Abstract
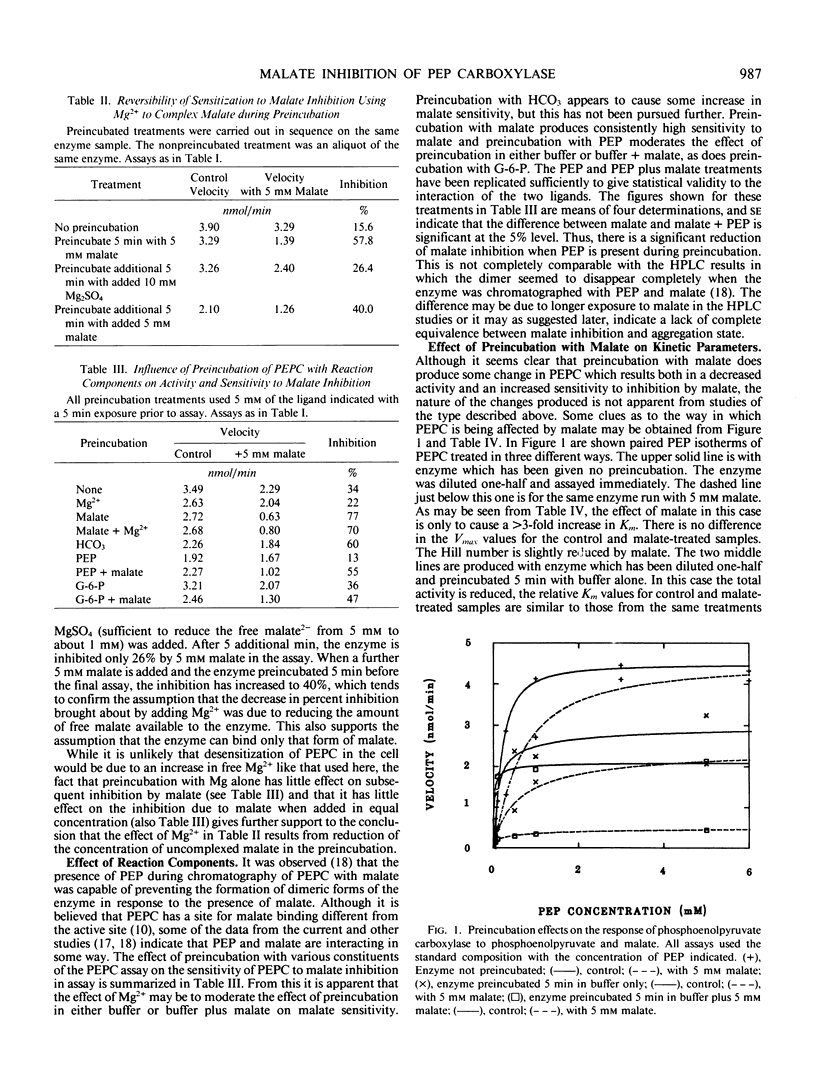
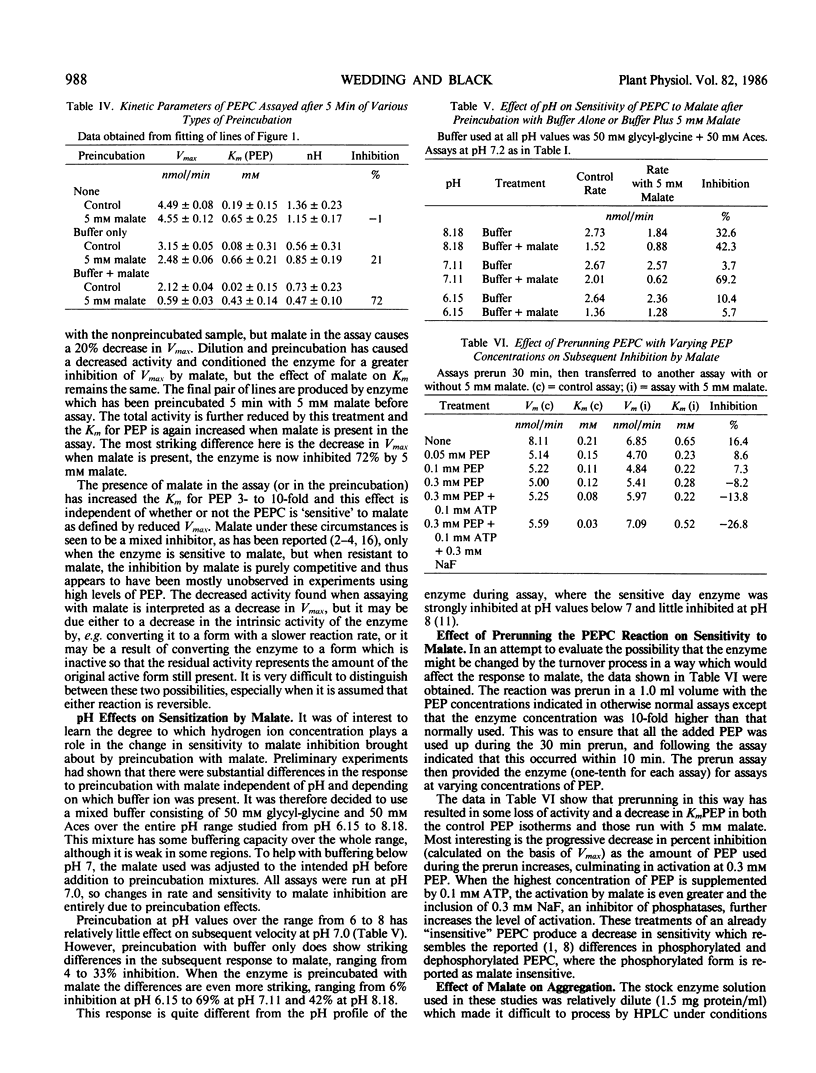
Phosphoenolpyruvate carboxylase partially purified from leaves of Crassula and rendered insensitive to malate by storage without adjuvants can be altered to the form sensitive to malate inhibition by brief, 5-minute preincubation with 5 millimolar malate. The induction of malate sensitivity is reversible by lowering the malate2− concentration. Of the reaction components only HCO3− increases the sensitivity to malate in subsequent assay. Phosphoenolpyruvate (PEP), which itself tends to lower sensitivity to subsequent malate inhibition, also reduces the effect of malate in the assay, as does glucose-6-phosphate. PEP isotherms showed that the insensitive or unpreincubated enzyme, responds to the presence of 5 millimolar malate during assay with a 3-fold increase in Km, but no effect on Vmax. Enzyme preincubated with malate shows the same effect of malate on Km, but in addition Vmax is inhibited 72%. It thus appears that both sensitive and insensitive forms of PEP carboxylase are subject to K-type inhibition by malate, but only the sensitive form also shows V-type inhibition. Preincubation with malate at different pH values showed that at pH 6.15, the inhibition by malate in subsequent assay at pH 7 was much lower than at pH 7 or 8. When the reaction is prerun for 30 minutes with increasing concentrations of PEP, subsequent assay with malate shows progressively less inhibition due to malate. When 0.3 millimolar PEP either alone or with 0.1 millimolar ATP and 0.3 millimolar NaF is present during preincubation, the effect of malate in a following assay is to activate the reaction. These results may indicate an effect of phosphorylation of the enzyme on sensitivity to malate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brulfert J., Vidal J., Le Marechal P., Gadal P., Queiroz O., Kluge M., Kruger I. Phosphorylation-dephosphorylation process as a probable mechanism for the diurnal regulatory changes of phosphoenolpyruvate carboxylase in CAM plants. Biochem Biophys Res Commun. 1986 Apr 14;136(1):151–159. doi: 10.1016/0006-291x(86)90889-2. [DOI] [PubMed] [Google Scholar]
- Walker G. H., Ku M. S., Edwards G. E. Catalytic activity of maize leaf phosphoenolpyruvate carboxylase in relation to oligomerization. Plant Physiol. 1986 Apr;80(4):848–855. doi: 10.1104/pp.80.4.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedding R. T., Black M. K. Physical and Kinetic Properties and Regulation of the NAD Malic Enzyme Purified from Leaves of Crassula argentea. Plant Physiol. 1983 Aug;72(4):1021–1028. doi: 10.1104/pp.72.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K. Day/Night Changes in the Sensitivity of Phosphoenolpyruvate Carboxylase to Malate during Crassulacean Acid Metabolism. Plant Physiol. 1980 May;65(5):792–796. doi: 10.1104/pp.65.5.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. X., Wedding R. T. Diurnal regulation of phosphoenolpyruvate carboxylase from crassula. Plant Physiol. 1985 Mar;77(3):667–675. doi: 10.1104/pp.77.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. X., Wedding R. T. Regulation of phosphoenolpyruvate carboxylase from Crassula by interconversion of oligomeric forms. Arch Biochem Biophys. 1985 Aug 1;240(2):655–662. doi: 10.1016/0003-9861(85)90073-6. [DOI] [PubMed] [Google Scholar]


