SUMMARY
Stem cell therapy shows promise for multiple disorders; however, the molecular crosstalk between grafted cells and host tissue is largely unknown. Here, we take a step toward addressing this question. Using translating ribosome affinity purification (TRAP) with sequencing tools, we simultaneously decode the transcriptomes of graft and host for human neural stem cells (hNSCs) transplanted into the stroke-injured rat brain. Employing pathway analysis tools, we investigate the interactions between the two transcriptomes to predict molecular pathways linking host and graft genes; as proof of concept, we predict host-secreted factors that signal to the graft and the downstream molecular cascades they trigger in the graft. We identify a potential host-graft crosstalk pathway where BMP6 from the stroke-injured brain induces graft secretion of noggin, a known brain repair factor. Decoding the molecular interplay between graft and host is a critical step toward deciphering the molecular mechanisms of stem cell action.
Graphical abstract
In brief
Stem cell therapy shows promise for neurological disorders; however, the molecular crosstalk between host and grafted cells is largely unknown. Azevedo-Pereira et al. apply biological and bioinformatic tools to simultaneously identify host and graft transcriptomes and their subsequent interactions; they highlight the significance of host microenvironment on the graft secretome.
INTRODUCTION
Stem cell transplantation is a promising therapeutic strategy moving toward the clinic for many diseases and injuries, including stroke.1,2 However, the molecular mechanisms of stem cell action remain largely unknown. A key step toward decoding the molecular crosstalk between graft and host is to identify the expression profiles of both the graft and its host environment, as these are fundamental to predict signaling cascades and downstream biological processes affected in both. A major challenge is to distinguish factors expressed by the transplanted cells from those of the surrounding host cells, particularly as the ratio of graft to host cells is very low. We overcome this hurdle by using translating ribosome affinity purification (TRAP) to enrich for graft mRNA.3 TRAP isolates cell-specific tagged ribosomes and their bound mRNA, thereby specifically enriching for translating RNAs and providing a gene expression profile closer to that of the protein expression profile. Additionally, rapid mRNA stabilization during extraction minimizes gene expression changes, providing an accurate readout of the in vivo graft transcriptome.
Here, we establish the use of TRAP, in combination with RNA sequencing (TRAP-seq) and bioinformatic tools, to separate and identify the transcriptomes of graft and host in our model system of human neural stem cells (hNSCs) grafted into the stroke-injured rat brain, which promotes stroke recovery.4,5 Using pathway tools, we investigate the interactions between the two transcriptomes to predict the molecular crosstalk between host and graft. This unbiased approach enables us to build a molecular picture of factors that potentially signal between host and graft, and link these signals with downstream molecular cascades. In doing so, we develop testable hypotheses regarding the molecular interactions between graft and host, a critical first step toward defining the molecular mechanisms of stem cell action. As proof of concept, we focus on paracrine signaling from the host to the grafted cells; through the interactions of host Bmp6 with graft-expressed genes, we demonstrate the validity of our approach to build testable hypotheses with which we can start to decode the molecular interplay between graft and host.
RESULTS
Combining TRAP-seq and bioinformatic tools to analyze graft and host transcriptomes
To separate graft and host transcriptomes, we first performed TRAP-seq on hNSCs transplanted in naive and stroke-injured rat brains to enrich for graft mRNA (Figures 1A and S1A). TRAP is based on the isolation of cell type-specific tagged ribosomes and their bound translating mRNA.3 We genetically engineered our hNSCs for TRAP using a lentiviral vector to ubiquitously express a GFP-tagged ribosomal protein RPL10a in hNSCs (Figure S1B). We detected GFP in 60% hNSCs by flow cytometry (Figure S1C); nestin staining confirmed maintenance of stemness in GFP+ neurospheres (Figures 1B and S1D). TRAP-compatible hNSCs retained efficacy; rats that received hNSCs exhibited significant behavior recovery in the whisker-paw reflex test at 7 days post-transplantation compared with vehicle group (Figure 1C).
Figure 1. TRAP-seq facilitates separation and identification of graft and host transcriptomes.
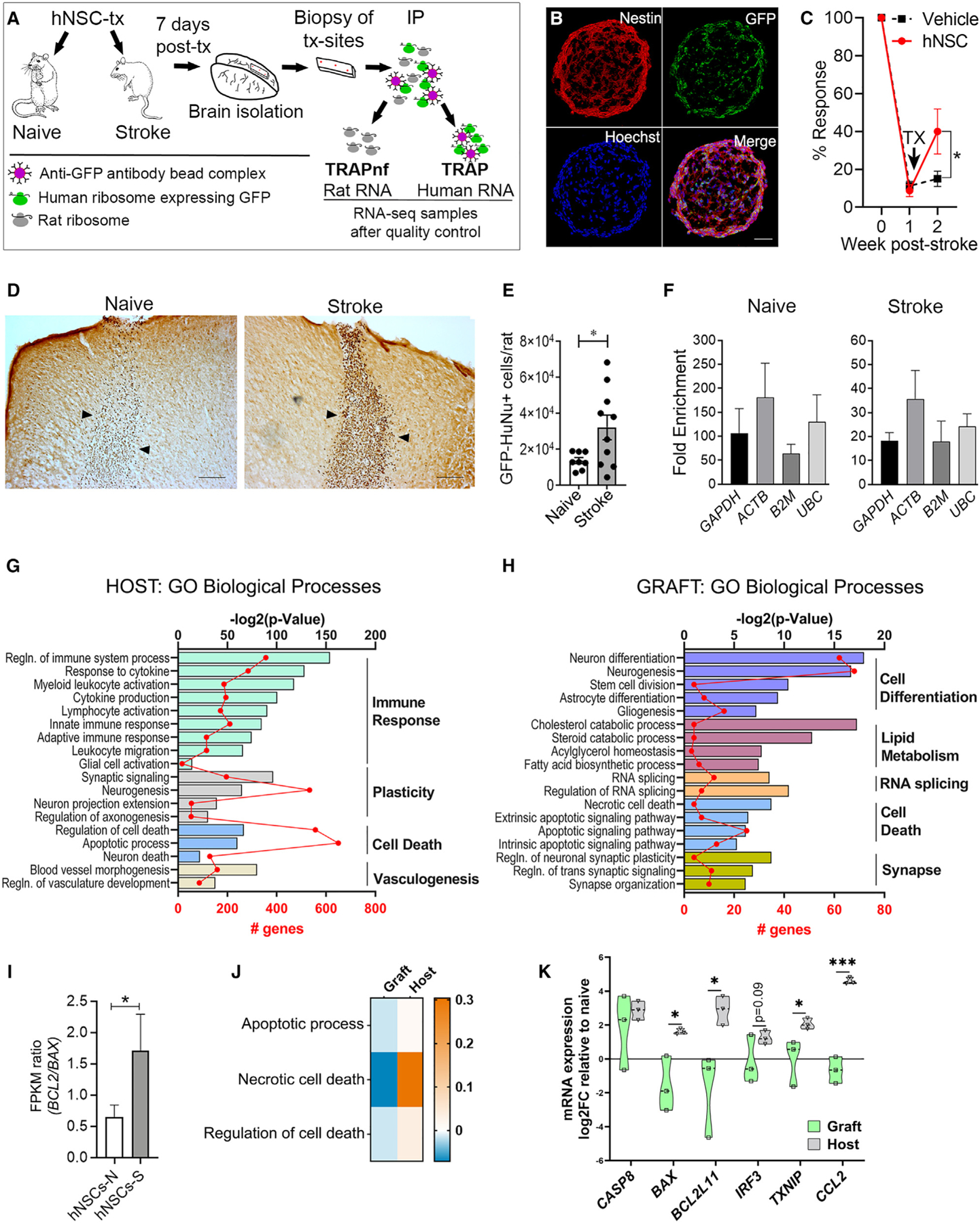
(A) TRAP-seq method overview: hNSCs were transplanted into brain of naive and stroke rats (7 days post-stroke) and biopsy of transplantation sites taken 7 days later. GFP-tagged ribosomes were immunoprecipitated (IP) using anti-GFP antibody. mRNA of graft (TRAP) and host (TRAP-negative fraction [TRAPnf]) were isolated for sequencing.
(B) hNSC neurospheres express nestin and GFP. Hoechst-stained nuclei (blue). Scale: 50 µm.
(C) Whisker-paw reflex behavioral test following stroke and treatment. Tx, time of treatment. *p < 0.05; two-way ANOVA-Sidak; n = 4/group.
(D) hNSC survival 7 days post-transplantation into naive and stroke-injured brains. hNSCs (dark brown) are stained for GFP plus human nuclear marker. Arrowheads indicate graft borders. Scale: 100 µm.
(E) Quantification of hNSCs in naive (n = 8) and stroke (n = 10) brains (*p < 0.05; unpaired Welch’s t test).
(F) Degree of graft RNA enrichment by TRAP (compared with using conventional RNA isolation; n = 4).
(G) Gene set enrichment analysis of host tissue DEGs.
(H) Gene set enrichment analysis of graft DEGs.
(I) FPKM ratio of BCL-2 and BAX expressed in hNSCs grafted in naive (hNSC-N) or stroke (hNSC-S) brains. n = 3; *p = 0.05; Mann-Whitney, one-tailed.
(J) Heatmap of gene ratio indicating whether GO term enrichment is driven by downregulated genes (blue), upregulated genes (orange), or both (white).
(K) qPCR validation showing differential regulation of pro-death genes in graft and host. Data shown as log2 fold change (FC) of stroke versus naive for host (gray) and for graft in stroke versus naive environment (green). Violin plot showing all points, n = 3; *p < 0.05, ***p < 0.001 by one-tailed t test of host versus graft.
All graph error bars: SEM.
See also Figures S1 and S2 and Table S1.
As TRAP requires living cells, we confirmed graft survival at 7 days post-transplantation in naive and stroke rat brains, with significantly greater hNSC survival in stroke than naive tissue (Figures 1D and 1E). We next performed TRAP on a separate cohort of rats using an anti-GFP antibody to pull down tagged ribosomes from the grafted hNSCs; both the immunoprecipitated fraction (i.e., the TRAP fraction), which is enriched for hNSC transcripts, and the flow-through fraction (i.e., the TRAP-negative fraction), which predominantly contains the host rat transcripts, were collected (Figure 1A). In the TRAP fraction, the amount of mRNA isolated was greater from hNSCs grafted in stroke versus naive brains (Table S1). To determine the enrichment of graft mRNA in the TRAP fraction, we compared housekeeping gene expression in TRAP and control non-TRAP samples using human-specific probes (Figure S1E). The TRAP method isolated human mRNA from grafted hNSCs in naive and stroke brains with a similar efficiency (Figure S1F), reaching average enrichments of 101- and 21.5-fold for graft in naive and stroke brain, respectively (Figure 1F). Rat housekeeping genes were detected in all TRAP samples, as expected6 (Figure S1G). Therefore, to further separate graft and host transcriptomes, we used RNA sequencing followed by RSEM (RNA-Seq by Expectation Maximization),7 an aligner tool successfully applied to define interspecies transcriptomes.8 The mapping accuracy of RSEM to a human-rat pooled reference transcriptome was tested using samples containing solely hNSC reads or rat brain reads; hNSC reads had a 0.7% ± 0.96% mis-assignment rate, and rat brain reads had a 3.2% ± 0.17% mis-assignment rate (mean ± SD), confirming that RSEM can differentiate between human and rat genes. Using this strategy, we found that the percentages of mapped reads mapping to the human transcriptome were 46% ± 14.9% and 24% ± 15.1% for grafts in naive and stroke brains, respectively (mean ± SD; Figure S1H). This difference indicates greater contamination from host RNA in the stroke TRAP samples. However, the extrapolated total number of human reads in the stroke TRAP samples was 3.4 times higher than from naive brains, consistent with greater graft survival in the stroke environment (mean total human reads ± SEM: stroke: 3 × 1012 ± 1.5 × 1012; naive: 9 × 1011 ± 5 × 1011).
Next, we assessed the gene expression profiles of grafted hNSCs and their respective host tissues. Analysis of the TRAP-negative, i.e., the host transcriptome, identified 5,396 genes differentially expressed between stroke and naive brain environments (Figure S2A). Principal-component analysis (PCA) of these differentially expressed genes (DEGs) showed distinct clusters between the two groups (Figure S2B). Gene set enrichment analysis revealed that these genes are enriched in biological processes associated with stroke,9 with Gene Ontology (GO) terms associated with the immune response being the dominant category; other biological processes were related to cell death, brain plasticity, and vasculogenesis (Figure 1G). Analysis of the TRAP fractions, i.e., the graft transcriptome, identified 11,008 graft genes, of which 460 genes were differentially expressed between hNSCs grafted in the stroke-injured versus naive brains (Figure S2A), with distinct clustering between the two groups observed by PCA (Figure S2B). These findings are consistent with previous reports showing that NSC gene expression is environment dependent.10–12 Gene set enrichment analysis of the graft DEGs identified five overarching biological processes: (1) cell differentiation, including neurogenesis, gliogenesis, and stem cell division; (2) cell death: intrinsic and extrinsic apoptosis and necrosis; (3) RNA splicing; (4) synaptic plasticity; and (5) lipid metabolism, including cholesterol and fatty acid metabolism (Figure 1H). Most notably, GO terms relating to the immune response—the dominant category identified in the host brains—are absent, implying successful separation of graft and host transcriptomes. Enrichment of cell death-related genes is consistent with our observation of greater graft survival in the stroke-injured versus naive brain (Figures 1D and 1E). This biological difference is further supported by an increased pro-survival BCL2:BAX ratio (Figure 1I) and significant decreased expression of cell death-related genes in hNSCs grafted in the stroke versus naive environment (Figures 1J and 1K). In contrast, host expression of cell death-related genes increased in stroke versus naive brains (Figure 1J). The reciprocal regulation of the most prominent pro-death DEGs by the host and graft was confirmed by qPCR analysis (Figure 1K), except for the procaspase-8 gene Casp8; caspase-8 may be regulated more at the post-translational level. Together, immune response GO terms identified in host but not graft, cell death-related genes regulated in opposite directions in graft and host, and a predicted increase in graft survival in the stroke environment, which we confirm, provide validation of successful separation of the graft and host transcriptomes. Thus, by combining TRAP-seq with RSEM, we can successfully distinguish the transcriptome of the grafted hNSCs from that of the host rat brain and subsequently identify graft biology affected by the host environment.
Predicting the molecular signals by which the host brain modulates the graft
For proof of concept that interaction analyses of graft and host transcriptomes can identify molecular signaling between the two entities, we investigated how the host microenvironment modulates the graft at the molecular level. We focused on the host secretome as paracrine signaling by secreted factors is a major mechanism of cellular communication. Of the host brain DEGs, 390 encoded for secreted proteins. The majority of these secretome DEGs encoded for extracellular matrix and other proteins (58.2%), followed by cytokines (10.5%), peptidases (9.0%), growth factors (8.7%), enzymes (8.2%), transporters (4.4%), phosphatases (0.5%), and kinases (0.5%; Figure 2A).
Figure 2. Graft and host transcriptome interactome analyses predict signaling from the host brain to the graft.
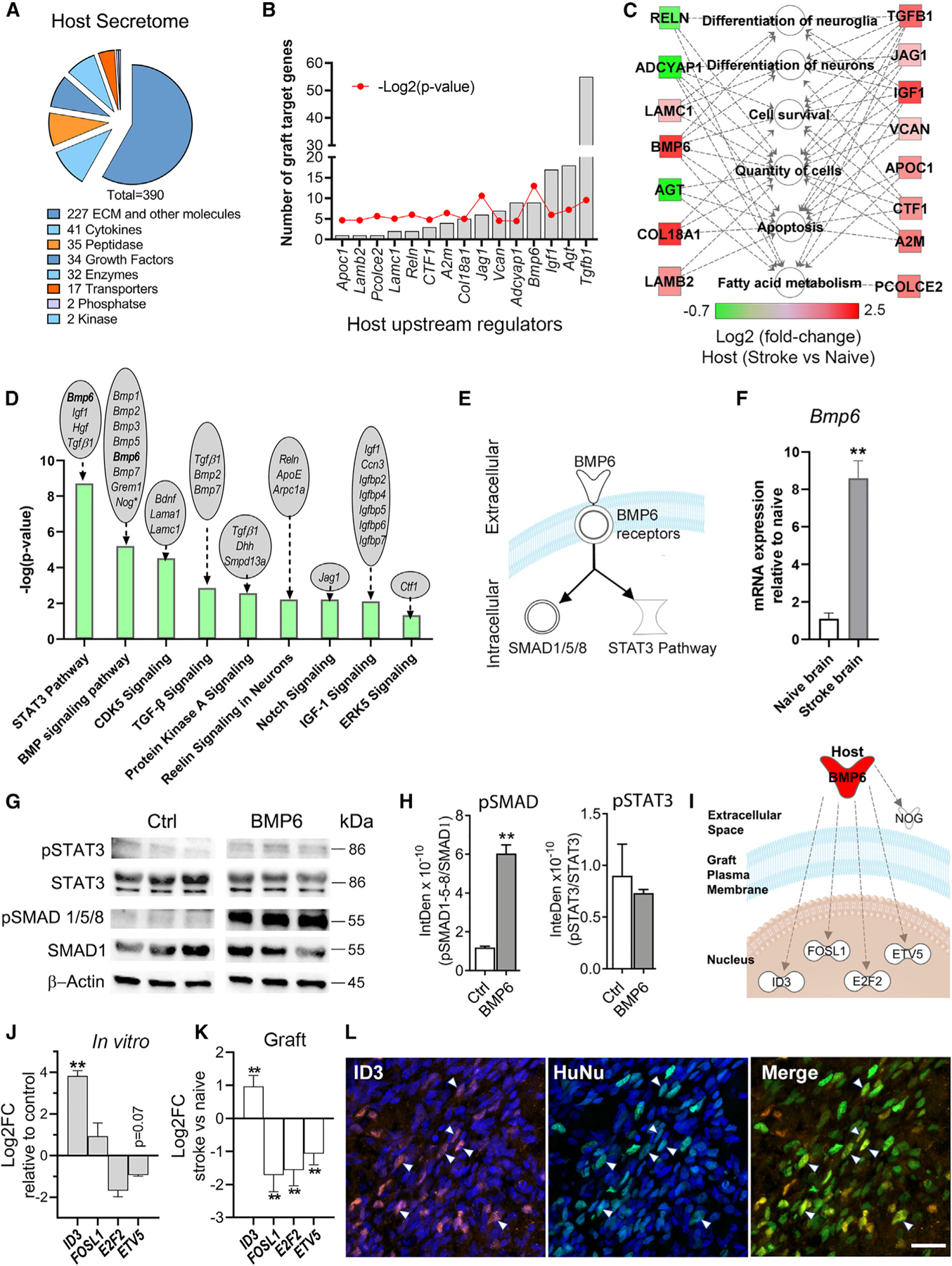
(A) Host secretome signature of differentially expressed secretome genes in naive versus stroke-injured brains.
(B) Host-expressed upstream regulators (URs) of grafted hNSC DEGs.
(C) Molecular functions of host URs identified in (B).
(D) Graft canonical pathways predicted to be affected by host environment. Host brain DEGs that signal to these pathways indicated above each bar.
(E) BMP6 signaling pathways schematic.
(F) qPCR validation of increased host Bmp6 expression in stroke versus naive brains. n = 3; **p < 0.01; unpaired t test.
(G) Immunoblotting for pSTAT3 and pSMAD1/5/8 in BMP6-treated hNSCs. SMAD1, STAT3, and β-actin used as internal controls. Membranes were digitally cut at the molecular weight of each protein. Space between control (Ctrl) and BMP6 images indicates lanes removed relating to BDNF-treated hNSCs (data not shown).
(H) Intensity density (InteDen) quantification of western blots for pSMAD 1/5/8 and pSTAT3. n = 3; **p < 0.01; Welch’s t test.
(I) Schematic of graft DEGs (white symbols) predicted to be downstream of host-secreted BMP6 (red).
(J) qPCR analysis showing BMP6 (50 ng/mL) treatment affects expression of its predicted downstream genes in cultured hNSCs. n = 3; **p < 0.01 compared with non-stimulated (control); multiple t tests with Holm-Sidak multiple comparisons correction.
(K) Graph showing log2FC of predicted downstream genes of BMP6, in hNSCs grafted in stroke versus naive brains, as determined by DESeq2 analysis of RNA-seq data. n = 3, **p < 0.01.
(L) Confocal projection image showing graft cells (green) at the stroke lesion border express ID3 (red), a downstream target of pSMAD signaling. Arrowheads indicate some ID3+ graft cells. HuNu, human nuclei marker. Blue: Hoechst nuclei stain. Scale: 20 µm.
All graph error bars: SEM.
See also Figures S3 and S4 and Table S2.
To predict which host-secreted factors signal to the grafted hNSCs and to identify the downstream graft genes that might be regulated by them, we used IPA upstream regulator (UR) analysis13 to identify differentially expressed host secretome genes that are URs of graft DEGs. We identified 15 such host brain genes (Figure 2B), which associated with 89 target graft DEGs. Thirteen of these graft genes, including NOG, BAX, and seven transcription factors, were targets of three or more host URs (Figure S3), suggesting these could be key graft genes involved in converting host signals into changes in graft biology. The molecular functions of the host URs were enriched for terms relating to cell differentiation (e.g., differentiation of neurons and neuroglia), cell survival, and fatty acid metabolism (Figure 2C). Notably, these terms align with the key biological processes affected in the graft, as determined by the graft transcriptome analysis (Figure 1H), providing confidence in this analysis approach and highlighting how we can begin to link changes in graft biology with host molecular signals that could drive these biological changes. The hNSC graft expresses receptors for 11 of the identified host URs (Table S2), confirming the potential of these host factors to directly signal to the graft. Furthermore, canonical pathway analysis of graft DEGs identified signaling pathways in the graft activated by several host URs, notably Bmp6, Igf1, Jag, Reln, and TGFβ1 (Figure 2D), further strengthening the hypothesis that these host-secreted factors signal to the graft.
To validate our analysis approach, we investigated the effects of BMP6 on hNSCs. BMP6 is a host brain factor predicted to be a UR of two graft signaling pathways identified by our canonical pathway analysis (Figures 2D and 2E), namely ‘‘BMP signaling,’’ which is predicted to be the most significantly activated pathway identified (Z score: 2.14), and ‘‘STAT3 signaling,’’ which has a low activation score (Z score: 0.65), implying a lower probability of activation. Bmp6 encodes bone morphogenetic protein 6, and the grafted hNSC expresses five BMP receptor genes (Table S2). qPCR analyses confirmed increased Bmp6 expression in the stroke (versus naive) brain both in the presence (Figure 2F) and absence of grafted hNSCs (relative quantitation ± SEM: naïve 1.2 ± 0.3; stroke 22 ± 8.1; p < 0.05, n = 6), indicating that Bmp6 elevation is a brain injury response as previously reported.14 The activity of these two signaling pathways in cultured hNSCs treated with recombinant BMP6 was determined by immunoblotting analysis of SMAD1/5/8 phosphorylation, the downstream signal transducer of the BMP pathway,15 and STAT3 phosphorylation (Figures 2E, 2G, and 2H). BMP6 increased phosphorylation of SMAD1/5/8, but not STAT3 (Figures 2G and 2H), indicating activation of the canonical BMP signaling pathway, but not the STAT3 pathway, consistent with our analysis predictions. To further validate our analysis approach, we tested whether BMP6 acts as a UR to other graft genes as predicted (Figure 2I). BMP6 stimulation of cultured hNSCs significantly increased expression of ID3 and showed a trend for decreased expression of E2F2 and ETV5 (Figure 2J). This mirrored the in vivo gene expression pattern observed for hNSCs grafted in stroke versus naive brains (Figure 2K), supporting the idea that BMP6 modulates these graft genes in vivo. FOSL1 gene expression changes were opposite in vitro versus in vivo (Figures 2J and 2K), suggesting that other host genes modulate graft FOSL1 expression in the stroke brain. We also confirmed that BMP6 significantly increased graft expression of NOG, at both the RNA and protein level, as predicted (Figures 4); this is discussed in more detail later.
Confirmation of increased graft SMAD signaling in vivo was achieved by staining for pSMAD and the transcription factor ID3, a known downstream target of BMP6 and pSMAD16 identified by our RNA-seq data (Figures 2I and 2J). Both pSMAD+ and ID3+ graft cells (Figures 2L and S4A–S4E) were more prominent in the stroke environment than in the naive brain. As the pSMAD signal was relatively weak, potentially due to the inherent difficulties in detecting phospho-proteins, we quantified ID3+ graft cells and found they were almost 7-fold more abundant in the stroke versus naive brain (% ID3+ graft cells: stroke 8.8% ± 3.2%; naive 1.4% ± 0.6%; mean ± SEM; n = 3; Figure S4E). These data agree with our analyses-based prediction of increased BMP6/pSMAD signaling in the stroke graft. Notably, graft (and host) cells were only pSMAD+ or ID3+ in areas of injury (i.e., stroke lesion and border; top of the needle track), identifying a very environment-specific effect; most graft ID3+ cells in the stroke brain were at the lesion border (81% ± 8.7%: mean ± SEM; n = 3). O’Shea et al.12 also observed this and suggested that ID3+ stroke border cells are wound repair astrocytes. Graft cells were more responsive to the stroke environment than host cells, with 13.8% ± 3% of graft cells in the lesion border expressing ID3 versus 6.8% ± 2% of host cells (mean ± SEM; n = 3). Host BMP6 levels were also highest at the lesion border, expressed by astrocytes but not microglia (Figures S4F–S4H), consistent with our hypothesis that host BMP6, upregulated by injury, increases graft pSMAD signaling.
In summary, by analyzing the interaction between the host secretome and graft transcriptome, we start to elucidate the molecular communication from host to graft (e.g., ligand-receptor interactions) and the downstream genes and signaling pathways altered in the graft by these host URs. In this way, we can begin to predict the response of the graft to its environment.
Host microenvironment modulates graft secretome: Host and graft crosstalk mediated by BMP6-NOG interaction
As the graft is expected to exert its therapeutic effects via paracrine factors,1,5,17 the effect of the host on the graft secretome is of interest, as the host environment could modulate graft efficacy. Of the 371 secretome genes expressed by hNSCs in the stroke-injured brain, 13 were significantly upregulated by hNSCs in the stroke versus naive brain, indicating a conditional response of these graft secretome genes to their environment (Figure 3A). Consistent with the potential for these graft factors to influence graft efficacy, the known functions of these graft secretome genes are enriched for biological processes associated with stroke recovery, including plasticity (e.g., regeneration of axons and plasticity of synapses), and effects on the vasculature, blood brain barrier, and immune response4,5,18,19 (Figure 3B). Notably, expression levels of two of these genes, NOG and MATN2, positively correlated with the extent of recovery at 1 week post-transplantation (Figure 3C), suggesting that these factors could be important determinants of graft efficacy. Indeed, NOG and MATN2 have been associated with enhanced stroke recovery20,21; only a high dose of noggin exhibited biological activity,20 consistent with our observed correlation between NOG levels and functional recovery.
Figure 3. Graft secretome is modulated by the host environment.
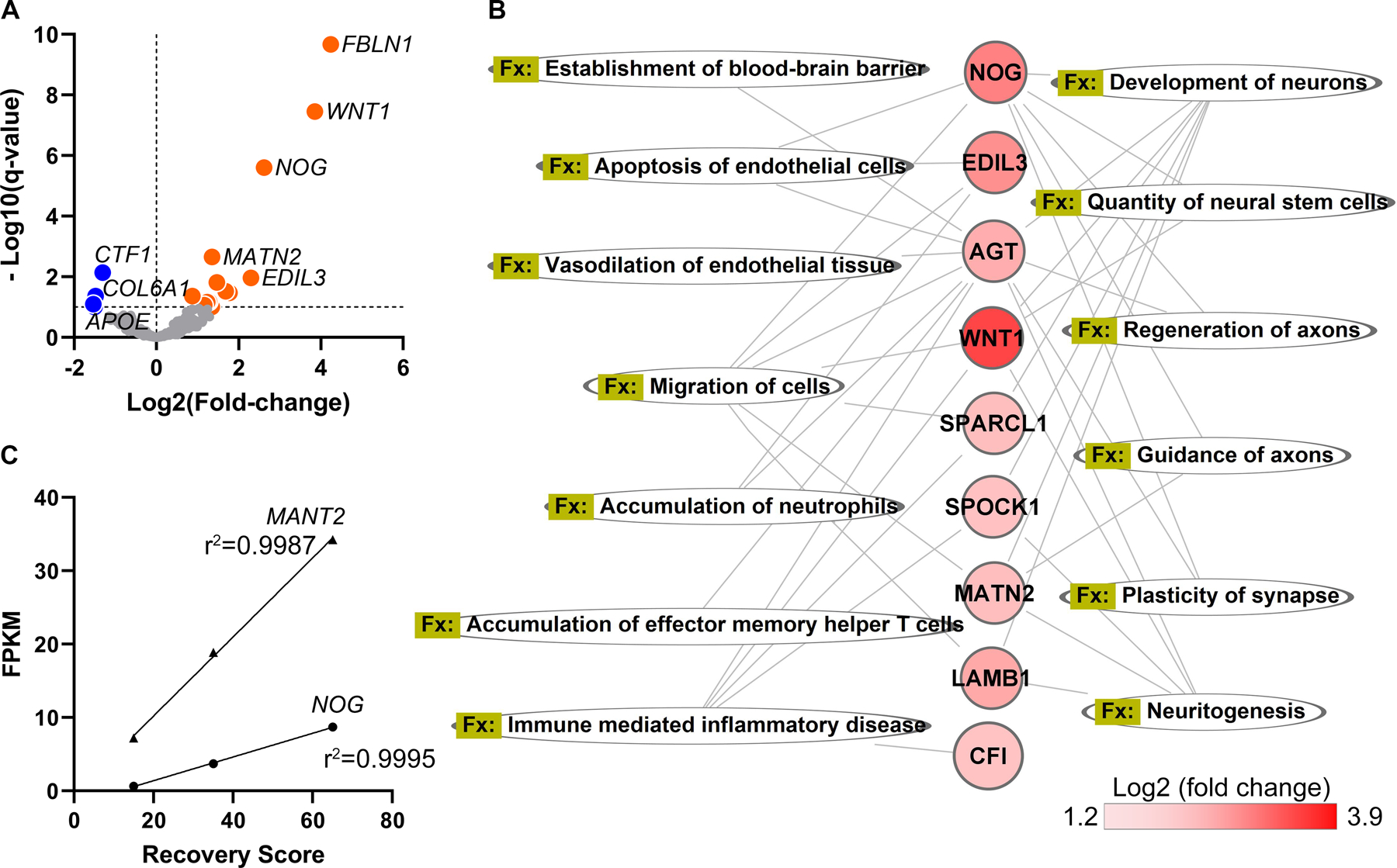
(A) Volcano plot of graft secretome genes. Orange: graft genes upregulated by the stroke environment; blue: downregulated genes.
(B) Molecular functions of graft secretome genes upregulated by the stroke environment.
(C) Linear regression showing positive correlation between graft expression levels of NOG and MATN2 with the extent of graft-induced behavior recovery. p < 0.05, q = 0.15 by the Benjamini-Hochberg procedure.
To further validate our crosstalk analyses, we focused on NOG, the most highly upregulated graft secretome gene in the stroke brain environment (versus naive) that positively correlates with behavior recovery. NOG encodes noggin, an antagonist of transforming growth factor-β (TGF-β) superfamily members,22 and is associated with brain plasticity, neurogenesis, and inflammation.20,23,24 We confirmed graft expression of NOG by qPCR (Figure 4A) and by in situ hybridization (ISH) (Figure 4B) using human-specific probes. UR analysis, to determine host paracrine factors that might modulate graft expression of NOG, identified three host-expressed genes (Bmp6, Igf1, and Tgfb1) associated with NOG (Figures 4C and S3) and upregulated in stroke tissue (Figure 4D). BMP6 is predicted to activate NOG, IGF1 to inhibit NOG, and TGF-β−1 to mirror NOG expression changes in response to various stimuli. To test if BMP6 increases NOG expression, we added recombinant BMP6 (rBMP6) to cultured hNSCs and used recombinant TGF-β−1 as a UR control. As predicted, only BMP6 significantly upregulated gene expression of NOG (Figure 4E). Furthermore, BMP6-induced noggin protein expression by hNSCs was confirmed by ELISA (Figure 4F). Notably, our RNA-seq data suggested a positive correlation between the expression of host Bmp6 and graft NOG (Figure 4G), which we confirmed in vitro by showing that rBMP6 increased hNSC NOG expression in a dose-dependent manner (Figure 4H).
Figure 4. Host and graft crosstalk mediated by BMP-NOG interplay.

(A) qPCR of TRAP samples confirming increased expression of NOG by hNSCs grafted into stroke (hNSC-S; n = 4) versus naive brain (hNSC-N; n = 3). ***p < 0.001; Welch’s t test.
(B) Immunohistochemistry (IHC) and ISH of hNSCs in stroke brain expressing mCherry protein (mCh; red) and NOG mRNA (green). Hoechst-stained nuclei (blue); scale: 50 µm. White box in first panel indicates region magnified in subsequent panels.
(C) URs of NOG predicted by IPA and STRING. Colored edges represent action type: activation (green); inhibition (red); transcriptional regulation (yellow); comentioned in previous reports (gray); unspecified reaction (black).
(D) Expression levels, by RNA-seq analyses, of host secretome genes encoding URs of NOG. n = 3–4, ****p < 0.0001 by DESeq2 analysis of stroke versus naive tissue.
(E) qPCR of NOG in cultured hNSCs after stimulation with recombinant BMP6 or TGFb1. n = 3; ****p < 0.001 compared with non-stimulated (Ctrl); one way ANOVA with Tukey post-hoc.
(F) ELISA quantification of tissue culture conditioned media showing Bmp6 treatment significantly increased noggin expression by hNSCs in vitro (n = 3; *p < 0.05; unpaired t test).
(G) Pearson correlation between Bmp6 expression in stroke tissue and corresponding graft NOG expression based on RNA-seq data.
(H) qPCR analysis showing BMP6 induces NOG expression in cultured hNSCs in a dose-dependent manner. n = 3; ****p < 0.0001; one way ANOVA with Tukey post-hoc.
(I) Schematic of potential graft and host crosstalk mediated by the BMP6 pathway.
All graph error bars: SEM.
These data imply that the host microenvironment modulates the graft secretome and thereby modulates graft efficacy. Taken together with our data showing that BMP6 activates the hNSC BMP canonical signaling pathway (Figures 2G–2L), we predict the following molecular crosstalk between the host stroke brain and grafted hNSCs (Figure 4J): BMP6 secreted by the stroke-injured brain activates the BMP signaling pathway in the graft, increasing graft expression of NOG in a dose-dependent manner. In turn, graft-secreted noggin acts on the host brain, altering various biological processes (as indicated in Figure 3B) that induce stroke recovery, in a dose-dependent manner. Thus, by looking at the interaction between graft and host transcriptomes, we can build hypotheses to start to decode the molecular crosstalk between graft and host.
DISCUSSION
Here, we present an approach, applicable to many stem cell transplantation paradigms, to predict the molecular crosstalk between grafted stem cells and their host tissue. We used TRAP-seq with RSEM analysis to simultaneously decode the transcriptomes of the graft and its host tissue, followed by UR and canonical pathway analyses to predict the molecular cascades linking graft and host genes. We applied this to our model system of hNSCs transplanted into the stroke-injured brain and identified a potential host-graft crosstalk pathway whereby BMP6 from the stroke-injured brain induces canonical BMP signaling in the graft and hNSC secretion of noggin, a factor known to promote stroke brain recovery.20
Having both host and graft transcriptomes offers, for the first time, an unbiased way to decode the molecular interactions between host and graft. It enables us to build a picture of the molecular cascade of events that occur when host and graft interact, from URs that signal between the host and graft to downstream signaling pathways and biological processes these URs might affect. In essence, we can generate data-driven hypotheses with which to interrogate the complex molecular interactions between graft and host. In our proof-of-concept study, we focused on communication from the host to the graft, but the same approach can be used to investigate communication from the graft to the host to predict signals from the grafted stem cells, and the host molecular cascades they activate, that drive recovery. This is a critical step toward defining the molecular mechanisms of stem cell action and host tissue recovery.
The important concept of the host environment modulating the graft is just starting to be addressed.12,25 Here, we demonstrate that the environment greatly affects the host-graft molecular crosstalk. The graft received different molecular signals and responded differently (e.g., better graft survival, different graft signaling pathways activated) in the stroke versus naive brain environment. Notably, we observed stroke-dependent effects on graft secretome gene expression (e.g., NOG). This is significant because the graft can exert therapeutic effects via paracrine signaling,1,5,17 irrespective of whether the cells are in a progenitor or differentiated state, implying that the host environment can affect graft efficacy by modulating the graft secretome. Within the stroke brain, there are different microenvironments depending on proximity to the lesion, suggesting that different regions of the graft undergo different molecular interactions with the host.12 Indeed, we observed that graft (and host) cells very close to the lesion responded with upregulated expression of the transcription factor ID3, while those further away did not; this was also observed by O’Shea et al.12 This implies that different regions of the graft, in different microenvironments, have unique molecular interactions with the host and elicit distinct effects on the brain. Notably, not all graft cells close to the lesion exhibited increased ID3 expression, potentially due to different microenvironments around the lesion or due to graft heterogeneity, with only certain cell types responding in this manner.12 Taken together, the location and timing of cell transplantation and the types of cells being transplanted will all affect the host-graft molecular crosstalk and, ultimately, graft efficacy. Moreover, since the host environment and the graft mature over time,12,26 the crosstalk between them is likely to change too, with the graft exerting different mechanisms of action in a temporal fashion.5
Deciphering the molecular crosstalk between graft and host has significant therapeutic implications. Identifying host factors that modulate graft efficacy can help identify an optimal time window for cell transplantation and provide potential biomarkers for selecting patients that would be good responders to stem cell therapy. For example, in our model system, host BMP6 is a potential biomarker as it modulates graft expression of noggin, a secreted protein positively linked to stroke recovery.20 Patients expressing BMP6 may respond better to cell therapy than those without because graft-secreted noggin, and thus graft efficacy, would be enhanced in these patients. Furthermore, decoding the molecular mechanism of stem cell action can lead to better therapeutic strategies: key graft-secreted factors identified to promote recovery can be used therapeutically instead of stem cells,27 and identifying key host molecular pathways triggered by the stem cells can offer innovative drug targets for stroke recovery.
Limitations of the study
This study describes an unbiased approach to identify the molecular crosstalk between graft and host, a critical first step toward decoding the molecular mechanisms of grafted stem cell action. However, by using ribosome-bound RNA, this method does not address changes in microRNA or post-translational protein modifications, which also affect biological processes. Our bulk RNA-seq approach gives a broad picture of potential graft-host molecular interactions, but spatial and single-cell transcriptomics are needed to decipher the finer details of neighborhood- and cell type-specific interactions. While our study successfully predicts molecular pathways linking graft and host genes, future investigations require blocking or knockdown studies to identify which interactions are important for graft efficacy.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Gary K. Steinberg (gsteinberg@stanford.edu).
Materials availability
Vectors generated in this study are available upon request.
Data and code availability
The RNAseq data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus33 and are accessible through GEO Series accession number GSE150710: (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE150710). Other primary data, such as Fastqc files, raw PCR data, western blot data, or microscopy images, are available from the authors upon reasonable request.
This paper does not report original code.
Any additional information required, related to this paper, is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals, stroke model, and cell transplantation
Distal Middle Cerebral Artery Occlusion (dMCAO) stroke model and cell transplantation surgeries were approved by Stanford University’s Administrative Panel on Laboratory Animal Care. T cell-deficient adult (10–12wk old) male nude rats (NCI at Frederick; Cr:NIH-RNU 250–350 g) were subjected to permanent dMCAO with 0.5 hour bilateral CCA occlusion as described34 under isoflurane anesthesia. Ampicillin was administered in the drinking water (1 mg/ml) starting 3 days before stroke surgery to 7 days post-transplantation surgery.
For cell transplantation, hNSC neurospheres (detailed in the section below) were dissociated to single cells. Briefly, neurospheres were washed with PBS pH 7.4 (Invitrogen, Carlsbad, CA) and incubated with TrypLE (Invitrogen) for 30 min at 37°C, washed 1x with NSC medium, and dissociated into single cells with a fire-polished Pasteur pipette. Cells were counted, centrifuged at 300g for 5 min and resuspended in appropriate volume. The cells were resuspended in Leibovitz 15 Media (Gibco, Thermo Fisher Scientific, Waltham, MA) supplemented with 0.3% glucose (Sigma-Aldrich, St. Louis, MO) and B27 without vitamin A (Gibco) at 1 × 105 cells/ml. Three sites of cell deposits (1 × 105 cells per deposit) or vehicle were injected into the ipsilesional cortex at 7 days post-dMCAo as described in34: (a) anterior–posterior (A-P) +1.0 mm; medial–lateral (M–L), +1.2 mm; dorsal–ventral (D–V), −1.4 mm; (b) A–P, −0.3 mm; M–L, +1.2 mm; D–V, −1.4 mm; (c) A–P, −1.8 mm; M–L, +1.2 mm; D–V, −1.4 mm from the Bregma. The cell solution and vehicle were kept on ice during the injection procedure. All animals used for RNAseq were transplanted within 4 days of each other using the same cell passage.
Human stem cell culture, dissociation, and in vitro assay
Human fetal NSC (G010 clone)35 were a kind gift from Dr. Clive Svendsen, Cedars-Sinai, USA. The cells were cultured at 37°C and 5% CO2 as neurospheres in NSC medium consisting of StemlineTM Neural Stem Cell Expansion Medium (Sigma) supplemented with 100 ng/ml epidermal growth factor (Gibco) and 10 ng/ml Leukaemia inhibitory factor (Gibco) as previously described.35,36
Neurospheres were dissociated into single cells for lentivirus infection, transplantation, and recombinant protein screening. Neurospheres were washed with PBS pH 7.4 (Invitrogen) and incubated with TrypLE (Invitrogen) for 30 min at 37°C, washed 1x with NSC medium, and dissociated into single cells with a fire-polished Pasteur pipette. Cells were counted, centrifuged at 300g for 5 min and resuspended in appropriate volume.
To test the effects of recombinant proteins on the hNSCs, 1.0 × 105 cells/mL were seeded on tissue culture plates previously coated with Matrigel (BD, Franklin Lakes, NJ), and cells were maintained in Neurobasal medium supplemented with L-glutamine, B27 and N2 (all reagents from Gibco) for 7 days with different recombinant proteins: BMP6 (1.0 – 50 ng/mL; 10 ng/ml used in Figure 4E; Peprotech, Rocky Hill, NJ), TGF-beta 1 (10 ng/mL; R&D Systems, Inc., Minneapolis, MN). Medium was changed every other day for a total of 7 days.
METHOD DETAILS
Behavioral test
The animals were trained for three days to perform ten trials of the vibrissae-evoked forelimb placing test for each forelimb as described.37 In brief, animals were tested for their reflex response to extend a forepaw in response to vibrissae being brushed against a table edge. Ten trials were performed per testing day, and the number of trials in which a vibrissae-evoked forelimb response was observed was recorded. Only animals that reached 100% response during training were included in the study. The animals were tested one day before dMCAO (baseline), 7 days post-dMCAO, and 14 days post-dMCAO, by testers blinded to the treatment groups. Animals at day 7 post-dMCAo with a response of greater than 30% of baseline were excluded for insufficient stroke deficit. Following the day 7 post-dMCAo testing, animals were divided into two groups with similar mean deficit and standard deviation, prior to treatment.
Vector information and lentivirus infection
All lentiviral particles were produced by Stanford Neuroscience Gene Vector and Virus Core and handled in class II biosafety laboratory. To construct the TRAP lentiviral vector, a plasmid containing the eGFP-L10a transgene (C2-eGFP-L10a) was received as a kind gift from Dr. Nathaniel Heintz, from the Rockefeller University, USA, and taken through a series of cloning steps. First, the sequence for the Mus musculus ribosomal protein L10a was replaced with an in-frame construct for human L10a (hL10a, hNCBI Ref Seq NM_007104.4, nucleotides 38-688). The eGFP-hL10a transgene was then inserted into a third-generation lentiviral vector FUGW, which was received as a gift from Dr. Craig Garner (Stanford University, USA) and contained the human ubiquitin promotor for constitutive transgene expression. A map of the resulting TRAP lentiviral vector (FUG-hL10a-W) is provided in the Figure S1B.
For lentiviral transduction, hNSC were dissociated to single cells as described above and 2 × 106 cells were transduced for 48 hours in 35mm non-adherent plate containing NSC medium supplemented with B27 minus vitamin A (Gibco). A multiplicity of infection (MOI) of 4 virus units/cell was applied for all lentivirus transduction procedures. After transduction hNSC were grown as neurospheres as described above.
Red Fluorescent Protein (RFP) was cloned under cytomegalovirus promoter into pLEX-MCS vector (Thermo Scientific Open Biosystem #OHS4735) and LV-GFP plasmid contained histone–fluorescent protein gene fusions (Hist2h2be-eGfp) was acquired from Addgene (plasmid #25999; Addgene, Watertown, MA).28
Flow cytometry
Neurospheres of hNSC expressing GFP under ribosomal proteins were dissociated into single cells as described above (cell transplantation). Cells were filtered through a 70 µm strainer (BD Falcon) in PBS. For negative-selection, cells were pre-stained with LIVE/DEAD Fixable Aqua Dead Cell Stain kit (Thermo Fisher Scientific) for 30 min in PBS at room temperature. Analysis was done using an BD FACSCanto II (BD Biosciences) and analyzed using FlowJo™ software (Tree Star; Becton, Dickinson, and Company Ashland, OR).
Brain tissue dissection
Animals were euthanized at 7d post-transplantation (equivalent to 14d post-stroke) according to Stanford University’s Administrative Panel on Laboratory Animal Care protocol. All the surgical instruments and labware glass material were previously sterilized. After euthanizing, the hair on the scalp was trimmed followed by decapitation and scalp remove to expose the scull. The scull and meninges were removed, and the brain was rinsed in cold sterile and RNase-free PBS solution. The brain was moved to a glass tissue culture plate with sterile and RNase-free PBS solution. Blood vessels from the top of the brain were removed and four dorso-ventral incisions in a rectangle around the transplantation sites were made, including one at the midline of the brain. The two hemispheres were separated by sagittal incision at the midline exposing the corpus callosum. A medial-lateral planar incision above the corpus callosum was made separating the cortical tissue and dislocating the cortex with the three transplantation sites from the rest of the brain. The cortical biopsy containing the three transplantation sites was moved to a 2 mL dounce tissue grinder in 1.5 mL lysis buffer and processed as described below in the TRAP Method. The cortical biopsies taken for qPCR analysis of BMP6 expression in the absence of transplantation (‘no transplantation, no TRAP’) were first put in RNAlater™ Stabilization Solution (Thermo Fisher Scientific) overnight at 4°C, and then stored at −80°C until RNA extraction (described below).
Translating ribosome affinity purification: TRAP method
TRAP was performed as described previously,3,38 with slight modifications. Briefly, 500 uL magnetic Dynabeads Protein G (Invitrogen) were washed 3x with Low-salt buffer (150 mM KCl, 20 mM HEPES KOH pH7.4, 10 mM MgCl2, 1% NP-40, 0.5 mM DTT and 100 ug/mL cycloheximide in RNase-free water) using a DynaMag-2 magnet (Invitrogen). Beads were incubated with anti-GFP monoclonal antibodies (18C8 and 19F7; Memorial Sloan-Kettering Monoclonal Antibody Facility) for 2 hours at room temperature in Low-salt buffer. Next, immunocomplex bead-anti-GFP antibodies were washed 3x with Low-salt buffer and stored on ice. Biopsy of brain cortex (~20 mm3) containing the three transplantation sites was excised into cold PBS pH7.4 (Invitrogen). The biopsy was dissociated with a 2 mL dounce tissue grinder in 1.5 mL Lysis buffer (150 mM KCl, 20 mM HEPES KOH pH7.4, 10 mM MgCl2, 0.5 mM DTT and 100 ug/mL cycloheximide, EDTA-free protease inhibitors cocktail, 10 uL/mL RNasin in RNase-free water) followed by centrifugation at 2,000g for 10 minutes at 4°C. The post-nuclear supernatant (S2) was transferred and incubated with DHPC (150 mM) and NP-40 (150 mM) at 4°C for 5 min. Next, S2 was centrifuged at 16,000g for 10 min at 4°C and the post-mitochondrial supernatant (S20) was removed and incubated overnight at 4°C with immunocomplex bead-anti-GFP antibodies with end-over-end mixing. After incubation, the S20 fraction containing the immunocomplex bead-anti-GFP-polyribosomes was placed on DynaMag-2 magnet and non-bound product (TRAP negative fraction; TRAPnf) was removed and stored at −80°C for RNA extraction as described below. Immunocomplex bead-anti-GFP-polyribosomes were gently washed 6x with High-salt buffer (350 mM KCl, 20 mM HEPES KOH pH7.4, 10 mM MgCl2, 1% NP-40, 0.5 mM DTT and 100 ug/mL cycloheximide in RNase-free water) on ice. To elute RNA, the immunocomplex was incubated with RLT buffer (Qiagen) for 5 min at room temperature and RLT buffer containing the released RNA was removed carefully from the beads for immediate RNA extraction as described below.
RNA extraction
TRAP-enriched RNA samples were immediately extracted with the RNeasy Micro Kit (Qiagen, Hilden, Germany) per the manufacturer’s protocol. TRAPnf fractions were lyophilized using the freezer dryer system (Labconco, Kansas City, MO) and resuspended in 300 uL RLT buffer. RNA purification was performed with the RNeasy Mini Kit (Qiagen) per the manufacturer’s protocol. The quality of TRAP-enriched and TRAPnf RNAs was assessed by the Stanford Protein and Nucleic Acid Facility using a Bioanalzyer system and the Pico and Nano chips (Agilent Technologies, Santa Clara, CA). Samples with an RNA Integrity Number lower than 7.8 were excluded.
For RNA extraction from in vitro samples, medium was removed and RLT buffer was immediately added for RNA extraction with the RNeasy Micro kit. For RNA extraction for the ‘no transplantation, no TRAP’ BMP6 qPCR experiment, RNAlater solution was removed from the cortical biopsies, RLT buffer added, the tissue homogenized, and RNA extracted using the RNeasy Plus Mini kit. RNA concentration and quality were assessed with Infinite M200 Pro-reader (TECAN, Mä nnedorf, Switzerland) and samples with a 260/280 ratio less than 1.8 were excluded.
cDNA synthesis, library construction, and RNA sequencing
RNA from TRAP samples and TRAPnf samples were converted to cDNA using the Ovation RNA-seq v2 (NuGEN Technologies, Inc., Redwood City, CA) and cDNA libraries were prepared by Ovation Ultralow System V2 (NuGEN). Briefly, ribosomal RNA was depleted from 2 ng of total RNA using the RiboMinus Eukaryote Kit RNA-Seq (Invitrogen). Ribosomal RNA-depleted samples were lyophilized (Freeze Dry System, Labconco) and resuspended in RNase-free water for cDNA synthesis. cDNA was cleaned up using the MiniElute Reaction Cleanup Kit (Qiagen) and 500 ng of cDNA were fragmented by sonication into a 200 bp peak (Covaris, Inc., Woburn, MA). A total of 500 ng of fragmented cDNA was used for library preparation. The quality of fragmented cDNA and cDNA libraries were assessed by the Stanford Protein and Nucleic Acid Facility using a Bioanalyzer system with the High-sensitivity DNA chip (Agilent). RNA sequencing was performed on an Illumina HiSeq 2500 system (Elim Biopharmaceuticals, Inc., Hayward, CA), with TRAP samples from engrafted tissue run as single lane 125 bp paired-end reads, and TRAPnf and TRAP from in vitro samples as multiplexed (4 samples per lane), 50 bp paired-end reads.
For quantitative PCR 300 ng of RNA (500 ng for the ‘No transplantation, No TRAP’ BMP6 experiment) was converted to cDNA using the High Capacity RNA-to-cDNA kit (Thermo Fisher Scientific) according to the manufacturer.
TRAP: Enrichment and efficiency analysis
For TRAP efficiency and enrichment evaluations we compared TRAP samples with non-TRAP control samples which were samples where RNA was extracted from the transplantation site biopsy by conventional extraction methods. To obtain the non-TRAP samples, brains from naïve (n = 4) and stroke rats (7-days post-ischemia; n = 4) that received hNSC transplantation (as described above), were isolated 7 days post-transplantation. The transplantation site biopsy was removed, and RNA purification was performed with the RNeasy Mini Kit (Qiagen) per the manufacturer’s protocol. Next, we performed qPCR on both TRAP and non-TRAP control samples applying the same amount of input RNA for all samples and using human-specific primers for four housekeeping genes (GAPDH, ACTB, B2M and UBC). TRAP efficiency was assessed by the average 2dCT of the two TRAP samples (grafted hNSC in näve vs stroke) and compared with the two non-TRAP samples (biopsy of grafted hNSC in näıve vs stroke)]. Enrichment was assessed by comparing the average of log-fold change for each housekeeping gene between TRAP versus non-TRAP samples; see equation below.
Equations
Efficiency:
Enrichment:
(TN): Ct TRAP of graft hNSC in naïve; (TS): Ct TRAP in graft hNSC in stroke; (CN): Ct control non-TRAP of graft hNSC in naïve; (CS) Ct control non-TRAP of graft hNSC in stroke.
RSEM and bioinformatics analysis
TRAP samples obtained from hNSC-engrafted tissue generated an average of 230 million reads; rat host tissue samples (TRAPnf) generated an average of 30 million reads; TRAP samples from in vitro hNSC generated an average of 41 million reads. FastQC (version 0.11.4; Babraham Institute, Cambridge, UK) was used for sequencing quality assessment and FASTX-Toolkit (version 0.0.14; http://hannonlab.cshl.edu/fastx_toolkit/) was used to trim the first 10 bp from both reads. The trimmed reads were then aligned to the pooled transcriptome of the human (hg19) and rat (rn5) using Bowtie (version 2.2.7; https://sourceforge.net/projects/bowtie-bio/files/bowtie2/2.2.7/) with splice junctions being defined in GTF file for both genomes (obtained from UCSC). To calculate the total number of human reads in each sample we took the number of human reads in the fraction of sample that was sequenced and extrapolated that using the total amount of RNA extracted, see Table S1, and the known fraction of RNA, cDNA-, fragmented-, and bar-coded libraries taken for sequencing.
Gene level expression was determined by calculating fragments per kilo base per million mapped fragments (FPKM) as well as raw count using RNA-Seq by Expectation Maximization7 (RSEM; version 1.2.30) with a human-rat pooled reference transcriptome. To test the RSEM accuracy of interspecies mapping (rat and human), TRAP-seq with RSEM analysis was run on samples that contained only human RNA i.e., hNSCs in culture, or only rat RNA i.e., brain samples from stroke-injured rats that received vehicle transplants.
Differently expressed genes (DEGs) with fold change were further calculated by DESeq2 version 1.10.1 (https://bioconductor.org/packages/release/bioc/html/DESeq2.html). DEGs were considered statistically significant using a false discovery rate (FDR) threshold of 10%, i.e., q-value < 0.1. Host or graft genes with an average FPKM<1 in both stroke and naïve groups were excluded from the analysis. Principal component analysis (PCA) was conducted using normalized counts.
To identify enriched Gene Ontology (GO) terms, we used the limma goana package32 for gene enrichment analysis. For all enrichment analyses, DEGs (as defined above) were used as target genes, and all genes listed in their respective transcriptomes (hg19 or rn5), with FPKM≥1 in at least one group being tested, were used as background. Furthermore, to take advantage of the more highly curated human databases, all rat genes were converted to their human homologues prior to enrichment analysis. Over-represented biological processes were included with annotation p-value threshold < 0.05. Cytoscape 3.8.0. with ClueGO (v2.5.7) plus Clupedia (v1.5.7) plugins31 was used to visualize clustering of the generated GO terms; GO terms are clustered based on gene overlap between GO terms. For Figures 1H and 1J, the clusters were used to help identify representative GO terms to include in the graphs.
Ingenuity Pathway Analysis (IPA; QIAGEN Inc., https://www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis) was used to identify genes encoding secreted proteins (Location: Extracellular Space), Upstream Regulators (URs; p < 0.05), canonical pathways (p<0.05), and to link graft and host genes with each other and to their known molecular functions (using the Pathway Explorer tool). STRING (https://string-db.org) was also used to assess the association activity between genes.
Quantitative PCR
Gene expression was assessed by quantitative PCR using Taqman Gene Expression Assays (Applied Biosystems, Foster City, CA). For full primer details see key resources table and Table S3. Human housekeeping and target genes primers used in TaqMan assay: glyceraldehyde dehydrogenase (GAPDH, Hs03929097_g1 and Hs99999905_m1); beta-2-microglobulin (B2M, Hs00984230_m1); beta actin (ACTB, Hs03023943_g1); ubiquitin C (UBC, Hs00824723_m1); Transcription factor E2F2 (E2F2, Hs00918090_m1); ETS variant transcription factor 5 (ETV5, Hs00927557_m1); FOS Like 1, AP-1 Transcription Factor Subunit (FOSL1, Hs00759776_s1); Inhibitor Of DNA Binding 3, HLH Protein (ID3, Hs00171409_m1); caspase 8 (CASP8, Hs01018151_m1); Bcl2 associated X (BAX, Hs00180269_m1); Bcl-2 Interacting mediator of cell death (BCL2L11, Hs00197982_m1); interferon regulatory factor 3 (IRF3, Hs01547282_m1); thioredoxin interacting protein (TXNIP, Hs01006900_g1); C-C Motif chemokine ligand 2 (CCL2, Hs00234140_m1) and noggin (NOG, sense: CCTGGAGTAATTTCGGATG; anti-sense: GGAAGAAAGGCACACAAG; anti-sense (TaqMan probe): ACTCCTCTCCCGGGTCTACT). Rat housekeeping and target genes used in TaqMan assay: glyceraldehyde dehydrogenase (Gapdh, Rn01775763_g1), beta-2-microglobulin (B2m, Rn00560865_m1); beta actin (Actb, Rn01412977_g1); Small nuclear ribonucleoprotein Sm D3 (Snrp3, Rn01428248_g1); bone morphogenic protein 6 (Bmp6, Rn00432095_m1); caspase 8 (Casp8, Rn00574069_m1); Bcl2 associated X (Bax, Rn02532082_g1); Bcl-2 Interacting mediator of cell death (Bcl2l11, Rn00674175_m1); interferon regulatory factor 3 (Irf3, Rn01764369_m1); thioredoxin interacting protein (Txnip, Rn06170057_s1); C-C Motif chemokine ligand 2 (Ccl2, Rn00580555_m1). Samples were run in duplicate using 50 ng of cDNA per reaction. qPCR data was analyzed using ddCt method.39 For qPCR validation of reciprocal regulation of death genes in graft vs host, fragmented cDNA (made during the preparation of the cDNA libraries for RNAseq) was used at a 1:100 in dilution; the qPCR data was analyzed using Pfaffl method40 and expressed as log2FC values. Negative control reactions for each assay (i.e., cDNA template excluded) produced no detectable signal within 45 cycles of amplification.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse anti-GFP 19C8 | Memorial Sloan-Kettering Monoclonal Antibody Facility | Htz-GFP-19C8; RRID: AB_2716737 |
| Mouse anti-GFP 19F7 | Memorial Sloan-Kettering Monoclonal Antibody Facility | Htz-GFP-19F7; RRID: AB_2716736 |
| Rabbit anti-GFP | Abcam | Cat#ab6556; RRID: AB_305564 |
| Mouse anti-Human Nuclei | Millipore | Cat#MAB1281; RRID: AB_94090 |
| Rabbit anti-vimentin | Cell Signaling | Cat#5741; RRID: AB_10695459 |
| Rabbit anti-mcherry | Abcam | Cat#ab167453; RRID: AB_2571870 |
| Mouse anti-hNestin | Abcam | Cat#ab18102; RRID: AB_444246 |
| Rabbit anti-Sox2 | Cell Signaling | Cat#2748; RRID: AB_823640 |
| Guinea pig anti-SLC1a3 | Millipore | Cat#AB1783; RRID: AB_90949 |
| Chiken anti-Iba1 | Abcam | Cat#ab139590; RRID: AB_2728648 |
| Rabbit anti-GFAP | Dako | Cat#Z0334; RRID: AB_10013382 |
| Rabbit anti-NeuN | Abcam | Cat#ab177487; RRID: AB_2532109 |
| Rabbit anti-phospho SMAD1/SMAD5/SMAD8 | Millipore | Cat#ab3848-I; RRID: AB_177439 |
| Mouse anti-SMAD1 | Santa Cruz | Cat#sc7965; RRID: AB_628261 |
| Rabbit anti-p-STAT3 | Cell Signaling | Cat#9131; RRID: AB_331586 |
| Mouse STAT3 | Santa Cruz | Cat#sc8019; RRID: AB_628293 |
| Goat Alexa-488 anti-mouse | Invitrogen | Cat#A11001; RRID: AB_2534069 |
| Goat Alexa-488 anti-rabbit | Invitrogen | Cat#A11034; RRID: AB_2576217 |
| Goat Alexa-546 anti-rabbit | Invitrogen | Cat#A11035; RRID: AB_143051 |
| Goat Alexa-647 anti-mouse | Invitrogen | Cat#A21236; RRID: AB_141725 |
| Goat Alexa-546 anti-Guinea pig | Invitrogen | Cat#A11074; RRID: AB_2534118 |
| Anti-rabbit IgG HRP linked | Cell Signaling | Cat#7074; RRID: AB_2099233 |
| Anti-mouse IgG HRP linked | Cell Signaling | Cat#7076; RRID: AB_330924 |
| β-Actin (8H10D10) Mouse mAb (HRP Conjugate) | Cell Signaling | Cat#12262; RRID: AB_2566811 |
| β-Actin (13E5) Rabbit mAb (HRP Conjugate) | Cell Signaling | Cat#5125; RRID: AB_1903890 |
| Rabbit anti-phospho SMAD1/SMAD5/SMAD9 | Cell Signaling | Cat#13820; RRID: AB_2493181 |
| Rabbit anti-ID3 | Cell Signaling | Cat#9837; RRID: AB_2732885 |
| Mouse anti-BMP6 | BMA Biomedicals | Cat#T-3206, RRID: AB_1227246 |
| Goat anti-rabbit IgG (Biotin conjugate) | Invitrogen | Cat#31820, RRID: AB_228340 |
| Goat Alexa-546 anti-chicken | Invitrogen | Cat# A-11040, RRID: AB_2534097 |
| Goat Alexa-647 anti-rabbit | Invitrogen | Cat# A-21245, RRID: AB_2535813 |
|
Recombinant DNA | ||
| pLEX-MCS vector | Thermo Scientific Open Biosystem | #OHS4735 |
| LV-GFP plasmid | Beronja et al.28 | Addgene; Plasmid #25999 |
| C2-eGFP-L10a plasmid | Gift from Dr. Nathaniel Heintz, Rockefeller University, USA | N/A |
| lentiviral vector FUGW | Gift from Dr. Craig Garner, Stanford University, USA | N/A |
|
Chemicals, peptides, and recombinant proteins | ||
| Recombinant Human BMP-6 | PeproTech | Cat#120-06 |
| Recombinant Human TGFb1 | R&D System | Cat#240-B |
| Recombinant human EGF | Invitrogen | Cat#PH60311 |
| Recombinant human LIF | Millipore | Cat#LIF1010 |
| B27 supplement without vitamin A (50x) | Gibco | Cat#12587-010 |
| B27 supplement (50x) | Gibco | Cat#17504-040 |
| N2 supplement (50x) | Gibco | Cat#17502-048 |
| Non-tissue culture treat plate, 6 well | Falcon | Cat#351146 |
| Stemline™ Neural Stem Cell Expansion Medium | Sigma | Cat#S3194 |
| Recovery Cell Culture Freezing Medium | Gibco | Cat#12648-010 |
| TryPLE | Gibco | Cat#12563-029 |
| Neurobasal Medium | Gibco | Cat#21103-049 |
| BD Matrigel Matrix | BD Biosciences | Cat#356234 |
| Leibovitz 15 Media | Gibco | Cat#21083027 |
| Normal Goat Serum | Jackson Immunoresearch | Cat#005-000-121 |
| Normal Donkey Serum | Jackson Immunoresearch | Cat#017-000-121 |
| Hoechst 33342 | Thermo Fisher Scientific | Cat# H3570 |
| Fluoromount-G | SouthernBiotech | Cat#0100-01 |
| Complete Protease Inhibitors Tablet, EDTA-free | Roche | Cat#11873580001 |
| Cyclohexamide, power | Sigma | Cat#0110-1G |
| Rnasin, recombinant | Promega | Cat#N2511 |
| 10% NP-40 | Thermo Fisher Scientific | Cat#28324 |
| DHPC | Avanti Polar Lipids | Cat#850306P |
| Antigen retrieval solution | BD Pharmingen | Cat# 550524 |
| Richard-Allan Scientific Cytoseal XYL | Thermo Fisher Scientific | Cat#8312-4 |
| DynaMag™-2 Magnet | Invitrogen | Cat# 12321D |
| N-PER Neuronal Protein Extraction Reagent | Thermo Fisher Scientific | Cat#87792 |
| Halt protease and phosphatase inhibitor cocktail | Thermo Fisher Scientific | Cat#78440 |
| 12% Mini-PROTEAN TGX Stain-Free Precast Gels | Bio-Rad | Cat#4561046 |
| 4x Laemmli Sample Buffer | Bio-Rad | Cat#1610747 |
| SuperBlock (TBS) Blocking Buffer | Thermo Fisher Scientific | Cat# 37581 |
| Tween-20 | Bio-Rad | Cat#170-6531 |
| Midi format, 0.2μm nitrocellulose, single application | Bio-Rad | Cat#1704159 |
| Restore™ PLUS Western Blot Stripping Buffer | Thermo Fisher Scientific | Cat# 46430 |
| Falcon Cell Strainers | Corning Life Science | Cat# 352350 |
| Streptavidin, Alexa Fluor 546 conjugate | Invitrogen | Cat# S11225 |
| Antibody diluent / block | Akoya Biosciences | Cat# SKU ARD1001EA |
| RNAlater™ Stabilization Solution | Thermo Fisher Scientific | Cat# AM7020 |
| Streptavidin, Alexa Fluor 647 conjugate | Invitrogen | Cat# S32357 |
|
Critical commercial assays | ||
| Vectastain Universal Elite ABC Kit | Vector Laboratories | Cat#PK-6200 |
| DAB Peroxidase HRP Substrate kit | Vector Laboratories | Cat#SK-4100 |
| Dynabeads Protein G | ThermoFisher Scientific | Cat# 10003D |
| MinElute Reaction Cleanup Kit | QIAGEN | Cat# 28204 |
| Rneasy Plus Mini kit | QIAGEN | Cat#74104 |
| Rneasy Plus Micro kit | QIAGEN | Cat#74034 |
| Human NOG / Noggin ELISA Kit | LifeSpan BioScience | LS-F12164 |
| Bradford assay | Bio-Rad | Cat#5000006 |
| Clarity ECL kit | Bio-Rad | Cat# #1705060 |
| LIVE/DEAD™ Fixable Aqua Dead Cell Stain Kit | ThermoFisher Scientific | Cat#L34957 |
| TaqMan™Gene Expression Master Mix | Applied Biosystems | Cat# 4369016 |
| High-Capacity RNA-to-cDNA™ | Kit Applied Biosystems | Cat# 4387406 |
| Ovation RNA-seq system V2 | NuGEN Technologies, Inc; sold by Tecan | Cat # 7102 |
| Ovation Ultralow System V2 | NuGEN Technologies, Inc; sold by Tecan | Cat# 0344NB |
| RiboMinus Eukaryote Kit for RNA-Seq | Invitrogen/ThermoFisher Scientific | Cat# A1083708 |
|
Deposited data | ||
| Raw and analyzed data | This paper | GEO: GSE150710 |
|
Experimental models: Organisms/strains | ||
| NCI Cr:NIH-RNU Rats | NCI Frederick | NIH-RNU Straincode#568 |
|
Oligonucleotides | ||
| BA-Hs-NOG-noX-Rn-2zz-st | Advanced Cell Diagnostics, Inc | Customized-709631 |
| BA-Rn-Bmp6-No-XHs-3zz-st | Advanced Cell Diagnostics, Inc | Customized-848911 |
| ACTB | Thermo Fisher Scientific | Hs03023943_g1 |
| NOG | Thermo Fisher Scientific | Customized-AP7DP33 Sense: CCTGGAGTAATTTCGGATG Anti-sense: GGAAGAAAGGCACACAAG Anti-sense (TaqMan sequence): ACTCCTCTCCCGGGTCTACT |
| Tgln3 | Thermo Fisher Scientific | Rn00581563_m1 |
| Angptl2 | Thermo Fisher Scientific | Rn07313407_m1 |
| Adamts12 | Thermo Fisher Scientific | Rn04418968_m1 |
| Dspp | Thermo Fisher Scientific | Rn02132391_s1 |
| GAPDH-Sybr green | Elim Biopharmaceuticals Inc | Reverse primer GTCAAAGGTGGAGGAGTGG GRn04418968_m1 Forward primer GCATCCTGGGCTACACTGAG |
| NOG-Sybr | Elim Biopharmaceuticals Inc | green Reverse primer CATGAAGCCTGGGTCGTAGT Forward primer CCTCATCGAACACCCAGAC |
| N/A | For additional Oligonucleotides, see Table S3 | |
|
Software and algorithms | ||
| GraphPad Prism v 8.4 | GraphPad Software, LLC | N/A |
| Fiji v 2.0.0 | Schneider et al.29 | N/A |
| StarDist plugin for Fiji-ImageJ | Schmidt et al.30 | N/A |
| RSEM v 1.2.30 | Li and Dewey7 | N/A |
| Cytoscape (v3.8.0) with ClueGO (v2.5.7) plus Clupedia (v1.5.7) plugins | Bindea et al.31 | N/A |
| limma goana R package | Young et al.32 | N/A |
| QIAGEN IPA | QIAGEN | N/A |
| FastQC (v0.11.4) | https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ | N/A |
| Bowtie (v 2.2.7) | https://sourceforge.net/projects/bowtie-bio/files/bowtie2/2.2.7/ | N/A |
| DESeq2 (v 1.10.1) | https://bioconductor.org/packages/release/bioc/html/DESeq2.html | N/A |
| FASTX-Toolkit (v 0.0.14) | http://hannonlab.cshl.edu/fastx_toolkit/ | N/A |
| STRING (v10.5) | https://string-db.org | N/A |
| FlowJo software v9.6.2 | Tree Star | N/A |
ELISA
Noggin levels in the supernatant of hNSC culture were determined with a sandwich ELISA kit following the manufacture’s protocols (LifeSpan BioSciences, Inc., Seattle, WA). Briefly, supernatants of hNSC treated with recombinant protein BMP6 (20 ng/ml, Peprotech) or vehicle control were collected and centrifuged for 20 minutes at 1000xg. On the ELISA plate, 100 μL of supernatant was incubated for 2 hours at 37°C. The liquid in each well was removed and 100 μL of Detection Reagent A was added. After incubation for 1 hour at 37°C, samples were washed 3 times and 100 μL of Detection Reagent B were added followed by incubation for 1 hour at 37°C. The samples were washed 5 times and 90 μL of TMB Substrate were added. After incubation for 10 minutes at 37°C 50 μL, optical density (OD value) was determined using a microplate reader set to 450nm.
Immunoblotting assay
hNSC were lysed in N-PER Neuronal Protein Extraction Reagent (Thermo Fisher Scientific) supplemented with 1:100 of Halt protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific) on ice for 5 min and centrifuged at 14000 rpm for 15 min at 4°C. The supernatant protein concentrations were measured by the Bradford assay (Bio-Rad Laboratories, Hercules, CA) and 5 μg of total protein was loaded in 12% Mini-PROTEAN TGX Stain-Free Precast Gels (Bio-Rad) and transferred onto 0.2μm nitrocellulose membranes (Bio-Rad). Membranes were blocked with SuperBlock Blocking Buffer (Thermo Scientific) for 10 min, and then incubated overnight with primary antibody in 0.1% Tween-20 in TBS (TBS-T) at 4°C with continuous shaking. Primary antibodies were directed against phosphorylated SMAD1/SMAD5/SMAD8 (1:500; EMD Millipore, Burlington, MA) and phospho-STAT3 (1:1000; Cell Signaling Technology, Danvers, MA). Total relative protein expression was normalized by SMAD1 (1:1000, Santa Cruz Biotechnology, Inc., Dallas, TX) and/or STAT3 (1:1000; Santa Cruz Biotechnology, Inc.). Following three washes with TBS-T, blots were incubated at room temperature for 1 h with secondary HRP-linked anti-rabbit IgG (1:1000; Cell Signaling Technology) or HRP-linked anti-mouse (1:1000; Cell Signaling Technology) in TBS-T. After each round of chemiluminescence detection, nitrocellulose membranes were washed three times with TBS-T and antibody stripped with Restore Western Blot Stripping Buffer (Thermo Fisher Scientific) at room temperature for 10 min. This was followed with 5 min TBS-T wash and SuperBlock Blocking Buffer (Thermo Fisher Scientific) incubation for 10 min at room temperature. The complex was revealed by chemiluminescence system (Clarity ECL kit; Bio-Rad). The protein loading was verified by the expression of β-actin (1:1000; Cell Signaling Technology). Measurement of protein phosphorylation was performed by Fiji-ImageJ software (NIH).29
Immunohistochemistry
Animals were euthanized 7 days post-transplantation and fixed by transcardial perfusion with PBS, followed by 4% paraformaldehyde on ice. Brains were removed, immersed in 20% sucrose in 4% PFA overnight at 4°C and then cryopreserved at −80°C. Coronal sectioning at 40 μm was performed in a cryostat (Leica) and sections were preserved at −20°C in anti-freezing buffer (30% ethylene glycol and 30% of glycerol in PBS pH 7.4). For all staining except pSMAD, ID3 and BMP6 (see below), sections were mounted on superfrost plus slides (Thermo Fisher Scientific), and samples were blocked with 5% normal goat serum (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) in PBS with 0.3% Triton X-100 (Sigma-Aldrich) for 30 min at room temperature (RT). The primary antibodies used were: rabbit anti-GFP (1:500; Abcam, Cambridge, UK), mouse anti-Human nuclei (1:100; MilliporeSigma, Burlington, MA), rabbit anti-Vimentin (1:100; Cell Signaling), rabbit anti-mCherry (1:500; Abcam), mouse anti-human-Nestin (1:100; Abcam), rabbit anti-SOX2 (1:100; Cell Signaling), guinea pig anti-SLC1a3 (1:100; Millipore), rabbit anti-GFAP (1:1000; DAKO), rabbit anti-NeuN (1:250; Abcam), and chicken anti-IBA1 (1:500; Abcam). The slices were washed 3x with PBS with 0.3% Triton X-100 (Sigma) and incubated with respective secondary antibodies overnight at 4°C. The secondary antibodies used were Alexa Fluor 488-conjugated goat anti-rabbit (1:1000; Invitrogen), Alexa Fluor 488-conjugated goat anti-mouse (1:1000; Invitrogen), Alexa Fluor 647-conjugated goat anti-mouse (1:1000; Invitrogen), Alexa Fluor 546-conjugated goat anti-guinea pig (1:1000; Invitrogen), Alexa Fluor 546-conjugated goat anti-rabbit (1:1000; Invitrogen), Cy3-conjugated goat anti-rabbit (1:1,000; Jackson Immunoresearch Laboratories, Inc.). Followed by 3x washes with PBS with 0.3% Triton X-100 for 5 min each, nuclei were labeled with Hoechst 33342 (Thermo Fisher Scientific) for 5 min and then were mounted with Fluoromount-G (SouthernBiotech, Birmingham, AL).
Phospho-SMAD, ID3 and BMP6 immunostaining was performed on floating sections. Sections were washed with TBS in a 12-well plate and antigen retrieval was done by incubating the sections with pre-heated (83°C) antigen retrieval solution (BD Biosciences Pharmingen, San Jose, CA) for 20 minutes at 63°C followed by 3x washing with distilled water. The sections were then incubated with blocking buffer (Akoya Biosciences) for 1 hour at room temperature followed by overnight incubation with primary antibodies at 4°C. Primary antibodies used: rabbit anti-phospho-SMAD1/SMAD5/SMAD9 (1:50; Cell signaling), rabbit anti-ID3 (1:500; Cell signaling), mouse anti-BMP6 (1:20; BMA Biomedicals), chicken anti-IBA1 (1:250; Abcam), rabbit anti-GFAP (1:2000; DAKO) and mouse anti-Human nuclei (1:100; MilliporeSigma, Burlington, MA). Next day, the sections were washed 3× 5 minutes with TBS-T and incubated with respective secondary antibodies for 2h at room temperature. The secondary antibodies used were: Alexa Fluor 488-conjugated goat anti-mouse (1:1000; Invitrogen) Alexa Fluor 546-conjugated goat anti-chicken (1:1000; Invitrogen), Alexa Fluor 647-conjugated goat anti-rabbit (1:1000; Invitrogen), biotin conjugated goat anti-rabbit IgG (1:1000; Invitrogen) followed by streptavidin conjugated Alexa Fluor 647 (1:100; Invitrogen) or streptavidin conjugated Alexa Fluor 546 (1:100; Invitrogen). This was followed by 3× 5 minutes washing with TBS-T and labelling of nuclei with Hoechst 33342 (Thermo Fisher Scientific) incubation for 5 min before mounting on superfrost plus slides with Fluoromount-G (SouthernBiotech, Birmingham, AL). Fluorescence images were recorded using a digital camera attached to confocal microscope (LSM 800, Zeiss, Germany). Image processing were performed by Fiji-ImageJ software (NIH).29 StarDist plugin for Fiji-ImageJ was applied with the default fluorescent nuclei model to perform nuclei segmentation for cell counting.30 Antibody specificity and tissue background were tested by using only secondary antibodies for each slice stained.
Cell survival analysis
To quantify hNSC survival in naive and stroke host tissue, we used anti-human nuclei and anti-GFP labeling and colorimetric development with the Vectastain Universal Elite ABC Kit and Vector’s DAB Substrate Kit (Vector Laboratories Inc., Burlingame, CA). A series of brain sections (40 μm), each 200 mm apart, were mounted onto Superfrost slides antigen retrieval performed according to the manufacturing instructions (BD Biosciences Pharmingen, San Jose, CA). Briefly, the antigen retrieval solution was pre-heated in a water bath to 89°C and the slices incubated for 10 minutes, then rinsed with distilled water. To quench the endogenous peroxidase, brain slices were incubated with 0.3% hydrogen peroxide solution in PBS supplemented with 0.3% horse serum for 5 minutes at room temperature and then washed 1x in PBS. The sections were incubated with 5% normal donkey serum (Jackson Immunoresearch) in PBS with 0.3% Triton X-100 (Sigma-Aldrich) for 30 minutes at room temperature, followed by incubation overnight at 4°C with the following primary antibodies: mouse anti-Human nuclei (1:100; Millipore) and rabbit anti-GFP (1:500; Abcam). The slices were washed in 3x PBS in 0.3% Triton X-100 (Sigma-Aldrich), then incubated with biotinylated universal secondary antibody for 2 hours at room temperature. The slices were washed 3x in PBS with 0.3% Triton X-100 (Sigma-Aldrich) and then incubated in the ABC reagent for 30 minutes at room temperature. After 3x washes in PBS with 0.3% Triton X-100 (Sigma-Aldrich), the staining was developed with DAB-peroxidase kit for 7 minutes and then deactivated with distilled water. After fully drying, the sections were mounted in Cytoseal XYL. Images were acquired using a digital camera attached to inverted microscope (Axio Observer. A1, Zeiss, Oberkochen, Germany). Image J was used to count hNSC number in the acquired images, in a blinded manner.
In situ hybridization
Animals were euthanized and perfused as described in the previous section (Immunohistochemistry). Coronal sections (20 μm) were cut using a cryostat (Leica Biosystems, Wetzlar, Germany) and kept in RNAase-free water. Sections containing grafted hNSC were identified by detection of the mCherry fluorescent protein using an inverted epifluorescent microscope (Axio Observer. A1, Zeiss). The sections were mounted onto Superfrost plus slides (Thermo Fisher Scientific) and stored at −80°C. In situ hybridization was performed using BaseScope™ assay red (ACD Bio, Newark, CA) and following the manufacture’s protocols. Briefly, sections were pre-treated by baking at 60°C for 30 min and incubated for 20 min with hydrogen peroxide at room temperature (RT). Target retrieval was performed at 95°C for 5 min in RNAscope Target Retrieval solution, rinsed with water and incubated for 3 min with 100% of ethanol at room temperature. After drying, sections were incubated with Proteinase Plus at 40°C for 30 min and immediately used in the BaseScope assay according to the manufacturer’s protocols. NOG (BA-Hs-NOG-noX-Rn-2zz-st) and Bmp6 (BA-Rn-Bmp6-No-Hs-3zz-st) probes were designed for the BaseScope assay to detect human specific and rat specific transcript, respectively. After the last step for signal detection, sections were incubated with normal goat serum (Jackson Immunoresearch) for 30 min at room temperature and immunohistochemistry was performed as described in the Immunohistochemisrty section. Positive and negative probes were used to test ISH specificity.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analyses for the RNAseq data are given in the ‘RSEM and Bioinformatic Analyses’ section above. All other statistical analyses, detailed in the figure legends, were performed using GraphPad Prism version 8.4 (GraphPad Software, San Diego, California USA, www.graphpad.com). Shapiro-Wilk test was used to test the normality (alpha = 0.05). The significance of differences between two groups was calculated by unpaired Student’s t-test or Mann Whitney test as indicated in the figure legends. Comparison of multiple groups used analysis of variance (ANOVA) comparison and Tukey post hoc tests, except analysis of the vibrissae-evoked forelimb placing which used two-way ANOVA-Sidak. Correlation analysis was evaluated by Pearson r with 95% confidence interval, with the Benjamini-Hochberg Procedure used to adjust for multiple testing. All tests are two-tailed unless otherwise stated in the figure legends. Graphs are shown as mean with SEM unless otherwise indicated.
Supplementary Material
Highlights.
Graft hNSCs and host transcriptomes simultaneously identified using TRAP-seq
Host microenvironment modulates the graft secretome
Interactome analyses between host/graft transcriptomes predict molecular crosstalk
BMP6-noggin is a potential host-graft crosstalk pathway for stroke brain recovery
ACKNOWLEDGMENTS
We wish to sincerely thank Clive Svendsen for his G010 cell line; Drs. Nathaniel Heintz and Craig Garner for their help with the TRAP lentiviral vector; Dr. Guohua Sun for stroke surgeries; Javi Fernandez-Alcudia and Stanford Neuroscience Gene Vector and Virus Core for lentivirus production; Marten Wing and Andrew Spencley for bioinformatic analysis advice; Lina Jansson for ISH troubleshooting; Michelle Cheng for critical reading of the manuscript and scientific discussion; Gabriela Bindea for ClueGo advice; Drs. An Zwijsen and Danny Huylebroeck for pSMAD signaling advice; and Christine Plant for editing and formatting the manuscript. This work utilized computing resources provided by the Stanford Genetics Bioinformatics Service Center. The graphical abstract was created with Biorender.com. This work was funded by a National Institute of Health (NIH) grant (R01 NS058784) and a California Institute for Regenerative Medicine (CIRM) grant (RB5-07363) to G.K.S. Data availability: GEO: GSE150710.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2023.112353.
DECLARATION OF INTERESTS
The authors declare no competing interests.
INCLUSION AND DIVERSITY
We support inclusive, diverse, and equitable conduct of research.
REFERENCES
- 1.Trounson A, and McDonald C (2015). Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell 17, 11–22. 10.016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Coburn ML, Billigen JB, Kim AS, Johnson JN, Bates D, King B, et al. (2016). Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: a phase 1/2a study. Stroke 47, 1817–1824. 10.1161/STROKEAHA.116.012995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heiman M, Schaefer A, Gong S, Peterson JD, Day M, Ramsey KE, Suárez-Fariñas M, Schwarz C, Stephan DA, Surmeier DJ, et al. (2008). A translational profiling approach for the molecular characterization of CNS cell types. Cell 135, 738–748. 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andres RH, Horie N, Slikker W, Keren-Gill H, Zhan K, Sun G, Manley NC, Pereira MP, Sheikh LA, McMillan EL, et al. (2011). Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain 134, 1777–1789. 10.1093/brain/awr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horie N, Pereira MP, Niizuma K, Sun G, Keren-Gill H, Encarnacion A, Shamloo M, Hamilton SA, Jiang K, Huhn S, et al. (2011). Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells 29, 274–285. 10.1002/stem.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okaty BW, Sugino K, and Nelson SB (2011). Cell type-specific transcriptomics in the brain. J. Neurosci 31, 6939–6943. 10.1523/JNEUROSCI.0626-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li B, and Dewey CN (2011). RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinf 12, 323. 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avraham R, Haseley N, Brown D, Penaranda C, Jijon HB, Trombetta JJ, Satija R, Shalek AK, Xavier RJ, Regev A, and Hung DT (2015). Pathogen cell-to-cell variability drives heterogeneity in host immune responses. Cell 162, 1309–1321. 10.1016/j.cell.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George PM, and Steinberg GK (2015). Novel stroke therapeutics: unraveling stroke pathophysiology and its impact on clinical treatments. Neuron 87, 297–309. 10.1016/j.neuron.2015.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hallmann AL, Araúzo-Bravo MJ, Zerfass C, Senner V, Ehrlich M, Psathaki OE, Han DW, Tapia N, Zaehres H, Schöler HR, et al. (2016). Comparative transcriptome analysis in induced neural stem cells reveals defined neural cell identities in vitro and after transplantation into the adult rodent brain. Stem Cell Res 16, 776–781. 10.1016/j.scr.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Kumamaru H, Ohkawa Y, Saiwai H, Yamada H, Kubota K, Kobayakawa K, Akashi K, Okano H, Iwamoto Y, and Okada S (2012). Direct isolation and RNA-seq reveal environment-dependent properties of engrafted neural stem/progenitor cells. Nat. Commun 3, 1140. 10.1038/ncomms2132. [DOI] [PubMed] [Google Scholar]
- 12.O’Shea TM, Ao Y, Wang S, Wollenberg AL, Kim JH, Ramos Espinoza RA, Czechanski A, Reinholdt LG, Deming TJ, and Sofroniew MV (2022). Lesion environments direct transplanted neural progenitors towards a wound repair astroglial phenotype in mice. Nat. Commun 13, 5702. 10.1038/s41467-022-33382-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krämer A, Green J, Pollard J Jr., and Tugendreich S (2014). Causal analysis approaches in ingenuity pathway analysis. Bioinformatics 30, 523–530. 10.1093/bioinformatics/btt703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Z, Trautmann K, Artelt M, Burnet M, and Schluesener HJ (2006). Bone morphogenetic protein-6 is expressed early by activated astrocytes in lesions of rat traumatic brain injury. Neuroscience 138, 47–53. 10.1016/j.neuroscience.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 15.Wrighton KH, Lin X, and Feng XH (2009). Phospho-control of TGF-beta superfamily signaling. Cell Res 19, 8–20. 10.1038/cr.2008.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyazono K, and Miyazawa K (2002). Id: a target of BMP signaling. Sci. STKE 2002, pe40. 10.1126/stke.2002.151.pe40. [DOI] [PubMed] [Google Scholar]
- 17.Martino G, and Pluchino S (2006). The therapeutic potential of neural stem cells. Nat. Rev. Neurosci 7, 395–406. 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- 18.Carmichael ST (2016). The 3 Rs of stroke biology: radial, relayed, and regenerative. Neurotherapeutics 13, 348–359. 10.1007/s13311-015-0408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee ST, Chu K, Jung KH, Kim SJ, Kim DH, Kang KM, Hong NH, Kim JH, Ban JJ, Park HK, et al. (2008). Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain 131, 616–629. 10.1093/brain/awm306. [DOI] [PubMed] [Google Scholar]
- 20.Shin JA, Lim SM, Jeong SI, Kang JL, and Park EM (2014). Noggin improves ischemic brain tissue repair and promotes alternative activation of microglia in mice. Brain Behav. Immun 40, 143–154. 10.1016/j.bbi.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Sozmen EG, DiTullio DJ, Rosenzweig S, Hinman JD, Bridges SP, Marin MA, Kawaguchi R, Coppola G, and Carmichael ST (2019). White matter stroke induces a unique oligo-astrocyte niche that inhibits recovery. J. Neurosci 39, 9343–9359. 10.1523/JNEUROSCI.0103-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brazil DP, Church RH, Surae S, Godson C, and Martin F (2015). BMP signalling: agony and antagony in the family. Trends Cell Biol 25, 249–264. 10.1016/j.tcb.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Tassew NG, Mothe AJ, Shabanzadeh AP, Banerjee P, Koeberle PD, Bremner R, Tator CH, and Monnier PP (2014). Modifying lipid rafts promotes regeneration and functional recovery. Cell Rep 8, 1146–1159. 10.1016/j.celrep.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Lim DA, Tramontin AD, Trevejo JM, Herrera DG, García-Verdugo JM, and Alvarez-Buylla A (2000). Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron 28, 713–726. 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 25.Khazaei M, Ahuja CS, Nakashima H, Nagoshi N, Li L, Wang J, Chio J, Badner A, Seligman D, Ichise A, et al. (2020). GDNF rescues the fate of neural progenitor grafts by attenuating Notch signals in the injured spinal cord in rodents. Sci. Transl. Med 12, eaau3538. 10.1126/scitranslmed.aau3538. [DOI] [PubMed] [Google Scholar]
- 26.Anthony S, Cabantan D, Monsour M, and Borlongan CV (2022). Neuroinflammation, stem cells, and stroke. Stroke 53, 1460–1472. 10.1161/STROKEAHA.121.036948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.George PM, Oh B, Dewi R, Hua T, Cai L, Levinson A, Liang X, Krajina BA, Bliss TM, Heilshorn SC, and Steinberg GK (2018). Engineered stem cell mimics to enhance stroke recovery. Biomaterials 178, 63–72. 10.1016/j.biomaterials.2018.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beronja S, Livshits G, Williams S, and Fuchs E (2010). Rapid functional dissection of genetic networks via tissue-specific transduction and RNAi in mouse embryos. Nat. Med 16, 821–827. 10.1038/nm.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider CA, Rasband WS, and Eliceiri KW (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt U, Weigert M, Broaddus C, and Myers G (2018). Cell Detection with Star-Convex Polygons. In Medical Image Computing and Computer Assisted Intervention – MICCAI 2018. MICCAI 2018. Lecture Notes in Computer Science, vol 11071. (Springer, Cham: Springer International Publishing; ), pp. 265–273. 10.1007/978-3-030-00934-2_30. [DOI] [Google Scholar]
- 31.Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, Kirilovsky A, Fridman WH, Pagès F, Trajanoski Z, and Galon J (2009). ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 25, 1091–1093. 10.1093/bioinformatics/btp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young MD, Wakefield MJ, Smyth GK, and Oshlack A (2010). Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol 11, R14. 10.1186/gb-2010-11-2-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edgar R, Domrachev M, and Lash AE (2002). Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30, 207–210. 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, Masel J, Yenari MA, Weissman IL, Uchida N, et al. (2004). Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc. Natl. Acad. Sci. USA 101, 11839–11844. 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Svendsen CN, ter Borg MG, Armstrong RJ, Rosser AE, Chandran S, Ostenfeld T, and Caldwell MA (1998). A new method for the rapid and long term growth of human neural precursor cells. J. Neurosci. Methods 85, 141–152. 10.1016/s0165-0270(98)00126-5. [DOI] [PubMed] [Google Scholar]
- 36.Nelson AD, Suzuki M, and Svendsen CN (2008). A high concentration of epidermal growth factor increases the growth and survival of neurogenic radial glial cells within human neurosphere cultures. Stem Cell 26, 348–355. 10.1634/stemcells.2007-0299. [DOI] [PubMed] [Google Scholar]
- 37.Woodlee MT, Asseo-Garćıa AM, Zhao X, Liu SJ, Jones TA, and Schallert T (2005). Testing forelimb placing “across the midline” reveals distinct, lesion-dependent patterns of recovery in rats. Exp. Neurol 191, 310–317. 10.1016/j.expneurol.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Heiman M, Kulicke R, Fenster RJ, Greengard P, and Heintz N (2014). Cell type-specific mRNA purification by translating ribosome affinity purification (TRAP). Nat. Protoc 9, 1282–1291. 10.1038/nprot.2014.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Livak KJ, and Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Pfaffl MW (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29, e45. 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNAseq data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus33 and are accessible through GEO Series accession number GSE150710: (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE150710). Other primary data, such as Fastqc files, raw PCR data, western blot data, or microscopy images, are available from the authors upon reasonable request.
This paper does not report original code.
Any additional information required, related to this paper, is available from the lead contact upon request.


