Abstract
The microbial community composition of five distinct thermophilic hot springs was effectively described in this work, using broad-coverage nanopore sequencing (ONT MinION sequencer). By examining environmental samples from the same source, but from locations with different temperatures, bioinformatic analysis revealed dramatic changes in microbial diversity and archaeal abundance. More specifically, no archaeal presence was reported with universal bacterial primers, whereas a significant archaea presence and also a wider variety of bacterial species were reported. These results revealed the significance of primer preference for microbiomes in extreme environments. Bioinformatic analysis was performed by aligning the reads to 16S microbial databases for identification using three different alignment methods, Epi2Me (Fastq 16S workflow), Kraken, and an in-house BLAST tool, including comparison at the genus and species levels. As a result, this approach to data analysis had a significant impact on the genera identified, and thus, it is recommended that use of multiple analysis tools to support findings on taxonomic identification using the 16S region until more precise bioinformatics tools become available. This study presents the first compilation of the ONT-based inventory of the hydrogen producers in the designated hot springs in Türkiye.
Subject terms: Next-generation sequencing, Environmental biotechnology
Introduction
Many thermophiles have been found in hot springs, where geothermally heated groundwater is discharged from fissures in the Earth’s crust. Since the first phylogenetic characterization of an environmental microbial population by 16S rDNA sequencing at Yellowstone National Park, USA1, a steadily increasing number of studies have been conducted on metagenomics of extreme environments2–7. According to the scientists conducting these investigations, common bacterial primers designed from the genomic sequences of laboratory-grown bacteria are insufficient to represent and distinguish uncultured prokaryotic isolates from environmental samples taken under challenging circumstances, which are heavily influenced by seasonal fluctuations. In this study, comparative genomic data sets from the scientific literature were carefully examined in order to design effective primer sets for the investigation of the target microbial communities in extreme conditions. A wide range of bacteria remains unculturable under lab conditions, thus, their sensitive detection within their habitats, where they are already found in low abundances and in dormant states, rely on primers whose design is based on up-to-date and comprehensive 16S databases. Metagenomics is a cutting-edge technique for screening bacterial and archaeal species independent of cultures and serves as a crucial tool for research on the variety of microbial communities in extreme environments8,9. This tool allows the detection of all genes present in an organism, and their structures, functions, distribution and diversity in the context of their community by simultaneously sequencing, or arraying, genetic fragments obtained from the complete microbiome10–12. Natural water sources with extreme temperatures and their residences of thermophilic microorganisms remain highly unexplored, and unable to be cultivated in lab conditions. However, metagenomic investigations shed light into these wild microbial communities and help environmental microbiologists to better understand and uncover the great potential and diversity held in these rare-biospheres.
Sequencing technologies are mainly classified for their sequencing length in base pairs (bp), commonly used methods cover only a portion of the whole region (the V3–V4 region of the 16S rDNA gene13), however, the 16S region can be covered by all V1–V9 hypervariable regions in the newly developed long-read sequencing platforms allows for better taxonomical classification and functional profiling of uncultured organisms. Unlike many other methods, the Oxford Nanopore MinION technology (ONT) relies on direct detection and sequencing of a single-stranded DNA (ssDNA) molecule with characteristic electrical current changes of bases, rather than nucleotide incorporation14,15. DNA sequencing with nanopore sequencing distinguishes it from other next-generation sequencing (NGS) and third generation sequencing (TGS) platforms, as it enables on-field applications with rapid library preparation and real-time acquisition of genomic data, as well as cost-efficiency16. Considering these, nanopore sequencing has become an appealing tool for genomic characterization of complex environmental samples, improving the detection of unknown organisms, owing to the development of rapid protocols and analysis pipelines for metagenomic profiling17.
In this study, it was aimed to compare the capabilities of different pipelines in bioinformatics in nanopore studies by sequencing samples of thermal water sources, one of the habitats of extremophile microorganisms. We hypothesized that the microbial populations of five distinct hot springs in Izmir, Türkiye, may contain extreme thermophiles, such as carboxydotrophic hydrogenogenic profiles, which alter with the evolution of molecular and environmental growth conditions. In our study, using two separate 16S rDNA primers (universal and custom designed), we identified bacterial and archaeal communities associated with those that use carbon monoxide to produce hydrogen. In Türkiye, an inventory of hydrogen producers in the designated hot springs was first assembled using ONT technology.
Results
Metagenomic analysis of Doğanbey, Çeşme, and Bergama hot springs
Our data is presented using a sequential approach, which encompassed the steps of sample gathering and processing. Biohydrogen production capacities and SEM images are also presented to evaluate the sequencing results, and the practical outcomes.
Between the dates of 25/05/2021 and 23/08/2021, sampling of the microbial communities at the Izmir hot spring ecosystem revealed variations in temperature and ORP values across the sample location. For the Doğanbey hot spring, the following were observed: Upstream (US) 77.3 °C; 43.8 mV, midstream (MS) 52 °C; − 13.2 mV and downstream (DS) 49.2 °C; − 23.1 mV, with a slight change in pH values between 6.42 (US) to 7.43 (DS). On the other hand, Çeşme and Dikili-Bademli hot springs reported mesophilic conditions (38 °C; pH 7.2 and 43.2 °C; pH 6.75) whereas Dikili-Nebiler and Bergama hot springs reported thermophilic conditions (56.2 °C; pH 7.88 and 56.2 °C; pH 6.86).
According to taxonomic classification data obtained from all hot springs, bacteria (95%) with 6,376,218 reads, and archaea with 85,786 reads (1%) were classified as superkingdom in the taxonomic classification.
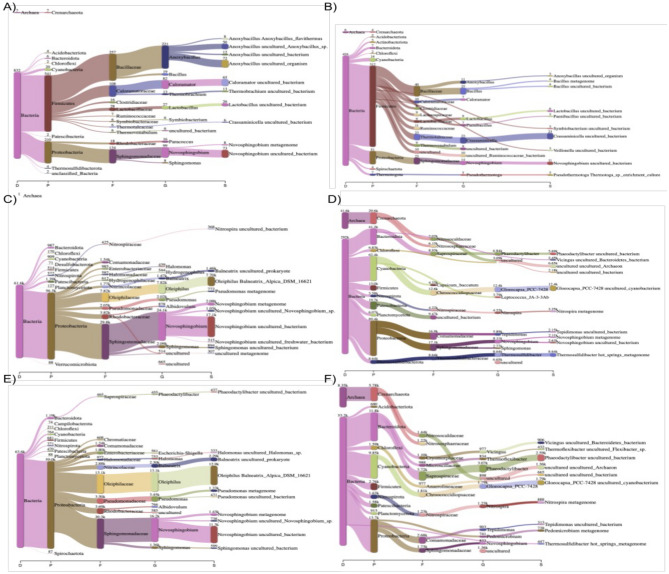
Three different tools were used to compare the samples analyzed using archaea-specific primers. Overall, the analysis of Doğanbey samples indicated that the choice of primer pair has a significant impact on the range identified microorganisms and their abundances. Interestingly, when analyzed using Kraken (version number,citation), upstream samples showed no significant change with use of archaea primers at domain or phylum levels (Fig. 1). Crassaminicella, Paenibacillus and Pseudothermotoga at genus level were reported. Epi2Me (v3.6.2) tool also showed similar results. Using universal primers, Nitrosopumilus was the only archaeal genus detected at Doğanbey upstream, with no sign of archaea in the other locations. However, within all Doğanbey samples, the use of the archaea primers resulted in increased abundance of this genus, as well as the discovery of other archaea species. Newly detected archaea genera were Methanothermobacter for the upstream, and for the mid and downstream regions, Nitrososphaera, Archaeoglobus, Methanospirillum (Fig. 2). The in-house BLAST tool was used as an alternative for identification of genera (Figs. 3, 4). Using this tool obtained a similar microorganism profile as found by using Kraken and Epi2Me. In all samples analyzed using universal primers, reads were much more aligned to Novoshingobium species than in those analysed using archaea-specific primers. Moreover, using archaea-specific primers, the archaea genus Haloferax, rather than Nitrososphaera was identified as the dominant genus.
Figure 1.
Representation of species level classification comparing archaeal distribution among samples collected from 3 different parts of the Doğanbey hot spring and amplified with two different 16S rDNA primers (universal and custom-built) using Kraken tool as indicated in the (Supplementary Information) including the Python codes and BLAST data. Samples were collected from upstream (A,B), midstream (C,D) and downstream (E,F) regions, and analyzed using universal (A,C,E) and archaea (B,D,F) primers.
Figure 2.
Representation of species level classification of samples collected from Doğanbey hot spring using the Epi2Me tool. Samples were collected from upstream (A,B), midstream (C,D) and downstream (E,F) regions, and analyzed using universal (A,C,E) and archaea (B,D,F) primers.
Figure 3.
Representation of genus level classification of samples collected from Doğanbey hot spring using the in-house BLAST. Samples were collected from upstream (a,b), midstream (c,d) and downstream (e,f) regions, and analyzed using universal (a,c,e) and archaea (b,d,f) primers.
Figure 4.
Representation of species level classification of samples collected from Doğanbey hot spring using the in-house BLAST. Samples were collected from upstream (a,b), midstream (c,d) and downstream (e,f) regions, and analyzed using universal (a,c,e) and archaea (b,d,f) primers.
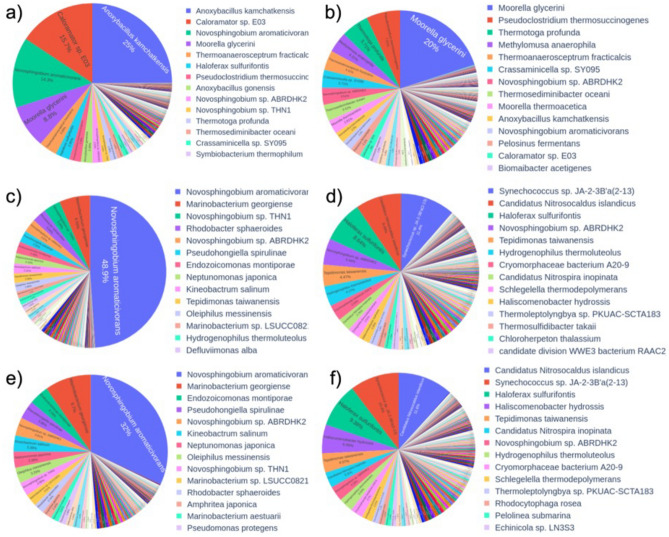
In-house BLAST classifications were compared to Kraken and Epi2me platforms, and indicated that these three different platforms were compatible, and result in very similar performances of bacteria as far as genus level classification. However, in species level classification, there are few differences, concerning most abundant species in the samples. For example, in Doğanbey downstream sample (E in Figs. 1, 4), the most abundant species, according to Kraken classification, is Oleiphilus balneatrix, and according to in-house BLAST classification, it is Novosphingobium aromaticivorans.
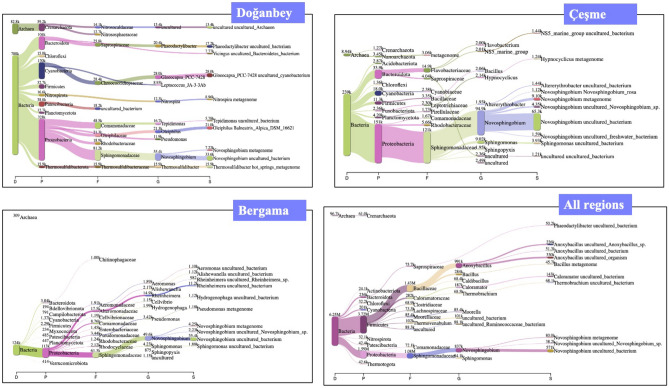
Based on the species obtained as a result of 16S rDNA metagenomic sequencing using universal primers depicted in Fig. 5, Doğanbey hot spring hosts dominantly Novospingobium species belonging in Proteobacteria genus, which is frequently encountered in thermal water resources18. Novospingobium sp., which live in milder temperature conditions (30–45 °C), also dominate the Çeşme hot spring community19. This was an expected finding considering the temperature of the hot spring was measured as 38–43 °C.
Figure 5.
Metagenomic analysis and taxonomic classification of Doğanbey, Çeşme, Bergama hot springs and comparison of all regions in Izmir province using universal 16S rDNA bacterial primers (prepared using Kraken).
Among the 5 hot spring water communities, the archaea population was most evident in the Doğanbey thermal spring, from which more than 80 k reads were obtained. Archaea population in the hot spring was determined as a result of 16S analysis, and the three most common archaeal species were Nitrosopumilus ureiphilus, Nitrosopumilus maritimus, Nitrososphaera viennensis.
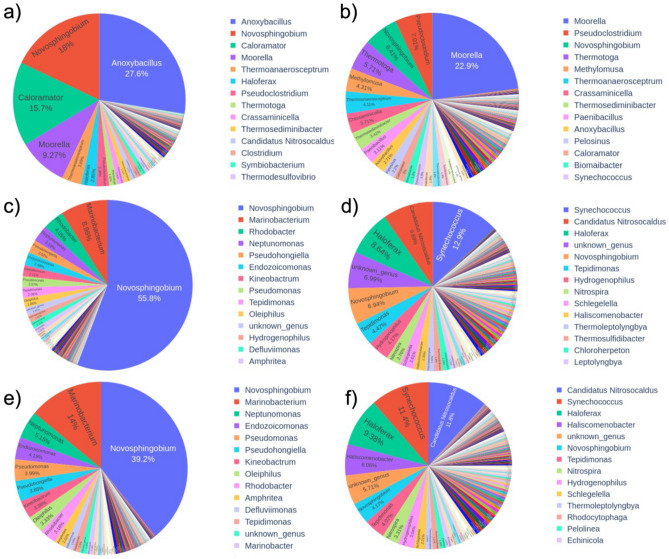
Only 4% of the reads could not be classified. Novosphingobium species capable of degrading aromatic compounds were found to be dominant (17%), but these bacterial species are moderately thermophilic (37–45 °C)20 and aerobic, and therefore not considered as one of the species of interest in terms of H2 production within the scope of the study.
Among the bacterial communities, Moorella (Moorella humiferrea, Moorella thermoacetica, Moorella gylcerini), a gram-positive thermophilic and anaerobic bacterium, was reported as the second most dominant genus after Novospingobium species (15%). Thermophilic, anaerobic and gram positive Anoxybacillus species and gram positive, facultative anaerobic and moderately thermophilic Caloramator species with 3% population density were determined. Moorella, Anoxybacillus and Caloramator genera, which dominate the community, are species with spore-forming properties, which have already been isolated from hot springs21–23.
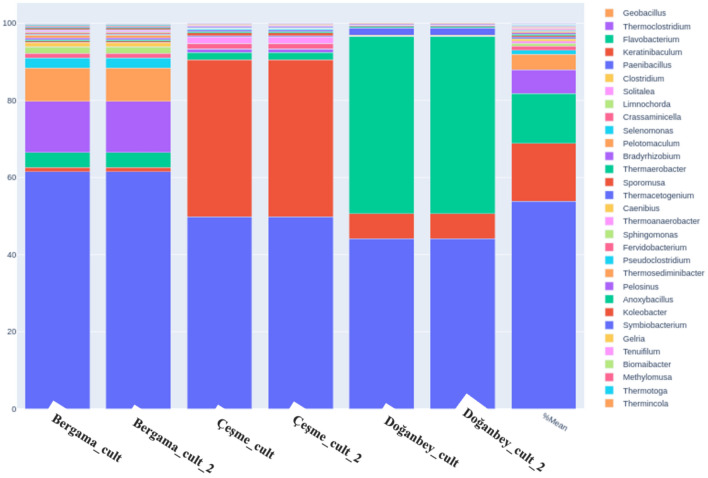
Figure 6 demonstrates the diversity of the bacterial and archaeal specimens at the genus level, highlighting the variation of distinct organisms in three different hot springs (Bergama, Çeşme, Doğanbey) using both isolated directly from the spring water and cultivated isolates amplified with universal bacterial 16S rDNA primers.
Figure 6.
Percentage representation of microbial community diversity and quantity plots of samples at genus level bacteria and archaea in Bergama, Çeşme, and Doğanbey hot springs from enriched cultures.
Previously, reports were made of the presence of carboxydotrophic hydrogenogenic organisms in different hot springs of Izmir province: these were Methanosarcina acetivorans24, Thermincola ferriacetica25, Moorella thermoacetica26, Thermosinus carboxydivorans Nor1 and Thermosinus carboxydivorans27–29, Carboxydothermus pertinax30, Carboxydocella ferrireducens and Carboxydocella thermautotrophica and Carboxydocella spp.31,32, Caldanaerobacter caldanaerobacter subterraneus and Caldanaerobacter thermoanaerobacter sp._RH080433–35 with low abundances, with the most dominant being M. stamsii by % 0.254945 presence26. Consequently, in our study, the reported low abundances of these microorganisms were in agreement with the known rarity of carbon monoxide (CO)-oxidizing and H2-producing microorganisms in nature (< 0.1%), and these are usually found in dormant state, associated with little to zero growth36–38. In order to test their capacity to convert CO into H2, the enrichment of these CO-utilizing consortia was experimented under carboxydotrophic growth-specific media and CO gas as the substrate, suitable growth conditions were supplied for thermophilic hot spring microorganisms to cultivate under lab conditions, increase their abundances in the culture and produce hydrogen using necessary enzymes in their metabolisms.
A significant increase in the genus of Moorella was achieved, with their abundance reaching up to 5% in cultivated samples, Caldanoerobacter species up to 0.6% however Carboxydocella and Carboxythermus genuses reached only 0.02% and 0.01% in abundance, respectively (Fig. 6). A comparative analysis of Doğanbey isolates cultured on different gas substrates showed a decrease in the number of species in the enriched mixed culture in lab conditions, and a significant difference between growth in syngas (see “Methods” section) and 100% CO. Caloramator sp., the species of greatest interest for hydrogen production in culture, is unable to grow in syngas-fed culture medium (less than 5% in abundance) and thus allowing the dominant growth of Novosphingobium (up to 60%), but grows dominantly in 100% CO gas-fed culture medium (up to 50%), with the remaining culture consortia consisting mainly of Novosphingobium aromaticivorans (38%) and Moorella glycerini (6%) (Fig. 6). Environmental and chemical growth conditions were adjusted for the thermophilic hot spring microorganisms to cultivate them under lab conditions. These conditions were applied in order to increase their abundances in the culture and simultaneously produce hydrogen with the help of the relevant enzymes in their metabolisms. Microorganisms were grown until hydrogen production was detected representing the end of their logarithmic phase as shown in our previous study39. The comparative results of taxonomic profiling in direct water isolations and in cultivated samples demonstrated that bacterial species belong to the Firmicutes genus Anoxybacillus and Caloramator alongside other thermophilic carboxydotrophic hydrogenogens (Moorella stamsii, Tepidimicrobium ferriphilum, Thermodesulfovibrio, Geobacillus, Thermosediminibacter)40–42 have been dominantly observed, and their abundances increased after being taken into anaerobic and thermophilic culture medium and feeding with 100% CO gas. Hydrogen production and CO utilizing activities of the cultivated samples were reported in our previous study39 and the results indicated that the presence of thermophiles together with anaerobes in the microbial community constructed an adequate mixed culture consortium for efficient hydrogen production.
Scanning electron microscopy (SEM) imaging of cultivated hot spring isolates
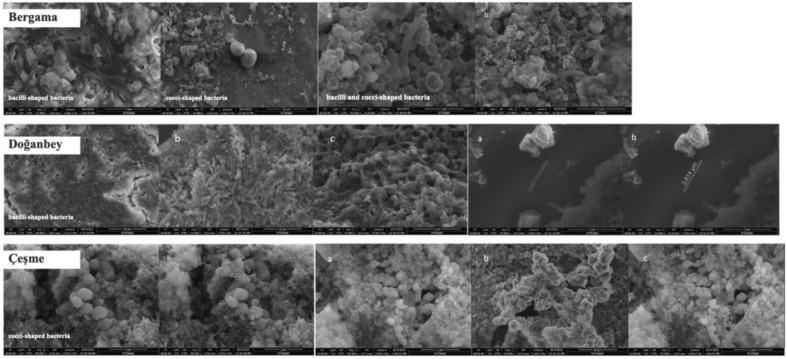
SEM imaging of 3 different H2-producing hot spring microbial communities showed that these communities consist of bacilli and cocci-shaped bacteria of various sizes and morphological features (Fig. 7). A dense population of a single bacilli-shaped bacteria 3.814 µm long and 362.8 nm wide was observed in Doğanbey. This single thermophilic and anaerobic microorganism showed significant morphological similarity to the genera of Thermoanaerobacter, Carboxydocella, and Bacillus associated with CO conversion to hydrogen and organic acids43,44. Çeşme samples exhibited mainly cocci-shaped bacterium with sizes ranging from 1.60 to 2.127 µm in length and 307.6 to 620.1 nm in width. In Bergama samples, both bacilli and cocci-shaped bacterium were observed simultaneously; the Bacilli-shaped bacteria were 1.850 µm in length and the cocci-shaped bacterium were 344.3 to 455.3 nm in width.
Figure 7.
SEM images and dimensions of bacilli and cocci-shaped bacteria isolated from Bergama, Doğanbey, and Çeşme hot springs.
A recent publication by the authors reported the biohydrogen production capacities of the isolated microorganisms from the hot springs of Izmir province; the highest was found in Bergama, with a yield of 0.18 ΔH2 (mmol)/ΔCO (mmol), followed by Doğanbey (0.13 ΔH2 (mmol)/ΔCO (mmol)) and Çeşme (0.12 ΔH2 (mmol)/ΔCO (mmol))39.
Discussion
The abundances of microorganisms were determined by nanopore sequencing, and the findings were compared in accordance with the comparative bioinformatic analysis methods, sampling sites, sequencing primer selections, and culturing techniques.
Following the quality check and barcoding, the reads were aligned to 16S microbial databases for identification using three different alignment tools, Epi2Me (Fastq 16S workflow), Kraken and in-house BLAST tool. A comparison of the differences at genus and species levels shows that there is agreement between these tools at genus level classification, but this agreement disappears at species level in all cases. While Kraken identifies Oleiphilus species among samples in mid- and low-course samples, the other tools would align these reads to Marinobacterium. It is important to note that both belong to the same Oceanospirillales order. On the other hand, unlike the other two tools, in-house BLAST showed a significant proportion of reads (up to 15%) aligning to Haloferax sulfurifontis in samples studied with Archaea-specific primers. Such differences in the three analysis tools we have employed suggests that the way the data is analyzed has a significant impact on the species level identification, however, shows a significant compatibility in identified genus of hot spring microbial communities. These differences in species level identifications might be due to the fact that the 16S region where classification is based, is too short a stretch of DNA, resulting in significant alignment score fluctuations by the algorithms, but it is also very likely to be due to the differences in the available databases they utilize. For that reason, until more precise tools are developed, use of more than one analysis tool is beneficial to support findings on species level taxonomic identification using the 16S region. Cumulative contribution of the scientific community to excel sequencing analysis of microbiota may eventually result in one of these or many other tools to be commonly accepted, however, it should be remembered that the methods and databases utilized by different tools may be more sensitive over the others in particular conditions or sample sources. For this reason, it may not be possible to claim any tool to be superior to others and the use of multiple tools will remain the optimal approach. The problem with such an approach is how to evaluate the disagreements. While different approaches may be utilized to overcome this, the choice is still a question of statistics and beyond the scope of this work.
Besides the choice of tool, there were significant differences between the results obtained using different primer pairs. Accordingly, the archaea-specific primers classified both archaea and bacteria, while universal bacterial primers showed fewer reads aligned to any archaeal species. It is crucial to note that to generate the archaea-specific primers, only archaea genomes were used to identify a consensus sequence, and primers were selected at highly conserved regions. Despite this, the bacterial sequences made up the greater portion of the reads, even with archaea-specific primers. This may indicate that, despite the PCR bias for archaeal species, the abundance of bacteria may be well above the archaea species in the sample and make up more than the abundance observed by the archaeal species. Supporting this, universal primers resulted in a marginal number of reads aligned to the archaea. Read numbers are a result of PCR amplification thus not represent the reads obtained directly from microorganisms and this PCR bias could always effect the abundance of microorganisms frequency. For this study to minimize the PCR bias we have implemented PCR with minimum cycles to obtain target DNA concentration.
Despite a general agreement between the studies at the genus level and above, there is no agreement at the species level. Even in pure culture samples, there are conflicts regarding the classification at the species level, for example the species of Novosphingobium and Anoxybacillus are detected and in every sample, but there is a distinct bias towards Novosphingobium. It is argued that microbial genomes from natural environments exhibit trait biases resulting from phylogeny-based approaches, and lacking whole-genome sequences of uncultured bacteria, thus the available reference genome datasets may exhibit biases towards more abundant organisms45. In pure cultures from Doğanbey hot spring, there is a bias towards this species in bioinformatic analyzes, especially in Epi2Me workflows. Considering that the Novosphingobium species live in moderately thermophilic environments18,20, this bias is explained by the frequent classification of this species (about 15%) in the Doğanbey hot spring samples, where water temperatures reach 75 °C, and where the growth of Novosphingobium, a mesophilic species, is usually very limited20.
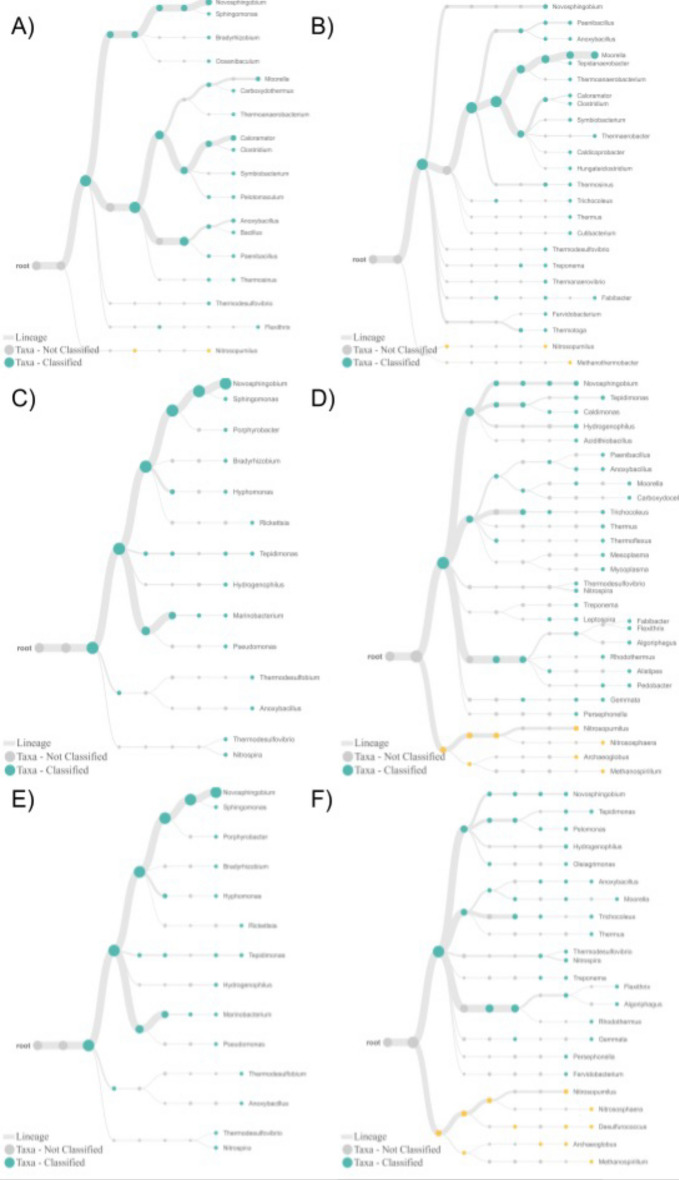
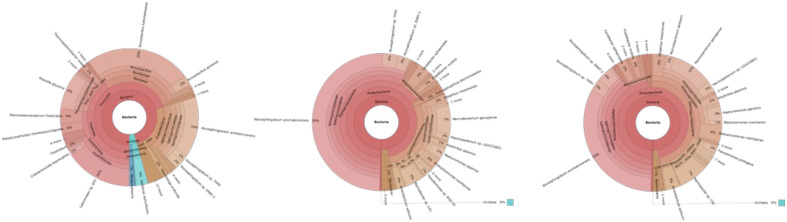
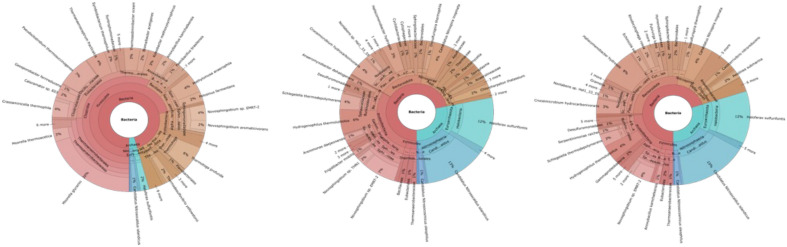
Figure 8 shows a Krona plot analysis of the comparative classification for different parts of the hot spring stream with temperatures decreasing with distance from the source. This revealed that sequencing with universal bacterial primers, archaea and bacteria species was the most common in terms of microbial variety in the most thermophilic part of the source (upstream), then, as the temperature decreased downstream, the archaea disappeared, the bacterial diversity of species decreased and Novosphingobium species became the more dominant (up to 50%). However, this finding runs counter to investigations reported in the literature, where an increase in temperature results in decreasing microbial variety, due to mainly temperature stress and low adaptation rates of the microorganisms to the stressful thermophilic conditions46,47. This suggests that universal bacterial primers did not represent an accurate phylogenetic model of the hot spring in terms of microbial variety. On the other hand, using custom designed primers in this study, the phylogenetic model of the three parts of the hot spring suggested a reasonably accurate representation in terms of microbial diversity, showing a decline in microbial diversity with increasing temperatures (Fig. 8). Even though the custom primers were designed using archaeal genomes, these still demonstrate a better representation in terms of the detection and dissociation of bacterial species.
Figure 8.
Krona plot analysis of three different points of decreasing temperatures of the hot spring: upstream, midstream and downstream, demonstrating significant loss of both archaeal presence and microbial variety using universal 16S rDNA bacterial primers.
Custom-made primers worked well on direct DNA isolation from water samples (uncultured); however, it was not possible to classify archaeal presence with universal bacterial primers. The amount of archaea increases in the midstream, and at the beginning, downstream distancing from the hot spring source. This can be explained by the lower number of reads in the upstream samples, while the sensitivity may also have decreased. These primers detected 4% of archaea at the most highly thermophilic part of the stream, 28% at the middle part, and 38% at the most highly mesophilic part (downstream). With the universal bacterial primers, the presence of archaea was highest at the source (upstream) with 4%, but no archaea were detected at the mid and downstream points (Fig. 9).
Figure 9.
Krona plot analysis of three different points of decreasing temperatures of the hot spring: upstream, midstream and downstream, demonstrating significant loss of both archaeal presence and microbial variety using custom designed archaeal 16S rDNA primers.
The designed archaea primers were sensitive in detecting the presence of archaea only at mild temperatures, while, at higher temperatures, no difference was observed in the classification of archaea between universal primers and custom-designed archaea primers. The use of two different primers in the upstream sample showed that the archaea primers were able to classify a wider variety of species, but the universal primers dominantly classified Anoxybacillus and Novosphingobium species.
A study48 demonstrated a custom-primer design for archaea, which included a wider variety of species that live in psychrophilic (4 °C from freshwater samples) to mesophilic (37 °C from bioreactor samples) and temperatures and involved alignment of 8500 of sequences. This study reported at least 38% higher coverage of archaea compared to more commonly used universal primers. Another study49, involved distinctive environmental conditions (1 to 4 °C and high pressure) and aimed for higher detection of microbial communities living in niche conditions, similar to the this current study. While as a difference, that study claimed that short read sequencing and V3–V4 hypervariable region of 16S gene in terms of taxonomic assignment of oceanic consortia, and the better performance of the archaeal primer designed in that study in terms of diversity of archaeal communities, especially on Thaumarchaeota (Crenarchaeota), compared to universal primers, and reported up to 70% coverage for archaea. The current study includes niche growth conditions (thermophilic temperatures 60 °C and above, anaerobic and CO-utilizing) of archaea with hot springs of similar ecological conditions, therefore we acknowledge that our custom-built primers are not more comprehensive in terms of the range of archaea covered, however, these primers offer a more sensitive and robust detection, and are able to capture thermophilic and anaerobic hot spring archaea where they can be found in very small abundances. Another study50 targeted pig fecal samples using a short read NGS sequencer, Illumina MiSeq, and designed universal prokaryotic primers based on V3–V4 hypervariable regions of 16S gene. In their study, custom designed primers matched to 94.6% of Archaea rRNA gene sequences in the database used as a reference. It was additionally shown, similar to the current study, that, where a primer bias existed, archaeal species were also detected with the bacterial universal primer. Custom designed prokaryotic primers increased performance by 0.8% for Archaea compared to previously described universal primer sequences in the database used in that study, and they successfully lowered the bias with the custom designed prokaryotic primers when compared to commonly used universal prokaryotic primers.
Some 16S rDNA primer sets have been reported to include biases, thus it is important to minimize these, and obtain an even coverage of both overall and desired microorganisms for an optimized resolution within primer sets. To achieve this, firstly, regarding 16S primers and their relative performance regarding microbial representation, it would be wise to conduct preliminary investigations with less complex samples or mock microbial communities before implementations on environmental extreme areas, especially for the investigation of archaeal species51,52. Imitation restrictions of these unknown areas in extreme conditions require sensitive and comprehensive studies to achieve maximum precision with microbial community profiling. Sequencing platforms directly affect the outcome of the study, as they differ greatly in terms of protocol directions (such as PCR conditions), and differences between MiSeq and HiSeq sequencing platforms were reported with their respective protocols using mock microbial communities53. High throughput sequencing platforms (Nanopore and PacBio technologies) present greater depths; however, another study52 suggested that observed biases using the MiSeq platform between different primers could not be solved by greater depths, but it is possible to resolve biases by the comparative approach, using mock communities and field samples. A study extensively comparing both platform and primer choice effects on 16S sequencing reported that primer choice had a greater biological effect than sequencing platforms and emphasized the importance of experimental methods for achieving accurate representation of abundance in microbial communities54.
This current study explored hot spring thermophilic communities with investigational parameters, including the comparison of the community structure in different parts of the hot spring waterflow where water temperature ranged from 38.0 to 77.3 °C. Furthermore, we have compared several bioinformatic pipelines and demonstrated unalike prokaryotic profiles with the utilization of conventional and novel primers (universal vs. custom design) for PCR amplification of the samples. Custom design primers indicated better results in terms of decreasing microbial diversity among increased water temperatures of hot springs while sequencing results with universal primers suggested the opposite. Study results also highlighted that both custom designed and universal prokaryotic primers have a certain bias towards Novosphingobium species, this was indicated by the samples where water temperature was too high for this mesophilic species (30–45 °C) to live and still showed an abundance of Novosphingobium species in bioinformatic analysis. Overall, this study is the crucial to report the effectiveness of different bioinformatic pipelines for nanopore sequencing studies together with representing valuable data with the custom design primers used.
As a conclusion, among the two methologies of using targeted custom-design primers and using different bioinformatic analysis pipelines for the classification of hot spring microorganisms, the primer choice had a critical effect on detection of archaeal species and also identification of the variety of bacterial species. Sequencing results revealed that enriched cultures demonstrated a significant increase of carboxydotrophic hydrogenogenic bacteria in abundance due to the manipulation of operational parameters in their favor. This approach has led to the discovery of a methodology for the detection of hydrogen producers from extreme environments. The current study also highlights the advantages of the nanopore sequencing tool for improving the feasibility of molecular workflows in microbial metagenomic studies and the proposed methodology could also be used in future works to investigate the roles of industrially important bacteria and archaea in similarly extreme environments.
Methods
Archaeal primer design for custom-built 16S rDNA primers
Custom-built primers were specifically designed for PCR amplification of 16S rDNA regions of thermophilic archaea that are likely to be isolated in Izmir province. Based on literature investigations, thermophilic and anaerobic archaea species reported in hot spring microbial community studies were identified and selected. Archaea sequences were downloaded from the NCBI database in FASTA format (Table 1).
Table 1.
Archaeal species that are likely to be found in thermal waters in and around İzmir, living at a temperature of 50–65 °C, around pH 6–7.
| Organism | Accession code | Reference |
|---|---|---|
| Thermococcus barophilus | NC_014804 | 4 |
| Thermococcus guaymasensis DSM11113 | NZ_CP007140 | |
| Thermococcus onnurineus NA1 | NC_011529 | |
| Thermococcus paralvinellae | NZ_CP006965 | |
| Thermofilum adornatum | NC_022093 | |
| Methanothermobacter thermautotrophicus | NC_000916 | |
| Methanomethylovorans thermophila strain L2FAW | NR_043089 | 59 |
| Methanomassiliicoccus luminyensis | HQ896499 | |
| Methanococcus aeolicus | (NC_009635) | |
| Methanocaldococcus jannaschii | NC_000909 | 60 |
| Archaeoglobus fulgidus | NZ_CP006577 | |
| Thermosphaera aggregans | NC_014160 | 61 |
| Methanocaldococcus vulcanius | NC_013407 | |
| Methanocella conradii | NC_017034 | |
| Methanothermobacter marburgensis | NC_014408 | 62 |
| Methanosarcina thermophila | NZ_CP009501 |
In order to detect potential species, archaea species and their entire genomes were combined on the same text file. As an exemplary, it was decided to select a thermophilic archaeal species isolated from a hot spring Thermococcus celer and its 16s rDNA partial sequence (NCBI Reference Sequence: NR_113295.1) because species growth conditions showed similarity to the investigated hot springs in this study55. Archaeal sequence and the FASTA file were downloaded through the NCBI database.
For the alignment of the 16S rDNA sequence (query) and the merged archaeal sequences (subject) via BLAST + (BLASTN 2.10.1+), a Python code was programmed to give only the accession codes of the merged archaeal sequences. The program printed the access codes of the archaea, and codes were also transferred to BLAST+, so that it could load archaea sequences from its own database. The command ‘blastn -query “.\Methanocaldococcus 16S.txt” -subject Merged_archaea.fasta -outfmt 7 > output.tsv’ was entered to the BLAST + program. Tabular with comment lines was selected as a formatting option to save the results as output.tsv format in an Excel file. The start and end of the sequences in common to the 16 s sequence, and the archaea sequence in the tsv file were determined as the query start (q.start) and end points (q.end).
A Python program called SeqExtractor was coded in order to detect the archaea name and sequences from the ‘merged_archaea.fasta’ file, which was initially given in the Python program to detect the 8th (query start) and 9th (query end) rows in the query table in tsv file given by the BLAST program. Query start and end points from the archaea sequences were extracted and printed as alignments. Archaea accession codes and alignment sequences were recorded in a file named ‘16ssequences.fasta’. The script receives two files, a list of fasta files containing the genomic sequences of selected archaea species and a BLAST output where a reference 16S sequence was aligned to these sequences. The script extracts 16S sequences from each genome and assembles them in a single file in fasta format, ready to be aligned.
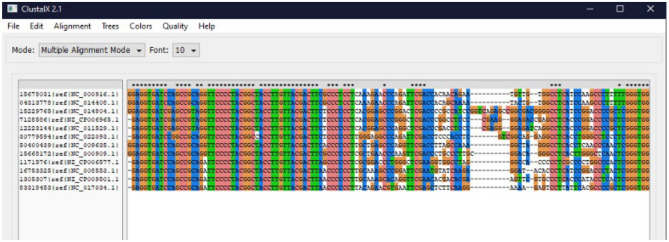
These sequences were aligned with the UGENE Multiple Sequence Alignment program (v.33)56. Some sequences were found to be inverted, otherwise the sequences were very similar and aligned. 16S sequences were also aligned with the ClustalX Multiple Sequence Alignment program (v2.0)57. Reverse complementary sequences of reversed sequences were obtained (via were loaded into the ClustalX program together with the sequences, and realigned (Fig. 10). Forward and reverse archaeal primers were determined according to the obtained alignment results (Table 2).
Figure 10.
Alignment results on ClustalX for the archaea species likely to be found in the İzmir hot springs.
Table 2.
Thermophilic bacterial and archaeal 16S rDNA primers.
| Primers | Forward | Reverse |
|---|---|---|
| Bacteria primers (Universal) | Tailed-27For: 5ʹ TTTCTGTTGGTGCTGATATTGCAGAGTTTGATCMTGGCTCAG 3ʹ | Tailed-1492Rev: 5ʹ ACTTGCCTGTCGCTCTATCTTCCGGTTACCTTGTTACGACTT 3ʹ |
| Archaea primers (custom designed) | For27: ACTTGCCTGTCGCTCTATCTTCTACGGCTACCTTGTTACGAC |
Rev621: TTTCTGTTGGTGCTGATATTGCCTGAAACTTAAAGGAATTGGC Rev1413: TTTCTGTTGGTGCTGATATTGCACKGCTCAGTAACACGTG |
Universal 16S rDNA Bacterial primers were supplied by Nucleus Genetic Inc. In order to obtain the same Tm (melting temperature) value of the primers to perform PCR under the same conditions as the primers to be used in bacteria, the Tm value of the specific bacterial primers was first found on the website (https://tmcalculator.neb.com) and the Archaea primer sequences were shortened accordingly (Table 2). PCR trial conducted with custom designed 16S rDNA primers for archaea showed the success of the amplification of 16S region of the DNA samples isolated from hot springs.
Sampling and cultivation of hot spring isolates
Five different hot springs located in Doğanbey, Çeşme, Dikili Bademli, Dikili Nebiler, and Bergama, Izmir, Türkiye (Table 3) were field-visited to collect samples between 25/05/2021 to 23/08/2021 (Fig. 11). Samples were collected in sterile plastic bottles and transferred to the laboratory in a heat-insulated container. pH, temperature, and oxidation–reduction potential (ORP) measurements were made from each collection point with a portable pH meter (MW 105 Max, Milwaukee, USA). The samples were processed in two different workflows:
Direct DNA isolation of hot spring samples was performed (5 L of volume) according to the manufacturer’s instructions with Thermo Fisher GeneJET Genomic DNA Purification Kit (Thermo Fisher Scientific, USA) in triplicate, without any enrichment. Isolated DNA was stored at − 20 °C for further 16S rDNA analysis.
The collected samples were transferred into sterile 50 mL falcon tubes and centrifuged at 1398×g for 10 min (Hanil Science Industrial, South Korea). The enrichment media content including macronutrients, micronutrients, and vitamins, was prepared as indicated in Ref.32. pH of the nutrient medium was fixed to 6.8 with 0.5 M HCl and 54 mL of prepared basal medium was transferred to glass bottles with a total volume of 100 mL. The bottles were closed with gas-tight rubber stoppers and metal caps, and autoclaved at 121 °C and 15 min. O2 in the headspace of the closed bottles was removed by purging with N2 gas. Following sterilization, 0.1 mL of sterile 10× vitamin solution was added to sealed vials containing 54 mL of sterile anaerobic thermophilic medium, and 6 mL (10%) collected pellet was inoculated. Bottles were fed with two different gaseous substrates: syngas (H2 (5%), O2 (5%), CO (10%), CH4 (5%), CO2 (20%) and N2 (40%)) and 100% CO gas for 30 s. The bottles were inoculated and placed in an incubator at a temperature appropriate to the isolates’ isolation temperatures (45, 55, 60 or 65 °C). Following serial cultures grown to the logarithmic phase, DNA isolation was performed according to the manufacturer’s instructions, and isolates were stored at − 20 °C for further 16S rDNA analysis.
Table 3.
Summary of the 16S rDNA metagenomic investigation of the classification of 5 distinct hot springs in Izmir area.
| Isolation source | Isolation technique | Number of raw reads | Classified reads (%) | Unclassified reads (%) | Microbial reads (%) | Bacterial reads (%) |
|---|---|---|---|---|---|---|
| All samples | Isolated cells + cultured cells | 6.391.436 | 99.4 | 0.641 | 99.3 | 97.8 |
| Doğanbey | Isolated cells | 877.400 | 99.3 | 0.699 | 99.2 | 89.8 |
| Cultured cells | 1.902.906 | 99.8 | 0.196 | 99.8 | 99.7 | |
| Çeşme | Isolated cells | 248.028 | 99.9 | 0.0867 | 99.9 | 96.3 |
| Cultured cells | 291.211 | 98.2 | 1.82 | 98.2 | 98.1 | |
| Dikili Bademli | Isolated cells | 50.438 | 99.8 | 0.151 | 99.8 | 99.4 |
| Cultured cells | 258.004 | 98.2 | 1.75 | 98.2 | 98.2 | |
| Dikili Nebiler | Isolated cells | 1.148 | 12 | 88 | 12 | 11.9 |
| Bergama | Isolated cells | 125.354 | 99.5 | 0.534 | 99.5 | 99.2 |
| Cultured cells | 999.771 | 99.6 | 0.436 | 99.6 | 99.4 |
Isolated cells describe the uncultured microorganisms directly analyzed from the water samples, and cultured cells describe the microorganisms grown under CO and enriched media.
Figure 11.
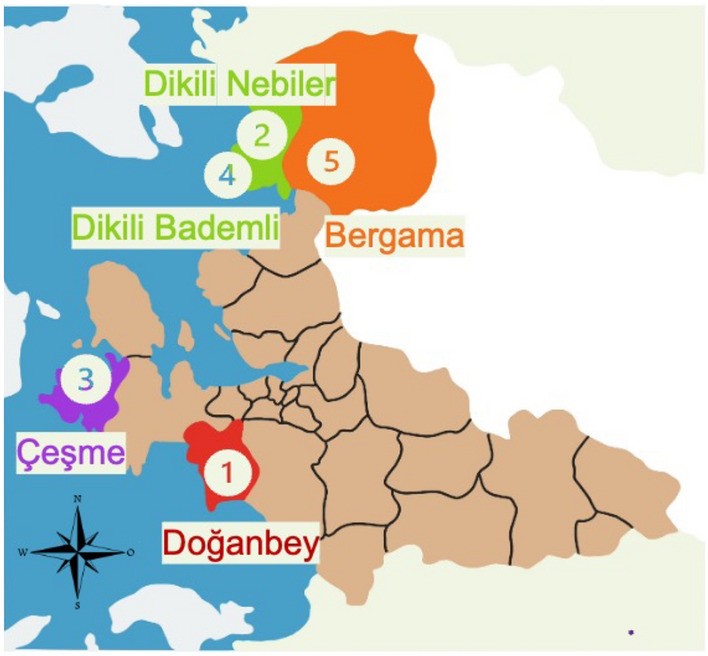
Schematic map locating the hot springs visited in this study in İzmir, Türkiye (The figure was created by the authors using Microsoft Office Power Point Tool (version 16.73; https://www.microsoft.com/tr-tr/microsoft-365/business/).
DNA library preparation
DNA libraries were prepared using consecutive PCR, to both amplify the 16S region and barcoding. 16S rDNA genomic sequences were amplified in two separate ways, using a universal primer set, and an in-house thermophilic archaea-specific primer. Sequencing bacterial 16S rDNA regions was conducted using two primers with barcoding tag in the upstream, 5ʹ TTT CTG TTG GTG CTG ATA TTG CAG AGT TTG ATC MTG GCT CAG 3ʹ and 5ʹ ACT TGC CTG TCG CTC TAT CTT CCG GTT ACC TTG TTA CGA CTT 3ʹ. To amplify archaeal 16S sequences, custom-designed thermophilic-specific primer set (from Table 2), the For27 and Rev1413 primers (5ʹ ACT TGC CTG TCG CTC TAT CTT CTA CGG CTA CCT TGT TAC GAC 3ʹ and 5ʹ TTT CTG TTG GTG CTG ATA TTG CAC KGC TCA GTA ACA CGT G 3ʹ) were used.
16S regions were amplified using the above-mentioned primers by standard PCR with 12 cycles. The amplicon sizes were confirmed on agarose gel and isolated using AMPure XP (Beckman Coulter) nucleic acid purification beads. Barcoding was performed according to the manufacturer’s protocol (Oxford Nanopore Technologies: SQK-LSK109 with EXP-PBC096). Briefly, samples were barcoded by PCR using the barcoding primers. Amplicons were purified using AMPure XP beads and quantified using a Qubit fluorometer (Thermo Fisher, Germany). The samples were pooled to yield 1 μg in 47 μL. Using Ultra II End Prep (NEB) 3ʹ ends were adenylated and 5ʹ ends were phosphorylated before AMPure XP clean-up. The adapters were ligated to pooled DNA using Quick T4 Ligase (NEB).
Nanopore 16S sequencing and bioinformatic analysis
Nanopore sequencing was performed using an ONT MinION device connected to a workstation running MinKNOW software under Windows. Sequencing was stopped after 48 h without synchronous base calling and a minimum score threshold of 7.
After sequencing was completed, base calling, barcoding and trimming was performed using Guppy under Ubuntu 18 on a workstation equipped with a GTX 1050 for GPU acceleration. Barcoded reads were analyzed and taxonomically classified using three different bioinformatic pipelines: Kraken (v2.1.2), Epi2Me (v3.6.2), and Mothur platform58; these three approaches were conducted separately.
For the Kraken classification pipeline, barcoded samples classified with Kraken software were visualized employing Sankey visualization, using the Pavian tool, and these were demonstrated with default parameters (number of taxa at each level:10, scale distance between nodes: 0.9, figure height: 400, node border-width: 0, the opacity of links: 0.6, node label margins: 1).
For the in-house BLAST (Mothur) classification and visualization pipeline, for targeting the 16S rDNA region, the obtained reads between 1250 and 1750 bp in length were filtered using Trimmomatic and the remaining reads were excluded from the analysis. Filtered reads were analyzed with a customized workflow by matching each sequence with the BLAST algorithm during the filtering process. In the matching results, an operational taxonomic unit (OTU) was created by taking the taxonomic data of sequences with more than 60% reference coverage and 80% pairwise similarity. Mothur platform was used to demonstrate taxonomic classifications and Krona charts. Graphs and tables in the analyses were generated with Python libraries.
Scanning electron microscopy (SEM) imaging
Hydrogen-producing isolates from Çeşme, Doğanbey, and Bergama mixed cultures were screened using Scanning Electron Microscopy (SEM) (FEI Quanta 250 FEG Philips, Netherlands) imaging for the detailed examination of their sizes and shapes. 15 mL of liquid culture were transferred to sterile falcon tubes and then centrifuged 1789×g for 10 min, and the supernatant was discarded. The pellet was suspended in a phosphate-buffered saline (PBS) solution (4.43 g NaCl, 0.546 g of Na2HPO4, and 0.138 g of NaH2PO4 in 500 mL of UP water) with a pH of 7.5 and centrifuged (1789×g for 10 min). The supernatant was discarded and the pellet was re-suspended in 2.5% (v/v) formaldehyde solution for 1 h for cell fixation. Following the formaldehyde fixation, the falcon tubes were centrifuged three times for final pellet collection. The obtained pellet was fixed by dropping the final pellet onto glass slides and the matte side of a piece of aluminum foil, and air dried in an incubator at a temperature of 37 °C, followed by gold coating39.
Supplementary Information
Acknowledgements
This research was financially supported by The Scientific and Technological Research Council of Turkey (TUBITAK-CAYDAG) with Project Number 120Y069.
Author contributions
M.G. designed and supervised the project; İ.A., Ş.E. and O.D. performed experiments; İ.A., Ş.E., O.D. and M.G. analyzed the data; İ.A., Ş.E., O.D. and M.G. wrote the manuscript. All authors read and approved the final manuscript.
Data availability
The datasets generated and analysed during the current study are available in the NCBI repository with the SRA Bioproject Accession Number [PRJNA954581].
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: İlayda Akaçin and Şeymanur Ersoy.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-44357-3.
References
- 1.Brock TD. Life at high temperatures. Science. 1967;158:1012–1018. doi: 10.1126/science.158.3804.1012. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt TM, DeLong EF, Pace NR. Analysis of a marine picoplankton community by 16S rRNA gene cloning and sequencing. J. Bacteriol. 1991;173:4371–4378. doi: 10.1128/jb.173.14.4371-4378.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panda AK, Bisht SS, De Mandal S, Kumar NS. Microbial diversity of thermophiles through the lens of next generation sequencing. In: Panda AK, Bisht SS, De Mandal S, Kumar NS, editors. Microbial Diversity in the Genomic Era. Elsevier; 2018. pp. 217–226. [Google Scholar]
- 4.Omae K, et al. Diversity and distribution of thermophilic hydrogenogenic carboxydotrophs revealed by microbial community analysis in sediments from multiple hydrothermal environments in Japan. Arch. Microbiol. 2019;201:969–982. doi: 10.1007/s00203-019-01661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roy C, et al. Microbiome and ecology of a hot spring-microbialite system on the Trans-Himalayan Plateau. Sci. Rep. 2020;10:1–22. doi: 10.1038/s41598-020-62797-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowers RM, et al. Dissecting the dominant hot spring microbial populations based on community-wide sampling at single-cell genomic resolution. ISME J. 2022;16:1337–1347. doi: 10.1038/s41396-021-01178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peach JT, et al. Longitudinal analysis of the Five Sisters hot springs in Yellowstone National Park reveals a dynamic thermoalkaline environment. Sci. Rep. 2022;12:1–15. doi: 10.1038/s41598-022-22047-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sambo F, et al. Optimizing PCR primers targeting the bacterial 16S ribosomal RNA gene. BMC Bioinform. 2018;19:1–10. doi: 10.1186/s12859-018-2360-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klindworth A, et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41:1–11. doi: 10.1093/nar/gks808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Handelsmanl J, Rondon MR, Goodman RM, Brady SF, Clardy J. Molecular biological access to the chemistry of unknown soil microbes: A new frontier for natural products. Chem. Biol. 1998;5:R245–R249. doi: 10.1016/s1074-5521(98)90108-9. [DOI] [PubMed] [Google Scholar]
- 11.Horgan RP, Kenny LC. ‘Omic’ technologies: Genomics, transcriptomics, proteomics and metabolomics. Obstetr. Gynaecol. 2011;13:189–195. [Google Scholar]
- 12.Latorre-Pérez A, Pascual J, Porcar M, Vilanova C. A lab in the field: Applications of real-time, in situ metagenomic sequencing. Biol. Methods Protoc. 2021 doi: 10.1093/biomethods/bpaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeong J, et al. The effect of taxonomic classification by full-length 16S rRNA sequencing with a synthetic long-read technology. Sci. Rep. 2021;11:1. doi: 10.1038/s41598-020-80826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hisham MB, et al. Bacterial communities associated with silage of different forage crops in Malaysian climate analysed using 16S amplicon metagenomics. Sci. Rep. 2022;12:1–17. doi: 10.1038/s41598-022-08819-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buck D, et al. Comprehensive comparison of Pacific Biosciences and Oxford Nanopore Technologies and their applications to transcriptome analysis. F1000Research. 2017;6:100. doi: 10.12688/f1000research.10571.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charalampous T, et al. Nanopore metagenomics enables rapid clinical diagnosis of bacterial lower respiratory infection. Nat. Biotechnol. 2019;37:783–792. doi: 10.1038/s41587-019-0156-5. [DOI] [PubMed] [Google Scholar]
- 17.Akaçin İ, Ersoy S, Doluca O, Güngörmüşler M. Comparing the significance of the utilization of next generation and third generation sequencing technologies in microbial metagenomics. Microbiol. Res. 2022;264:127154. doi: 10.1016/j.micres.2022.127154. [DOI] [PubMed] [Google Scholar]
- 18.Walker CB, et al. Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc. Natl. Acad. Sci. U.S.A. 2010;107:8818–8823. doi: 10.1073/pnas.0913533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyeon JW, et al. Novosphingobium humi sp. nov., isolated from soil of a military shooting range. Int. J. Syst. Evol. Microbiol. 2017;67:3083–3088. doi: 10.1099/ijsem.0.002089. [DOI] [PubMed] [Google Scholar]
- 20.Xian WD, et al. Novosphingobium meiothermophilum sp. nov., isolated from a hot spring. Int. J. Syst. Evol. Microbiol. 2019;69:1737–1743. doi: 10.1099/ijsem.0.003384. [DOI] [PubMed] [Google Scholar]
- 21.Nepomnyashchaya YN, et al. Moorella humiferrea sp. nov., a thermophilic, anaerobic bacterium capable of growth via electron shuttling between humic acid and Fe(III) Int. J. Syst. Evol. Microbiol. 2012;62:613–617. doi: 10.1099/ijs.0.029009-0. [DOI] [PubMed] [Google Scholar]
- 22.Ogg CD, Patel BKC. Caloramator australicus sp. nov., a thermophilic, anaerobic bacterium from the Great Artesian Basin of Australia. Int. J. Syst. Evol. Microbiol. 2009;59:95–101. doi: 10.1099/ijs.0.000802-0. [DOI] [PubMed] [Google Scholar]
- 23.Panosyan H, Margaryan A, Birkeland NK. Anoxybacillus karvacharensis sp. nov., a novel thermophilic bacterium isolated from the karvachar geothermal spring in nagorno-karabakh. Int. J. Syst. Evol. Microbiol. 2021;71:5035. doi: 10.1099/ijsem.0.005035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rother M, Metcalf WW. Anaerobic growth of Methanosarcina acetivorans C2A on carbon monoxide: An unusual way of life for a methanogenic archaeon. Proc. Natl. Acad. Sci. U.S.A. 2004;101:16929–16934. doi: 10.1073/pnas.0407486101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zavarzina DG, et al. Thermincola ferriacetica sp. nov., a new anaerobic, thermophilic, facultatively chemolithoautotrophic bacterium capable of dissimilatory Fe(III) reduction. Extremophiles. 2007;11:1–7. doi: 10.1007/s00792-006-0004-7. [DOI] [PubMed] [Google Scholar]
- 26.Diender M, Stams AJM, Sousa DZ. Pathways and bioenergetics of anaerobic carbon monoxide fermentation. Front. Microbiol. 2015;6:1–18. doi: 10.3389/fmicb.2015.01275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sokolova TG, et al. Thermosinus carboxydivorans gen. nov., sp. nov., a new anaerobic, thermophilic, carbon-monoxide-oxidizing, hydrogenogenic bacterium from a hot pool of Yellowstone National Park. Int. J. Syst. Evol. Microbiol. 2004;54:2353–2359. doi: 10.1099/ijs.0.63186-0. [DOI] [PubMed] [Google Scholar]
- 28.Techtmann SM, et al. Evidence for horizontal gene transfer of anaerobic carbon monoxide dehydrogenases. Front. Microbiol. 2012;3:1–16. doi: 10.3389/fmicb.2012.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toshchakov SV, et al. Genomic insights into energy metabolism of Carboxydocella thermautotrophica coupling hydrogenogenic co oxidation with the reduction of Fe(III) minerals. Front. Microbiol. 2018;9:1–20. doi: 10.3389/fmicb.2018.01759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoneda Y, et al. Carboxydothermus pertinax sp. nov., a thermophilic, hydrogenogenic, Fe(III)-reducing, sulfur-reducing carboxydotrophic bacterium from an acidic hot spring. Int. J. Syst. Evol. Microbiol. 2012;62:1692–1697. doi: 10.1099/ijs.0.031583-0. [DOI] [PubMed] [Google Scholar]
- 31.Slobodkin AI, Sokolova TG, Lysenko AM, Wiegel J. Reclassification of Thermoterrabacterium ferrireducens as Carboxydothermus ferrireducens comb. nov., and emended description of the genus Carboxydothermus. Int. J. Syst. Evol. Microbiol. 2006;56:2349–2351. doi: 10.1099/ijs.0.64503-0. [DOI] [PubMed] [Google Scholar]
- 32.Sokolova TG, et al. Carboxydocella thermautotrophica gen. nov., sp. nov., a novel anaerobic, CO-utilizing thermophile from a kamchatkan hot spring. Int. J. Syst. Evol. Microbiol. 2002;52:1961–1967. doi: 10.1099/00207713-52-6-1961. [DOI] [PubMed] [Google Scholar]
- 33.Bao Q, et al. A complete sequence of the T. tengcongensis genome. Genome Res. 2002;12:689–700. doi: 10.1101/gr.219302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fardeau, M., Ollivier, B. & Cayol, J. Caldanaerobacter. In Bergey’s Manual of Systematics of Archaea and Bacteria. 10.1002/9781118960608.gbm00745 (2015).
- 35.Kozina IV, Kublanov IV, Kolganova TV, Chernyh NA, Bonch-Osmolovskaya EA. Caldanaerobacter uzonensis sp. nov., an anaerobic, thermophilic, heterotrophic bacterium isolated from a hot spring. Int. J. Syst. Evol. Microbiol. 2010;60:1372–1375. doi: 10.1099/ijs.0.012328-0. [DOI] [PubMed] [Google Scholar]
- 36.Brady AL, Sharp CE, Grasby SE, Dunfield PF. Anaerobic carboxydotrophic bacteria in geothermal springs identified using stable isotope probing. Front. Microbiol. 2015;6:1–10. doi: 10.3389/fmicb.2015.00897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fukuyama Y, Inoue M, Omae K, Yoshida T, Sako Y. Anaerobic and hydrogenogenic carbon monoxide-oxidizing prokaryotes: Versatile microbial conversion of a toxic gas into an available energy. Adv. Appl. Microbiol. 2020;110:99. doi: 10.1016/bs.aambs.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 38.Yoneda Y, et al. Detection of anaerobic carbon monoxide-oxidizing thermophiles in hydrothermal environments. FEMS Microbiol. Ecol. 2015;91:1–9. doi: 10.1093/femsec/fiv093. [DOI] [PubMed] [Google Scholar]
- 39.Ersoy Ş, Akaçin İ, Güngörmüşler M. Comparative evaluation of the biohydrogen production potential of thermophilic microorganisms isolated from hot springs located in Izmir. Int. J. Hydrogen Energy. 2023 doi: 10.1016/j.ijhydene.2023.02.092. [DOI] [Google Scholar]
- 40.Hniman A, Prasertsan P, O-Thong S. Community analysis of thermophilic hydrogen-producing consortia enriched from Thailand hot spring with mixed xylose and glucose. Int. J. Hydrogen Energy. 2011;36:14217–14226. [Google Scholar]
- 41.Alves JI, van Gelder AH, Alves MM, Sousa DZ, Plugge CM. Moorella stamsii sp. nov., a new anaerobic thermophilic hydrogenogenic carboxydotroph isolated from digester sludge. Int. J. Syst. Evol. Microbiol. 2013;63:4072–4076. doi: 10.1099/ijs.0.050369-0. [DOI] [PubMed] [Google Scholar]
- 42.Liu C, et al. Microbial insights of enhanced anaerobic conversion of syngas into volatile fatty acids by co-fermentation with carbohydrate-rich synthetic wastewater. Biotechnol. Biofuels. 2020;13:1–14. doi: 10.1186/s13068-020-01694-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balk M, et al. Isolation and characterization of a new CO-utilizing strain, Thermoanaerobacter thermohydrosulfuricus subsp. carboxydovorans, isolated from a geothermal spring in Turkey. Extremophiles. 2009;13:885–894. doi: 10.1007/s00792-009-0276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slepova TV, et al. Carboxydocella sporoproducens sp. nov., a novel anaerobic CO-utilizing/H2-producing thermophilic bacterium from a Kamchatka hot spring. Int. J. Syst. Evol. Microbiol. 2006;56:797–800. doi: 10.1099/ijs.0.63961-0. [DOI] [PubMed] [Google Scholar]
- 45.Albright S, Louca S. Trait biases in microbial reference genomes. Sci. Data. 2023;10:84. doi: 10.1038/s41597-023-01994-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharp CE, et al. Humboldt’s spa: Microbial diversity is controlled by temperature in geothermal environments. ISME J. 2014;8:1166–1174. doi: 10.1038/ismej.2013.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lavrentyeva EV, et al. Bacterial diversity and functional activity of microbial communities in hot springs of the Baikal rift zone. Microbiol. (Russ. Fed.) 2018;87:272–281. [Google Scholar]
- 48.Gantner S, Andersson AF, Alonso-Sáez L, Bertilsson S. Novel primers for 16S rRNA-based archaeal community analyses in environmental samples. J. Microbiol. Methods. 2011;84:12–18. doi: 10.1016/j.mimet.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 49.Yang N, et al. Novel primers for 16S rRNA gene-based archaeal and bacterial community analysis in oceanic trench sediments. Appl. Microbiol. Biotechnol. 2022;106:2795–2809. doi: 10.1007/s00253-022-11893-3. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi S, Tomita J, Nishioka K, Hisada T, Nishijima M. Development of a prokaryotic universal primer for simultaneous analysis of bacteria and archaea using next-generation sequencing. PLoS ONE. 2014;9:e105592. doi: 10.1371/journal.pone.0105592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fadeev E, et al. Comparison of two 16S rRNA primers (V3–V4 and V4–V5) for studies of arctic microbial communities. Front. Microbiol. 2021;12:1–11. doi: 10.3389/fmicb.2021.637526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parada AE, Needham DM, Fuhrman JA. Every base matters: Assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ. Microbiol. 2016;18:1403–1414. doi: 10.1111/1462-2920.13023. [DOI] [PubMed] [Google Scholar]
- 53.Yeh Y-C, Needham DM, Sieradzki ET, Fuhrman JA. Taxon disappearance from microbiome analysis reinforces the value of mock communities as a standard in every sequencing run. mSystems. 2018;3:1–9. doi: 10.1128/mSystems.00023-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tremblay J, et al. Primer and platform effects on 16S rRNA tag sequencing. Front. Microbiol. 2015;6:1–15. doi: 10.3389/fmicb.2015.00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barns SM, Fundyga RE, Jeffries MW, Pace NR. Remarkable archaeal diversity detected in a Yellowstone National Park hot spring environment. Proc. Natl. Acad. Sci. U.S.A. 1994;91:1609–1613. doi: 10.1073/pnas.91.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okonechnikov K, et al. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics. 2012;28:1166–1167. doi: 10.1093/bioinformatics/bts091. [DOI] [PubMed] [Google Scholar]
- 57.Larkin MA, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 58.Schloss PD, et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chan CS, Chan K, Tay Y, Chua Y, Goh KM. Diversity of thermophiles in a Malaysian hot spring determined using 16S rRNA and shotgun metagenome sequencing. Front. Microbiol. 2015;6:1–15. doi: 10.3389/fmicb.2015.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sokolova TG, et al. Diversity and ecophysiological features of thermophilic carboxydotrophic anaerobes. FEMS Microbiol. Ecol. 2009;68:131–141. doi: 10.1111/j.1574-6941.2009.00663.x. [DOI] [PubMed] [Google Scholar]
- 61.Canganella F, Wiegel J. Anaerobic thermophiles. Life. 2014;4:77–104. doi: 10.3390/life4010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wagner ID, Wiegel J. Diversity of thermophilic anaerobes. Ann. N. Y. Acad. Sci. 2018;1125:1–43. doi: 10.1196/annals.1419.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are available in the NCBI repository with the SRA Bioproject Accession Number [PRJNA954581].