dosage of 1 g of deltamethrin/m2 for each net. The second re-treatment was carried out after one year of the distribution and after six months of the first treatment. The health worker with the assistance of an Anganwadi worker and villager conducted the subsequent re-treatment in the three study villages. Advance information was sent to the villagers through village leaders. The re-treatment was done free of charge as per the protocol.
Re-treatment coverage
The first re-treatment coverage was assessed from the cross-sectional survey of 35% of houses. The second and third cross-sectional survey covered 35% houses on each occasion was also utilized to confirm the coverage of the 1st re-treatment.
Cone bioassays for determination of insecticide persistence
The WHO standard cone bioassay procedure was conducted to assess the efficacy and persistence of insecticide incorporated in nylon nets on prevailing mosquito vectors Three minutes exposure tests were carried out using An. stephensi and An. subpictus s.l. after 6, 12 and 18 months. Bioassays were conducted with WHO cones on netting samples, using wildly caught mosquitoes. Three sets of ten wild-caught mosquitos were used exposed per replicate. After the 3 minutes exposures, mosquitoes were aspirated from the cones and held in paper cups and provided with a 10% glucose solution. Mortality was recorded after 24 h. All the tests were carried out at 25±2 °C and 70±10% relative humidity and were replicated 2–3 times. Corrected mortality was calculated by Abbott’s formula.
Data analysis
The data collected through cross-sectional surveys were encoded and analyzed in a computer using M. S. Excel. The control mortality of mosquitoes recorded during the cone bioassays was corrected using the formula of Abbott (16).
Results
Base line survey
The population of the six study villages, the annual parasite index, baseline data on mosquito density and malaria cases of the study villages are shown in Table 1. Five species of Anopheles were collected from the study villages and were mentioned below. The other disease transmitting mosquitoes collected from the study villages were Culex quinquefasciatus and Aedes aegypti.
The entomological and malaria fever surveys were conducted during the baseline, intervention, and post-intervention periods. Mosquito collections were done at the interval of every three months in the control villages and insecticide-treated net-distributed villages. The mosquito density in the houses of the insecticide-treated net-distributed villages was reduced significantly (t-test, P= 0.016) than the houses of the control villages (Fig. 1). During the first evaluation of the intervention, the percentage of reduction of pmh of female anophe-line mosquito in Nathusar (T-1), That (T-2) andand Bilia (T-3) villages were 72.7%, 58.3% and 75% respectively (Fig. 1). During the second impact evaluation of intervention, the percentage of reduction in T-1, T-2 and T-3 villages were 81.8%, 75% and 87.5% respectively. Among the control villages, 33.3% pmh of female anopheline mosquito was increased in Kelawa (C-1) and Devalpura (C-3), whereas in Ujjalaa (C-2) village 18% deceased of pmh was reported during 1st post intervention survey. During the 2nd post intervention survey in the control villages, 87.5% increase of pmh of Anopheles was recorded in the village C-1 and no changes recorded in the village C-3. During third post intervention survey, pmh of female Anopheles was found to be increased than the second post intervention survey. The effectiveness of the insecticide treatment was found for six months, and this was concluded from the increase of pmh. Thereafter, a retreatment of nets was conducted and because of this, mosquito density in the houses of the insecticide-treated net-distributed villages were lower than in the houses of the control villages (Fig. 1).
Fig. 1.
The per man-hour density (pmh) of female anopheline mosquitoes in the deltamethrin-treated nets distributed villages (T-1, T-2, T-3) and control villages (C-1, C-2, C-3) of Rajasthan State of India (2013–2015) during the pre-intervention, intervention and post-intervention period
The pmh of female anopheline mosquito was reduced to 50% (T-1), 55.5% (T-2) and 37.5% (T-3) in the three-insecticide treated net-distributed villages after six months of second re-treatment of the nets. Similarly, after third retreatment of the nets, the pmh of female anopheline mosquito in the insecticide treated net-distributed villages was reduced to 30% (T-1), 45.4% (T-2) and 27.2% (T-3) after three months of treatment. The pmh of female anopheline was not reduced more than 27.2% in the control villages. The densities of mosquitoes differ significantly (t-test, P< 0.01) between the rooms where the impregnation nets were present and rooms without net.
Cone bioassays results
The efficacies (KD50) of the treated bed nets collected in different intervals from the field against An. stephensi and An. subpictus s.l. are mentioned in Fig. 2. The insecticide activity was decreased slowly, and the knockdown time (KD50) were found to be increased with duration of usage of net. The knock down time (KD50) of An. subpictus s.l. was found to be more than the An. stephensi.
Fig. 2.
The knockdown (KD50) times in seconds of Anopheles stephensi and Anopheles subpictus s.l. exposed against the treated nets at various interval of time
Excitorepellency
The density of the mosquito was recorded in the rooms where treated nets were kept and rooms where no treated nets were placed. When the pmh among the type of rooms were compared, rooms where ITNs were present found to be with significantly (P= 0.006) lower pmh of mosquitoes. The species of Anopheles mosquitoes reported from the study villages in decreasing order were An. subpictus s.l., An. stephensi, An. culicifacies s.l., An. annularis and An. pulcherimus.
Malaria surveillance
Malaria parasites, Plasmodium vivax and P. falciparum were reported from the study villages. The slide positivity rate (SPR) in the study villages T-1 and T-2 was higher than C-1 and C-2 during the pre-intervention period (Fig. 4). The slide positivity rate in T-1, T-2 and T-3 was 20, 50 and 12% during the introduction of ITNs and during 1st post evaluation no malaria case was recorded. The SPR in the study (ITN distributed) village T-2 and T-3 was 6 and 8% during the 2nd post evaluation. In the control villages, SPR was reported between 12.5–50% in C-1, 15–40% in C-3 and up to 20% in C-2. After the re-treatment of the ITNs, no malaria cases were reported in the village T-2 and T-3 and 7% SPR in the village T-1. During the 1st surveillance of malaria after retreatment, 5% and 4% SPR were reported from T-2 and T-3 and no cases in T-1. Thereafter, cases were found to be increased in T-1. After the retreatment of nets, malaria cases were found to be declined in T-3, but the cases were found to be increased in T-2. The percentage of reduction of malaria cases in the insecticide-treated net-distributed villages was higher than the control villages.
Discussions
Malaria is the most important tropical and parasitic disease in the world. Indoor residual spraying and long-lasting insecticidal nets have contributed greatly to malaria control over the past decade, but with an estimated 619000 deaths due to the disease in 2021, there is a clear need for additional interventions (1). Due to the emergence and spread of insecticide resistance in malaria vector mosquitoes in Africa and Asia, interventions not relying on insecticide have an important role to play (2).
During the present study, we have examined the effects of ITNs impregnated with deltamethrin 2.5% suspension concentration a synthetic pyrethroid bought from Bayer India limited with the brand name K-Ornithrine flow with a view to determine the effectiveness of the insecticide in alternate forms like the use of ITNs beneath the bed and insecticide-treated door and window curtains. Our study indicates that deltamethrin treated nets provided protection against Anopheles mosquitoes. The effectiveness of ITNs was found to be six months and thereafter density of mosquitoes was found to be increased in the houses (Fig. 2). Similarly, the malaria cases were found to be increased after six months of introduction of ITNs (Fig. 4). In view of this, re-treatment was given after six months of introduction of ITNs. The cases were declined after introduction of ITNs up to 18 months and thereafter cases were found to be increased (Fig. 1 and 4). This may happen due to the non-adsorbent of insecticide by the ITNs. The KD50 was found to be decreased after 9 months (Fig. 2) and supported the hypothesis. Jambulingam et al. (17) have evaluated the effectiveness of ITNs for control of malaria in a primary health centre of Odisha, India, and recorded reduction of malaria incidence from 32.7% to 7.2% in cold and rainy season respectively. Similarly in the present study we have recorded 30% reduction in malaria incidence (Fig. 4). The mosquito bites and presence of mosquito inside houses declined more than 70% and 19% respectively (Fig. 3). Furthermore, reduction in pmh of mosquitoes was recorded more than 70% (Fig. 3).
Fig. 3.
Per man-hour density of Anopheles stephensi and Anopheles subpictus s.l. in the roooms where treated nets were kept and rooms where bed without treated nets were kept
Sahu et al. (18) reported decline in API about 65% in the villages where treated nets were used. However, in present study it was observed 30% (Fig. 4). The aforementioned investigations also reported bioassay with 3 minutes exposure to treated bed nets and found 100% mortality of An. culicifacies s.l. for 2 months and An. fluviatilis s.l. for 4.5 months. However, in present study we observed KD50 for both An. stephensi and An. culicifacies s.l. seven seconds for 9-month-old ITNs (Fig. 2). Wu et al. (19) evaluated the bed nets impregnated with deltamethrin for malaria control in hypoendemic region of China and recorded mosquitos resting on the surface of the bed nets decreased significantly. The results also showed that malaria incidence decreased significantly both in areas where impregnated bed nets were used and in areas where residual spraying was undertaken. The observations made by Wu et al. (19) were like the present study (Fig. 4).
Rafinejad et al. (20) evaluated the bioefficacy of bed nets impregnated with various pyrethroids after repeated washings and recorded the deltamethrin and permethrin impregnated ITNs were more effective than etofenprox and bifenthrin. In the present investigation, the ITNs were impregnated with deltamethrin and found to be effective for a period of six months without re-treatment (Fig. 2 and 4). Kroeger et al. (21) evaluated the malaria control by the application of ITNs in 11 intervention localities and 11 control localities and recorded the decline of malaria incidence from 6.5 to 2.3%. In this study, malaria incidence in the control villages was increased. This finding was like the present study and up to 15% increase of slide positive rate was recorded in the control villages (Fig. 4). Xavier (22) evaluated the effectiveness of the deltamethrin treated ITNs against the malaria vector An. darlingi and recorded a decrease of the malaria incidences from 40 to 4%. The malaria incidence in the present investigation was decreased to 46%, 20% and 4% in the three study villages after the intervention of the ITNs (Fig. 4). The findings of the present study resemblance with the study of Xavier et al. (22). Rozendaal et al. (23) evaluated the effectiveness of the permethrin treated nets and recorded a 14% lower incidence of malaria in the villages of Suriname supplied with ITNs. This finding was similar to our study (Fig. 4). They also reported 58% mortality among mosquitoes leaving the hut with impregnated nets versus 27% mortality among those leaving a control hut.
Malaria is transmitted by Anopheles mosquitoes, and because there is currently no vaccine available, vector control is one of the most important means of malaria prevention. ITNs introduced over 20 years ago (24, 25), are an important tool to protect individuals against the morbidity and mortality caused by malaria (26, 27). ITNs can also decrease local malaria transmission by mass killing and decreasing the survival of anopheline vectors, thereby protecting those in the community without ITNs (28). The low cost, lack of need for special equipment, lack of logistical problems and compatibility with local customs are the advantages of the ITNs.
ITNs not only confer personal protection against infectious bites but can also reduce the survival, feeding frequency, feeding success, and density of vector mosquito populations (29, 30). Recent evidence suggests behavioral changes by malaria mosquito populations to avoid contact with ITNs by either feeding predominantly outdoors or in the early part of the evening. Such changes can drastically reduce the level of personal protection conferred by ITNs for obvious reasons.
Conclusion
ITNs have the potential to be used as an effective intervention tool against malaria vector not only in complex emergency situations but also in remote and inaccessible areas. The use of the ITNS in alternative places like beneath the bed and wardrobe was found to be effective. The use of ITNs in alternative places and ways were accepted by the population and the efficacy of the net was found to be effective beyond six months.
Acknowledgements
The authors are thankful to Mr. S. K. Dhawal, Mr. Rohit Prasad Joshi, Mr. Trilok Kumar and Mr. Mahavir Prasad for providing the technical assistance in the study. The first author is grateful to Indian Council of Medical Re-search, New Delhi for providing the fund as Intramural-Grant.
Footnotes
Ethical consideration
The Institutional Ethics Committees of the Indian Council of Medical Research National Institute of Implementation Research on Non-Communicable Diseases, Jodhpur approved the study protocol (DMRC/IEC/2013/4/NP/4).
Conflict of interest statement
The authors declare there is no conflict of interests.
References
- WHO (2022) World malaria report 2022. World Health Organization, Geneva, 1–14. [Google Scholar]
- WHO (2019) World malaria report 2019. World Health Organization, Geneva, 1–232. [Google Scholar]
- Ministry of Health and Family Welfare (2014) Strategic action plan for malaria control in India 2007–2012. Directorate of Health Services, National Vector Borne Disease Control Programme, New Delhi, India.
- Tyagi BK. (2004) A review of the emergence of Plasmodium falciparum-dominated malaria in irrigated areas of the Thar Desert, India. Acta Trop. 89(2): 227–239. [DOI] [PubMed] [Google Scholar]
- Singh N. (2009) A new global malaria eradication strategy: implications for malaria research from an Indian perspective. Trans R Soc Trop Med Hyg. 103(12): 1202–1203. [DOI] [PubMed] [Google Scholar]
- Singh N, Chand SK, Bharti PK, Singh MP, Chand G, Mishra AK, Shukla MM, Mahulia MM, Sharma RK. (2013) Dynamics of forest malaria transmission in Balaghat District, Madhya Pradesh, India. PLoS One. 8(9): e73730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SN, Subbarao SK, Choudhury DS, Pandey KC. (1993) Role of An. culicifacies and An. stephensi in malaria transmission in urban Delhi. Indian J Malariol. 30(3): 155–168. [PubMed] [Google Scholar]
- Chakraborty S, Ray S, Tandon N. (1998) Seasonal prevalence of Anopheles stephensi larvae and existence of two forms of the species in an urban garden in Calcutta City. Indian J Malariol. 35(1): 8–14. [PubMed] [Google Scholar]
- Nagpal BN, Sharma VP. (1995) Indian Anophelines. Oxford and IBH Publishing, New Delhi. [Google Scholar]
- Korgaonkar NS, Kumar A, Yadav RS, Kabadi D, Dash AP. (2012) Mosquito biting activity on humans and detection of Plasmodium falciparum infection in Anopheles stephensi in Goa, India. Indian J Med Res. 135(1): 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophers SR. (1933) The fauna of British India including Ceylon and Burma. Diptera Vol. IV, Family Culicidae, Tribe Anophelini. Taylor and Francis, London. [Google Scholar]
- Barraud PJ. (1934) The fauna of British India, including Ceylon and Burma. Diptera. Vol. V. Family Culicidae, Tribes Megarhinini and Culincini, Taylor and Francies, London.
- Belkin JN. (1962) Mosquitoes of the South Pacific (Diptera, Culicidae). 2 Volumes. Univ Calif Press. Berkeley. [Google Scholar]
- Reuben R, Tewari SC, Hiriyan J, Akiyama J. (1994) Illustrated keys to species of Culex (Culex) associated with Japanese encephalitis in Southeast Asia (Diptera: Culicidae). Mosq Syst. 26(2): 75–96. [Google Scholar]
- Rattanarithikul R, Harbach RE, Harrison BA, Panthusiri P, Jones JW, Coleman RE. (2005) Illustrated keys to the mosquitoes of Thailand. II. Genera Culex and Lutzia. Southeast Asian J Trop Med Public Health. 36(Suppl 2): 1–97. [PubMed] [Google Scholar]
- Abbott WS. (1987) A method of computing the effectiveness of an insecticide. J Am Mosq Control Assoc. 3(2): 302–3. [PubMed] [Google Scholar]
- Jambulingam P, Gunasekaran K, Sahu S, Vijayakumar T. (2008) Insecticide treated mosquito nets for malaria control in India-experience from a tribal area on operational feasibility and uptake. Mem Inst Oswaldo Cruz. 103(2): 165–171. [DOI] [PubMed] [Google Scholar]
- Sahu SS, Jambulingam P, Vijayakumar T, Subramanian S, Kalyanasundaram M. (2003) Impact of alphacypermethrin treated bed nets on malaria in villages of Malkangiri district, Orissa, India. Acta Trop. 89(1): 55–66. [DOI] [PubMed] [Google Scholar]
- Wu N, Qin L, Liao G, Zhou W, Geng W, Shi Y, Tan Y, Zhao K. (1993) Field evaluation of bednets impregnated with deltamethrin for malaria control. Southeast Asian J Trop Med Public Health. 24(4): 664–671. [PubMed] [Google Scholar]
- Rafinejad J, Vatandoost H, Nikpoor F, Abai MR, Shaeghi M, Duchen S, Rafi F. (2008) Effect of washing on the bioefficacy of insecticide-treated nets (ITNs) and long-lasting insecticidal nets (LLINs) against main malaria vector Anopheles stephensi by three bioassay methods. J Vector Borne Dis. 45(2): 143–50. [PubMed] [Google Scholar]
- Xavier PA, Lima JENS O. (1986) Uso de cortinas impregnadas com deltametrina no control da malária em garimpos no Território Federal do Amapá: nota prévia. Rev Bras Malariol Doencas Trop. 38: 137–139. [PubMed] [Google Scholar]
- Kroeger A, Mancheno M, Pesse K. (1995) Métodos paramejorar el control de la malaria en Ecuador y Colombia. Cayambe, Ecuador: Abya-Yala. Med Hyg. 50: 72–81. [Google Scholar]
- Rozendaal JA, Voorham J, Van Hoof JPM, Oostburg BFJ. (1989) Efficacy of mosquito nets treated with permethrin in Suriname. Med Vet Entomol 3: 353–365. [DOI] [PubMed] [Google Scholar]
- Lines JD, Myamba J, Curtis CF. (1987) Experimental hut trials of permethrin-impregnated mosquito nets and eave curtains against malaria vectors in Tanzania. Med Vet Entomol. 1(1): 37–51. [DOI] [PubMed] [Google Scholar]
- Miller JE, Lindsay SW, Armstrong JR. (1991) Experimental hut trials of bed-nets impregnated with synthetic pyre-throid or organophosphate insecticide for mosquito control in The Gambia. Med Vet Entomol. 5(4): 465–476. [DOI] [PubMed] [Google Scholar]
- Phillips-Howard PA, Nahlen BL, Kolczak MS, Hightower AW, Ter Kuile FO, Alaii JA, Gimnig JE, Arudo J, Vulule JM, Odhacha A, Kachur SP, Schoute E, Rosen DH, Sexton JD, Oloo AJ, Hawley WA. (2003) Efficacy of permethrin-treated bed nets in the prevention of mortality in young children in an area of high perennial malaria transmission in western Kenya. Am J Trop Med Hyg. 68(4 Suppl): 23–29. [PubMed] [Google Scholar]
- Alonso PL, Lindsay SW, Armstrong JR, Conteh M, Hill AG, David PH, Fegan G, de Francisco A, Hall AJ, Shenton FC, Greenwood BM, Conteh M, Cham K, Hill AG, David PH, Fegan G, Hall AJ. (1991) The effect of insecticide-treated bed nets on mortality of Gambian children. Lancet. 337(8756): 1499–1502. [DOI] [PubMed] [Google Scholar]
- Takken W. (2002) Do insecticide-treated bednets have an effect on malaria vectors? Trop Med Int Health. 7(12): 1022–1030. [DOI] [PubMed] [Google Scholar]
- Ordóñez González J, Kroeger A, Aviña AI, Pabón E. (2002) Wash resistance of insecticide-treated materials. Trans R Soc Trop Med Hyg. 96(4): 370–375. [DOI] [PubMed] [Google Scholar]
- Cibulskis RE, Alonso P, Aponte J, Aregawi M, Barrette A, Bergeron L, Fergus CA, Knox T, Lynch M, Patouillard E, Schwarte S, Stewart S, Williams R. (2016) Malaria: Global progress 2000–2015 and future challenges. Infect Dis Poverty. 5 (1): 61. [DOI] [PMC free article] [PubMed] [Google Scholar]