Abstract
Purpose
Lower respiratory tract infections (LRTI) are the most frequent infectious complication in patients admitted to the intensive care unit (ICU). We aim to report the clinical characteristics of ICU-admitted patients due to nosocomial LRTI and to describe their microbiology and clinical outcomes.
Methods
A prospective observational study was conducted in 13 countries over two continents from 9th May 2016 until 16th August 2019. Characteristics and outcomes of ventilator-associated pneumonia (VAP), ventilator-associated tracheobronchitis (VAT), ICU hospital-acquired pneumonia (ICU-HAP), HAP that required invasive ventilation (VHAP), and HAP in patients transferred to the ICU without invasive mechanical ventilation were collected. The clinical diagnosis and treatments were per clinical practice and not per protocol. Descriptive statistics were used to compare the study groups.
Results
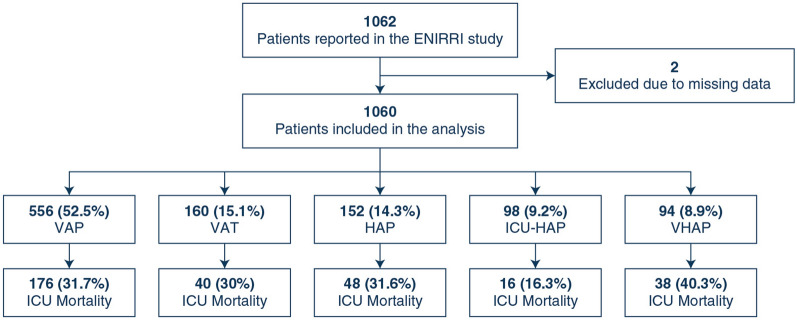
1060 patients with LRTI (72.5% male sex, median age 64 [50–74] years) were included in the study; 160 (15.1%) developed VAT, 556 (52.5%) VAP, 98 (9.2%) ICU-HAP, 152 (14.3%) HAP, and 94 (8.9%) VHAP. Patients with VHAP had higher serum procalcitonin (PCT) and Sequential Organ Failure Assessment (SOFA) scores. Patients with VAP or VHAP developed acute kidney injury, acute respiratory distress syndrome, multiple organ failure, or septic shock more often. One thousand eight patients had microbiological samples, and 711 (70.5%) had etiological microbiology identified. The most common microorganisms were Pseudomonas aeruginosa (18.4%) and Klebsiella spp (14.4%). In 382 patients (36%), the causative pathogen shows some antimicrobial resistance pattern. ICU, hospital and 28-day mortality were 30.8%, 37.5% and 27.5%, respectively. Patients with VHAP had the highest ICU, in-hospital and 28-day mortality rates.
Conclusion
VHAP patients presented the highest mortality among those admitted to the ICU. Multidrug-resistant pathogens frequently cause nosocomial LRTI in this multinational cohort study.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00134-023-07210-9.
Keywords: VAT, VAP, VA-LRTI, Sepsis ICU
Take-home message
| Respiratory infections remain a frequent complication in patients admitted to the intensive care unit (ICU). In our study, multidrug-resistant pathogens were more frequently isolated in ventilator-associated pneumonia patients than in other nosocomial lower respiratory tract infections. Notably, patients with hospital-acquired pneumonia which require invasive ventilation have the highest mortality rate among patients with nosocomial LRTI in the ICU. |
Introduction
The primary focus of most studies conducted on critically ill patients has been to ascertain the diagnosis, occurrence rates, and clinical outcomes among individuals who acquired ventilator-associated pneumonia (VAP) [1]. However, recent data have shown that not all patients with lower respiratory tract infections (LRTI) admitted to the intensive care unit (ICU) are alike and may have different clinical characteristics and clinical outcomes [2–4]. Still, other less-known entities are frequent in the ICU, such as ventilator-associated tracheobronchitis (VAT) and hospital-acquired pneumonia (HAP) happening on the ward or in the ICU patients with spontaneous breathing but needing mechanical ventilation after diagnosis—(so-called ventilated HAP [VHAP]) [5, 6]. Most of the cumulative incidence of these entities is extracted from clinical trials and administrative observational datasets that allow us to determine the effect of medical interventions, often of new antibiotics [7]. However, these data might not represent the real world, and more data is needed.
Over the last twenty years, the profile of patients cared for in the ICU has profoundly changed [8–10]. Additionally, there has been an increase in ICU beds in some healthcare systems, making the admission criteria currently different from what they used to be. More importantly, these criteria are different among countries [11, 12]. A critical remark is that some patients on the ward might have a longer hospital stay, exposing them to hospital-specific pathogens, making them more susceptible to clinical complications than those treated in the ICU, where better access to multidisciplinary teams (e.g., infectious diseases and respiratory physicians, pharmacists, and even physiotherapists) with precise guidelines and goals of care are available [13, 14].
The European Network for ICU-Related Respiratory Infections (ENIRRI) network aimed to determine the clinical outcomes of patients who developed nosocomial LRTI while admitted to the ICU [5, 15]. We hypothesised that patients with VHAP in this cohort would show the highest mortality rates, as has been repeatedly published before; however, to our knowledge, no observational studies have been published in Europe and South America evaluating this critical clinical issue. Therefore, the primary goal of this study is to report the clinical characteristic of patients with nosocomial LRTI admitted to the ICU and to describe microbiology and the clinical outcomes of these patients using a prospective multinational cohort.
Methods
We carried out a prospective, multicentric, and observational cohort study at 28 selected ICUs in 13 countries across Europe and Latin America (Argentina, Belgium, Colombia, Croatia, France, Germany, Ireland, Italy, Netherlands, Poland, Portugal, Spain, Turkey) with critically ill patients admitted from 9th May 2016 until 16th August 2019. The time frame was determined by the enrolment period at each site (enrolment was performed during a 12-month continuous period in each participant site). We recruited consecutive patients aged 18 years or older who developed LRTI 48 h after admission (i.e., nosocomial LRTI), who were later admitted to ICU, and/or who developed LRTI during the ICU stay. Then, a follow-up until hospital discharge was performed. This study is the primary analysis of the ENIRRI study. The study received approval from the institution's Internal Review Board (Comité Ètic d'Investigació Clínica, registry number HCB/2020/0370). And registered at ClinicalTrials.gov Identifier: NCT03183921. We obtained informed consent for patients where this was required per local regulations. All clinical data were anonymised and transferred to the coordinating centre for data curation and analysis. Additionally, each one of the thirteen participating sites presented the project to its institutional ethics committee, and it was approved.
Patients
Patients were eligible if they had all three conditions below: (1) aged 18 years or older; (2) admitted to ICU; and (3) having a nosocomial LRTI. We excluded re-admitted patients.
Data collection
Recorded data included demographic characteristics, comorbidities, the time course of illness, treatments administered, laboratory and microbiologic data, complications during ICU stay, and outcomes. We determined disease severity by Simplified Acute Physiology Score (SAPS) II [16] and assessed organ failure using the Sequential Organ Failure Assessment (SOFA) score [17] calculating both within the first day of ICU admission. The study protocol of this project has been previously published and was shared with all the local investigators before beginning the enrolment [5, 15].
Ventilatory management strategies, treatments and microbiological assessments were not standardised among centres. They were left to the discretion of the attending clinician, based on local guidelines and recommendations and supported by international guidelines [1, 4].
LRTI definitions
A nosocomial LRTI was based on clinical criteria (i.e., new or progressive pulmonary infiltrates on chest radiographs, except for VAT, and at least two of the following: temperature > 38 °C or < 36 °C; leucocytosis > 12,000 mm3 or leukopenia < 4000 mm3; or purulent respiratory secretions). We classified the LRTI patients at the ICU as follows: (1) HAP: LRTI acquired outside the ICU at least ≥ 48 h after admission, not requiring invasive mechanical ventilation; (2) VHAP: LRTI acquired outside the ICU at least ≥ 48 h after the admission, requiring invasive mechanical ventilation due to the LRTI; (3) ICU-HAP: LRTI acquired at least ≥ 48 h after the ICU admission, not requiring invasive mechanical ventilation due to the LRTI; (4) VAP: patients admitted to the ICU who develop LRTI at least after ≥ 48 h of tracheal intubation/tracheostomy and (5) VAT: patients admitted to the ICU who develop LRTI at least ≥ 48 h after tracheal intubation/tracheostomy without a new or progressive radiological pulmonary infiltrate.
The following diagnostic thresholds were used to confirm the microbiological diagnosis: bronchial alveolar lavage (BAL)/mini-BAL/protected specimen brush (PSB) ≥ 104 colony-forming units per mL and sputum/endotracheal aspirate (ETA) ≥ 105 colony-forming units per mL or any threshold if the patient had concomitant antibiotic treatment when the sample was collected.
We used the Berlin definition to classify patients with acute respiratory distress syndrome (ARDS) [18]. The acute kidney injury use was established using the Kidney Disease Improving Global Outcomes score ≥ 2 [19]. As recommended by international guidelines, septic shock was defined as sepsis-induced hypotension persisting despite adequate fluid resuscitation [20]. Sepsis-induced tissue hypoperfusion was defined as infection-induced hypotension, elevated lactate, or oliguria. Septic shock was identified by the following clinical features sepsis with persisting hypotension requiring vasopressors to maintain median arterial pressure (MAP) ≥ 65 mmHg and having a serum lactate level > 2 mmol/L (18 mg/dL) despite adequate volume resuscitation [21]. Multiple organ dysfunction was determined when three or more organ systems failed after the nosocomial LRTI diagnosis [17, 22].
Clinical outcomes at the end of antibiotic treatment were defined as (1) Cure: all infection-related signs and symptoms have disappeared or have returned to the pre-infection state, and chest X-ray findings show improvement or stabilisation at an acceptable level. (2) Failure: all infection-related signs and symptoms were not improved, or one or more antibiotics were added due to lack of clinical improvement; the patient died while on antibiotic treatment. (3) Unknown: the patient was discharged before the end of the treatment evaluation. (4) Recurrence was defined as a new nosocomial LRTI episode (i.e., new clinical signs compatible with pneumonia) confirmed by significant growth in quantitative culture after the first diagnosis of LRTI was made; it includes time until hospital discharge [23, 24]. The study protocol has been previously published elsewhere with all the definitions [15].
Objectives
The primary objective of our study was to determine the clinical and laboratory characteristics, microbiologic features and outcomes of patients diagnosed with nosocomial LRTI in critically ill patients. The secondary aims included describing the clinical impact of these entities and comparing the different study groups.
Statistical analysis
We reported categorical variables as numbers and frequencies (%), normally distributed continuous variables as means (standard deviation [SD]) and skewed continuous variables as medians (interquartile ranges [IQR]). We performed χ2 tests or Fisher's exact tests to compare qualitative variables, Student's t tests, ANOVAs or Mann–Whitney U, and non-parametric Kruskal–Wallis tests to compare normally distributed or skewed continuous variables, whenever appropriate. We used SPSS (version 28) for data analysis.
Results
A total of 1060 patients were included in the study. The most frequent nosocomial LRTI was VAP (52.5% [556/1060]), followed by VAT (15.1% [160/1060]), hospital-acquired pneumonia not receiving invasive mechanical ventilation (HAP) (14.3% [152/1060]), ICU-acquired pneumonia not receiving invasive mechanical ventilation (ICU-HAP) (9.2% [98/1060]), and VHAP (8.9% [94/1060]) (Fig. 1). Most patients were male (72.5% [769/1060]) with a median (IQR) age of 64 (50–74) years. The most prevalent comorbidities were diabetes mellitus (20.3% [215/1060]), chronic heart disease (27% [286/1060]), and chronic lung disease (22.5% [230/1060]). The sociodemographic characteristics of each study group were balanced in age, sex, and body mass index (BMI, Table 1). Notably, most patients received enteral nutrition (71.5% [758/1060]), previous muscle relaxants (26.8% [284/1060]), systemic steroids (24.1% [255/1060]) and have had surgery during the hospitalisation (43.2% [458/1060]). The list of characteristics evaluated in the cohort and its frequencies are presented in Table 1.
Fig. 1.
Study flow chart
Table 1.
Characteristics and comorbid conditions stratified by the study groups
| Characteristic | All cohort | VAT | VAP | ICU-HAP | VHAP | HAP |
|---|---|---|---|---|---|---|
| n = 1060 | n = 160 | n = 556 | n = 98 | n = 94 | n = 152 | |
| Age, years | 64 (50–74) | 63 (49–76) | 62 (47–73) | 64.5 (53–75) | 65 (55–73) | 68 (59–75) |
| Male | 769 (72.5) | 114 (71.3) | 405 (72.8) | 72 (73.5) | 70 (74.5) | 108 (71.1) |
| Weight, kg | 75 (65–85) | 65 (70–80) | 65 (70–80) | 80 (65–90) | 76 (67–89) | 65 (75–85) |
| Height, cm | 170 (165–175) | 170 (163.5–175) | 170 (165–176) | 170 (163–176) | 170 (165–177.5) | 170 (165–175) |
| BMI, kg/m2 | 26 (23.2–29.4) | 25.4 (22.9–29) | 26.1 (23.4–29.4) | 27.8 (23.4–29.7) | 26.1 (23–30.8) | 24.8 (22.1–29.4) |
| Overweight | 343 (36) | 50 (34.7) | 186 (37.2) | 37 (42) | 29 (33) | 41 (30.8) |
| Obese | 206 (21.6) | 27 (18.8) | 112 (22.4) | 19 (21.6) | 23 (26.1) | 25 (18.8) |
| Chronic heart disease | 286 (27) | 47 (29.4) | 139 (25) | 36 (36.7) | 23 (24.5) | 41 (27) |
| Diabetes mellitus | 215 (20.3) | 42 (26.3) | 103 (18.6) | 16 (16.3) | 22 (23.4) | 32 (21.1) |
| Chronic lung disease | 239 (22.5) | 29 (18.1) | 107 (19.2) | 34 (34.7) | 33 (35.1) | 36 (23.7) |
| Chronic renal failure | 120 (11.3) | 23 (14.4) | 55 (9.9) | 10 (10.2) | 5 (5.3) | 27 (17.8) |
| Active solid neoplasia | 149 (14.1) | 19 (11.9) | 53 (9.5) | 24 (24.5) | 19 (20.2) | 34 (22.4) |
| Alcohol abuse | 94 (8.9) | 12 (7.5) | 55 (9.9) | 6 (6.1) | 8 (8.5) | 13 (8.6) |
| Chronic liver disease | 66 (6.2) | 11 (6.9) | 30 (5.4) | 7 (7.1) | 8 (8.5) | 10 (6.6) |
| Immunosuppressed | 100 (9.4) | 9 (5.6) | 48 (8.6) | 15 (15.3) | 7 (7.5) | 21 (13.8) |
| Active smoker | 60 (5.7) | 8 (5) | 38 (6.8) | 2 (2) | 7 (7.5) | 5 (3.3) |
| Ex-smoker | 67 (6.3) | 6 (3.8) | 31 (5.6) | 10 (10.2) | 8 (8.5) | 12 (7.9) |
| Active autoimmune disease | 57 (5.4) | 6 (3.8) | 33 (6) | 6 (6.1) | 1 (1.1) | 11 (7.2) |
| Cirrhosis | 36 (3.4) | 5 (3.1) | 17 (3.1) | 3 (3.1) | 5 (5.3) | 6 (4) |
| Chemotherapy | 49 (4.6) | 3 (1.9) | 15 (2.7) | 9 (9.2) | 3 (3.2) | 19 (12.5) |
| Enteral nutrition | 758 (71.5) | 133 (83.1) | 435 (78.2) | 60 (61.2) | 54 (57.5) | 76 (50) |
| Continuous enteral feeding | 679 (64.1) | 123 (76.9) | 416 (74.8) | 45 (45.9) | 49 (52.1) | 46 (30.3) |
| Previous surgery | 458 (43.2) | 66 (41.3) | 247 (44.4) | 52 (53.1) | 33 (35.1) | 60 (39.5) |
| Coma | 250 (23.6) | 56 (35) | 148 (26.6) | 10 (10.2) | 19 (20.2) | 17 (11.2) |
| Parenteral nutrition | 196 (18.5) | 32 (20) | 103 (18.5) | 18 (18.4) | 22 (23.4) | 21 (13.8) |
| Systemic steroids | 255 (24.1) | 32 (20) | 143 (25.7) | 24 (24.5) | 22 (23.4) | 34 (22.4) |
| Surgical trauma | 101 (9.5) | 20 (12.5) | 63 (11.3) | 7 (7.1) | 8 (8.5) | 3 (2) |
| Previous NIV | 151 (14.3) | 14 (8.8) | 80 (14.4) | 30 (30.6) | 11 (11.7) | 16 (10.5) |
| Therapeutic hypothermia | 28 (2.6) | 9 (5.6) | 16 (2.9) | 0 (0) | 1 (1.1) | 2 (1.3) |
| Previous HFNO | 93 (8.8) | 7 (4.4) | 39 (7) | 23 (23.5) | 7 (7.5) | 17 (11.2) |
| Oral feeding | 71 (6.7) | 7 (4.4) | 15 (2.7) | 15 (15.3) | 5 (5.3) | 29 (19.1) |
BMI body mass index, HAP hospital-acquired pneumonia, HFNO high flow nasal oxygen, ICU intensive care unit, NIV non-invasive ventilation, VAP ventilator-associated pneumonia, VAT ventilator-associated tracheobronchitis, VHAP ventilated hospital-acquired pneumonia
Characteristics of the intensive care units and admissions
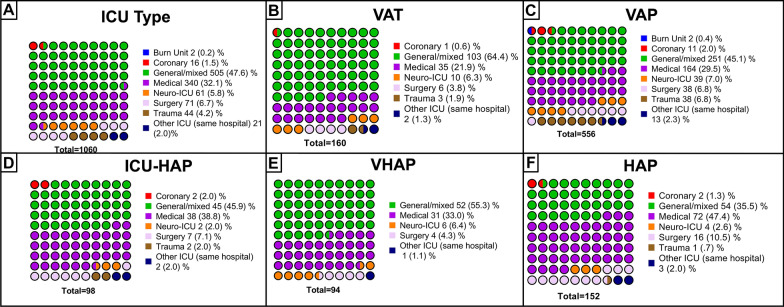
Most of the patients were admitted to the ICU due to medical diagnoses (66% [700/1060]), followed by emergent surgery (15.4% [153/1060]), trauma (9.6% [102/1060]), and elective surgery (9% [95/1060]) (Fig. 2). The most frequent cause of ICU admission was hypoxemic acute respiratory failure (28.4% [301/1060]), followed by postoperative (13% [138/1060]) and altered consciousness (12.1% [128/1060]). Most of these patients came from the emergency department (27.4% [290/1060]) and the general ward (26.8% [284/1060]). Of those who developed hypoxemic acute respiratory failure, a higher proportion developed VAP (VAT: 11.29% [34/301] vs VAP: 37.8% [114/301] vs ICU-HAP: 9.6% [29/301] vs VHAP: 16.3% [49/301] vs HAP: 25% [75/301]).
Fig. 2.
Characteristics of the ICUs stratified per study groups A Cohort distribution by ICU type. B VAT distribution by ICU type. C VAP distribution by ICU type. D ICU-HAP distribution by ICU type. E VHAP distribution by ICU type. F HAP distribution by ICU type
Laboratories results, severity scores, and systemic complications
When comparing the laboratory results, we found that several organ dysfunctions and biomarkers of systemic inflammation differed among the study groups (Table 2). For instance, we found that patients diagnosed with VHAP had higher serum procalcitonin (PCT) concentrations at ICU admission (Table 2). We also found that the PaO2/FiO2 ratio was lower in patients with VHAP, HAP, and VAP.
Table 2.
Scores, laboratories, and inflammatory markers ICU admission, stratified by the study groups
| Score or biomarker at ICU admission | VAT | VAP | ICU-HAP | VHAP | HAP | All cohort |
|---|---|---|---|---|---|---|
| n = 160 | n = 556 | n = 98 | n = 94 | n = 152 | n = 1060 | |
| SAPS II | 48 (35–66) | 48 (38–58) | 37 (28–47) | 49 (39–60) | 45 (33–57) | 47 (36–58) |
| SOFA | 8 (6–10) | 8 (5–10) | 5 (3–8) | 9 (7–12) | 7 (5–9) | 7 (5–10) |
| CPIS | 4 (3–6) | 6 (5–7) | 6 (5–7) | 6 (4–7) | 6 (5–7) | 6 (4–7) |
| Leucocytes, 109/L | 12 (8.9–15.6) | 13 (9.8–17.5) | 12.4 (7.3–17) | 13 (9.3–18.2) | 13.7 (8.4–18.5) | 12.8 (9.3–17.5) |
| CRP, mg/dL | 16.5 [6.7–80] | 23 [9.6–130] | 33.3 [9.9–152] | 54 [7.6–205.5] | 54 [20.1–180] | 24.7 [9.5–137.8] |
| PCT at ICU, ng/mL | 0.9 (0.2–2.4) | 0.7 (0.2–3) | 0.6 (0.2–3.1) | 2.9 (0.6–10) | 1.2 (0.3–3.7) | 0.8 (0.2–3.4) |
| PaO2/FiO2 ratio | 228.5 (177–284.5) | 198 (144–267) | 185 (134–267) | 133 (96.1–218) | 165.9 (113–240) | 190 (138–266) |
CRP C-reactive protein, CPIS clinical pulmonary infection score, HAP hospital-acquired pneumonia, ICU intensive care unit, PCT procalcitonin, SAPS II Simplified Acute Physiology Score II, SOFA Sequential Organ Failure Assessment, VAP ventilator-associated pneumonia, VAT ventilator-associated tracheobronchitis, VHAP ventilated hospital-acquired pneumonia
Patients diagnosed with VHAP had a more severe disease when evaluated by different severity scores at ICU admission (Table 2). For instance, patients diagnosed with VHAP showed higher scores in SOFA (VHAP: 9 [7–12], vs VAP: 8 [5–10], vs ICU-HAP: 5 [3–8], vs HAP: 7 [5–9], vs VAT: 8 [6–10]) (Table 2, Supplemental Table 1). Notably, we found that the Clinical Pulmonary Infection Score (CPIS) was not different among the groups (in ventilated patients). Microbiological and etiological findings are described below and are reported in Table 3. Systemic complications are presented in Table 4, stratified by the study groups. We found that patients diagnosed with VAP and VHAP had a higher prevalence of acute kidney injury, ARDS, multiple organ failure, and septic shock diagnosis.
Table 3.
Microbiological diagnosis and antibiotic resistance patterns
| VAT n = 160 |
VAP n = 556 |
ICU-HAP n = 98 |
VHAP n = 94 |
HAP n = 152 |
All cohort n = 1060 |
|
|---|---|---|---|---|---|---|
| Samples collected during LRTI diagnosis | 152 (95) | 531 (95.5) | 92 (93.8) | 92 (97.8) | 141 (92.7) | 1008 (95) |
| Blood culture | 122 (76.3) | 452 (81.3) | 75 (76.5) | 71 (75.5) | 112 (73.7) | 832 (78.5) |
| ETA-Sputum | 110 (68.8) | 249 (44.8) | 50 (51) | 37 (39.4) | 67 (44.1) | 513 (48.4) |
| BAL/miniBAL/PSB | 75 (46.9) | 352 (63.3) | 49 (50) | 59 (62.8) | 85 (55.9) | 620 (58.5) |
| Real-time PCR in respiratory samples | 26 (16.3) | 39 (7) | 8 (8.2) | 2 (2.1) | 12 (7.9) | 87 (8.2) |
| Respiratory virus testing | 15 (9.4) | 65 (11.7) | 15 (15.3) | 23 (24.5) | 16 (10.5) | 134 (12.6) |
| Pleural fluid | 3 (1.9) | 22 (4) | 9 (9.2) | 11 (11.7) | 7 (4.6) | 52 (4.9) |
| Microorganisms isolated | 121 (75.6) | 419 (75.4) | 52 (53.1) | 39 (41.5) | 80 (52.6) | 711 (67.1) |
| Acinetobacter baumanii | 9 (5.6) | 66 (11.9) | 5 (5.1) | 4 (4.3) | 12 (7.9) | 96 (9.1) |
| Aspergillus spp. | 0 (0) | 3 (0.5) | 1 (1) | 3 (3.2) | 1 (0.7) | 8 (0.8) |
| Corynebacterium | 1 (0.6) | 0 (0) | 0 (0) | 0 (0) | 1 (0.7) | 2 (0.2) |
| Citrobacter spp. | 0 (0) | 5 (0.9) | 1 (1) | 0 (0) | 0 (0) | 6 (0.6) |
| E. coli | 5 (3.1) | 35 (6.3) | 7 (7.1) | 7 (7.4) | 9 (5.9) | 63 (5.9) |
| Enterobacter spp. | 5 (3.1) | 24 (4.3) | 4 (4.1) | 1 (1.1) | 8 (5.3) | 42 (4) |
| Haemophilus spp. | 6 (3.8) | 15 (2.7) | 1 (1) | 2 (2.1) | 5 (3.3) | 29 (2.7) |
| Klebsiella spp. | 22 (13.8) | 57 (10.3) | 6 (6.1) | 8 (8.5) | 12 (7.9) | 105 (9.9) |
| Legionella spp. | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.7) | 1 (0.1) |
| Moraxella catarrhalis | 0 (0) | 2 (0.4) | 0 (0) | 0 (0) | 2 (1.3) | 4 (0.4) |
| Morganella morgagni | 0 (0) | 4 (0.7) | 0 (0) | 1 (1.1) | 0 (0) | 5 (0.5) |
| MRSA | 7 (4.4) | 21 (3.8) | 8 (8.2) | 6 (6.4) | 13 (8.6) | 55 (5.2) |
| MSSA | 16 (10) | 56 (10.1) | 7 (7.1) | 2 (2.1) | 4 (2.6) | 85 (8) |
| P. aeruginosa | 33 (20.6) | 101 (18.2) | 7 (7.1) | 4 (4.3) | 9 (5.9) | 154 (14.5) |
| Proteus spp. | 2 (1.3) | 11 (2) | 0 (0) | 0 (0) | 1 (0.7) | 14 (1.3) |
| S. pneumoniae | 6 (3.8) | 8 (1.4) | 4 (4.1) | 1 (1.1) | 2 (1.3) | 21 (2) |
| Serratia spp. | 9 (5.6) | 11 (2) | 1 (1) | 0 (0) | 0 (0) | 21 (2) |
| Antibiotic resistance patterns identified | 64 (40) | 201 (36.1) | 19 (19.4) | 32 (34) | 15 (9.9) | 320 (30.2) |
| MDR | 24 (15) | 74 (13.3) | 9 (9.2) | 14 (14.9) | 11 (7.2) | 132 (12.5) |
| ESBL | 11 (6.9) | 43 (7.7) | 8 (8.2) | 7 (7.4) | 4 (2.6) | 73 (6.9) |
| Carbapenemase resistant | 8 (5) | 44 (7.9) | 1 (1) | 3 (3.2) | 0 (0) | 56 (5.3) |
| XDR | 7 (4.4) | 37 (6.7) | 1 (1) | 7 (7.4) | 0 (0) | 52 (4.9) |
| PDR | 3 (1.9) | 3 (0.5) | 0 (0) | 1 (1.1) | 0 (0) | 7 (0.7) |
All the characteristics are presented in counts (%)
ETA endotracheal aspirate, BAL bronchial alveolar lavage, LRTI lower respiratory tract infections, MRSA methicillin-resistant Staphylococcus aureus, MSSA methicillin-sensitive Staphylococcus aureus, PCR polymerase chain reaction, MDR multidrug-resistant, PDR pandrug-resistant, XDR extensively-drug resistant, ESBL extended spectrum-beta-lactamase, VAT ventilator-associated tracheobronchitis, VAP ventilator-associated pneumonia, ICU-HAP Intensive Care Unit (ICU) hospital-acquired pneumonia, VHAP ventilated hospital-acquired pneumonia, HAP hospital-acquired pneumonia
Table 4.
Systemic complications upon diagnosis and clinical outcomes stratified the study groups
| Complications at ICU admission | VAT n = 160 |
VAP n = 556 |
ICU-HAP n = 98 |
VHAP n = 94 |
HAP n = 152 |
All cohort n = 1060 |
|---|---|---|---|---|---|---|
| Acute kidney injury | 23 (14.4) | 106 (19.1) | 12 (12.2) | 16 (17) | 22 (14.5) | 179 (16.9) |
| ARDS | 20 (12.5) | 116 (20.9) | 16 (16.3) | 32 (34) | 45 (29.6) | 229 (21.6) |
| Septic shock | 19 (11.9) | 148 (26.6) | 22 (22.5) | 38 (40.4) | 31 (20.4) | 258 (24.3) |
| Multiple organ failure | 14 (8.8) | 60 (10.8) | 6 (6.1) | 16 (17) | 8 (5.3) | 104 (9.8) |
| Clinical cure, n (%) | 96 (60) | 331 (59.5) | 62 (63.3) | 45 (47.9) | 85 (55.9) | 619 (58.4) |
| Treatment failure, n (%) | 52 (32.5) | 184 (33.1) | 22 (22.5) | 44 (46.8) | 52 (34.2) | 354 (33.4) |
| ICU mortality, n (%) | 48 (30) | 176 (31.7) | 16 (16.3) | 38 (40.3) | 48 (31.6) | 326 (30.8) |
| Hospital mortality, n (%) | 59 (36.9) | 210 (37.8) | 24 (24.5) | 47 (50) | 57 (37.5) | 397 (37.5) |
| 28-days mortality, n (%) | 51 (31.8) | 143 (25.7) | 14 (14.3) | 39 (41.5) | 44 (28.9) | 291 (27.4) |
| ICU LOS, median (IQR) | 20.5 (11.5–30) | 25 (15–41) | 20 (11–35) | 12 (6–27) | 12 (6–19.5) | 20 (11–35) |
| Hospital LOS, median (IQR) | 31 (19–57) | 44 (24–70) | 38 (28–72) | 34.5 (20–61) | 34 (21–54.5) | 38 (22–65) |
ARDS acute respiratory distress syndrome, ICU intensive care unit, LOS lenght of stay, VAP ventilator-associated pneumonia, VAT ventilator-associated tracheobronchitis, VHAP ventilated hospital-acquired pneumonia
Etiological diagnosis of nosocomial LRTI
Any microbiological test was performed in 95% [1008/1060] of patients. 5% (52/1060) of the cohort had no microbiological sample. A total of 70.5% (711/1008) had a microbial diagnosis. A total of 16.07% (162/1008) had ETA-Sputum and BAL/miniBAL/PSB. The 96.3% (971/1008) had ETA-Sputum or BAL/miniBAL/PSB. The 97.8% (986/1008) ETA-sputum, BAL/miniBAL/PSB, respiratory virus testing, or real-time polymerase chain reaction (PCR) in respiratory samples. The most frequent sample collected during the diagnosis of nosocomial LRTI was blood culture in 82.5% [832/1008], followed by ETA and sputum culture (Table 3). Notably, the etiological pathogen was more frequently identified in patients diagnosed with VAP (75.4% [419/556]).
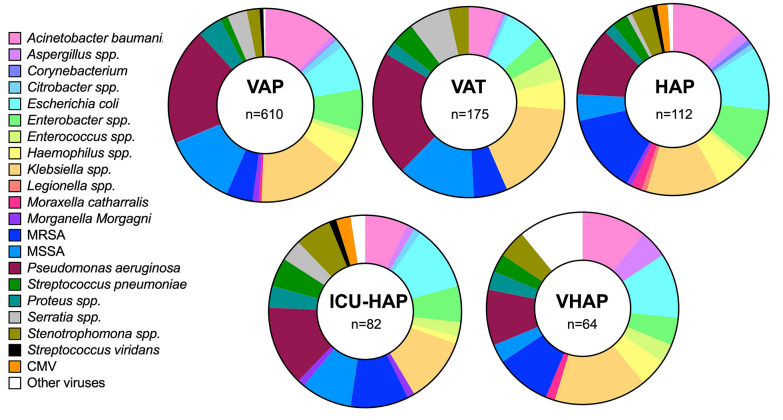
The most frequently identified microorganisms were P. aeruginosa (18.4% [186/1008]), Klebsiella spp. (14.4% [145/1008]), A. baumanii (11.0% [111/1008]), methicillin-susceptible S. aureus (MSSA) (10.81% [109/1008]) and E. coli (8.5% [86/1008]). We found that patients with VHAP and HAP had a lower prevalence of P. aeruginosa, Klebsiella spp., and methicillin-resistant Staphylococcus aureus (MRSA) than patients in the other groups. In contrast, patients with VAT, VAP, and ICU-HAP had comparable etiological pathogens, with a high prevalence of P. aeruginosa, Klebsiella spp., and MRSA (Fig. 3).
Fig. 3.
Etiological diagnosis stratified by the study groups
Multidrug-resistant pathogens
Interestingly, we found that 31% (320/1008) of the patients included in the study had an etiological pathogen that could be classified either as multidrug-resistant (MDR), pan-drug-resistant (PDR), extensively drug-resistant (XDL), extended spectrum-beta-lactamase (ESBL), or carbapenem-resistant. Most resistant pathogens were classified as MDR (13.1% [132/1008]). The total proportion of Gram-negative roots isolated was 50.3% (507/1008). Among these Gram-negative roots (i.e., A. baumanii, Citrobacter spp., E. coli, Legionella spp., Enterobacter spp., Klebsiella spp., M. morgagni, P. aeruginosa, Proteus spp., Serratia spp.) we found ESBL in 14.4% (73/507) and carbapenem-resistant in 11% (56/507). When analysing the distribution of these pathogens per study group, we found that VAP patients had a statistically significant higher prevalence of MDR pathogens (23.1% [74/320]), carbapenem-resistant pathogens (13.7% [44/320]), ESBL (13.43% [43/320]) and XDR (11.6% [37/320]) in comparison to the other study groups (Table 3).
Clinical outcomes
A total of 58.4% [619/1060] of the patients were reported to have resolved the infection. 33.4% [354/1060] patients presented treatment failure, and the remaining 7.9% [84/1060] had an unknown outcome. Recurrence of nosocomial LRTI was found in 10.7% [84/1060] of the whole cohort. VAT patients progress to VAP in 7.5% (12/160).
In-hospital mortality was 37.5% (397/1060), ICU mortality was reported in 30.8% (326/397) of the patients, and 28-day mortality was 27.5% (291/1060). Patients diagnosed with VHAP had higher hospital mortality (50% [47/94]), ICU mortality (40.3% [38/94]) and 28-day mortality (41.5% [39/94]) when compared to the other groups (Fig. 1; Table 4). The median (IQR) ICU length of stay (LOS) in survivors was 20 [11–35] days, and the hospital LOS was 38 [22–65] days. Patients with VAP had longer hospital and ICU LOS compared with other groups (VAT: 20.5 [11.5–30] vs VAP: 25 [15–41] vs ICU-HAP: 20 [11–35] vs VHAP: 12 [6–27] vs HAP:12 [6–19.5]) and (VAT: 31 [19–57] vs VAP: 44 [24–70] vs ICU-HAP: 38 [28–72] vs VHAP: 34.5 [20–61] vs HAP: 34 [21–54.5]) respectively (Table 4).
Discussion
This study represents a contemporary multinational observational perspective aiming to describe all the possible nosocomial pneumonia presentations in a critical care setting. The main finding is the high mortality, especially among those with VHAP. Additionally, these patients presented not only a worse unadjusted outcome for mortality but also for morbidity with longer duration of their stay in both hospital and ICU. We also found that the etiological pathogens in patients admitted with nosocomial pneumonia in the ICU were similar among groups. However, we found that multidrug-resistant pathogens were more frequently identified in VAP patients and were infrequent in the other groups. These findings have important therapeutic implications that should be further explored.
Patients admitted to the ICU often present pneumonia as an infectious complication during their stay (i.e., nosocomial pneumonia). However, most of the work done has been continuously focused on only one entity: VAP. Nevertheless, in recent years, VAT has emerged as a new relevant infection in patients under invasive mechanical ventilation. Some authors have hypothesised that VAT is an intermediate infection before affecting the lung parenchyma [25–27]. In contrast, others have proposed VAT as an independent entity that does not need antibiotic treatment [4, 28]. Current international guidelines have contradictory recommendations about differentiating VAP and VAT and how to treat these infections [4]. On the other hand, as more patients are being admitted to the ICU without requiring invasive mechanical ventilation, different types of nosocomial pneumonia are now a growing global problem in the ICU [29, 30]. Notably, the clinical characteristics and outcomes of these new kinds of pneumonia in the ICU have yet to be appropriately described.
An important finding was the higher frequency of infection due to P. aeruginosa in the study groups. Over the last 5–10 years, epidemiological studies have reported increased rates of Klebsiella pneumonia and other non-fermentative Gram-negative pathogens [10, 31–34]. This has also resulted in resistant strains, especially carbapenem-resistant pathogens [35–37], our findings remind us that P. aeruginosa should still be considered a common pathogen in patients with nosocomial pneumonia [33]. Yet, we had a low rate of aetiology identification in patients without invasive mechanical ventilation, which could underestimate the prevalence of other etiological pathogens [4]. This is a common feature being shown in studies including patients that were not invasively ventilated and opens the question of how we could improve diagnostic yields in patients without an artificial airway in place [38]. An alternative to this could be PCR-based technologies; however, further studies are needed to prove the utility of these new technologies.
Attributable pneumonia-related mortality has been and will be a matter of debate. Studies have been published to determine this effect, but the results remain unclear. One of the most cited studies was published almost ten years ago from a meta-analysis of individual patient data from randomised studies. Melsen et al. reported an overall attributable mortality of 13% in patients with VAP [39]. The authors reported attributable avoidable mortality in patients with mid-range severity and after surgery. However, data obtained by us suggest higher rates of mortality. In our study, higher mortality rates were linked with severity scores, especially in patients with VHAP. As the clinical characteristics and outcomes are different among the different kinds of nosocomial types of pneumonia, we propose to analyse attributable mortality based on the various clinical trajectories associated with ICU admission. In other words, pneumonia is not a static process, and dynamic changes apply and might better understand the attributable mortality dilemma in patients with nosocomial pneumonia.
Etiological diagnosis of pneumonia represents a cornerstone element for adequate medical treatment. Not only to obtain and to identify the responsible pathogen promptly but also a valid identification with a significant sample avoids overtreatment and helps in early de-escalation [40]. As our results showed, patients with VHAP yielded poor microbiological confirmation. This can be interpreted in two ways. One, patients have received previous antibiotic treatment, which made antibiotic levels not allow the growth, or two, we should propose a more specific and invasive technique for this type of patient (i.e., bronchoscopy at intubation). Unfortunately, previous studies have shown that the performance of bronchoscopy is not widely part of clinical practice in European ICUs [41]. Yet, bronchoscopy is a valuable technique, and international pneumonia guidelines currently recommend it to improve diagnosis accuracy [1, 4].
Our study has some limitations that are important to mention. Although the study is a multicentre, multinational study conducted in Europe and Latin America, some other countries still need to be included to allow the generalisability of the results. There needs to be more representation from the Scandinavian countries where low resistance rates have been published. However, this study finds the differences and aetiology in places with potentially higher resistance rates, and this information would be valuable. Second, the diagnosis and treatment of the patients were not standardised per the study protocol among the different participating ICUs; thus, some fungal and virus tests were not performed, which might represent a potential bias. However, our results provide an insightful analysis of current clinical practice. Third, this study only recruited patients with nosocomial LRTI admitted to the ICU. This does not allow us to estimate the prevalence or incidence rate of LRTI among patients admitted to the hospital. Nevertheless, the proportion of nosocomial LRTI are well described and subcategorised in the VAT/VAP/ICUHAP/VHAP/HAP groups.
In conclusion, with a prospective design analysis, this cohort study provides contemporary data on all the different types of nosocomial pneumonia that need to be treated in the critically ill setting. We found that VHAP mortality was the highest among patients admitted to the ICU, confirming what has been reported in previous clinical trials. However, the reason these patients have a higher mortality rate is still being determined and should be further explored in upcoming studies. Finally, we found that multidrug-resistant pathogens more frequently infected patients with VAP than other nosocomial pneumonia, which might have important clinical implications.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Collaborators of the European Network for ICU-Related Respiratory Infections (ENIRRIs) European Respiratory Society-Clinical Research Collaboration Investigators: Yuli Viviana Fuentes, Francesco Blasi, Marta Di Pasquale, Paolo Maurizio Soave, Giorgia Spinazzola, Anselmo Caricato, Serena Silva, Mariachiara Ippolito, Federico Longhini, Andrea Bruni, Eugenio Garofalo, Vittoria Comellini, Luca Fasano, Angelo Pezzi.
Funding
Open Access funding provided by the IReL Consortium. European respiratory Society Clinical Research Collaboration (Prof Ignacio Martin-Loeches) for the The European Network for ICU-Related Respiratory Infections (ENIRRIs), Universidad de La Sabana [Grant no. MED-244-2018].
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Conflicts of interest
All authors have no conflict of interest.
Footnotes
European Network for ICU-Related Respiratory Infections (ENIRRIs) European Respiratory Society-Clinical Research Collaboration Investigators are listed in acknowledgements section.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ignacio Martin-Loeches and Luis Felipe Reyes: Co-first authors.
Contributor Information
Ignacio Martin-Loeches, Email: drmartinloeches@gmail.com, Email: imartinl@tcd.ie.
Antoni Torres, Email: ATORRES@clinic.cat.
the European Network for ICU-Related Respiratory Infections (ENIRRIs) European Respiratory Society-Clinical Research Collaboration Investigators:
Yuli Viviana Fuentes, Francesco Blasi, Marta Di Pasquale, Paolo Maurizio Soave, Giorgia Spinazzola, Anselmo Caricato, Serena Silva, Mariachiara Ippolito, Federico Longhini, Andrea Bruni, Eugenio Garofalo, Vittoria Comellini, Luca Fasano, and Angelo Pezzi
References
- 1.Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H et al (2017) International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociacion Latinoamericana del Torax (ALAT). Eur Respir J 50(3) [DOI] [PubMed]
- 2.Ferrer M, Difrancesco LF, Liapikou A, Rinaudo M, Carbonara M, Li Bassi G, et al. Polymicrobial intensive care unit-acquired pneumonia: prevalence, microbiology and outcome. Crit Care. 2015;19:450. doi: 10.1186/s13054-015-1165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin-Loeches I, Povoa P, Rodriguez A, Curcio D, Suarez D, Mira JP, et al. Incidence and prognosis of ventilator-associated tracheobronchitis (TAVeM): a multicentre, prospective, observational study. Lancet Respir Med. 2015;3(11):859–868. doi: 10.1016/S2213-2600(15)00326-4. [DOI] [PubMed] [Google Scholar]
- 4.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Pascale G, Ranzani OT, Nseir S, Chastre J, Welte T, Antonelli M et al (2017) Intensive care unit patients with lower respiratory tract nosocomial infections: the ENIRRIs project. ERJ Open Res 3(4) [DOI] [PMC free article] [PubMed]
- 6.Rodriguez A, Povoa P, Nseir S, Salluh J, Curcio D, Martin-Loeches I, et al. Incidence and diagnosis of ventilator-associated tracheobronchitis in the intensive care unit: an international online survey. Crit Care. 2014;18(1):R32. doi: 10.1186/cc13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrer M, Torres A. Epidemiology of ICU-acquired pneumonia. Curr Opin Crit Care. 2018;24(5):325–331. doi: 10.1097/MCC.0000000000000536. [DOI] [PubMed] [Google Scholar]
- 8.De Pascale G, Bello G, Tumbarello M, Antonelli M. Severe pneumonia in intensive care: cause, diagnosis, treatment and management: a review of the literature. Curr Opin Pulm Med. 2012;18(3):213–221. doi: 10.1097/MCP.0b013e328351f9bd. [DOI] [PubMed] [Google Scholar]
- 9.Reyes LF, Garcia-Gallo E, Pinedo J, Saenz-Valcarcel M, Celi L, Rodriguez A, et al. Scores to predict long-term mortality in patients with severe pneumonia still lacking. Clin Infect Dis. 2021;72(9):e442–e443. doi: 10.1093/cid/ciaa1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carugati M, Aliberti S, Reyes LF, Franco Sadud R, Irfan M, Prat C et al (2018) Microbiological testing of adults hospitalised with community-acquired pneumonia: an international study. ERJ Open Res 4(4) [DOI] [PMC free article] [PubMed]
- 11.Smith G, Nielsen M. ABC of intensive care. Criteria for admission. BMJ. 1999;318(7197):1544–1547. doi: 10.1136/bmj.318.7197.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nates JL, Nunnally M, Kleinpell R, Blosser S, Goldner J, Birriel B, et al. ICU admission, discharge, and triage guidelines: a framework to enhance clinical operations, development of institutional policies, and further research. Crit Care Med. 2016;44(8):1553–1602. doi: 10.1097/CCM.0000000000001856. [DOI] [PubMed] [Google Scholar]
- 13.Rouze A, Nseir S (2022) Hospital-acquired pneumonia/ventilator-associated pneumonia and ventilator-associated tracheobronchitis in COVID-19. Semin Respir Crit Care Med [DOI] [PubMed]
- 14.Reyes LF, Rodriguez A, Bastidas A, Parra-Tanoux D, Fuentes YV, Garcia-Gallo E, et al. Dexamethasone as risk-factor for ICU-acquired respiratory tract infections in severe COVID-19. J Crit Care. 2022;69:154014. doi: 10.1016/j.jcrc.2022.154014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin-Loeches I, Torres A, Collaboration ENCR (2019) The European Network for ICU-Related Respiratory Infections (ENIRRIs) ERS clinical research collaboration. Eur Respir J 53(1) [DOI] [PubMed]
- 16.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–2963. doi: 10.1001/jama.1993.03510240069035. [DOI] [PubMed] [Google Scholar]
- 17.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 18.Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 19.Biyik M, Ataseven H, Biyik Z, Asil M, Cifci S, Sayin S, et al. KDIGO (Kidney Disease: Improving Global Outcomes) criteria as a predictor of hospital mortality in cirrhotic patients. Turk J Gastroenterol. 2016;27(2):173–179. doi: 10.5152/tjg.2016.15467. [DOI] [PubMed] [Google Scholar]
- 20.Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Executive summary: surviving sepsis campaign: international guidelines for the management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11):1974–1982. doi: 10.1097/CCM.0000000000005357. [DOI] [PubMed] [Google Scholar]
- 21.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181–1247. doi: 10.1007/s00134-021-06506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giunta V, Ferrer M, Esperatti M, Ranzani OT, Saucedo LM, Li Bassi G, et al. ICU-acquired pneumonia with or without etiologic diagnosis: a comparison of outcomes. Crit Care Med. 2013;41(9):2133–2143. doi: 10.1097/CCM.0b013e31828a453b. [DOI] [PubMed] [Google Scholar]
- 24.Esperatti M, Ferrer M, Giunta V, Ranzani OT, Saucedo LM, Li Bassi G, et al. Validation of predictors of adverse outcomes in hospital-acquired pneumonia in the ICU. Crit Care Med. 2013;41(9):2151–2161. doi: 10.1097/CCM.0b013e31828a674a. [DOI] [PubMed] [Google Scholar]
- 25.Koulenti D, Arvaniti K, Judd M, Lalos N, Tjoeng I, Xu E et al (2020) Ventilator-associated tracheobronchitis: to treat or not to treat? Antibiotics (Basel) 9(2) [DOI] [PMC free article] [PubMed]
- 26.Salluh JIF, Souza-Dantas VC, Martin-Loeches I, Lisboa TC, Rabello L, Nseir S, et al. Ventilator-associated tracheobronchitis: an update. Rev Bras Ter Intensiva. 2019;31(4):541–547. doi: 10.5935/0103-507X.20190079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nseir S, Di Pompeo C, Pronnier P, Beague S, Onimus T, Saulnier F, et al. Nosocomial tracheobronchitis in mechanically ventilated patients: incidence, aetiology and outcome. Eur Respir J. 2002;20(6):1483–1489. doi: 10.1183/09031936.02.00012902. [DOI] [PubMed] [Google Scholar]
- 28.Agrafiotis M, Siempos II, Falagas ME. Frequency, prevention, outcome and treatment of ventilator-associated tracheobronchitis: systematic review and meta-analysis. Respir Med. 2010;104(3):325–336. doi: 10.1016/j.rmed.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Niederman MS, Martin-Loeches I, Torres A. The research agenda in VAP/HAP: next steps. Intensive Care Med. 2017;43(9):1389–1391. doi: 10.1007/s00134-017-4695-2. [DOI] [PubMed] [Google Scholar]
- 30.Vallecoccia MS, Dominedo C, Cutuli SL, Martin-Loeches I, Torres A, De Pascale G (2020) Is ventilated hospital-acquired pneumonia a worse entity than ventilator-associated pneumonia? Eur Respir Rev 29(157) [DOI] [PMC free article] [PubMed]
- 31.Jones RN. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis. 2010;51(Suppl 1):S81–S87. doi: 10.1086/653053. [DOI] [PubMed] [Google Scholar]
- 32.Villafuerte D, Aliberti S, Soni NJ, Faverio P, Marcos PJ, Wunderink RG, et al. Prevalence and risk factors for Enterobacteriaceae in patients hospitalized with community-acquired pneumonia. Respirology. 2020;25(5):543–551. doi: 10.1111/resp.13663. [DOI] [PubMed] [Google Scholar]
- 33.Restrepo MI, Babu BL, Reyes LF, Chalmers JD, Soni NJ, Sibila O et al (2018) Burden and risk factors for Pseudomonas aeruginosa community-acquired pneumonia: a multinational point prevalence study of hospitalised patients. Eur Respir J 52(2) [DOI] [PubMed]
- 34.Gramegna A, Sotgiu G, Di Pasquale M, Radovanovic D, Terraneo S, Reyes LF, et al. Atypical pathogens in hospitalized patients with community-acquired pneumonia: a worldwide perspective. BMC Infect Dis. 2018;18(1):677. doi: 10.1186/s12879-018-3565-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarda C, Fazal F, Rello J. Management of ventilator-associated pneumonia (VAP) caused by resistant gram-negative bacteria: which is the best strategy to treat? Expert Rev Respir Med. 2019;13(8):787–798. doi: 10.1080/17476348.2019.1632195. [DOI] [PubMed] [Google Scholar]
- 36.Koulenti D, Tsigou E, Rello J. Nosocomial pneumonia in 27 ICUs in Europe: perspectives from the EU-VAP/CAP study. Eur J Clin Microbiol Infect Dis. 2017;36(11):1999–2006. doi: 10.1007/s10096-016-2703-z. [DOI] [PubMed] [Google Scholar]
- 37.Micek ST, Wunderink RG, Kollef MH, Chen C, Rello J, Chastre J, et al. An international multicenter retrospective study of Pseudomonas aeruginosa nosocomial pneumonia: impact of multidrug resistance. Crit Care. 2015;19(1):219. doi: 10.1186/s13054-015-0926-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caliendo AM, Gilbert DN, Ginocchio CC, Hanson KE, May L, Quinn TC, et al. Better tests, better care: improved diagnostics for infectious diseases. Clin Infect Dis. 2013;57(Suppl 3):S139–S170. doi: 10.1093/cid/cit578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melsen WG, Rovers MM, Groenwold RH, Bergmans DC, Camus C, Bauer TT, et al. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis. 2013;13(8):665–671. doi: 10.1016/S1473-3099(13)70081-1. [DOI] [PubMed] [Google Scholar]
- 40.Cilloniz C, Martin-Loeches I, Garcia-Vidal C, San Jose A, Torres A (2016) Microbial etiology of pneumonia: epidemiology, diagnosis and resistance patterns. Int J Mol Sci 17(12) [DOI] [PMC free article] [PubMed]
- 41.Menditto VG, Mei F, Fabrizzi B, Bonifazi M. Role of bronchoscopy in critically ill patients managed in intermediate care units—indications and complications: a narrative review. World J Crit Care Med. 2021;10(6):334–344. doi: 10.5492/wjccm.v10.i6.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.