Abstract
Objectives
Chronic total occlusion (CTO) of the right coronary artery (RCA) is common in patients with coronary artery disease. Although revascularization techniques and success rates have improved significantly in recent years, there are still no studies investigating possible effects of successful recanalization of RCA CTO on the right-ventricular (RV) function. With this study, we aimed to evaluate RV function after recanalization of the RCA by two-dimensional transthoracic echocardiography (2DE) and additional two-dimensional speckle-tracking echocardiography (2DSTE).
Methods and results
Our analysis included 102 patients undergoing successful RCA CTO recanalization at the University Medical Center of Mainz. All patients underwent 2DE and 2DSTE to assess RV function before PCI procedure and 6 months after successful revascularization. We found an altered RV function in our collective at baseline assessed by 2DSTE with a significant improvement at 6 month follow-up (baseline RV free wall strain: − 20.7 [− 6.3 to − 32.0] % vs. − 23.4 [− 8.3 to − 39.3] % at follow-up, p < 0.001 and baseline RV global strain − 15.9 [− 6.0 to − 25.7] % vs. − 17.9 [− 7.0 to − 29.5] % at follow-up, p < 0.001).
Conclusion
RV function was altered in patients with RCA CTO and showed significant improvement after successful recanalization. We also noticed an improvement in patient-reported clinical symptoms. Our study suggests that CTO procedure is a beneficial treatment option in symptomatic patients with RCA CTO.
Keywords: Chronic total occlusion (CTO), Percutaneous coronary intervention (PCI), Coronary artery disease, Right ventricle (RV), Two-dimensional speckle-tracking echocardiography (2DESTE)
Introduction
The complex anatomy and the volume and pressure dependency represent a challenge for accurate assessment of the right ventricle (RV) with the conventional cardiovascular imaging techniques. As a result, assessment of the RV was neglected for a long time and studies focused on the left ventricle [1]. Impaired RV function is associated with a worse outcome of patients with pulmonary hypertension, heart failure and coronary artery disease [2–8]. RV function not only has prognostic relevance for patients, but also has a decisive influence on clinical symptoms and exercise capacity [9, 10]. The RV is routinely assessed by two-dimensional echocardiography (2DE). The conventional functional parameters TAPSE (tricuspid annular plane systolic excursion), 2D RV-FAC (fractional area change), and TDI S’ (Doppler-derived tricuspid lateral annular systolic velocity) are recommended by the American Society of Echocardiography and the European Association of Cardiovascular Imaging to assess the systolic function of the RV [11, 12]. More recently, two-dimensional speckle-tracking echocardiography (2DESTE) is increasingly becoming the focus of interest as a reproducible, accessible, and accurate method to investigate RV function [13–15].
Coronary artery disease is one of the leading causes of death worldwide [16]. Register studies show that 15–25% of patients with coronary artery disease have a chronic total occlusion (CTO) of at least one coronary artery [17].
Several studies have investigated previously the effect of successful CTO percutaneous coronary intervention (PCI) on left-ventricular function, but data on the RV function are lacking [18, 19]. With this study, we aimed to investigate RV function in patients with RCA CTO assessed by two-dimensional echocardiography and two-dimensional speckle-tracking echocardiography and whether successful RCA CTO PCI affects the right-ventricular function.
Methods
Between August 2018 and May 2022, 124 patients with RCA CTO underwent successful recanalization at our institution. All patients signed an informed consent for this prospectively conducted study. 2DE and 2DESTE were performed at baseline (before RCA CTO PCI) and at 6 month follow-up after a surveillance coronary angiography confirmed a good result without the need of revascularization within the treated vessel.
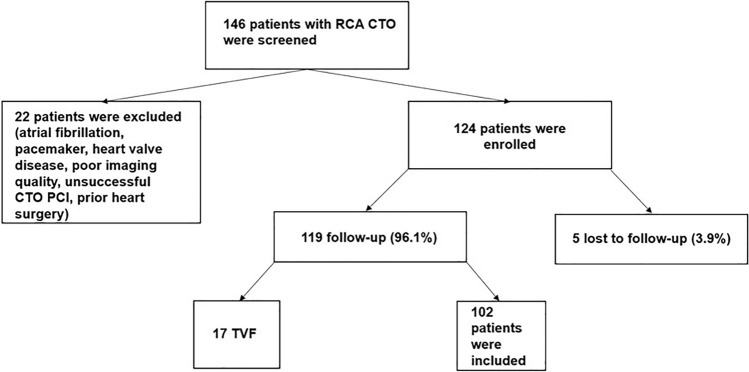
Patients with valve disease (moderate or severe valve regurgitation or stenosis), prior valve surgery or interventional repair, prior cardiac surgery, atrial fibrillation, paced rhythm, left or right bundle branch block, severe pulmonary, kidney (dialysis) or liver disease, pulmonary hypertension, cardiac surgery, or intervention in the period between study inclusion and follow-up were excluded. Inclusion criteria for our study were proof of viability, successful recanalization of RCA CTO with good angiographic result in the 6 months surveillance coronary angiography [absence of target vessel failure (TVF defined as presence of diameter restenosis > 50% by visual estimation, total re-occlusion, or any revascularization within the treated vessel at 6 months follow-up surveillance)], sinus rhythm, and sufficient 2-dimensional imaging quality. Only patients whose long-term medication had not changed by the time of the follow-up were included. 102 patients were included in the final analysis (Fig. 1). The local ethics committee approved the study protocol and registered it as a study in the DRKS.
Fig. 1.
Flowchart of enrollment. TVF = target vessel failure (defined as presence of diameter restenosis > 50% by visual estimation, total re-occlusion, or any revascularization within the treated vessel at 6 month follow-up surveillance coronary angiography)
Standard echocardiography and two-dimensional strain echocardiography
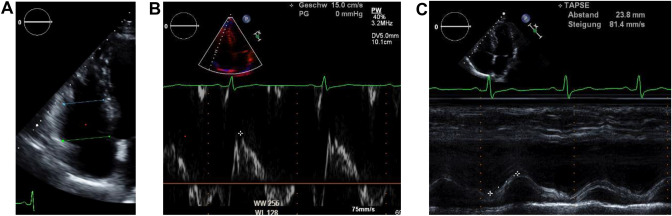
The measurements of the transthoracic echocardiography for quantification of the RV were selected based on the expert consensus document of the European Association of Cardiovascular Imaging. The right heart dimensions were evaluated using diameter at the base, diameter at the mid-level. Right ventricular function was assessed by TAPSE, 2D RV-FAC, and TDI S’ [20]. The assessment of these parameters was performed in accordance with the guidelines of the American Society of Echocardiography and the European Association of Cardiovascular Imaging [11, 12]. A representative example of these measurements is shown in Fig. 2A–C.
Fig. 2.
A RV diameter at base (green line): linear dimension measured end-diastolic at the basal one third of the RV; RV diameter mid-level (blue line): linear dimension measured end-diastolic in the middle third of the RV; B representative example of TAPSE (a parameter of RV longitudinal function and is measured from the tricuspid lateral annulus in M-Mode); C representative example of TDI S’ (the systolic velocity of lateral tricuspid annulus by pulsed tissue Doppler)
Conventional transthoracic echocardiography was performed at rest in a left lateral position using a Philips EPIQ 7 ultrasound system (Koninklijke Philips N.V., Amsterdam, The Netherlands). 2DE included two-dimensional views, including apical 4-chamber, apical 2-chamber, and parasternal long-axis views and Doppler imaging analysis. Two cardiac cycles were recorded and analyzed in each view.
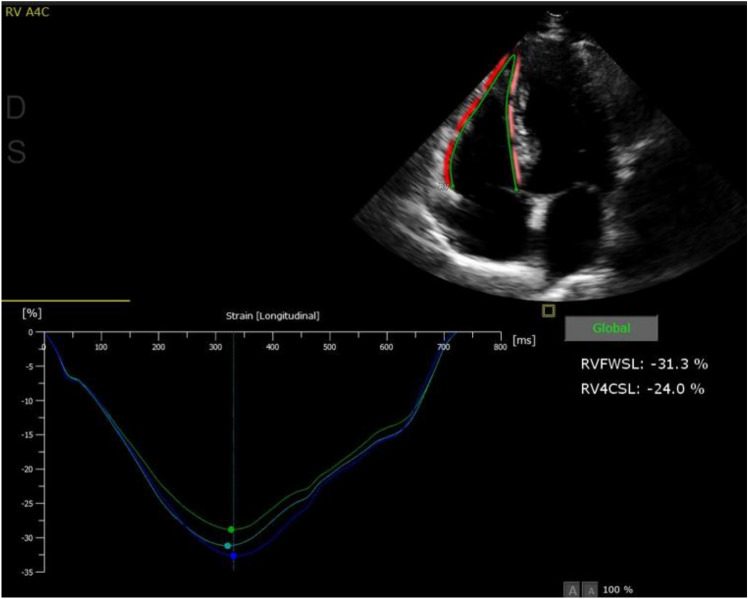
The RV strain analysis [RV free wall systolic strain (%) and RV global (septal and free wall) systolic strain (%)] was performed offline using Q-LAB 13 (PHILIPS Andover, MA Koninklijke Philips Electronics N.V. 2019). Based on the recommendations of the consensus EACVI/ASE/Industry Task Force to standardize deformation imaging, the RV focused 4-chamber view was used, to visualize the entire RV and avoid shortening. RV's region of interest (ROI) was traced along the endocardial border as follows: tricuspid valve annulus, RV free wall, RV apex, septum, and ending at the opposite tricuspid annulus. The ROI was generated automatically, was checked, and, if necessary, the contours were adjusted manually (Fig. 3).
Fig. 3.
Representative example of RV strain imaging
Angina was classified according to the Canadian Cardiovascular Society (CCS) classification and dyspnea based on NYHA (New York Heart Association).
Statistical analysis
Normal distribution of the variables was tested by QQ-plot analysis, the Kolmogorov–Smirnov test, and Shapiro–Wilk test. Categorical data are presented as frequency and percentage, and normally distributed data as mean ± standard deviation and not normally distributed variables are presented as median and interquartile range. To investigate possible differences between baseline and follow-up values, we used the Wilcoxon signed-rank test. Correlations were tested with Spearman's Rho. To investigate possible effects of the right-ventricular side branch (RVSB) on the RV function, we formed four groups (RVSB occluded before and after PCI, RVSB not occluded before and after PCI, RVSB occluded before and not occluded after PCI, and RVSB not occluded before and occluded after PCI) and investigated possible differences between the groups with the Kruskal–Wallis test. A two-sided p value of < 0.05 was statistically significant. The statistical analysis was performed using SPSS (Version 23, IBM SPSS Statistics).
Results
Demographic and clinical baseline characteristics
Of the 102 patients in our final analysis, 72.5% were male with a median age of 66 years (44–80). The mean follow-up period was 187 ± 12.65 days. Median J-CTO Score was 2 (1–3) and most of the patients (54.9%) had Rentrop grade 2 of coronary collateral circulation. Baseline demographical and CTO characteristics are listed in Table 1.
Table 1.
Baseline characteristics
| All patients (n = 102) | |
|---|---|
| Demographics characteristics | |
| Age (yrs) | 66 (44–80) |
| Male | 74 (72.5) |
| BMI (kg/m2) | 26.2 (20–42.4) |
| Diabetes mellitus | 25 (24.5) |
| Hypertension | 92 (90.2) |
| Hyperlipidemia | 90 (88.2) |
| Smoker | 36 (35.3) |
| Multivessel CAD | 90 (88.2) |
| GFR (ml/min) | 81.5 (9–117) |
| PAD | 10 (9.8) |
| Previous MI | 26 (25.5) |
| Previous PCI | 71 (69.6) |
| CTO characteristics | |
| Balanced coronary circulation | 71 (69.6) |
| Left dominant coronary circulation | 29 (28.4) |
| Right dominant coronary circulation | 2 (2.0) |
| J-CTO | 2 (1–3) |
| Rentrop classification | |
| 1 | 34 (33.3) |
| 2 | 56 (54.9) |
| 3 | 12 (11.8) |
| Werner classification | |
| 1 | 57 (55.9) |
| 2 | 35 (34.3) |
| 3 | 10 (9.8) |
Values are represented as n (%), median (minimum–maximum), or mean ± SD
yrs years, BMI body mass index, CAD coronary artery disease, GFR glomerular filtration rate, PAD peripheral artery disease, MI myocardial infarction, PCI percutaneous coronary intervention, RVSD dominant right-ventricular side branch
Clinical parameters at baseline are shown in Table 2. We found no difference when comparing BNP values at baseline and follow-up in our collective (p = 0.150). 65 of the enrolled patients reported an improvement in CCS class (63.7% and p < 0.001) and 51 reported an improvement in NYHA class (51.0% and p < 0.001). Complete freedom of angina was achieved in 72.5% of the patients in our collective and 58.8% of the patients reported to having no limitation of physical activity in daily life (NYHA stage 1) at follow-up.
Table 2.
Clinical parameters baseline and follow-up
| Baseline (n = 102) | Follow-up (n = 102) | p value | |
|---|---|---|---|
| NYHA | < 0.001 | ||
| 1 | 13 (12.7%) | 60 (58.8%) | |
| 2 | 69 (67.6%) | 35 (34.8%) | |
| 3 | 19 (18.6%) | 6 (5.9%) | |
| 4 | 1 (1.0%) | 1 (1.0%) | |
| CCS | < 0.001 | ||
| 0 | 12 (11.8) | 74 (72.5%) | |
| 1 | 27 (26.5) | 7 (6.9%) | |
| 2 | 48 (47.1) | 18 (17.6%) | |
| 3 | 15 (14.7) | 3 (2.9%) | |
| BNP | 70 (10–788) | 61.5 (10–892) | 0.150 |
| VCI | 1.9 (1.7–2.7) | 1.9 (1.6–2.9) | 0.223 |
Values are represented as n (%) and median (minimum–maximum)
p value comparison of baseline and follow-up values
NYHA New York Heart Association stages, CCS Canadian Cardiovascular Society Classification, BNP brain natriuretic peptide pg/ml, VCI vena cava inferior (cm)
Echocardiographic parameters
The measured RV diameters were within the normal ranges compared to the reference values recommended by the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Median RV basal diameter was 36 mm (30–46) at baseline (reference values 33 ± 4 mm without significant change in the follow-up) (p 0.203), RV mid-level diameter was 30 mm (22–36) (reference value 27 ± 4 mm, p 0.374), and RV longitudinal diameter was 70 mm (56–86) (reference value 71 ± 6 mm, p 0.348). The analysis of the functional parameters at baseline was also within the normal ranges: TAPSE was 20 mm (reference value TAPSE > 17 mm), TDI S’ was 12.3 cm/s (reference value 9.5 cm/s) and FAC was 35.01% (reference values > 35%) in our collective. In our collective, 19 of 102 patients had an impaired TAPSE, and we were able to determine an improvement in all 19 patients in the 6-month follow-up (p < 0.001). 73 out of 102 patients showed an RV-FAC lower than 35%, we observed an improvement in 59 patients after a successful RCA CTO recanalization at the follow-up echocardiography (p < 0.001). 14 out of 102 patients had an impaired TDI and an improvement could be observed in all patients (p < 0.001).
We found significant increase of the functional parameter at follow-up, as shown in Table 3 [12]. Left-ventricular function assessed by left-ventricular ejection fraction (LVEF) and global longitudinal strain (GLS) showed no improvement after successful CTO PCI. In our collective, 77 (75.5%) patients had inferior or posterolateral hypokinesia prior to the recanalization. At follow-up, inferior or posterolateral hypokinesia was found in 66 (64.7%) of the patients (p < 0.001).
Table 3.
Echocardiographic assessment of dimension and function
| Baseline (n = 102) | Follow-up (n = 102) | p value | |
|---|---|---|---|
| Ventricular function | |||
| RV basal diameter (mm) | 36 (30–46) | 36 (30–46) | 0.203 |
| RV mid diameter (mm) | 30 (22–36) | 30 (23–41) | 0.374 |
| RV longitudinal diameter (mm) | 70 (56–86) | 70 (57–88) | 0.348 |
| RV wall thickness (mm) | 5 (3–8) | 5 (3–8) | 0.185 |
| TDI S’ (cm/s) | 12.30 (5.7–16.6) | 12.75 (5.9–17.9) | < 0.001 |
| TAPSE (mm) | 20 (6.1–28) | 22 (7.2–31) | < 0.001 |
| FAC (%) | 35.01 (11.21–60.51) | 36.14 (17.54–64.06) | 0.002 |
| GLS (%) | − 15.65 ± 4.39 | − 16.05 ± 4.13 | 0.179 |
| LVEF (%) | 55 (20–60) | 55 (20–60) | 0.179 |
Values are represented as mean ± SD and median (minimum–maximum)
RV right ventricle, TAPSE tricuspid anular plane systolic excursion, FAC fractional area change, GLS global longitudinal strain of left ventricle, LVEF left-ventricular ejection fraction, mm millimeter, cm centimeters, s seconds
RV free wall strain was − 20.7 [− 6.3 to − 32.0]% at baseline and RV global strain was − 15.9 [− 6.0 to − 25.7]%, reference values being − 28.5 ± 4.8% for RV free wall strain, and − 24.5 ± 3.8% for RV global strain in healthy subjects [21]. The right-ventricular function of patients with RCA CTO was found to be altered assessed by speckle tracking in our analysis. We found a significant increase in both values at follow-up. The results of the RV function are presented in Table 4 and Figs. 4 and 5 (boxplot diagrams).
Table 4.
RV function
| Baseline (n = 102) | Follow-up (n = 102) | p value | |
|---|---|---|---|
| RV function | |||
| RV free wall strain (%) | − 20.7 [− 6.3 to − 32.0]% | − 23.4 [− 8.3 to 39.3]% | < 0.001 |
| RV global strain (%) | − 15.9 [− 6.0 to − 25.7]% | − 17.9 [− 7.0 to 29.5]% | < 0.001 |
Values are represented as mean and standard deviation
RV right ventricle
Fig. 4.
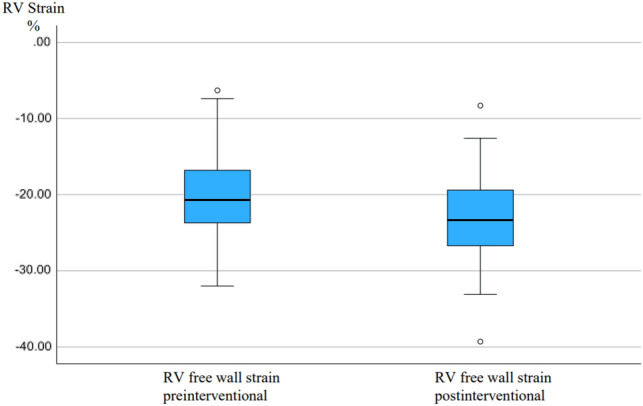
Boxplot diagram with RV free wall strain at baseline before RCA CTO PCI and 6 months after successful RCA CTO PCI
Fig. 5.
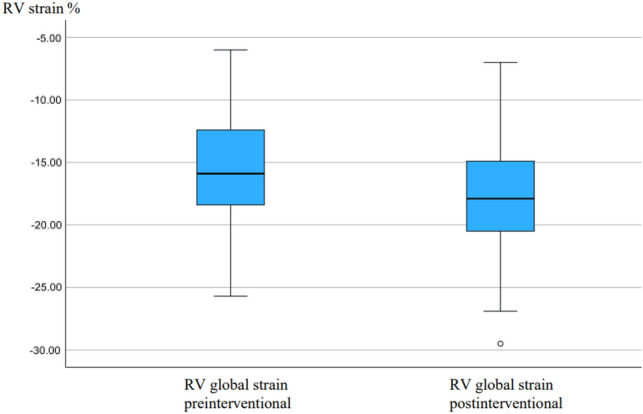
Boxplot diagram with RV global strain at baseline before RCA CTO PCI and 6 months after successful RCA CTO PCI
Coronary collateral circulation and right-ventricular side branch
Correlation of coronary collateral circulation (Rentrop und Werner classification) with right-ventricular function after successful CTO PCI was calculated using Spearman’s Rho. We found no correlation between Werner’s classification (CC grade) and right-ventricular function (RV free wall strain und CC grade: correlation coefficient − 0.102 and p 0.307; RV global strain and CC grade: correlation coefficient 0.011 and p 0.912; RV free wall strain and Rentrop classification: correlation coefficient − 0.103 and p 0.301; RV global strain and Rentrop classification: correlation coefficient 0.012 and p 0.906).
We investigated whether the location of the CTO [proximal vs. distal to the dominant right-ventricular side branch (RVSB)] influenced right-ventricular function and found no difference between the groups (RV free wall p 0.527 and RV global strain p 0.325).
Patients were divided into the following groups: RVSB occluded before and after PCI, RVSB not occluded before and after PCI, RVSB occluded before and not occluded after PCI, and RVSB not occluded before and occluded after PCI. We found no difference between the groups the patients benefited regardless of side branch anatomy. Effects of the dominant RVSB on the result were tested with the Kruskal–Wallis test. The results are listed in Table 5.
Table 5.
Effect of dominant right-ventricle side branch
| n = 102 | p value | |
|---|---|---|
| RVSB occluded before and after PCI | 7 | |
| RVSB not occluded before and after PCI | 87 | |
| RVSB occluded before and not occluded after PCI | 5 | |
| RVSB not occluded before and occluded after PCI | 3 | |
| RV global strain | 0.937 | |
| RV free wall strain | 0.300 |
Values are represented as n (%)
RVSB right-ventricle side branch, RV right ventricle, PCI percutaneous coronary intervention
Reproducibility
Inter-observer reproducibility was tested at baseline and follow-up values for RV free wall and global strain. Inter-observer reproducibility was good (intraclass correlation coefficient for RV free wall strain before PCI: 0.84, 95% CI 0.696–0.904, RV free wall strain after PCI: 0.825, 95% CI 0.683–0.902, RV global strain before PCI: 0.849, 95% CI 0.707–0.918, and RV global strain after PCI: 0.797, 95% CI 0.644–0.884) as determined by intraclass correlation coefficients.
Discussion
To the best of our knowledge, this is the first study analyzing RV function in patients with CTO of the RCA. We assessed RV function with longitudinal strain at baseline and 6 months after successful CTO PCI. Compared to other studies, we only included patients with a good angiographic result in the 6 month surveillance coronary angiography to ensure that the effect on RV function is mainly based on CTO PCI. The main findings of the study were: (1) right-ventricular function is altered in patients with RCA CTO, (2) right-ventricular function improved significantly after successful CTO PCI, and (3) the effect on right-ventricular function is independent of coronary collateralization circulation and the anatomy of the dominant right-ventricular side branch.
RV function is an essential parameter for the prognosis and treatment of patients with various diseases, especially after cardiac surgery or interventional therapies [2–4, 14]. On top of this, it is a relevant predictor for the functional status and exercise capacity of patients which underlines the importance of an accurate assessment of the RV [1]. The linear diameters and conventional parameters of RV function (FAC, TAPSE, and TDI S’) are recommended as part of clinical routine [20]. We found the quantitative parameters and the conventional measurement of the RV function to be within reference values in our collective. However, the assessment of the RV with the conventional parameters has some limitations due to its complex anatomy [thin and highly trabeculated RV wall, dependency of the RV function on preload and afterload, angle dependency of the examination, and the contraction pattern of the RV (longitudinal contraction accounts for 75% of RV contraction)] [1, 14, 22, 23]. Following these limitations, longitudinal strain analysis by 2D speckle tracking should be performed for accurate imaging of the RV. Longitudinal strain analysis already detects subclinical impairment and subtle changes in RV function and is less afterload dependent compared to the conventional parameters [14, 20, 22]. Park et al. demonstrated a good correlation of the right-ventricular longitudinal strain with invasive parameters (cardiac index and pulmonary vascular resistance) and functional parameters (BNP and 6-min walking test) [24]. Based on this scientific knowledge, longitudinal strain of the right ventricle is also recommended in pressure or volume overload conditions and provides valid assessment [22, 25]. To investigate the role of RCA CTO PCI on RV function precisely and minimize the effect of potential influencing factors (e.g., volume and pressure overload), we performed strain analysis and excluded patients with pulmonary hypertension and severe lung or valvular disease. Additionally, we monitored brain natriuretic peptide values and the diameter of the inferior vena cava at baseline and follow-up. We found no significant difference in these parameters.
The benefits of CTO PCI are still controversial. There is evidence that patients benefit from CTO PCI with improved clinical symptoms and quality of life [26–29]. In contrast, study results of the prognostic benefit and the effect on the left-ventricular function are still inconsistent [18, 19, 27, 29, 30]. One reason for the controversial results could be the different study conditions (examinations at rest vs. stress), as studies have especially revealed exercise-induced ischemia of the myocardium of the CTO territory. The REVASC Trial investigated the effect of CTO PCI on the left-ventricular function. The segmental wall thickening of the CTO territory was assessed by cardiac magnetic resonance imaging (CMR) at rest at baseline and 6 month follow-up. In about 60% of the patients, the CTO was localized in the RCA. The authors reported no improvement of the segmental wall thickening and other left-ventricular indices after 6 months [19]. In contrast to this study, Bucciarelli-Ducci et al. showed a significant improvement of LEVF in patients after successful CTO PCI assessed by stress perfusion imaging CMR [31]. The IMPACTOR-CTO trial focused on patients with RCA CTO to study the effects of CTO PCI vs. optimal medical therapy (OMT) on inducible myocardial ischemia burden in this special collective. Adenosine stress cardiac magnetic resonance, 6-min walk test, and Short Form-36 Health Survey were performed at baseline, after 2 and 12 months. Endpoints were defined as: decrease in inducible myocardial ischemia burden, changes in 6-min walk test distance, and quality of life. In the follow-up, inducible myocardial ischemia burden was lower and decreased significantly in the CTO PCI collective, 6-min walking distance significantly increased in the CTO PCI collective, and the scales of the Short Form-36 Health Survey improved compared to the OMT collective. These effects could not be shown in the OMT collective and no significance was reached in the OMT collective for the endpoints. The decrease of inducible myocardial ischemia burden indicates an improved perfusion of the CTO supplied territory after PCI [26]. These results underline the importance of studying the ventricular function not only at rest but also under stress conditions. Our analysis is in good agreement with the findings above. The comparison of the left-ventricular indices showed no difference between baseline and follow-up. The mean GLS was within the borderline range (16–18%) in our collective without improvement at follow-up [20, 32]. We expected these results as Pereztol-Valdes et al. already showed that none of the 17 segments of the LV is exclusively perfused by the RCA [33, 34]. Since the right ventricle is mainly supplied with blood by the RCA, we hypothesized that the improved perfusion of the CTO supplied territory after CTO PCI has a positive effect on the RV function. With our analysis, we were able to confirm this hypothesis using global and free wall strain assessment. In addition to improved perfusion, other reasons for our findings could be the improved microvascular function and the increased trainings capacity of our patients due to reduction of clinical symptoms (especially angina relief). These findings support the benefits of CTO PCI in patients with RCA CTO and clinical symptoms.
Conclusion
Our single-center experience demonstrates a significant improvement of the right-ventricular function assessed by two-dimensional echocardiography and longitudinal strain imaging in patients with RCA CTO after successful recanalization. We also found an improvement in patient-reported exercise tolerance in daily life (NYHA classification) and angina symptoms (CCS classification).
Limitations
This study has a single-center prospective design with a limited sample size and lacks of a control group. Our results, and their potential clinical impact, should be tested in a larger cohort. The longitudinal strain analysis is recommended for an accurate assessment of the right-ventricular function, but the results are dependent on the ankle and pre- and afterload conditions. We tried to reduce the influence of these factors by patients’ selection (exclusion criteria, e.g., pulmonary hypertension) and use of clinical parameters (BNP, VCI) to assess hemodynamic conditions. 2 DE was performed in patients with normotension, sinus rhythm, and no lung disease. However, the RV function is influenced by several factors, which should be taken into account when interpreting our data. Due to the size of our collective and the follow-up period of 6 months, we are not able to evaluate prognostic implications of RCA CTO PCI.
Abbreviations
- CTO
Chronic total occlusion
- BNP
Brain natriuretic peptide
- CAD
Coronary artery disease
- CCS
Canadian Cardiovascular Society Classification
- FAC
Fractional area change
- GFR
Glomerular filtration rate
- GLS
Global longitudinal strain
- LVEF
Left-ventricular ejection fraction
- MI
Myocardial infarction
- NYHA
New York Heart Association
- OMT
Optimal medical therapy
- PAD
Peripheral artery disease
- PCI
Percutaneous coronary intervention
- RCA
Right coronary artery
- ROI
Region of interest
- RV
Right ventricle
- RVSB
Right-ventricular side branch
- TVF
Target vessel failure
- VCI
Vena cava inferior
- 2DE
Two-dimensional transthoracic echocardiography
- 2DSTE
Two-dimensional speckle-tracking echocardiography
Author contributions
Conceptualization, ZD and TG; methodology, ZD and RB; software, RB; validation, ZD, TG, ID, and RB; formal analysis, RB; investigation, ZD; TG, PW, and RB; resources, TM; data curation, RB; writing—original draft preparation, RB; writing—review and editing, PW, ZD, TG, and TM ID; visualization, RB; supervision, ZD; project administration, TM; All authors have read and agreed to the published version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. There was no financial funding for the project.
Availability of data and materials
The dataset generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval and consent to participate
All patients signed informed consent. Study protocol was approved by the local Ethics Committee to be in accordance with the legal regulations and the Declaration of Helsinki # 2019-14197, and was registered as a study in the DRKS (DRKS #00025882).
Consent for publication
Not applicable.
Contributor Information
Recha Blessing, Email: recha.blessing@unimedizin-mainz.de.
Zisis Dimitriadis, Email: dimitriadiszisis@gmail.com.
References
- 1.Sanz J, Sánchez-Quintana D, Bossone E, Bogaard HJ, Naeije R. Anatomy, function, and dysfunction of the right ventricle: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(12):1463–1482. doi: 10.1016/j.jacc.2018.12.076. [DOI] [PubMed] [Google Scholar]
- 2.Tello K, Gall H, Richter M, Ghofrani A, Schermuly R. Rechtsventrikuläre Funktion bei pulmonaler (arterieller) Hypertonie. Herz. 2019;44(6):509–516. doi: 10.1007/s00059-019-4815-6. [DOI] [PubMed] [Google Scholar]
- 3.Schmeißer A, Rauwolf T, Groscheck T, Fischbach K, Kropf S, Luani B, et al. Predictors and prognosis of right ventricular function in pulmonary hypertension due to heart failure with reduced ejection fraction. ESC Heart Fail. 2021;8(4):2968–2981. doi: 10.1002/ehf2.13386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lejeune S, Roy C, Ciocea V, Slimani A, de Meester C, Amzulescu M, et al. Right ventricular global longitudinal strain and outcomes in heart failure with preserved ejection fraction. J Am Soc Echocardiogr. 2020;33(8):973–984.e2. doi: 10.1016/j.echo.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Ghio S, Guazzi M, Scardovi AB, Klersy C, Clemenza F, Carluccio E, et al. Different correlates but similar prognostic implications for right ventricular dysfunction in heart failure patients with reduced or preserved ejection fraction. Eur J Heart Fail. 2017;19(7):873–879. doi: 10.1002/ejhf.664. [DOI] [PubMed] [Google Scholar]
- 6.Dini FL, Carluccio E, Simioniuc A, Biagioli P, Reboldi G, Galeotti GG, et al. Right ventricular recovery during follow-up is associated with improved survival in patients with chronic heart failure with reduced ejection fraction. Eur J Heart Fail. 2016;18(12):1462–1471. doi: 10.1002/ejhf.639. [DOI] [PubMed] [Google Scholar]
- 7.Gorter TM, Lexis CPH, Hummel YM, Lipsic E, Nijveldt R, Willems TP, et al. Right ventricular function after acute myocardial infarction treated with primary percutaneous coronary intervention (from the glycometabolic intervention as adjunct to primary percutaneous coronary intervention in ST-segment elevation myocardial infarction III trial) Am J Cardiol. 2016;118(3):338–344. doi: 10.1016/j.amjcard.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Grothoff M, Elpert C, Hoffmann J, Zachrau J, Lehmkuhl L, de Waha S, et al. Right ventricular injury in ST-elevation myocardial infarction: risk stratification by visualization of wall motion, edema, and delayed-enhancement cardiac magnetic resonance. Circ Cardiovasc Imaging. 2012;5(1):60–68. doi: 10.1161/CIRCIMAGING.111.967810. [DOI] [PubMed] [Google Scholar]
- 9.Konstam MA, Kiernan MS, Bernstein D, Bozkurt B, Jacob M, Kapur NK, et al. Evaluation and management of right-sided heart failure: a scientific statement from the American Heart Association. Circulation. 2018;137(20):e578–e622. doi: 10.1161/CIR.0000000000000560. [DOI] [PubMed] [Google Scholar]
- 10.Ohara K, Imamura T, Ihori H, Chatani K, Nonomura M, Kameyama T, et al. Association between right ventricular function and exercise capacity in patients with chronic heart failure. J Clin Med. 2022;11(4):1066. doi: 10.3390/jcm11041066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713 (quiz 786–788). [DOI] [PubMed]
- 12.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2018;19(6):591–600. doi: 10.1093/ehjci/jey042. [DOI] [PubMed] [Google Scholar]
- 14.Dandel M, Hetzer R. Echocardiographic assessment of the right ventricle: Impact of the distinctly load dependency of its size, geometry and performance. Int J Cardiol. 2016;221:1132–1142. doi: 10.1016/j.ijcard.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Dutta T, Aronow WS. Echocardiographic evaluation of the right ventricle: clinical implications. Clin Cardiol. 2017;40(8):542–548. doi: 10.1002/clc.22694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ralapanawa U, Sivakanesan R. Epidemiology and the magnitude of coronary artery disease and acute coronary syndrome: a narrative review. J Epidemiol Glob Health. 2021;11(2):169–177. doi: 10.2991/jegh.k.201217.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galassi AR, Werner GS, Boukhris M, Azzalini L, Mashayekhi K, Carlino M, et al. Percutaneous recanalisation of chronic total occlusions: 2019 consensus document from the EuroCTO Club. EuroIntervention. 2019;15(2):198–208. doi: 10.4244/EIJ-D-18-00826. [DOI] [PubMed] [Google Scholar]
- 18.Henriques JPS, Hoebers LP, Råmunddal T, Laanmets P, Eriksen E, Bax M, et al. Percutaneous intervention for concurrent chronic total occlusions in patients with STEMI: the EXPLORE trial. J Am Coll Cardiol. 2016;68(15):1622–1632. doi: 10.1016/j.jacc.2016.07.744. [DOI] [PubMed] [Google Scholar]
- 19.Mashayekhi K, Nührenberg TG, Toma A, Gick M, Ferenc M, Hochholzer W, et al. A randomized trial to assess regional left ventricular function after stent implantation in chronic total occlusion: the REVASC trial. JACC Cardiovasc Interv. 2018;11(19):1982–1991. doi: 10.1016/j.jcin.2018.05.041. [DOI] [PubMed] [Google Scholar]
- 20.Galderisi M, Cosyns B, Edvardsen T, Cardim N, Delgado V, Di Salvo G, et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2017;18(12):1301–1310. doi: 10.1093/ehjci/jex244. [DOI] [PubMed] [Google Scholar]
- 21.Morris DA, Krisper M, Nakatani S, Köhncke C, Otsuji Y, Belyavskiy E, et al. Normal range and usefulness of right ventricular systolic strain to detect subtle right ventricular systolic abnormalities in patients with heart failure: a multicentre study. Eur Heart J Cardiovasc Imaging. 2017;18(2):212–223. doi: 10.1093/ehjci/jew011. [DOI] [PubMed] [Google Scholar]
- 22.Muraru D, Haugaa K, Donal E, Stankovic I, Voigt JU, Petersen SE, et al. Right ventricular longitudinal strain in the clinical routine: a state-of-the-art review. Eur Heart J Cardiovasc Imaging. 2022;23(7):898–912. doi: 10.1093/ehjci/jeac022. [DOI] [PubMed] [Google Scholar]
- 23.Nägele MP, Flammer AJ. Heart failure after right ventricular myocardial infarction. Curr Heart Fail Rep. 2022;19(6):375–385. doi: 10.1007/s11897-022-00577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park J-H, Kusunose K, Kwon DH, Park MM, Erzurum SC, Thomas JD, et al. Relationship between right ventricular longitudinal strain, invasive hemodynamics, and functional assessment in pulmonary arterial hypertension. Korean Circ J. 2015;45(5):398–407. doi: 10.4070/kcj.2015.45.5.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright L, Negishi K, Dwyer N, Wahi S, Marwick TH. Afterload dependence of right ventricular myocardial strain. J Am Soc Echocardiogr. 2017;30(7):676–684.e1. doi: 10.1016/j.echo.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Obedinskiy AA, Kretov EI, Boukhris M, Kurbatov VP, Osiev AG, Ibn Elhadj Z, et al. The IMPACTOR-CTO trial. JACC Cardiovasc Interv. 2018;11(13):1309–1311. doi: 10.1016/j.jcin.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 27.Werner GS, Martin-Yuste V, Hildick-Smith D, Boudou N, Sianos G, Gelev V, et al. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur Heart J. 2018;39(26):2484–2493. doi: 10.1093/eurheartj/ehy220. [DOI] [PubMed] [Google Scholar]
- 28.Sapontis J, Salisbury AC, Yeh RW, Cohen DJ, Hirai T, Lombardi W, et al. Early procedural and health status outcomes after chronic total occlusion angioplasty: a report from the OPEN-CTO Registry (outcomes, patient health status, and efficiency in chronic total occlusion hybrid procedures) JACC Cardiovasc Interv. 2017;10(15):1523–1534. doi: 10.1016/j.jcin.2017.05.065. [DOI] [PubMed] [Google Scholar]
- 29.Di Mario C, Mashayekhi KA, Garbo R, Pyxaras SA, Ciardetti N, Werner GS. Recanalisation of coronary chronic total occlusions. EuroIntervention. 2022;18(7):535–561. doi: 10.4244/EIJ-D-21-01117. [DOI] [PubMed] [Google Scholar]
- 30.Lee S-W, Lee PH, Ahn J-M, Park D-W, Yun S-C, Han S, et al. Randomized trial evaluating percutaneous coronary intervention for the treatment of chronic total occlusion. Circulation. 2019;139(14):1674–1683. doi: 10.1161/CIRCULATIONAHA.118.031313. [DOI] [PubMed] [Google Scholar]
- 31.Bucciarelli-Ducci C, Auger D, Di Mario C, Locca D, Petryka J, O'Hanlon R, et al. CMR guidance for recanalization of coronary chronic total occlusion. JACC Cardiovasc Imaging. 2016;9(5):547–556. doi: 10.1016/j.jcmg.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 32.Potter E, Marwick TH. Assessment of left ventricular function by echocardiography: the case for routinely adding global longitudinal strain to ejection fraction. JACC Cardiovasc Imaging. 2018;11(2 Pt 1):260–274. doi: 10.1016/j.jcmg.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 33.Pereztol-Valdés O, Candell-Riera J, Santana-Boado C, Angel J, Aguadé-Bruix S, Castell-Conesa J, et al. Correspondence between left ventricular 17 myocardial segments and coronary arteries. Eur Heart J. 2005;26(24):2637–2643. doi: 10.1093/eurheartj/ehi496. [DOI] [PubMed] [Google Scholar]
- 34.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105(4):539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset generated and/or analyzed during the current study are available from the corresponding author on reasonable request.