Abstract
We developed a model to test whether non-membrane-permeative therapeutic agents such as gentamicin could be delivered into mammalian cells by means of bacterial membrane vesicles. Many gram-negative bacteria bleb off membrane vesicles (MVs) during normal growth, and the quantity of these vesicles can be increased by brief exposure to gentamicin (J. L. Kadurugamuwa and T. J. Beveridge, J. Bacteriol. 177:3998–4008, 1995), which can be entrapped within the MVs. Gentamicin-induced MVs (g-MVs) were isolated from Shigella flexneri and contained 85 ± 2 ng of gentamicin per μg of MV protein. Immunogold electron microscopic labeling of thin sections with antibodies specific to S. flexneri lipopolysaccharide (LPS) demonstrated the adherence and subsequent engulfment of MVs by the human Henle 407 intestinal epithelial cell line. Further incubation of g-MVs with S. flexneri-infected Henle cells revealed that the g-MVs penetrated throughout the infected cells and reduced the intracellular pathogen by ∼1.5 log10 CFU in the first hour of incubation. Antibiotic was detected in the cytoplasms of host cells, indicating the intracellular placement of the drug following the penetration of g-MVs. Soluble antibiotic, added as a fluid to the tissue culture growth medium, had no effect on intracellular bacterial growth, confirming the impermeability of the cell membranes of the tissue to gentamicin. Western blot analysis of MVs with S. flexneri Ipa-specific antibodies demonstrated that the invasion protein antigens IpaB, IpaC, and IpaD were present in MVs. Being bilayered, with outer faces composed of LPS and Ipa proteins, these MVs were readily engulfed by the otherwise impermeable membranes and eventually liberated their contents into the cytoplasmic substance of the host tissue.
The prime objective of antimicrobial chemotherapy is to aid in eradicating invading microorganisms by delivering an optimal amount of active drug to the site of infection. The ability of a drug to reach effective concentrations at the site of infection depends on many physiochemical and pharmacological characteristics (4). Aminoglycoside antibiotics, such as gentamicin, are potent antimicrobial agents that are active against both gram-negative and gram-positive bacteria. However, these compounds are not effective against facultative intracellular pathogens such as Shigella spp., Listeria spp., and Salmonella spp. during their intracellular growth phase, because of their inability to effectively penetrate mammalian cells (9, 30, 32).
Recently we have demonstrated that gentamicin can be easily introduced into membrane vesicles (MVs) of Pseudomonas aeruginosa that naturally bleb off the bacterium throughout its growth cycle (12). In fact, by exposing the bacterium to the surface activity of this antibiotic (13), the level of gentamicin-containing MVs (g-MVs) can be increased threefold above normal levels of naturally forming MVs (12). g-MVs are able to deliver the antibiotic directly to other gram-positive and gram-negative pathogens (including permeation-resistant P. aeruginosa) (11).
Since both natural MVs (n-MVs) and g-MVs easily attach to other cellular systems (11, 15), we decided to investigate whether this non-membrane-permeative antibiotic could also be delivered into mammalian cells when it is associated with MVs. To test this hypothesis, we developed an in vitro model using a human intestinal epithelial cell line infected with the intracellular pathogen Shigella flexneri (14), after which we subjected the tissue cells to g-MVs and examined the intracellular multiplication of the parasite over time.
(Parts of this study were presented at the 97th General Meeting of the American Society for Microbiology [17].)
MATERIALS AND METHODS
Bacterial strains and growth conditions.
A virulent strain of S. flexneri serotype 5 (M90T) (26), kindly provided by P. J. Sansonetti, was grown in Trypticase soy broth (TSB) with shaking on an orbital shaker at 37°C as described previously by Kadurugamuwa et al. (14).
Antibiotic susceptibility test.
The MIC of gentamicin was determined by a dilution method with Mueller-Hinton broth and was 0.78 μg/ml (11).
Isolation of MVs.
MVs were isolated from M90T as previously outlined for P. aeruginosa PAO1 by Kadurugamuwa and Beveridge (12). Briefly, 1 liter of bacterial culture in early stationary growth phase was divided into two equal parts. To one part, gentamicin at a final concentration of 25 μg/ml was added; the other part served as a control. Both cultures were incubated for 30 min on an orbital shaker at room temperature. Cells were removed from suspension by centrifugation at 6,000 × g for 15 min. The supernatants were filtered sequentially through 0.45- and 0.22-μm-pore-size cellulose acetate membranes (MSI, Westbro, Mass.) to remove residual cells. MVs were removed from the filtrates by centrifugation at 150,000 × g for 3 h at 4°C, and the vesicle pellet was washed and resuspended in phosphate-buffered saline (PBS; pH 7.4).
Detection of gentamicin in g-MVs and Henle cell lysate.
The amount of gentamicin in g-MVs or Henle cell lysate following incubation with MVs was determined by an enzyme-linked immunoassay with antiserum to gentamicin, which was obtained from Sigma (St. Louis, Mo.). Experiments were performed to test the sensitivity, specificity, precision, and accuracy of the assay. The limits of detection were 0.2 to 3 μg/ml. Intrarun and interrun coefficients of variation (2.3 and 11.5%, respectively) were calculated and were comparable to those in similar assays. The assay’s correlation coefficient (r = 0.9882) demonstrated a statistically significant correlation between the optical density of the sample and the concentration of antibiotic in the sample.
Tissue culture methods and infection procedure.
The human intestinal epithelial cell line, Henle 407 (ATCC CCL-6), was maintained in Dulbecco’s modified Eagle medium (DMEM) (GIBCO BRL Laboratories, Burlington, Ontario, Canada) at 37°C in a 5% CO2 atmosphere and infected with S. flexneri as previously described by Kadurugamuwa et al. (14). Briefly, monolayers were infected with 4 × 108 CFU of bacteria per ml in DMEM without antibiotic. After allowing entry of bacteria into epithelial cells for 10 min, extracellular cells were removed by washing the cells three times with sterile PBS. At this point, DMEM containing 40 μg of gentamicin per ml was added to each tissue culture well or flask to kill extracellular bacteria and thereby prevent reinfection of cells. This antibiotic is unable to penetrate epithelial cells (4, 26, 32), so that intracellular pathogens (i.e., internalized S. flexneri) survived the treatment. Monolayers were incubated for an additional 2 to 3 h so as to allow the intracellular pathogen to multiply and grow. Minimum lysis without measurably harming the monolayers occurred during this time interval, as determined by the trypan blue exclusion test.
Effect of MVs on intracellular killing.
M90T-infected monolayers, incubated for 2 h at 37°C in a 5% CO2 atmosphere, were washed five times in PBS and overlaid with either n-MVs or g-MVs (100 μg of MV protein/ml for each monolayer) in DMEM. After a 10-min incubation at 37°C in 5% CO2, unbound MVs were removed by washing the monolayers three times with PBS and reincubating them with DMEM without antibiotic for the times indicated below. At 0.5, 1.0, and 1.5 h, the culture medium was removed and the Henle cells were lysed by adding 1.0 ml of ice-cold 1% Triton X-100 solution per tissue culture well for 5 min to release intracellular bacteria. The lysate was serially diluted in TSB, and the viable intracellular bacteria were enumerated by plating them onto Trypticase soy agar plates and incubating them at 37°C for 16 h. Control cultures consisted of Shigella-infected monolayers that were not exposed to MVs but that were incubated with DMEM containing 40 μg of gentamicin per ml throughout the experiment. The bacteria that survived this treatment and multiplied intracellularly were enumerated as described above. The effects of n-MVs, g-MVs, and soluble antibiotic on intracellular bacteria were determined simultaneously in triplicate per time point. The results are expressed as the mean log10 of viable bacteria per ml.
Assay for attachment of MVs and their entry into epithelial cells.
The integration and entry of MVs into Henle 407 cells were demonstrated by the immunolabeling of whole mounts or thin sections of epithelial cells with antibodies for S. flexneri M90T lipopolysaccharide (LPS) (14) as described previously by Kadurugamuwa and Beveridge (12).
TEM.
Negative stains, thin sectioning, and immunogold labeling were performed as described by Kadurugamuwa and Beveridge (12). Transmission electron microscopy (TEM) was performed with a Philips EM300 microscope under standard operating conditions at 60 kV with the anticontaminator (cold finger) in place.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis.
Outer membrane protein (OMP) from S. flexneri was prepared as described previously (6). Twenty micrograms of protein from MVs, OMPs, or whole-cell lysate was suspended in Laemmli sample buffer (18). Each protein was separated on 12.5% polyacrylamide gels and electrophoretically transferred onto nitrocellulose sheets according to the technique of Towbin et al. (31). The Ipa proteins were detected with mouse monoclonal antibodies or rabbit polyclonal antibodies specific for IpaB, IpaC, or IpaD protein (kindly provided by E. V. Oaks and C. Sasakawa). Immunoblots were developed with either alkaline phosphatase-labeled or horseradish peroxidase-conjugated secondary antibodies.
RESULTS
TEM, SDS-PAGE, and Western blot analysis of MVs.
Examination of intact isolated purified MVs from both natural and gentamicin-treated cultures in negative stains showed that they were spherical (ca. 80 nm in diameter), bilayered vesicles filled with a particulate substance (Fig. 1).
FIG. 1.
Electron micrograph of negatively stained g-MVs. The spherical MVs are 50 to 80 nm in diameter, possess an intact bilayer (small arrow), and enclose electron-dense material (large arrow). Bar = 100 nm.
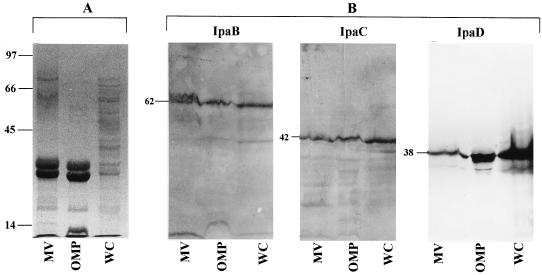
The protein profiles of MVs, OMPs extracted from whole cells, and whole-cell lysates were compared by SDS-PAGE. As seen in Fig. 2A, the banding patterns of MVs and OMPs were very similar; both appeared to have fewer bands than are normally present in whole-cell lysates. Prominently stained bands in MVs and OMP preparations included those of ∼70, 62, 37, 35, and 30 kDa. The proteins of 62, 42, and 38 kDa were identified as IpaB, IpaC, and IpaD by immunoblot analysis with antibodies specific for IpaB, IpaC, and IpaD (Fig. 2B). Interestingly, the 42- and 38-kDa bands, which were hardly detected with Coomassie blue stain, reacted strongly with IpaC and IpaD antibodies in Western blots. This result indicates that the sensitivity of immunoblotting is generally greater than that of conventional staining with Coomassie blue in detection of protein antigens. The 70-kDa protein seen by SDS-PAGE is most probably the IpaA protein. We were unable to confirm the identity of this protein by immunoblot analysis because of the unavailability of specific antibodies to the antigen, but the molecular mass of IpaA is 70 kDa (28). The ipa-encoded membrane proteins (which are products of the invasion plasmid) in S. flexneri are surface exposed and essential to the pathogen for penetrating epithelial cells and escaping phagocytic vacuoles (2, 8, 22, 23, 29, 33).
FIG. 2.
(A) SDS-PAGE protein profiles of MVs, OMPs, and whole-cell lysate (WC); (B) immunoblot detection of Ipa proteins in MVs, OMP, and WC after reaction with antibodies specific for IpaB, IpaC, and IpaD proteins. Numbers indicate positions and sizes (in kilodaltons) of standard proteins.
Adhesion and penetration into eucaryotic cells by MVs.
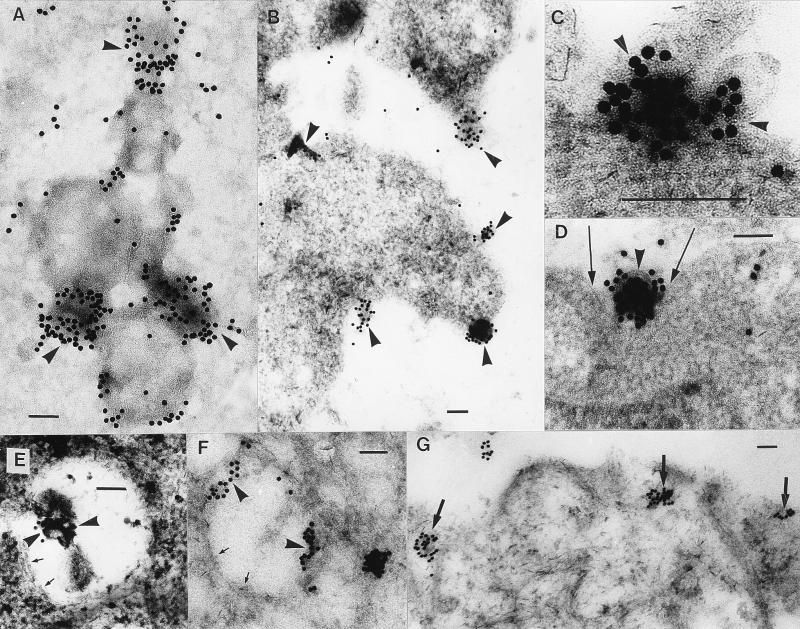
Immunogold labeling of MVs with LPS-specific antibodies provided information on the series of events leading to the internalization of the vesicles by the Henle cell line. The first five minutes were characterized by strong adsorption of MVs onto the cell membrane. This specific gold labeling of MVs showed the topographic distribution of MVs (usually several per tissue culture cell) attached to epithelial cells by both the whole-mount (Fig. 3A) and thin-section (Fig. 3B and C) techniques. Small invaginations within the cytoplasmic membrane of the tissue cells directly under the labeled MVs were next observed as the MVs were engulfed (Fig. 3D). The MVs were then seen in the cytoplasm in membrane-bound vacuoles, which appeared morphologically similar to phagosomes (Fig. 3E). Within 15 min of this event, MVs attached themselves to the phagosome membrane (Fig. 3F). Thirty minutes later, the vacuole membrane disintegrated and the MV constituents were found within the cytoplasm (Fig. 3G). The actual physical movement of MV components from phagosome to cytoplasm was never detected by TEM of thin sections, and we were left with the impression that it was a rapid physical transformation whereby the MV fused into the phagosomal membrane and escaped into the cytoplasm. In total, our evidence suggests that the chain of events leading to transepithelial migration involves the internalization of MVs by phagocytosis, with subsequent “exocytosis” of MV components (Fig. 4) from the phagosome to the host cytoplasm in a manner similar to that in which whole cells of S. flexneri operate (14, 26).
FIG. 3.
Fusion with and penetration into eucaryotic cells by MVs. Immunogold electron microscopic labeling of whole mounts (A) or thin sections (B to G) with antibodies to S. flexneri LPS demonstrates the sequence of events leading to the internalization of MVs by mammalian cells. Specific labeling of MVs with Shigella antigens (arrowheads) in whole mounts (A) or thin sections (B and C) shows several MVs adsorbed to epithelial cells. Small invaginations are seen in the cytoplasmic membrane (arrows) of the tissue containing the labeled MVs (arrowhead) (D). Engulfed MVs are seen within a phagosome (arrowheads) (E) and attached to the phagosome membrane (arrowheads) (F). Note that the partially dissolved plasma membrane of the phagosome is still barely visible (arrows) (E and F). (G) MV constituents can be seen within the cytoplasm (arrows). Bars = 100 nm.
FIG. 4.
Infection and growth of S. flexneri in Henle tissue. (A) S. flexneri infecting a Henle cell. Spongy material (arrows) in proximity to the bacterial attachment site is most probably polymerized actin. (B) Many actively dividing bacteria can be seen lying free in the cytoplasm. Bars = 100 nm.
Infection of Henle cells with S. flexneri and exposure of infected tissue to MVs.
Figure 4A shows S. flexneri infecting a Henle cell. The entry process into the mammalian cell also resembles phagocytosis as described previously (14, 26). The less densely stained spongy material seen in Fig. 4A at the site of bacterial entry is probably polymerized cytoskeletal actin, which has previously been seen during the internalization of Shigella (14, 26). Following infection (i.e., immediately after internalization) and as expected, bacteria were found lying within membrane-bound phagocytic vacuoles in cytoplasm (14, 26). The infection was allowed to proceed for 2 h until many actively dividing bacteria could be seen lying freely within cytoplasm (Fig. 4B); this result confirmed S. flexneri’s ability to migrate rapidly from the phagosome to the cytoplasm (8, 14, 21, 26, 35).
After this infection period, infected cells were exposed to MVs. Figure 5 shows a Shigella-infected Henle cell with internalized MVs in the cytoplasm.
FIG. 5.
Infected tissue after exposure to MVs. A Shigella-infected Henle cell with internalized MV in the cytoplasm (arrow) is shown. Also note the less densely stained material surrounding vesicle contents. Bar = 100 nm.
Association of gentamicin with g-MVs.
Enzyme-linked immunosorbent assays demonstrated that g-MVs contained 85 ± 2 ng of gentamicin per μg of MV protein. After penetration of g-MVs into the monolayer of tissue and the lysing of cells by detergent, the lysate contained 3.1 ± 0.6 ng of gentamicin per μg of epithelial cell protein. Epithelial cells incubated in tissue culture medium containing a bactericidal concentration of gentamicin in solution or n-MVs (no gentamicin is associated with these) had no detectable amount of antibiotic in the cell lysate. This indicated that gentamicin is indeed associated with g-MVs and that the antibiotic was able to penetrate the mammalian cells in our experiments only following internalization of g-MVs. Consistent with this conclusion were the results of electron microscopic observation of thin sections prepared from these cell cultures. The luminal location of the gentamicin and its transport into epithelial cells were demonstrated by immunogold labeling of thin sections with antibodies to the antibiotic. Gold particles were clearly seen in the cytoplasms of cells incubated with g-MVs but not in those incubated with n-MVs or tissue culture medium with antibiotic (data not shown). The detection of MVs and gentamicin within the cytoplasms of cells exposed to g-MVs plus the decrease in the number of intracellular S. flexneri organisms seen in viability studies suggested that the encapsulated MV antibiotic was, at least in part, responsible for the killing of the pathogen.
Effects of n- and g-MVs and soluble gentamicin on intracellular bacteria.
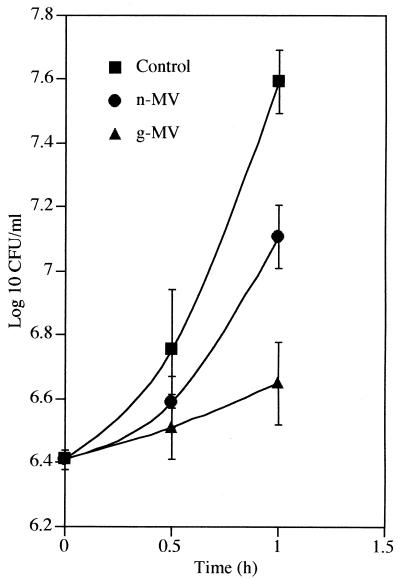
Two hours postinfection, gentamicin, n-MVs, or g-MVs were added to S. flexneri-infected epithelial cells, and viability was monitored over the next hour (Fig. 6). The soluble gentamicin concentration in the incubation medium was 40 μg/ml (which was determined to be approximately 50 times the MIC for S. flexneri). The viable number of bacteria in these cultures increased to reach ∼7.6 log10 CFU/ml. A similar concentration of free antibiotic in tissue culture medium or Henle cell lysate had a profound effect on the viability of free-living, external S. flexneri and produced a drop of ∼3.2 log10 CFU within 1 h at 37°C. These observations confirm that intracellular bacteria were protected against soluble gentamicin, since it was unable to penetrate the mammalian cells to exert its bactericidal action (32). The number of intracellular bacteria in monolayers exposed to n-MVs after 1.0 h of incubation was slightly lower (∼0.5 log10 CFU) than those of the control cultures. This difference was presumably due to autolysins found in MVs, which are capable of hydrolyzing the peptidoglycan layer and lysing bacteria (11). In contrast, a more dramatic killing was shown by the g-MVs. With g-MVs, the intracellular bacteria increased to only 0.2 log10 CFU/ml within a similar incubation period, indicating that the multiplication of Shigella in these cells was drastically suppressed. This inhibitory effect on g-MV-exposed cells was most likely due to the encapsulated antibiotic that penetrated these cells with the MVs. Once it is in phagosomes, gentamicin must be liberated into the cytoplasm, where it penetrates into the pathogen to inhibit the growth of intracellular bacteria. Presumably, this entire entry route for the antibiotic is similar to that outlined in Fig. 3; the gentamicin could breach the otherwise impermeable cytoplasmic membranes of the infected mammalian cells only because it was packaged into a bilayered transport device such as an MV.
FIG. 6.
Effects of n- and g-MVs and soluble gentamicin on intracellular bacteria. Two hours postinfection, gentamicin, n-MVs, or g-MVs were added to S. flexneri-infected epithelial cells, and viability was monitored over the next hour. The soluble gentamicin concentration used in the incubation medium was 40 μg/ml, and the MIC for free-living S. flexneri is 0.78 μg/ml. Data are averages of results from three separate experiments, with each being performed in triplicate. Means ± standard deviations of values from three replicates are shown.
DISCUSSION
Recently, we reported that P. aeruginosa releases MVs during normal growth (n-MVs) and that these MVs entrap gentamicin (g-MVs) if the bacterium is treated with the antibiotic during growth (12). These g-MVs contained not only gentamicin but also an extremely potent peptidoglycan hydrolase or autolysin. Both types of MVs from P. aeruginosa were capable of killing cultures of gram-negative and gram-positive pathogens, including a strain of P. aeruginosa with resistance to permeation by gentamicin (11). In that previous study, the fusion of g-MVs with the outer membranes of gram-negative pathogens allowed the vesicles to overcome surface permeability barriers and liberate both autolysin and antibiotic directly into the periplasm of the host bacterium to attack their target sites (i.e., peptidoglycan and ribosomes, respectively). The killing power of g-MVs was greater than that of n-MVs because of the synergistic effect of the antibiotic with autolysins. That study demonstrated that the entrapment of antibiotic within MVs could be a successful tool to both kill gram-negative and gram-positive pathogens and to overcome resistance to permeation by an antibiotic. Furthermore, the protection of entrapped drug from the action of external hydrolytic enzymes by the lipid bilayer of the MVs may become valuable in a clinical situation.
In the present study, we have demonstrated that MVs from S. flexneri were able to attach to and penetrate human epithelial cells. Once inside, these g-MVs were able to deliver the antibiotic to intracellular S. flexneri and inhibit the multiplication of the pathogen. External, soluble antibiotic had no effect on intracellular bacterial growth, confirming that gentamicin could enter mammalian cells only when it was packaged in MVs.
Many commercially available antibiotics are ineffective against intracellular infections, mainly because of poor penetration and retention of the drug or because of decreased intracellular drug activity (9, 30, 32). The development of methods to manipulate the chemical structures of antibiotics or the designs of carriers to enhance penetration is becoming important due to a growing appreciation of the importance of intracellular pathogens. One approach to increase the penetration of antibiotics into target cells is to use liposomes as transport vehicles (7, 24). MVs are subtly different from liposomes. They are natural agents produced by a variety of gram-negative bacteria (10), they have many of the antigenic attributes of their host bacteria (12), they are (therefore) considered foreign agents, and as a consequence, they are readily engulfed by mammalian cells. Accordingly, the use of MVs containing a drug active against intracellular pathogens has several advantages for efficient, direct drug delivery to the intracellular site of infection. Not all MVs are useful to combat intracellular pathogens of a specific type of tissue. For example, even though g-MVs from P. aeruginosa have much more peptidoglycan hydrolase than those from S. flexneri, we found that they did not penetrate into gut epithelial tissue as efficiently as those from the intestinal pathogen (16). It is possible, because specific pathogens have preferences for different tissues, that MVs from each will be preferentially engulfed by their respective tissue types. Consequently, it may be possible to make MV treatment partially selective. In general, we have found MVs from invasive pathogens to have more penetration efficiency than noninvasive varieties.
In the specific case of S. flexneri, we believe the high penetrability of its MVs is because of Ipa proteins. Previous studies have shown IpaB, IpaC, and IpaD proteins to be essential for successful infection and escape from the vacuole by this intestinal pathogen (2, 22, 27, 35). Ipa’s are surface associated (Fig. 2) (1, 2, 8, 19, 34) and present on the MVs (Fig. 2). In a previous study, Kadurugamuwa et al. (14) demonstrated that invasive S. flexneri was able to rearrange the cytoskeletal proteins actin and vinculin during the pathogen’s intracellular growth cycle and protrusion formation. Subsequently, other authors have demonstrated that both Ipa’s and vinculin are necessary for invasion and eventual escape from the phagocytic vacuole (5, 8, 25). Remarkably, we have demonstrated a similar reorganization of vinculin in epithelial cells when they are exposed to S. flexneri MVs (16). We also have unequivocally identified IpaB, IpaC, and IpaD proteins in MVs using monoclonal antibodies known to bind specific epitopes on these proteins. Previous work suggested that these surface-exposed Ipa proteins were released into the external medium during growth (1, 2, 8, 19, 20, 22, 27, 34, 35), and now we have clearly demonstrated in our present study that at least a certain proportion of these are released as MVs. We have not attempted to ascertain the proportions of soluble versus MV-associated Ipa’s in this study, but their proportions in MVs may be surprisingly high, as was true for certain proteins in P. aeruginosa (12). As the recognition of n-MVs being a general trait of gram-negative bacteria increases among researchers, the abundance of so-called soluble secreted proteins may have to be reevaluated.
The fact that Ipa proteins are immobilized in MVs has several ramifications. MVs are small and released into the surrounding environment. They are bilayered, are enriched with Ipa proteins, and accordingly, can meld into larger structures such as host cell membranes. In so doing, they trigger specific signal transduction pathways and elicit a host cell cytoskeletal rearrangement which is ultimately responsible for the pathogen’s internalization. We believe that these MVs might be ideal candidates to investigate the role of Ipa proteins in each stage of S. flexneri’s infection process without the interference or participation of whole cells, thus allowing definitive assignment of specific roles for each protein. A similar approach has been taken by Ménard et al. (21), who used cell-free culture supernatants to immuno-adsorb Ipa proteins to the surfaces of latex beads and demonstrated the uptake of these particles by epithelial cells. Most probably, these immunoprecipitated proteins were associated with MVs.
TEM provided an intimate glimpse of each stage of development as the MVs attached to and migrated throughout the epithelial tissue and suggested that many events were analogous to those of intact S. flexneri cells. For example, since we were never able to detect lysosomal fusion to phagosomes (containing MVs), penetration of MVs from the phagosome to the cytoplasm appeared to not require acidification by endosomes. At the same time, gentamicin (which was delivered by g-MVs) retained its antibiotic activity. The activity of aminoglycoside antibiotics is known to diminish in phagolysomes because of their acidic pH (pH 4 to 5) due to lysosome-phagosome fusion (3, 30, 32). This escape from acid inactivation by our MVs may be an added advantage for their use in delivering drugs into tissues, especially if the compound is acid labile.
In summary, we have been able to demonstrate that MVs can be used as delivery devices to transport foreign antigens and chemotherapeutic agents (including nonpenetrating compounds) into eucaryotic cells. We believe that this important observation can be applied to the delivery of a number of therapeutic agents to targeted tissues, either as single MV constituents or as a consortium of clinically active substances. Clearly our experimentation reveals that g-MVs can be used against intracellular parasites.
ACKNOWLEDGMENTS
We thank Anuradha Saxena and Dianne Moyles for their excellent technical assistance with this study.
This work was made possible by funding through the Canadian Bacterial Disease Network as a National Center of Excellence. The electron microscopy was performed in the National Sciences and Engineering Research Council of Canada (NSERC) Guelph Regional STEM Facility, which is partially funded by an NSERC Major Facilities Access grant to T.J.B.
REFERENCES
- 1.Allaoui A, Sansonetti P J, Parsot C. MxiD, an outer membrane protein necessary for the secretion of the Shigella flexneri Ipa invasins. Mol Microbiol. 1993;7:59–68. doi: 10.1111/j.1365-2958.1993.tb01097.x. [DOI] [PubMed] [Google Scholar]
- 2.Andrews G P, Hromockyj A E, Cocker J, Maurelli A T. Two novel virulence loci, mxiA and mxiB, in Shigella flexneri 2a facilitate excretion of invasion plasmid antigens. Infect Immun. 1991;59:1997–2005. doi: 10.1128/iai.59.6.1997-2005.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barber M, Waterworth P M. Activity of gentamicin against Pseudomonas and hospital Staphylococci. Br Med J. 1966;1:203–205. doi: 10.1136/bmj.1.5481.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown K N, Percival A. Penetration of antimicrobials into tissue culture cells and leukocytes. Scand J Infect Dis. 1978;14:251–260. [PubMed] [Google Scholar]
- 5.Dehio C, Prévost M-C, Sansonetti P J. Invasion of epithelial cells by Shigella flexneri induces tyrosine phosphorylation of cortactin by a pp60c-src-mediated signaling pathway. EMBO J. 1995;14:2471–2482. doi: 10.1002/j.1460-2075.1995.tb07244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filip C, Fletcher G, Wulff J L, Earhart C F. Solubilization of the cytoplasmic membrane of Escherichia coli. J Bacteriol. 1973;115:717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregoriadis G. The carrier potential of liposomes in biology and medicine. N Engl J Med. 1976;295:704–710. doi: 10.1056/NEJM197609232951305. [DOI] [PubMed] [Google Scholar]
- 8.High N, Mounier M, Prevost M C, Sansonetti P J. IpaB of Shigella flexneri causes entry into epithelial cells and escape from the phagocytic vacuole. EMBO J. 1992;11:1991–1999. doi: 10.1002/j.1460-2075.1992.tb05253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs R F, Wilson C B, Laxton J G, Haas J E, Smith A L. Cellular uptake and intracellular activity of antibiotics against Haemophilus influenzae type b. J Infect Dis. 1982;145:152–159. doi: 10.1093/infdis/145.2.152. [DOI] [PubMed] [Google Scholar]
- 10.Kadurugamuwa J L, Beveridge T J. Natural release of virulence factors in membrane vesicles by Pseudomonas aeruginosa and the effect of aminoglycoside antibiotics on their release. J Antimicrob Chemother. 1997;40:1–7. doi: 10.1093/jac/40.5.615. [DOI] [PubMed] [Google Scholar]
- 11.Kadurugamuwa J L, Beveridge T J. Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: conceptually new antibiotics. J Bacteriol. 1996;178:2767–2774. doi: 10.1128/jb.178.10.2767-2774.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kadurugamuwa J L, Beveridge T J. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J Bacteriol. 1995;177:3998–4008. doi: 10.1128/jb.177.14.3998-4008.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadurugamuwa J L, Clarke A J, Beveridge T J. Surface action of gentamicin on Pseudomonas aeruginosa. J Bacteriol. 1993;175:5798–5805. doi: 10.1128/jb.175.18.5798-5805.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadurugamuwa J L, Rohde M, Wehland J, Timmis K N. Intercellular spread of Shigella flexneri through a monolayer mediated by membranous protrusions and associated with reorganization of cytoskeletal protein vinculin. Infect Immun. 1991;59:3463–3471. doi: 10.1128/iai.59.10.3463-3471.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadurugamuwa J L, Saxena A. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. A novel mechanism to introduce surface antigens of pathogenic bacteria into a live attenuated vaccine strain of Salmonella typhi Ty21a, abstr. E-21; p. 270. [Google Scholar]
- 16.Kadurugamuwa J L, Saxena A, Beveridge T J. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Membrane vesicles from Shigella flexneri invade human epithelial cells efficiently, abstr. B-45; p. 34. [Google Scholar]
- 17.Kadurugamuwa J L, Saxena A, Beveridge T J. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Delivery of the membrane-impermeant antibiotic gentamicin into mammalian cells using membrane vesicles, abstr. A-28; p. 5. [Google Scholar]
- 18.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Lee C A. Type 111 secretion system: machines to deliver bacterial proteins into eukaryotic cells? Trends Microbiol. 1997;5:148–156. doi: 10.1016/S0966-842X(97)01029-9. [DOI] [PubMed] [Google Scholar]
- 20.Marquart M E, Picking W L, Picking W D. Soluble invasion plasmid antigen C (IpaC) from Shigella flexneri elicits epithelial cell responses related to pathogen invasion. Infect Immun. 1996;64:4182–4187. doi: 10.1128/iai.64.10.4182-4187.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ménard R, Prévost M-C, Gounon P, Sansonetti P J, Dehio C. The secreted Ipa complex of Shigella flexneri promotes entry into mammalian cells. Proc Natl Acad Sci USA. 1996;93:1254–1258. doi: 10.1073/pnas.93.3.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ménard R, Sansonetti P J, Parsot C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol. 1993;175:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oaks E V, Picking W L, Picking W D. Antibody response of monkeys to invasion plasmid antigen D after infection with Shigella. Clin Diagn Lab Immunol. 1996;3:242–245. doi: 10.1128/cdli.3.2.242-245.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostro M J. Liposomes. Sci Am. 1987;256:102–111. [PubMed] [Google Scholar]
- 25.Sansonetti P J, Mounier J, Prévost M-C, Mège R-M. Cadherin expression is required for the spread of Shigella flexneri between epithelial cells. Cell. 1994;76:829–839. doi: 10.1016/0092-8674(94)90358-1. [DOI] [PubMed] [Google Scholar]
- 26.Sansonetti P J, Ryter A, Clerc P, Murelli A T, Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect Immun. 1986;51:461–469. doi: 10.1128/iai.51.2.461-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasakawa C, Adler B, Tobe T, Okada N, Nagai S, Komatsu K, Yoshikawa M. Functional organization and nucleotide sequence of virulence region 2 on the large virulence plasmid of Shigella flexneri 2a. Mol Microbiol. 1989;3:1191–1201. doi: 10.1111/j.1365-2958.1989.tb00269.x. [DOI] [PubMed] [Google Scholar]
- 28.Sasakawa C, Buysse J M, Watanabe H. The large virulence plasmid of Shigella. Curr Top Microbiol Immunol. 1992;180:21–44. doi: 10.1007/978-3-642-77238-2_2. [DOI] [PubMed] [Google Scholar]
- 29.Sasakawa C, Komatsu K, Tobe T, Suzuki T, Yoshikawa M. Eight genes in region 5 that form an operon are essential for invasion of epithelial cells by Shigella flexneri 2a. J Bacteriol. 1993;175:2324–2346. doi: 10.1128/jb.175.8.2334-2346.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scwab J C, Mandell G L. The importance of penetration of antimicrobial agents into cells. Infect Dis Clin N Am. 1989;3:461–467. [PubMed] [Google Scholar]
- 31.Towbin H, Stachelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets. Procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaudaux P, Waldvogel F A. Gentamicin antibacterial activity in the presence of human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1979;16:743–749. doi: 10.1128/aac.16.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venkatesan M M, Buysse J M, Kopecko D J. Characterization of invasion plasmid antigen (ipaBCD) genes from Shigella flexneri: DNA sequence analysis and control of gene expression. Proc Natl Acad Sci USA. 1988;85:9317–9321. doi: 10.1073/pnas.85.23.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Venkatesan M M, Buysse J M, Oaks E V. Surface presentation of Shigella flexneri invasion plasmid antigens requires the products of spa locus. J Bacteriol. 1992;174:1990–2001. doi: 10.1128/jb.174.6.1990-2001.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watarai M, Tobe T, Yoshikawa M, Sasakawa C. Contact of Shigella with host cells triggers release of Ipa invasins and is an essential function of invasiveness. EMBO J. 1995;14:2461–2470. doi: 10.1002/j.1460-2075.1995.tb07243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]