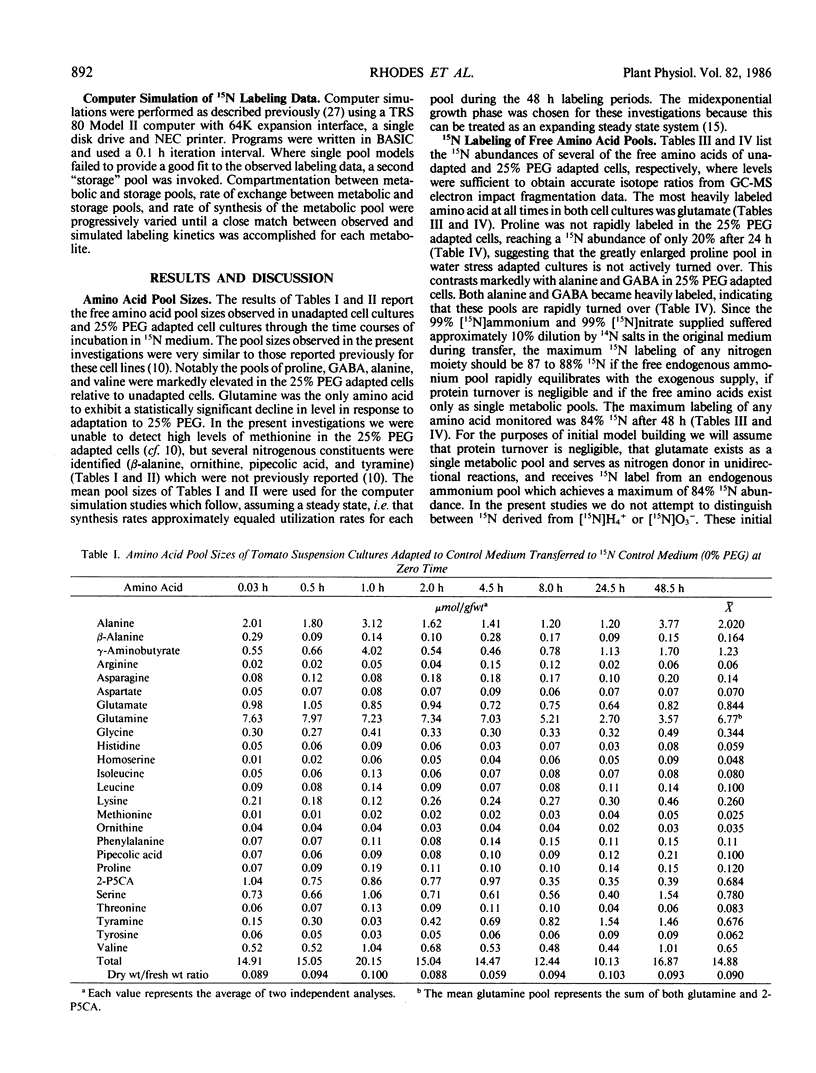
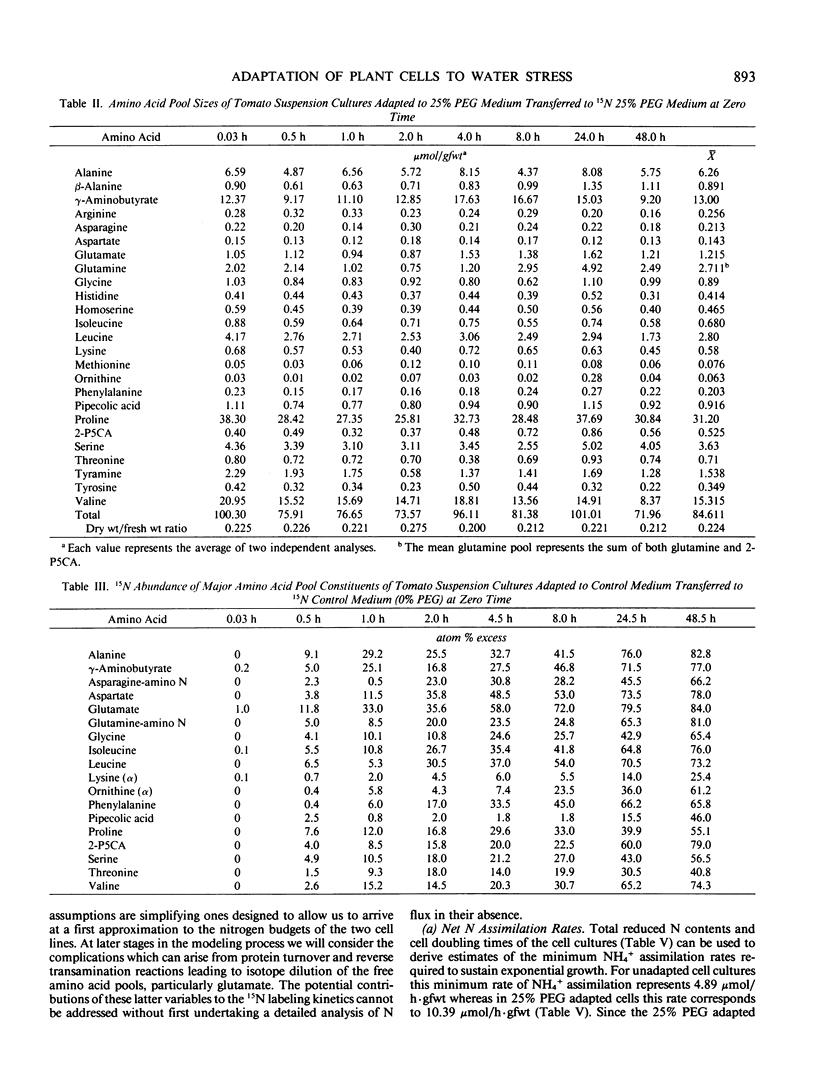
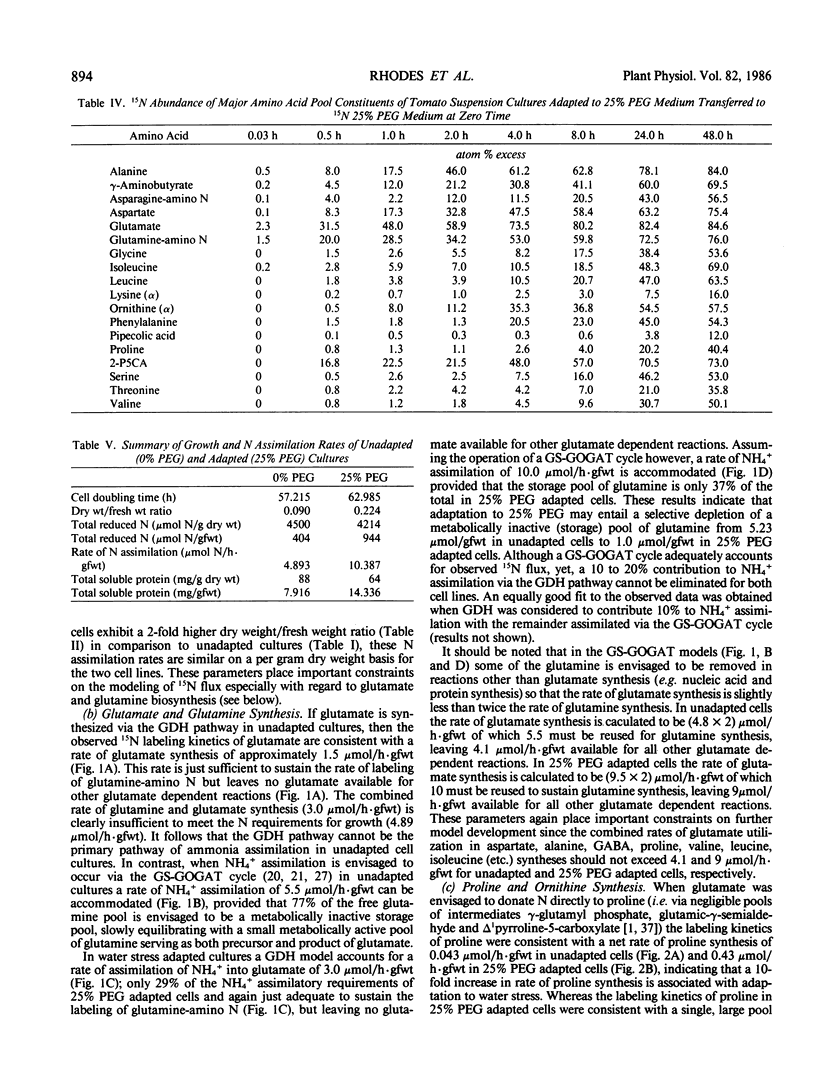
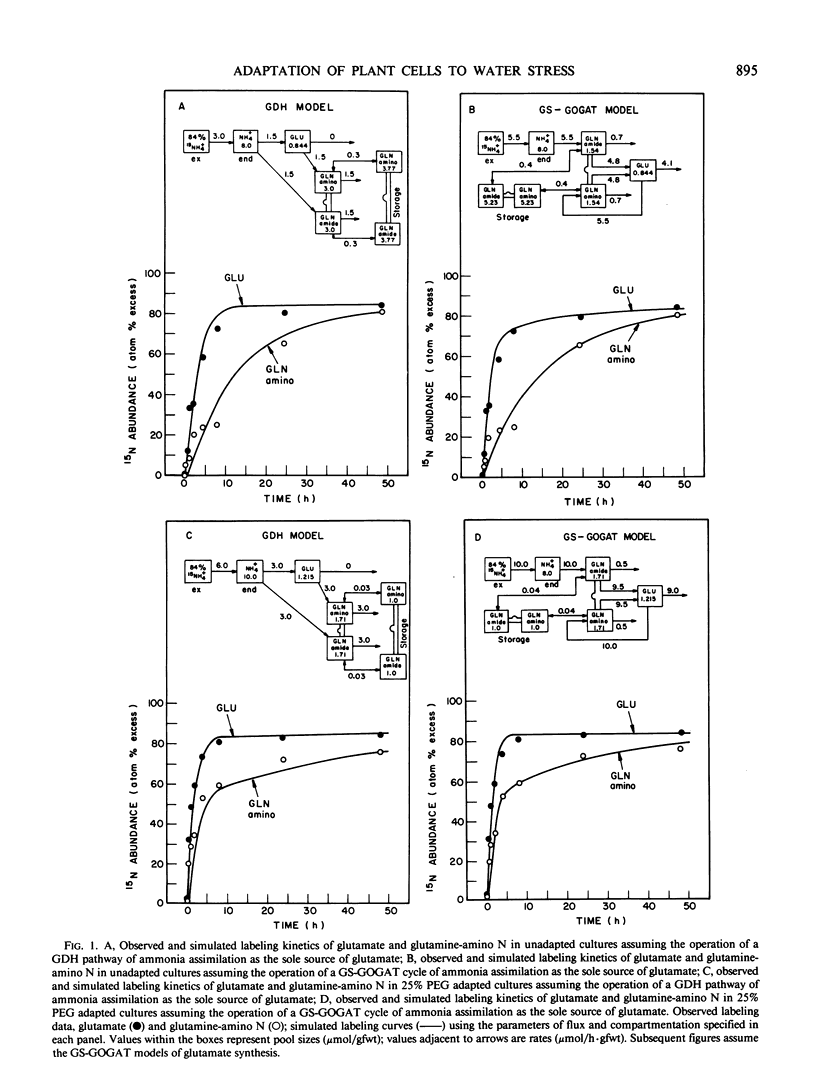
Abstract
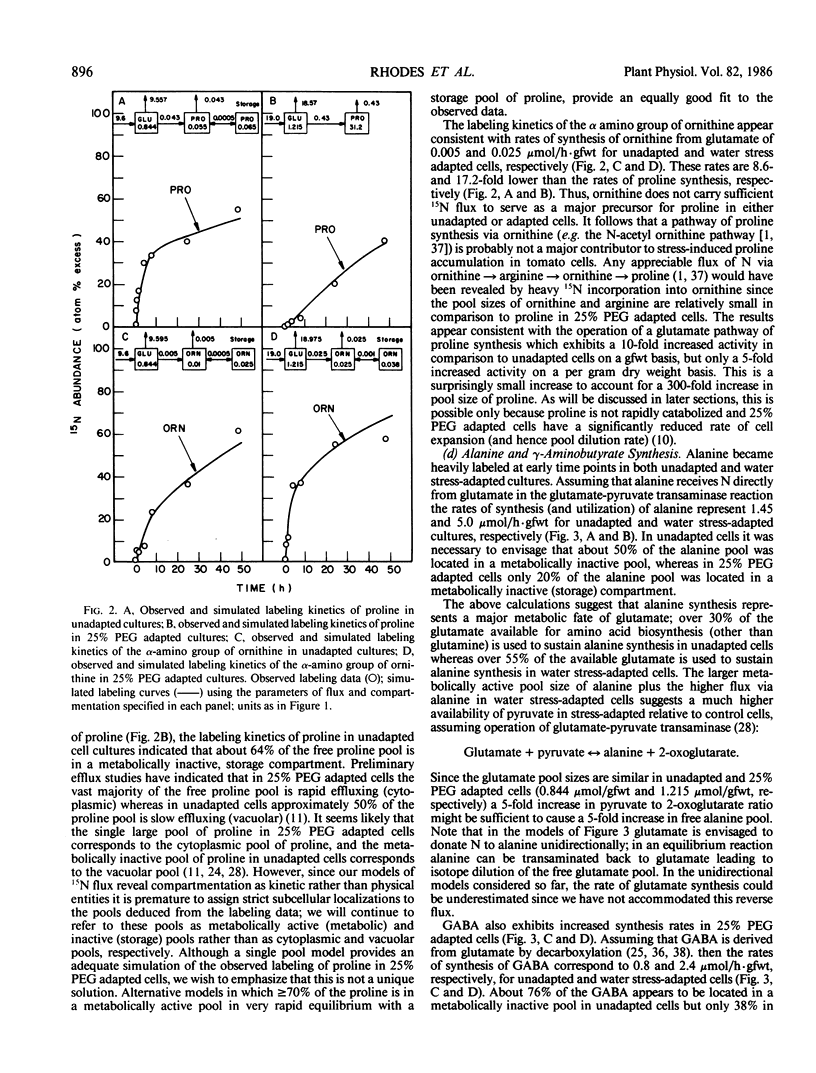
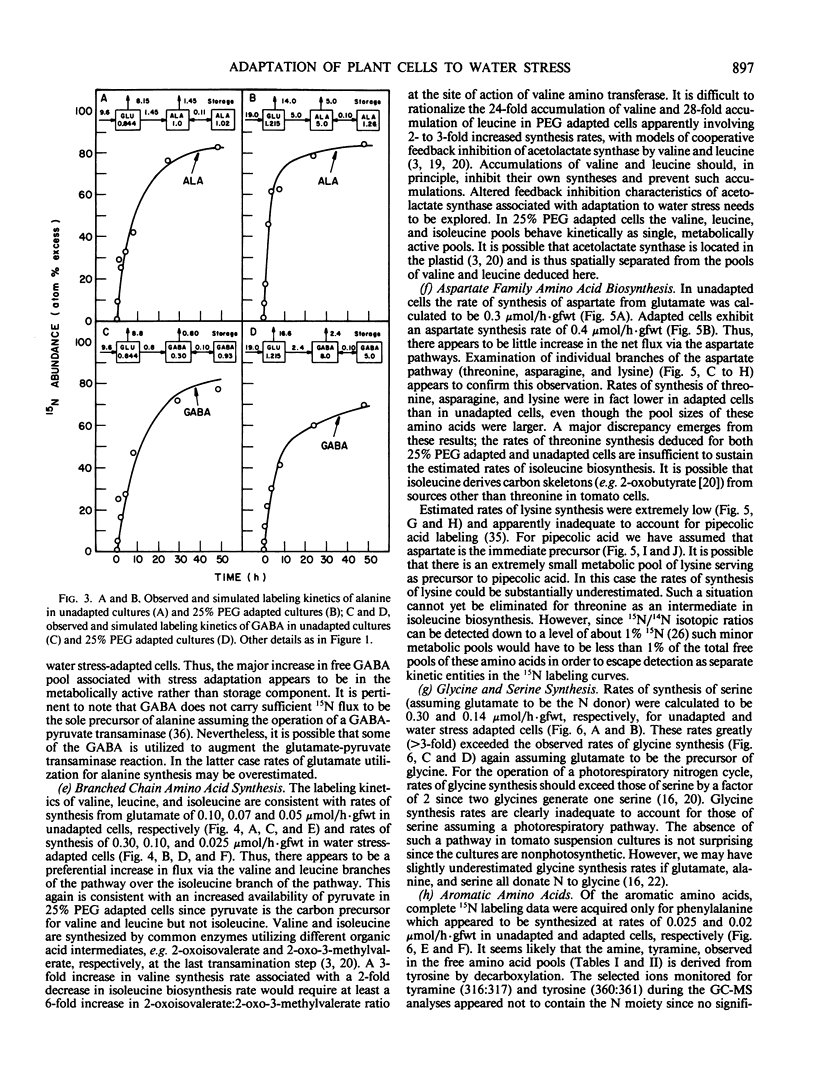
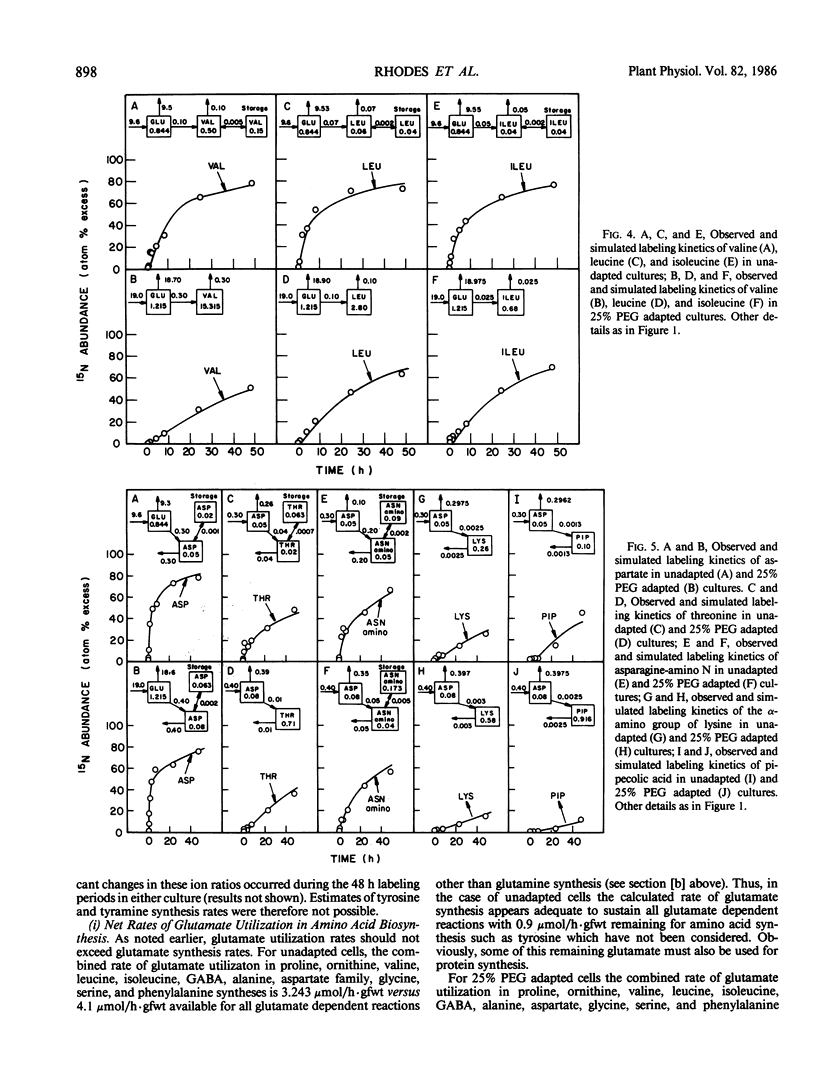
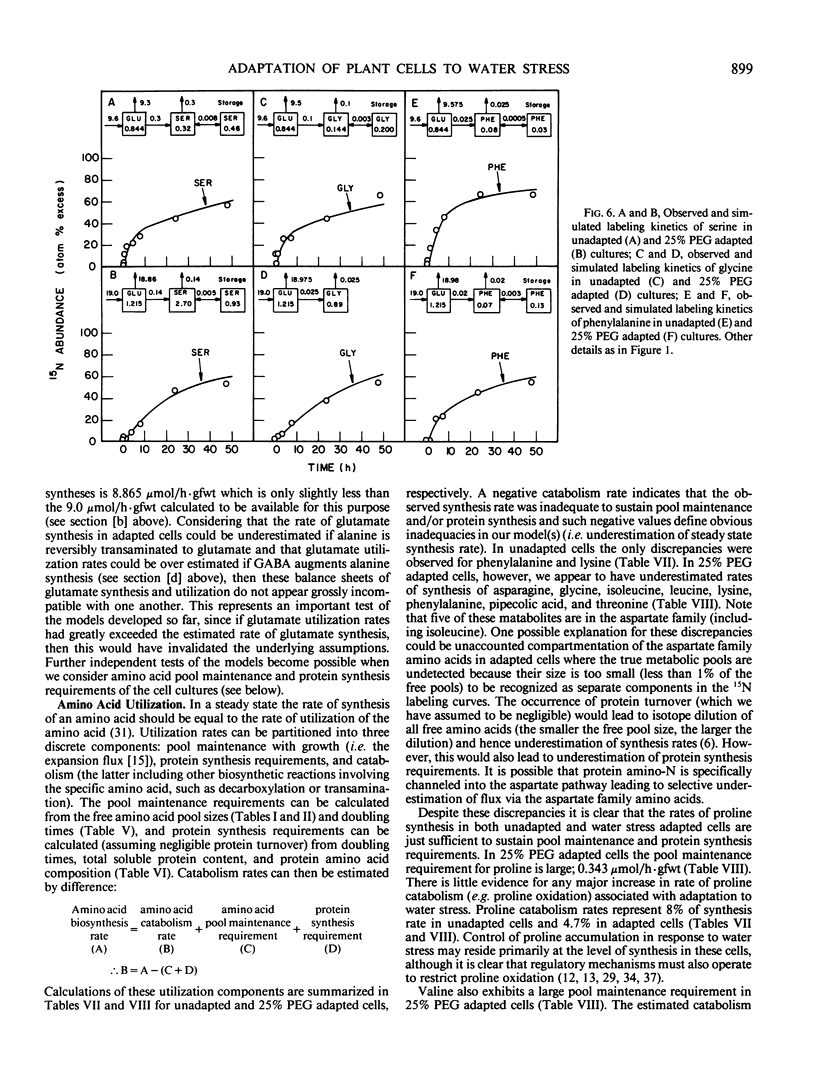
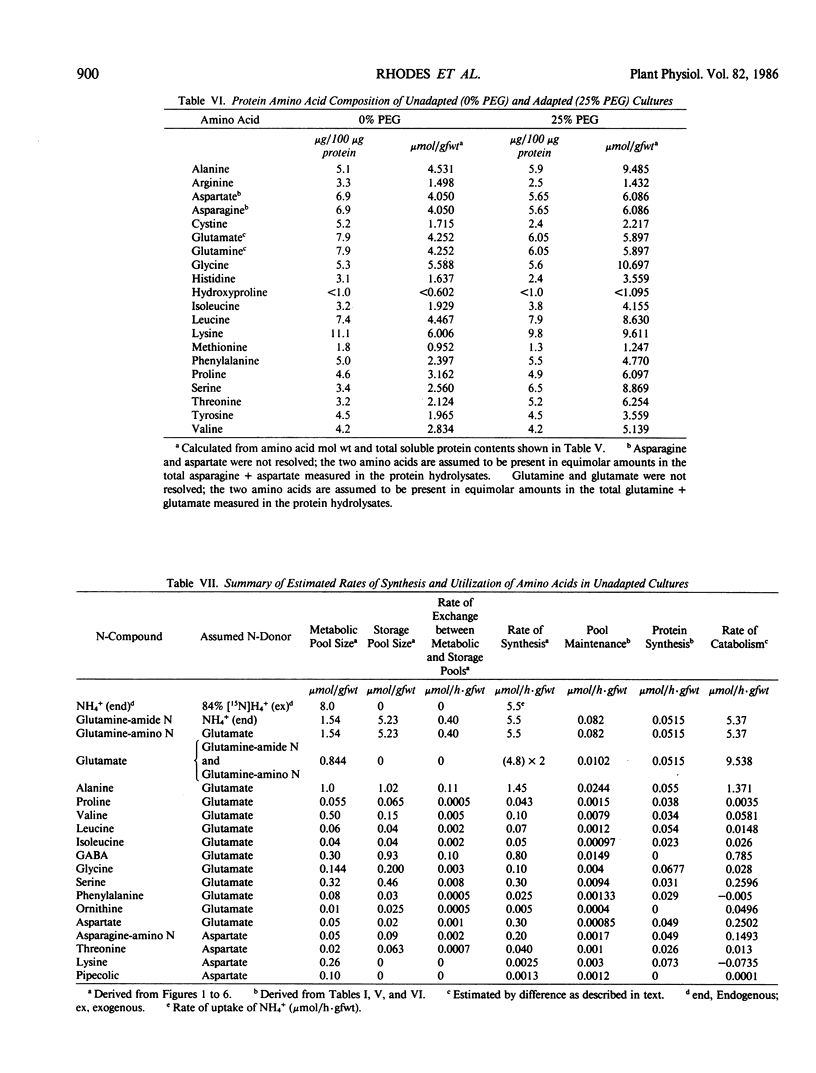
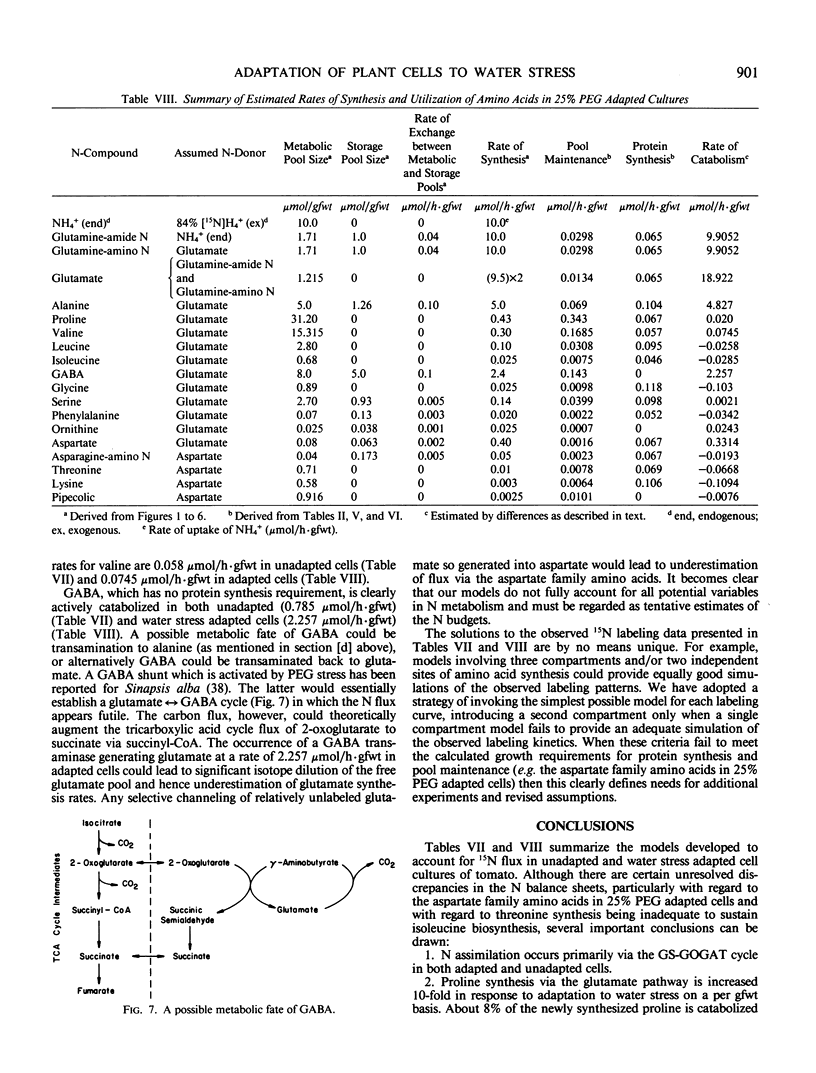
Suspension cultured cells of tomato (Lycopersicon esculentum Mill. cv VFNT Cherry) adapted to water stress induced with polyethylene glycol 6000 (PEG), exhibit marked alterations in free amino acid pools (Handa et al. 1983 Plant Physiol 73: 834-843). Using computer simulation models the in vivo rates of synthesis and utilization and compartmentation of free amino acid pools were determined from 15N labeling kinetics after substituting [15N]ammonium and [15N]nitrate for the 14N salts in the culture medium of cell lines adapted to 0% and 25% PEG. The 300-fold elevated proline pool in 25% PEG adapted cells is primarily the consequence of a 10-fold elevated rate of proline synthesis via the glutamate pathway. Ornithine was insufficiently labeled to serve as a major precursor for proline. Our calculations suggest that the rate of proline synthesis only slightly exceeds the rate required to sustain both protein synthesis and proline pool maintenance with growth. Mechanisms must operate to restrict proline oxidation in adapted cells. The kinetics of labeling of proline in 25% PEG adapted cells are consistent with a single, greatly enlarged metabolic pool of proline. The depletion of glutamine in adapted cells appears to be a consequence of a selective depletion of a large, metabolically inactive storage pool present in unadapted cultures. The labeling kinetics of the amino nitrogen groups of glutamine and glutamate are consistent with the operation of the glutamine synthetase-glutamate synthase cycle in both cell lines. However, we could not conclusively discriminate between the exclusive operation of the glutamine synthetase-glutamate synthase cycle and a 10 to 20% contribution of the glutamate dehydrogenase pathway of ammonia assimilation. Adaptation to water stress leads to increased nitrogen flux from glutamate into alanine and γ-aminobutyrate, suggesting increased pyruvate availability and increased rates of glutamate decarboxylation. Both alanine and γ-aminobutyrate are synthesized at rates greatly in excess of those simply required to maintain the free pools with growth, indicating that these amino acids are rapidly turned over. Thus, both synthesis and utilization rates for alanine and γ-aminobutyrate are increased in adapted cells. Adaptation to stress leads to increased rates of synthesis of valine and leucine apparently at the expense of isoleucine. Remarkably low 15N flux via the aspartate family amino acids was observed in these experiments. The rate of synthesis of threonine appeared too low to account for threonine utilization in protein synthesis, pool maintenance, and isoleucine biosynthesis. It is possible that isoleucine may be deriving carbon skeletons from sources other than threonine. Tentative models of the nitrogen flux of these two contrasting cell lines are discussed in relation to carbon metabolism, osmoregulation, and nitrogenous solute compartmentation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams E., Frank L. Metabolism of proline and the hydroxyprolines. Annu Rev Biochem. 1980;49:1005–1061. doi: 10.1146/annurev.bi.49.070180.005041. [DOI] [PubMed] [Google Scholar]
- Csonka L. N. Proline over-production results in enhanced osmotolerance in Salmonella typhimurium. Mol Gen Genet. 1981;182(1):82–86. doi: 10.1007/BF00422771. [DOI] [PubMed] [Google Scholar]
- Davies D. D. Control of and by pH. Symp Soc Exp Biol. 1973;27:513–529. [PubMed] [Google Scholar]
- Folkes B. F., Sims A. P. The significance of amino acid inhibition of NADP-linked glutamate dehydrogenase in the physiological control of glutamate synthesis in Candida utilis. J Gen Microbiol. 1974 May;82(1):77–95. doi: 10.1099/00221287-82-1-77. [DOI] [PubMed] [Google Scholar]
- Handa S., Bressan R. A., Handa A. K., Carpita N. C., Hasegawa P. M. Solutes contributing to osmotic adjustment in cultured plant cells adapted to water stress. Plant Physiol. 1983 Nov;73(3):834–843. doi: 10.1104/pp.73.3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H., Cavalieri A. J. Proline Oxidase and Water Stress-induced Proline Accumulation in Spinach Leaves. Plant Physiol. 1979 Mar;63(3):531–535. doi: 10.1104/pp.63.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacser H. The control of enzyme systems in vivo: elasticity analysis of the steady state. Biochem Soc Trans. 1983 Jan;11(1):35–40. doi: 10.1042/bst0110035. [DOI] [PubMed] [Google Scholar]
- Mexal J., Fisher J. T., Osteryoung J., Reid C. P. Oxygen availability in polyethylene glycol solutions and its implications in plant-water relations. Plant Physiol. 1975 Jan;55(1):20–24. doi: 10.1104/pp.55.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miflin B. J. Cooperative feedback control of barley acetohydroxyacid synthetase by leucine, isoleucine, and valine. Arch Biochem Biophys. 1971 Oct;146(2):542–550. doi: 10.1016/0003-9861(71)90159-7. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Tolbert N. E. Serine: glyoxylate, alanine:glyoxylate, and glutamate:glyoxylate aminotransferase reactions in peroxisomes from spinach leaves. J Biol Chem. 1983 Jun 25;258(12):7631–7638. [PubMed] [Google Scholar]
- Pahlich E., Kerres R., Jäger H. J. Influence of Water Stress on the Vacuole/Extravacuole Distribution of Proline in Protoplasts of Nicotiana rustica. Plant Physiol. 1983 Jun;72(2):590–591. doi: 10.1104/pp.72.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D., Myers A. C., Jamieson G. Gas Chromatography-Mass Spectrometry of N- Heptafluorobutyryl Isobutyl Esters of Amino Acids in the Analysis of the Kinetics of [N]H(4) Assimilation in Lemna minor L. Plant Physiol. 1981 Nov;68(5):1197–1205. doi: 10.1104/pp.68.5.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMS A. P., FOLKES B. F. A KINETIC STUDY OF THE ASSIMILATION OF (15N)-AMMONIA AND THE SYNTHESIS OF AMINO ACIDS IN AN EXPONENTIALLY GROWING CULTURE OF CANDIDA UTILIS. Proc R Soc Lond B Biol Sci. 1964 Feb 18;159:479–502. doi: 10.1098/rspb.1964.0015. [DOI] [PubMed] [Google Scholar]
- Sakano K., Tazawa M. Metabolic Conversion of Amino Acids Loaded in the Vacuole of Chara australis Internodal Cells. Plant Physiol. 1985 Aug;78(4):673–677. doi: 10.1104/pp.78.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sells G. D., Koeppe D. E. Oxidation of proline by mitochondria isolated from water-stressed maize shoots. Plant Physiol. 1981 Nov;68(5):1058–1063. doi: 10.1104/pp.68.5.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims A. P., Ferguson A. R. The regulation of glutamine metabolism in Candida utilis: studies with 15NH3 to measure in vivo rates of glutamine synthesis. J Gen Microbiol. 1974 Jan;80(1):143–158. doi: 10.1099/00221287-80-1-143. [DOI] [PubMed] [Google Scholar]
- Smith C. J., Deutch A. H., Rushlow K. E. Purification and characteristics of a gamma-glutamyl kinase involved in Escherichia coli proline biosynthesis. J Bacteriol. 1984 Feb;157(2):545–551. doi: 10.1128/jb.157.2.545-551.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. R. Inhibition of proline oxidation by water stress. Plant Physiol. 1977 May;59(5):930–932. doi: 10.1104/pp.59.5.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter J. G., Thompson J. F. Anaerobic Accumulation of gamma-Aminobutyric Acid and Alanine in Radish Leaves (Raphanus sativus, L.). Plant Physiol. 1972 Apr;49(4):572–578. doi: 10.1104/pp.49.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]


