Abstract
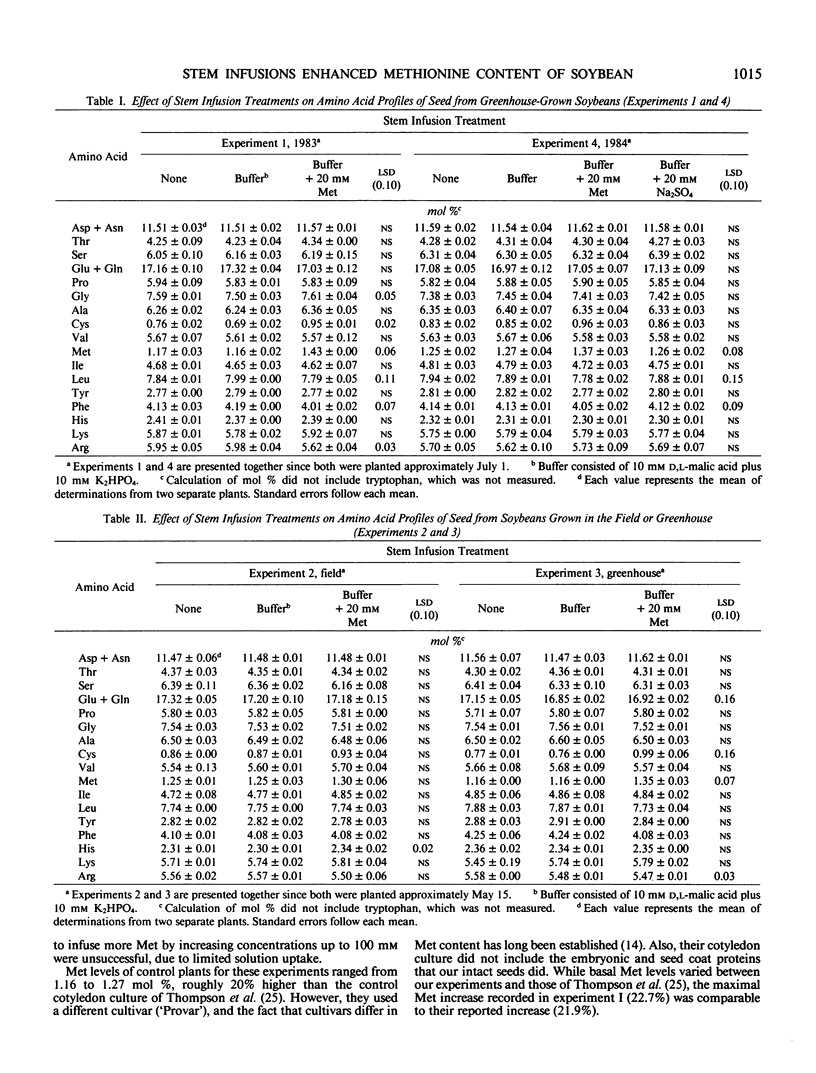
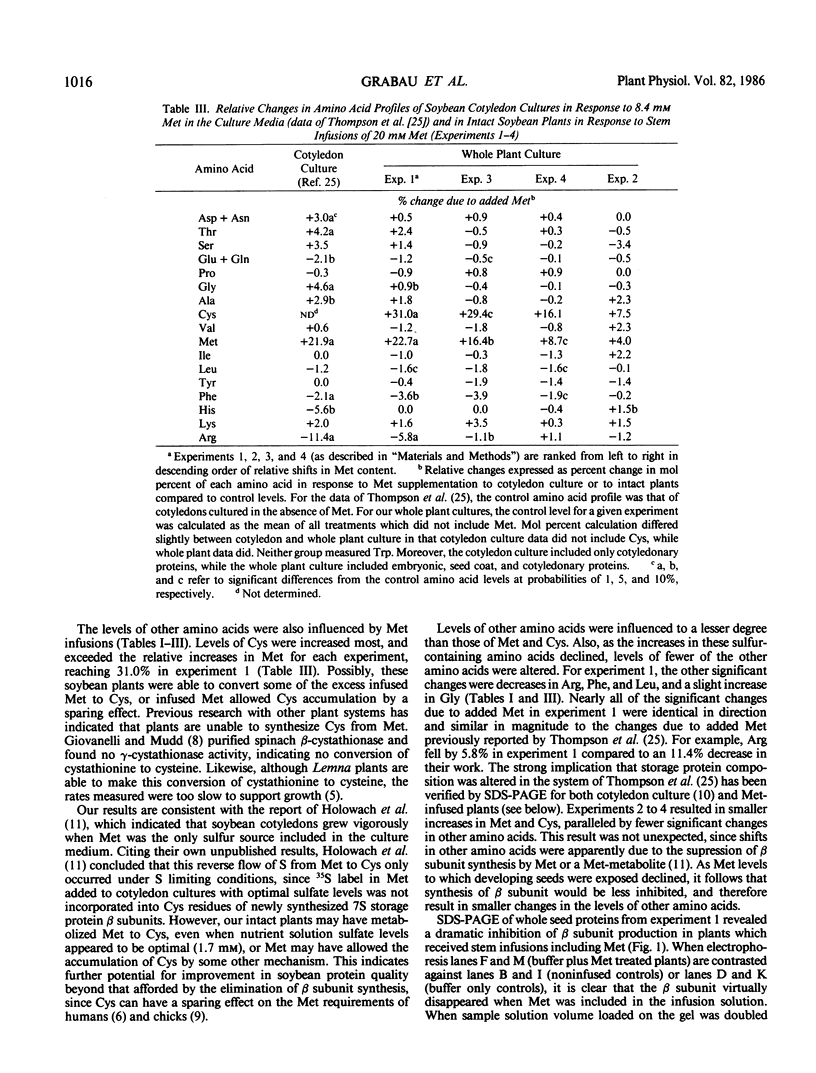
The quality of soybean (Glycine max [L.] Merrill) seed storage protein is limited by its low methionine (Met) content. Met supplementation of an in vitro soybean cotyledon culture has been shown to increase Met content by 21.9% due to an inhibition of the synthesis of the Met-devoid β subunit of 7S storage protein (JF Thompson et al. 1981, Phytochemistry 20: 941-945). The objective of this research was to determine if Met supplementation of intact plants would result in a similar improvement in soybean protein quality. A solution including 10 millimolar d,l malic acid plus 10 millimolar K2HPO4 with or without 20 millimolar d,l Met or 20 millimolar Na2SO4 was infused throughout seed development into lower stem internodes of soybeans (cv `Williams 79' or `Williams 82') grown under both greenhouse and field conditions. Pediatric intravenous kits were used to infuse an average of 51.2 milliliters per plant. Met content of whole soybean seeds from intact plants receiving Met infusions increased by as much as 22.7%. Even greater (up to 31.0%) increases in cysteine (Cys) content were noted, indicating that soybean plants are able to metabolize Met to Cys, or that supplemental Met allows Cys accumulation by some other mechanism. Electrophoretic patterns showed a dramatic decrease in the synthesis of the β subunit of 7S storage protein when Met was supplemented, and this effect was not confined to seeds at the lower nodes. In addition, seeds from upper compared to lower plant nodes (regardless of infusion treatment) had greater protein content (45.0 versus 41.6 w/w%), and different protein composition, as indicated by significantly different amino acid profiles. Methionine supplementation of intact soybean plants improved protein quality through an alteration in storage protein composition.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Creason G. L., Holowach L. P., Thompson J. F., Madison J. T. Exogenous methionine depresses level of mRNA for a soybean storage protein. Biochem Biophys Res Commun. 1983 Dec 28;117(3):658–662. doi: 10.1016/0006-291x(83)91647-9. [DOI] [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H. Methionine biosynthesis in lemna: inhibitor studies. Plant Physiol. 1982 May;69(5):1070–1076. doi: 10.1104/pp.69.5.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein J. D., Mudd S. H. Trans-sulfuration in mammals. The methionine-sparing effect of cystine. J Biol Chem. 1967 Mar 10;242(5):873–880. [PubMed] [Google Scholar]
- Fomon S. J., Ziegler E. E., Filer L. J., Jr, Nelson S. E., Edwards B. B. Methionine fortification of a soy protein formula fed to infants. Am J Clin Nutr. 1979 Dec;32(12):2460–2471. doi: 10.1093/ajcn/32.12.2460. [DOI] [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H. Transsulfuration in higher plants. Partial purification and properties of beta-cystathionase of spinach. Biochim Biophys Acta. 1971 Mar 10;227(3):654–670. doi: 10.1016/0005-2744(71)90015-5. [DOI] [PubMed] [Google Scholar]
- Halpin K. M., Baker D. H. Selenium deficiency and transsulfuration in the chick. J Nutr. 1984 Mar;114(3):606–612. doi: 10.1093/jn/114.3.606. [DOI] [PubMed] [Google Scholar]
- Holowach L. P., Thompson J. F., Madison J. T. Effects of exogenous methionine on storage protein composition of soybean cotyledons cultured in vitro. Plant Physiol. 1984 Mar;74(3):576–583. doi: 10.1104/pp.74.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowach L. P., Thompson J. F., Madison J. T. Storage Protein Composition of Soybean Cotyledons Grown In Vitro in Media of Various Sulfate Concentrations in the Presence and Absence of Exogenous l-Methionine. Plant Physiol. 1984 Mar;74(3):584–589. doi: 10.1104/pp.74.3.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu F. C., Bennett A. B., Spanswick R. M. Concentrations of sucrose and nitrogenous compounds in the apoplast of developing soybean seed coats and embryos. Plant Physiol. 1984 May;75(1):181–186. doi: 10.1104/pp.75.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Layzell D. B., Larue T. A. Modeling C and N transport to developing soybean fruits. Plant Physiol. 1982 Nov;70(5):1290–1298. doi: 10.1104/pp.70.5.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure P. R., Israel D. W. Transport of nitrogen in the xylem of soybean plants. Plant Physiol. 1979 Sep;64(3):411–416. doi: 10.1104/pp.64.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil D. L., Atkins C. A., Pate J. S. Uptake and Utilization of Xylem-borne Amino Compounds by Shoot Organs of a Legume. Plant Physiol. 1979 Jun;63(6):1076–1081. doi: 10.1104/pp.63.6.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D., Biddle G. N., Bauersfeld P. E., Jr, Cuppett S. L. Soybean meal diets supplemented with sulfate, methionine and fishery products. Poult Sci. 1974 Jan;53(1):226–234. doi: 10.3382/ps.0530226. [DOI] [PubMed] [Google Scholar]
- Potter L. M., Shelton J. R., Castaldo D. J. Supplementary inorganic sulfate and methionine for young turkeys. Poult Sci. 1983 Dec;62(12):2398–2402. doi: 10.3382/ps.0622398. [DOI] [PubMed] [Google Scholar]
- Rainbird R. M., Thorne J. H., Hardy R. W. Role of amides, amino acids, and ureides in the nutrition of developing soybean seeds. Plant Physiol. 1984 Feb;74(2):329–334. doi: 10.1104/pp.74.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter J. G. Allantoin and Allantoic Acid in Tissues and Stem Exudate from Field-grown Soybean Plants. Plant Physiol. 1979 Mar;63(3):478–480. doi: 10.1104/pp.63.3.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A. Purification and characterization of a soybean leaf storage glycoprotein. Plant Physiol. 1983 Sep;73(1):125–129. doi: 10.1104/pp.73.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]



