Abstract
Objective
This study was aimed to report the clinical characteristics of intramedullary schwannomas and discuss imaging findings and treatment strategies.
Methods
The inclusion criterion was consecutive patients with intramedullary schwannomas who were surgically treated at 8 centers between 2009 and 2020. Clinical characteristics included age, sex, clinical presentation, disease duration, and follow-up period. The modified McCormick scale was used to compare the preoperative and postoperative conditions. Pre- and postoperative magnetic resonance images (MRI) of each case were analyzed.
Results
The mean age of the total 11 patients at the operation was 50.2 years. The mean duration of the symptoms was 23 months, with limb paresthesia being the most common clinical presentation. The cervical spine was the most common localization level of the tumor in 6 cases. The mean follow-up duration was 49.4 months. Gross total resection (GTR) and subtotal resection (STR) was achieved in 9 and 2 cases, respectively. According to the modified McCormick scale at 6 months postoperatively, 7 cases (63.6%) had improved and 4 cases (36.3%) had unchanged grades. Typical MRI findings of the intramedullary schwannoma included ring-like enhancement, syringomyelia, cystic formation, intramedullary edema, and hemosiderin deposition. Gadolinium enhancement was homogenous in 8 cases (72.7%). The tumor margins were well demarcated in all cases.
Conclusion
Intramedullary schwannoma should be considered when sharp margins and well-enhanced tumors are present at the cervical spine level and the initial symptoms are relatively mild, such as dysesthesia. When GTR cannot be achieved, STR for tumor decompression is recommended.
Keywords: Intramedullary schwannoma, Magnetic resonance imaging, Gross total resection, Subtotal resection, Preoperative diagnosis
INTRODUCTION
Intramedullary schwannomas are rare tumors that contribute to 1.1% of spinal schwannomas and 0.3% of all intraspinal neoplasms [1-3].
Histologically, it is a benign tumor with a good prognosis if surgically removed. However, it is sometimes difficult to distinguish it from other intramedullary tumors, especially ependymomas and astrocytomas. In addition, there are cases in which intraoperative pathological examination does not reveal schwannoma, but the final pathological diagnosis reveals schwannoma [4]. These challenges make the determination of treatment strategy difficult.
Due to its rarity, the pathogenesis of intramedullary schwannoma is poorly understood. We report 11 cases of intramedullary schwannoma and discuss imaging findings and treatment strategies.
MATERIALS AND METHODS
This was a multicenter cohort study authorized by the Neurospinal Society of Japan. This study is a subanalysis of previous research and was approved by the Institutional Review Board of Tohoku University Hospital and the Ethics Committee (2021-1-130) of the relevant institution. This is a multicenter cohort study of 11 cases from 8 centers.
The inclusion criterion was consecutive patients with intramedullary schwannoma who were surgically treated at 8 centers between 2009 and 2020. Intramedullary schwannomas were defined as tumors that are pathologically diagnosed as schwannoma and present within the spinal cord, which can be identified on imaging; tumors with areas of exophytic extramedullary extension are also included.
Clinical characteristics included age, sex, clinical presentation, disease duration, and follow-up period. The modified McCormick scale was used to compare preoperative and postoperative conditions; mainly grades at discharge and 6 months postoperatively, were compared with those observed before surgery. Radiological data were collected from preoperative and postoperative images, lesion levels, and magnetic resonance imaging (MRI) findings. The degrees of excision and complications were evaluated from surgical records.
RESULTS
1. Patient Demographics
Eleven patients were identified. The mean patient age at the time of operation was 50.2 years (range, 13–73 years). The patient demographics are summarized in Table 1. Of the 11 patients, 5 (45%) were male and 6 (55%) were female. The mean duration of symptoms was 23± 18.8 months, with limb paresthesia (90.9%) being the most common clinical presentation, followed by limb weakness (66.6%), gait disturbance (63.6%), limb pain (45.4%), head, neck, or back pain (27.2%), and bladder and/or bowel disturbance (27.2%).
Table 1.
Clinical features of all cases with intramedullary schwannoma
| No. | Age (yr)/sex | Duration of illness (mo) | Symptom | Surgery | Modified McCormick scale |
Location/origin of tumor | FU (mo) | Preoperative diagnosis | NF | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Last FU | |||||||||
| 1 | 68/M | 24 | Limb pain, limb paresthesia, limb weakness, gait disturbance | GTR | 2 | 1 | 1 | T12–L1/NA | 26 | Myxopapillary ependymoma | No |
| 2 | 52/F | 36 | Limb pain, limb paresthesia | GTR | 2 | 2 | 1 | L1/dorsal root | 32 | schwannoma | No |
| 3 | 71/M | 36 | Limb paresthesia, limb weakness, gait disturbance, bladder/bowel disturbance | STR | 2 | 2 | 2 | T10–L1/dorsal root | 23 | Schwannoma | No |
| 4* | 13/M | None | GTR | 1 | 1 | 1 | C1–2/dorsal root | 22 | Schwannoma | NF1 | |
| 5 | 72/F | 2 | Limb pain, limb paresthesia, limb weakness, gait disturbance, bladder/bowel disturbance | GTR | 2 | 2 | 1 | C2–7/dorsal root | 78 | Schwannoma | No |
| 6 | 29/M | 2 | Limb pain, limb paresthesia, limb weakness, gait disturbance | GTR | 2 | 2 | 1 | C4–5/intramedullary | 19 | Schwannoma | No |
| 7* | 43/M | 60 | Neck and back pain, limb pain, limb paresthesia | GTR | 2 | 3 | 2 | C3–5/intramedullary | 24 | Ependymoma | NF |
| 8 | 48/F | 13 | Limb paresthesia, limb weakness | STR | 2 | 2 | 1 | C4–6/NA | 40 | Schwannoma | No |
| 9 | 47/M | 36 | Headache, neck and back pain, limb paresthesia, limb weakness, gait disturbance | GTR | 3 | 2 | 2 | C3–5/dorsal root | 38 | Extramedullary tumor | No |
| 10† | 37/M | 14 | Neck and back pain, limb pain, limb paresthesia, limb weakness, gait disturbance, bladder/bowel disturbance | GTR | 4 | 4 | 4 | T9–10/intramedullary | 70 | Ependymoma | NF2 |
| 11 | 73/F | 7 | Limb paresthesia, limb weakness, gait disturbance | GTR | 2 | 3 | 3 | T10–11/NA | 23 | Ependymoma | No |
FU, follow-up; NF, neurofibromatosis; NA, not available; GTR, gross total resection; STR, subtotal resection.
Neurofibromatosis type 1.
Neurofibromatosis type 2.
2. Tumor Levels, Surgical Treatment, and Postoperative Course
The localization level of the tumor was the cervical spine in 6 cases (46.1%), cervicothoracic spine in 1 case (7.6%), thoracic spine in 2 cases (15.3%), and thoracolumbar spine in 2 cases (15.3%), and the cervical spine being the most common site. The affected nerve root could be identified intraoperatively in 8 patients, all of whom were considered to have a posterior root origin. In 4 of these cases, the affected nerve root was confirmed to have been resected. Of these patients, 3 had postoperative improvement of neurological symptoms, and none had deterioration.
Surgical resection was performed in all cases: 9 patients (81.8%) underwent gross total resection (GTR) and 2 (18.1%) underwent subtotal resection (STR). In 5 cases, the nerve root of origin of the tumor was resected intraoperatively, and there were no cases of postoperative worsening of symptoms. In the case of the patient who failed to achieve STR, the motor evoked potential disappeared intraoperatively, and tumor removal was abandoned. In the other case, the tumor was strongly adherent near the dorsal root entry zone on the spinal cord surface, and there was potential for neurological symptoms after removal, so STR was performed.
Immediately post operation, symptoms improved from the preoperative state in 5 patients (45.4%), remained unchanged in 2 patients (18.8%), and worsened in 4 patients. Three of them had tumors without extramedullary lesions, and one had a tumor with extramedullary lesions that was strongly intramedullary embedded. On the modified McCormick scale, the grade improved in 2 cases (18.8%), remained unchanged in 7 cases (63.6%), and worsened in 2 cases (18.8%).
On the same scale at 6 months postoperatively, 7 (63.6%) and 4 (36.3%) cases showed improved and unchanged grades, respectively. The grades on the modified McCormick scale temporarily worsened in some patients immediately after surgery (at discharge); however, these patients showed functional improvement at 6 months postoperatively. The patients’ grades on the McCormick scale are shown before, immediately after, and 6 months after surgery. Complications occurred in 1 case, and the patient underwent reoperation due to postoperative intramedullary hemorrhage. The patients with postoperative intramedullary hemorrhage were those with a hemosiderin cap on preoperative MRI. One patient underwent STR at the time of initial surgery experienced local recurrence and underwent reoperation (Table 2).
Table 2.
Postoperative course and complications
| Characteristic | No. (%) |
|---|---|
| Symptom at discharge | |
| Improved | 5 (45.4) |
| Unchanged | 2 (18.8) |
| Worsened | 4 (36.3) |
| Modified McCormick scales at discharge | |
| Improved | 2 (18.8) |
| Unchanged | 7 (63.6) |
| Worsened | 2 (18.8) |
| Modified McCormick scales 6 months | |
| Improved | 7 (63.6) |
| Unchanged | 4 (36.3) |
| Worsened | 0 (0) |
| Surgical complication | |
| Cerebrospinal fluid leak | 0 (0) |
| Postoperative hematoma | 1 (9.0) |
| Surgical site infection | 0 (0) |
| Local recurrence | 1 (9.0) |
3. MRI Findings (Table 3)
Table 3.
Summary of magnetic resonance imaging findings of 11 cases
| Case No. | T1 | T2 | Enhancement | Edema | Cystic lesion | Extramedullary lesion | Hemosiderin deposition | Syringomyelia | Tumor length (vertebral segment) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Iso | Iso-high | Homogenous | - | + | + | - | - | 1 |
| 2 | Iso | Iso-high | Homogenous | + | + | + | - | + | 1 |
| 3 | Iso | Iso-high | Homogenous | - | - | + | - | - | 1 |
| 4* | Iso | High | N/A | - | - | + | - | - | 1 |
| 5 | Iso | High | Homogenous | - | + | + | - | - | 5 |
| 6 | Low-iso | Iso-high | Ring-like | + | + | - | - | - | 2 |
| 7 | Low-high | Iso-high | Heterogenous | + | + | - | + | - | 3 |
| 8 | N/A | Iso-high | Homogenous | + | + | + | + | - | 3 |
| 9 | Iso | Iso-high | Homogenous | + | - | + | - | - | 2 |
| 10† | Iso | Iso-high | Homogenous | - | + | - | - | + | 2 |
| 11 | N/A | Iso-high | Homogenous | + | + | - | - | - | 1 |
N/A, not available.
Neurofibromatosis type 1.
Neurofibromatosis type 2.
T1-weighted MRI scans showed iso intensity in 7 cases (63.6%) and a mixture of low to iso and high intensity in 2 cases (18.1%). T2-weighted images showed mixed iso-to high intensity in 9 cases (81.8%) and high intensity in 2 cases (18.1%). Tumor length (including cystic lesions) was 1 vertebra in 5 cases (45.4%), 2 vertebrae in 3 cases (27.2%), 3 vertebrae in 2 cases (18.1%), and 5 vertebrae in 1 case (5.5%). The border with the surrounding spinal cord was clear in all the cases. Gadolinium enhancement was homogenous in 8 cases (72.7%), heterogeneous enhancement in 1 (9.0%), and ring-like enhancement in 1 (9.0%).
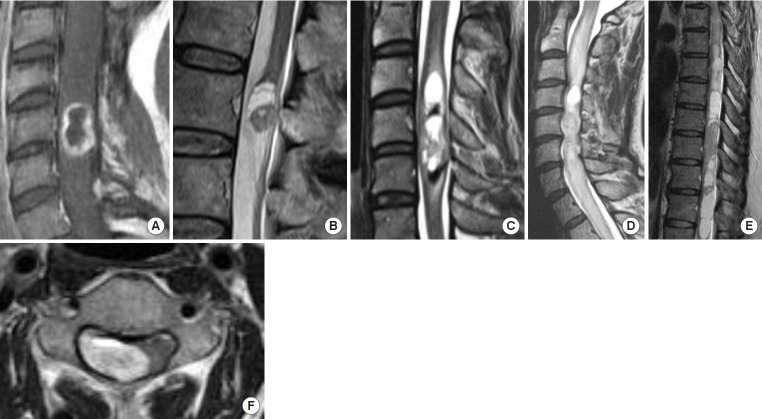
Associated syringomyelia was found in 2 cases (18.1%), cystic lesions in 8 (72.7%), intramedullary edema in 6 (54.5%), and hemosiderin deposition in 2 cases (18.1%). In the 2 cases with syringomyelia, the syringomyelia shrank with removal of the tumors. The extramedullary component of the tumor was found in 7 cases (63.6%), and the remaining 4 cases (36.3%) had an intramedullary component of the tumor (Fig. 1).
Fig. 1.
Representative magnetic resonance imaging findings of intramedullary schwannoma reviewed in this study. (A) Ring-like enhancement. (B) Cyst formation. (C) Hemosiderin. (D) Edema. (E) Syringomyelia. (F) Exophytic lesion.
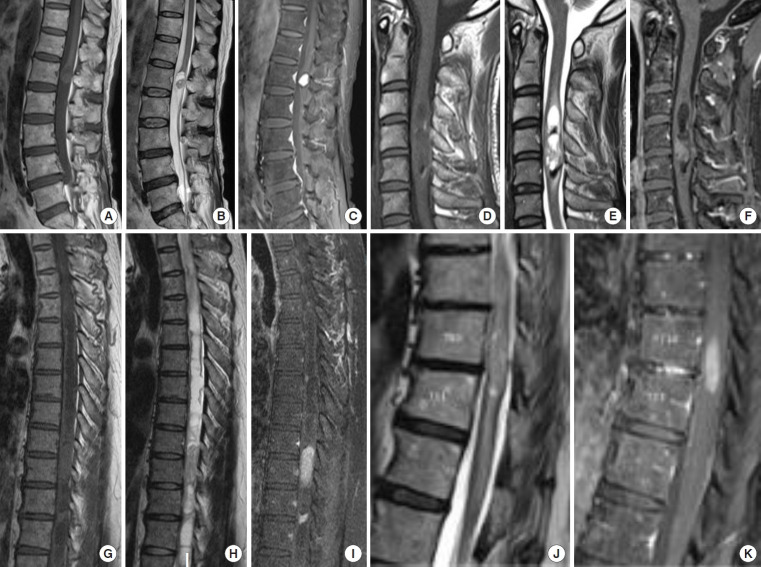
Preoperative diagnosis of schwannoma was possible in 6 cases, and 4 cases were diagnosed as ependymoma. These tumors were enhanced with gadolinium and were accompanied by cystic lesions, syringomyelia, and hemosiderin deposition, which are also seen in ependymomas. All 6 patients with preoperative diagnosis of schwannoma had extramedullary regions on preoperative MRI. Three patients diagnosed with ependymoma did not have exophytic lesions (Fig. 2, Table 1).
Fig. 2.
Preoperative magnetic resonance imaging (MRI) findings in patients with preoperative diagnosis of ependymoma. (A–C) Case 1. Preoperative MRI showing an isointense mass on the sagittal T1-weighted image (A), iso-high intense on T2 weighted image (B), and homogenously enhanced on T1-weighted image with gadolinium with cystic lesion at the conus medullaris (C). (D–F) Case 7. Preoperative MRI showing an isointense mass on the sagittal T1 weighted image (D), mixed intense on T2 weighted image with hemosiderin deposition (E), and irregularly enhanced on T1-weighted image with gadolinium at the C3–5 level (F). (G–I) Case 10. Preoperative MRI showing an isointense mass on the sagittal T1-weighted image (G), mixed intense on T2- weighted image with syringomyelia (H), and homogenously enhanced on T1-weighted image with gadolinium at the T9–10 level (I). (J, K) Case 11. Preoperative MRI showing an iso-high intense mass on T2-weighted image with edema (J) and homogenously enhanced on T1-weighted image with gadolinium at the T10–11 level (K).
Clinical features of all cases are shown in Table 1.
DISCUSSION
Intramedullary schwannomas have a frequency of 0.3% in all intraspinal neoplasms and 1.1% in spinal schwannomas, making it a very rare tumor [1-3]. They occur more frequently in men at a ratio of 3:1, and the average age of onset is reported to be 40 years, with very few pediatric cases [3,5]. The most common site of occurrence is the cervical spine, followed by the thoracic and lumbar spine [6]. In this study, the mean patient age at the operation was 50.2 years. The number of female patients (55%) was slightly different from that in previous reports, as 6 (55%) were predominant. The present study is a subanalysis of a previous multicenter study [7] which included 1,033 cases of intramedullary tumors. Among the 1,033 intramedullary spinal cord tumors, intramedullary schwannomas accounted for 1.06% of tumors during the same period. Based on previous reports, Ross et al. [1] estimated the frequency of intramedullary schwannomas to be 0.3% of all intramedullary neoplasms and 1.1% of all spinal cord schwannomas. The present study is significant because it directly presents the frequency of intramedullary schwannomas among spinal intramedullary tumors.
The unique feature of this study was not to report the status of treatment at a single institution, but to identify the actual treatment and status of intramedullary schwannoma in neurosurgical facilities in Japan, and to reveal the treatment and incidence of this disease currently being performed in Japan. Treatment results were generally comparable to previous reports, and the treatment system was considered to be basically well maintained on a nationwide basis.
The possible association between neurofibromatosis (NF) and intramedullary schwannoma has been reported from Japan, where it occurred in 2.2% of cases of NF type 1 (NF1) [8]. In addition, a literature review reported 13.6% of those occurring in NF1 and NF type 2 (NF2) [9]. In the present study, 1 case was observed in NF1 and 1 case in NF2, which suggests that intramedullary schwannoma can occur in NF as well as other types of schwannoma.
The pathogenesis of intramedullary schwannoma remains unclear. The following developmental mechanisms are thought to be responsible for the development of Schwann cell-derived tumors that exist in peripheral nerves within the spinal cord and central nervous system.
(1) Conversion of pial mesodermal cells into neuroectodermal Schwann cells [10].
(2) Schwannoma arises from Schwann cells in the dorsal root entry zone and extends intramedullary [11,12].
(3) Schwannoma arises from Schwann cells under the perivascular nerve plexus of spinal cord vessels, resulting in subpial extension [13].
(4) Schwannosis occurs in proximity of the anterior spinal artery [14].
(5) Schwannomas occur due to the intrusion of neural crest cells during embryogenesis [15].
(6) They result from inadequate regeneration of the spinal cord after trauma or chronic disease [16].
The localization and extension of the tumors in the cases included in this study can be divided into 2 main types: those that are completely intramedullary. One was completely intramedullary and the other was intramedullary to extramedullary extension. Considering these developmental mechanisms and tumor localization, each type may have a different developmental mechanism. The location within the spinal cord where the above mechanisms occur may determine the morphology of the tumor. That is, tumors that arise near the surface of the spinal cord are thought to have exophytic lesions, while tumors that arise in more central areas of the spinal cord are thought to have intramedullary lesions (Fig. 3).
Fig. 3.
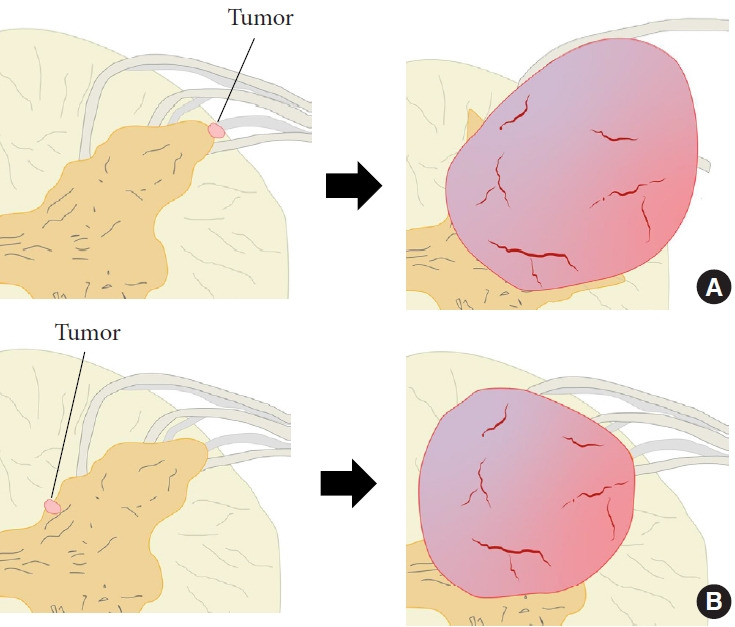
Classification of tumor form based on the location of tumor origin. (A) Tumor originates from the superficial layer within the spinal cord. (B) Tumor originates near the center within the spinal cord. Red area, tumor; brown area, gray matter; yellow area, white matter; gray area, root.
One of the problems in the treatment of intramedullary schwannomas is the difficulty of preoperative diagnosis. The problem with preoperative MRI is its inability to differentiate schwannomas from other intramedullary tumors, such as ependymomas and astrocytomas, which are relatively more common among intramedullary tumors. Since the surgical strategies for these tumors are different from those for schwannoma, it is worthwhile to identify imaging differentiators [17]. In addition, owing to the histological and structural characteristics of the tumor (Antony type A and type B), spinal schwannomas present various magnetic resonance (MR) appearances, such as cystic formation and ring-like enhancement [18].
In a report by Gupta et al. [19], MRI findings were a mixture of T2 hyperintensity changes and T2 isointense tumors. The tumor was strongly enhanced with the use of gadolinium. In addition, a cyst formed caudally to the tumor, which could be considered as a differential diagnosis for ependymomas or astrocytomas. The tumor was completely intramedullary, with edema around the tumor. Sekar et al. [20] reported a case of cervical intramedullary schwannoma with a hemosiderin cap sign that was difficult to distinguish from that observed in ependymoma. The causes of bleeding in schwannoma include spontaneous thrombosis of vessels with distal tumor necrosis, traction of vascular attachments to the nerve roots, neovascularization, and central ischemic necrosis [21]. In the present study, hemosiderin deposition was observed in 2 cases. This finding also makes differentiation from other intramedullary tumors difficult. Wu et al. [17] compared imaging findings and clinical symptoms in 8 intramedullary schwannoma cases and 243 glioma (ependymoma and astrocytoma) cases treated at the same time. In schwannomas, hypointensity on T1-weighted images, hyperintensity or mixed intensity on T2-weighted images, and associated syringomyelia and cysts were observed. However, these findings showed no significant differences between gliomas and intramedullary schwannomas. The authors suggest that the initial presentation of somatic pain or root pain is significantly common in patients with intramedullary schwannoma and that a preoperative diagnosis should be made based on the imaging findings and clinical course of the disease. In the present case series, as in previous reports, 90.9% of the cases showed limb paresthesia as the initial symptom. Many of our cases showed T1 iso intensity and T2 mixed iso-to high intensity on MRI. Similarly, syringomyelia and cystic formation were seen in some cases, and in addition, hemosiderin deposition was seen in some cases. These are common findings in ependymoma, thus the many similarities in imaging findings may be a factor that contributes to the difficulty in differentiating ependymoma from intramedullary schwannoma [20]. On the other hand, most of the tumor lengths including cystic lesions in the present study cases are less than 3 vertebral, and even shorter if only the enhanced region is considered. Ependymoma has been reported to have a tumor length of 3–4 vertebral levels [22,23], and the relatively short tumor length may be a point of differentiation between intramedullary schwannomas and ependymomas. We believe that the tumor is detected at a relatively small stage because schwannoma is a benign tumor and develops slowly, reflecting the nature of benign tumors. Tumors with extramedullary lesions may involve nerve roots relatively early in the process of tumor development and may develop and be diagnosed with dysesthesia or pain.
The characteristic findings of intramedullary schwannoma include a clear border between the tumor and the surrounding spinal cord on MRI and a strong enhancing effect [24]. In addition to intramedullary tumors, some studies have reported that contrast-enhanced MRI findings of enlarged roots contiguous with the tumor may be helpful in the diagnosis of intramedullary schwannoma [25,26].
Because schwannoma is a benign tumor, the dissection plane is usually clear, and GTR is likely to be achieved. If there is a strong adhesion between the tumor and the surrounding nerves or spinal cord, additional neurological symptoms may be exacerbated postoperatively if the tumor is removed for GTR. Therefore, if the dissection plane is unclear, STR should also be an option to avoid surgical complications [2,4,27]. For large tumors, staged operations should be considered to minimize the occurrence of postoperative neurological deficit [28]. In our case, one patient who underwent STR showed symptomatic improvement and one patient’s neurological symptoms remained unchanged postoperatively. As observed in the present study, long-term follow-up is important because of the possibility of recurrence and symptom relapse in patients who underwent STR [29]. Postoperative chemotherapy or radiation therapy is not recommended, and tumor resection is considered if the residual tumor progresses or symptoms develop [4].
CONCLUSION
Intramedullary schwannoma presents with a variety of imaging findings, making a preoperative diagnosis from imaging difficult, especially in the absence of exophytic lesions, which makes its differentiation from other intramedullary tumors difficult. Therefore, a differential diagnosis must be considered based on the clinical course and symptoms.
Schwannomas should be considered, especially when sharp margins and well-enhanced tumors are present at the level of the cervical spine and the initial symptoms are relatively mild, such as pain or dysesthesia. Schwannomas are benign tumors, and a good clinical outcome can be expected if GTR is achieved. However, it is possible to control the tumor without worsening neurological symptoms, even with STR; therefore, it is important to limit the treatment to STR if GTR is not feasible.
Acknowledgments
Dr Shinji Yamamoto: Provide case data; Dr Ryu Kurokawa: Provide case data.
Footnotes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: TH, MM, TE; Formal Analysis: TH; Methodology: TH, MM, TE; Project Administration: MM, KH, TE; Data curation: Toru S, YM, HU, HO, Taku S, YT, TE; Writing – Original Draft: TH; Writing – Review & Editing: MM, KH, Toru S, YM, HU, HO, Taku S, YT, YO, AK, TE.
REFERENCES
- 1.Ross DA, Edwards MS, Wilson CB. Intramedullary neurilemomas of the spinal cord: report of two cases and review of the literature. Neurosurgery. 1986;19:458–64. doi: 10.1227/00006123-198609000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Singh R, Chaturvedi S, Pant I, et al. Intramedullary schwannoma of conus medullaris: rare site for a common tumor with review of literature. Spinal Cord Ser Cases. 2018;4:99. doi: 10.1038/s41394-018-0134-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicácio JM, Rodrigues JC, Galles MH, et al. Cervical intramedullary schwannoma: a case report and review of the literature. Rare Tumors. 2009;1:e44. doi: 10.4081/rt.2009.e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SE, Chung CK, Kim HJ. Intramedullary schwannomas: long-term outcomes of ten operated cases. J Neurooncol. 2013;113:75–81. doi: 10.1007/s11060-013-1091-9. [DOI] [PubMed] [Google Scholar]
- 5.Landi A, Grasso G, Gregori F, et al. Isolated pediatric intramedullary schwannoma: case report and review of literature. World Neurosurg. 2018;115:417–20. doi: 10.1016/j.wneu.2018.04.220. [DOI] [PubMed] [Google Scholar]
- 6.Yang T, Wu L, Deng X, et al. Clinical features and surgical outcomes of intramedullary schwannomas. Acta Neurochir. 2014;156:1789–97. doi: 10.1007/s00701-014-2168-8. [DOI] [PubMed] [Google Scholar]
- 7.Endo T, Inoue T, Mizuno M, et al. Current trends in the surgical management of intramedullary tumors: a multicenter study of 1,033 patients by the Neurospinal Society of Japan. Neurospine. 2022;19:441–52. doi: 10.14245/ns.2244156.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yagi T, Ohata K, Haque M, et al. Intramedullary spinal cord tumour associated with neurofibromatosis type 1. Acta Neurochir. 1997;139:1055–60. doi: 10.1007/BF01411560. [DOI] [PubMed] [Google Scholar]
- 9.Riffaud L, Morandi X, Massengo S, et al. MRI of intramedullary spinal schwannomas: case report and review of the literature. Neuroradiology. 2000;42:275–9. doi: 10.1007/s002340050885. [DOI] [PubMed] [Google Scholar]
- 10.Swiatek VM, Stein KP, Cukaz HB, et al. Spinal intramedullary schwannomas-report of a case and extensive review of the literature. Neurosurg Rev. 2021;44:1833–52. doi: 10.1007/s10143-020-01357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Decharin P, Suvithayasiri S, Nivatpumin P, et al. Subpial schwannoma of the cervical spinal cord: a case report and its intraoperative finding supporting a theory of the pathogenesis of an intramedullary schwannoma. Asian J Neurosurg. 2022;17:108–11. doi: 10.1055/s-0042-1748785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Brien DF, Farrell M, Fraher JP, et al. Schwann cell invasion of the conus medullaris: case report. Eur Spine J. 2003;12:328–31. doi: 10.1007/s00586-002-0484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riggs HE, Clary WU. A case of intramedullary sheath cell tumor of the spinal cord; consideration of vascular nerves as a source of origin. J Neuropathol Exp Neurol. 1957;16:332–6. doi: 10.1097/00005072-195707000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Adelman LS, Aronson SM. Intramedullary nerve fiber and Schwann cell proliferation within the spinal cord (schwannosis) Neurology. 1972;22:726–31. doi: 10.1212/wnl.22.7.726. [DOI] [PubMed] [Google Scholar]
- 15.Ramamurthi B, Anguli VC, Iyer CG. A case of intramedullary neurinoma. J Neurol Neurosurg Psychiatry. 1958;21:92–4. doi: 10.1136/jnnp.21.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolman L. Post-traumatic regeneration of nerve fibres in the human spinal cord and its relation to intramedullary neuroma. J Pathol Bacteriol. 1967;94:123–9. doi: 10.1002/path.1700940116. [DOI] [PubMed] [Google Scholar]
- 17.Wu L, Yao N, Chen D, et al. Preoperative diagnosis of intramedullary spinal schwannomas. Neurol Med Chir. 2011;51:630–4. doi: 10.2176/nmc.51.630. [DOI] [PubMed] [Google Scholar]
- 18.Friedman DP, Tartaglino LM, Flanders AE. Intradural schwannomas of the spine: MR findings with emphasis on contrast-enhancement characteristics. AJR Am J Roentgenol. 1992;158:1347–50. doi: 10.2214/ajr.158.6.1590138. [DOI] [PubMed] [Google Scholar]
- 19.Gupta A, Nair BR, Chacko G, et al. Cervical intramedullary schwannoma mimicking a glioma. Asian J Neurosurg. 2015;10:42–4. doi: 10.4103/1793-5482.151509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sekar S, Vinayagamani S, Thomas B, et al. Haemosiderin cap sign in cervical intramedullary schwannoma mimicking ependymoma: how to differentiate? Neuroradiology. 2019;61:945–8. doi: 10.1007/s00234-019-02229-6. [DOI] [PubMed] [Google Scholar]
- 21.Kukreja S, Ambekar S, Sharma M, et al. Cauda equina schwannoma presenting with intratumoral hemorrhage and intracranial subarachnoid hemorrhage. J Neurosurg Spine. 2014;21:357–60. doi: 10.3171/2014.5.SPINE131014. [DOI] [PubMed] [Google Scholar]
- 22.Watts J, Box GA, Galvin A, et al. Magnetic resonance imaging of intramedullary spinal cord lesions: a pictorial review. J Med Imaging Radiat Oncol. 2014;58:569–81. doi: 10.1111/1754-9485.12202. [DOI] [PubMed] [Google Scholar]
- 23.Do-Dai DD, Brooks MK, Goldkamp A, et al. Magnetic resonance imaging of intramedullary spinal cord lesions: a pictorial review. Curr Probl Diagn Radiol. 2010;39:160–85. doi: 10.1067/j.cpradiol.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Kodama Y, Terae S, Hida K, et al. Intramedullary schwannoma of the spinal cord: report of two cases. Neuroradiology. 2001;43:567–71. doi: 10.1007/s002340100540. [DOI] [PubMed] [Google Scholar]
- 25.Colosimo C, Cerase A, Denaro L, et al. Magnetic resonance imaging of intramedullary spinal cord schwannomas. Report of two cases and review of the literature. J Neurosurg. 2003;99(1 Suppl):114–7. doi: 10.3171/spi.2003.99.1.0114. [DOI] [PubMed] [Google Scholar]
- 26.Ho T, Tai KS, Fan YW, et al. Intramedullary spinal schwannoma: case report and review of preoperative magnetic resonance imaging features. Asian J Surg. 2006;29:306–8. doi: 10.1016/S1015-9584(09)60108-1. [DOI] [PubMed] [Google Scholar]
- 27.Ohtonari T, Nishihara N, Ota T, et al. Intramedullary schwannoma of the conus medullaris complicated by dense adhesion to neural tissue. Neurol Med Chir. 2009;49:536–8. doi: 10.2176/nmc.49.536. [DOI] [PubMed] [Google Scholar]
- 28.Hida K, Yano S, Iwasaki Y. Staged operation for huge cervical intramedullary schwannoma: report of two cases. Neurosurgery. 2008;62(5 Suppl 2):ONS456–60. doi: 10.1227/01.neu.0000326035.54714.cc. discussion ONS460. [DOI] [PubMed] [Google Scholar]
- 29.Dhake RP, Chatterjee S. Recurrent thoracic intramedullary schwannoma: report of two cases with long term follow up. Br J Neurosurg. 2022;36:647–50. doi: 10.1080/02688697.2019.1566516. [DOI] [PubMed] [Google Scholar]